Neuronal Cell Differentiation of iPSCs for the Clinical Treatment of Neurological Diseases
Abstract
1. Introduction
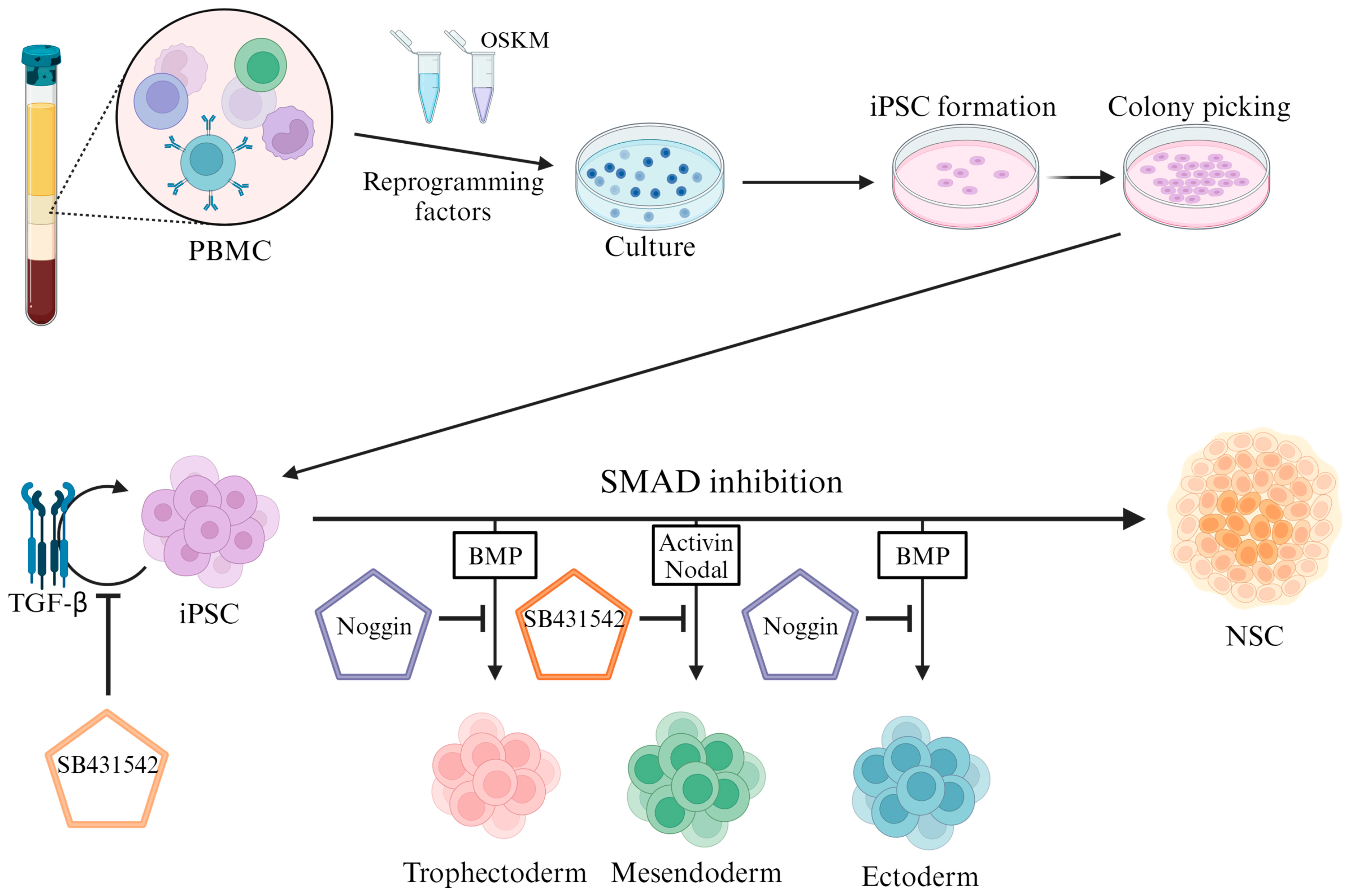
2. Inhibiting the SMAD Pathway in iPSCs for Neural Differentiation
2.1. SMAD Pathway Inhibition
2.2. TGFβ Signaling Pathway
2.3. BMP Signaling Pathway
2.4. RA Pathway
2.5. BDNF, GDNF, and NGF Pathway Regulation
3. Differentiation of Various Neural Cells from iPSCs
3.1. Differentiation into Cortical Neurons
3.2. Differentiation into Dopaminergic Neurons
3.3. Differentiation into Motor Neurons
3.4. Differentiation into Astrocytes
3.5. Differentiation into Oligodendrocytes
3.6. Differentiation into Hippocampal Neurons
3.7. Differentiation into Serotonergic Neurons
4. Therapeutic Research Using Neural Cells Derived from iPSCs
4.1. Dopaminergic Neuron Therapy in a Model of Parkinson’s Disease
4.2. In Vivo Transplantation and Survival of Astrocytes
4.3. Survival of Oligodendrocytes after Transplantation in Mice
4.4. Clinical Trials with iPSC Transplantation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
| BDNF | Brain-derived neurotrophic factor |
| BMP | Bone morphogenetic protein |
| ESC | Embryonic stem cell |
| GDNF | Glial-cell-line-derived neurotrophic factor |
| HLA | Human leukocyte antigen |
| iPSC | Induced pluripotent stem cell |
| NPC | Neural progenitor cell |
| NSC | Neural stem cell |
| PSC | Pluripotent stem cell |
| RA | Retinoic acid |
| RAR | Retinoic acid receptor |
| RXR | Retinoid X receptor |
| SMAD | Sma- and Mad-related protein |
| TGFβ | Transforming growth factor-beta |
References
- Tsao, C.W.; Aday, A.W.; Almarzooq, Z.I.; Anderson, C.A.M.; Arora, P.; Avery, C.L.; Baker-Smith, C.M.; Beaton, A.Z.; Boehme, A.K.; Buxton, A.E.; et al. Heart Disease and Stroke Statistics-2023 Update: A Report From the American Heart Association. Circulation 2023, 147, e93–e621. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Shu, B.; Zhang, D.; Huang, L.; Fu, Q.; Du, G. The Efficacy and Safety of Pharmacological Treatments for Post-stroke Aphasia. CNS Neurol. Disord. Drug Targets 2018, 17, 509–521. [Google Scholar] [CrossRef] [PubMed]
- Czlonkowska, A.; Lesniak, M. Pharmacotherapy in stroke rehabilitation. Expert Opin. Pharmacother. 2009, 10, 1249–1259. [Google Scholar] [CrossRef] [PubMed]
- Chollet, F.; Cramer, S.C.; Stinear, C.; Kappelle, L.J.; Baron, J.C.; Weiller, C.; Azouvi, P.; Hommel, M.; Sabatini, U.; Moulin, T.; et al. Pharmacological therapies in post stroke recovery: Recommendations for future clinical trials. J. Neurol. 2014, 261, 1461–1468. [Google Scholar] [CrossRef]
- Neaverson, A.; Andersson, M.H.L.; Arshad, O.A.; Foulser, L.; Goodwin-Trotman, M.; Hunter, A.; Newman, B.; Patel, M.; Roth, C.; Thwaites, T.; et al. Differentiation of human induced pluripotent stem cells into cortical neural stem cells. Front. Cell Dev. Biol. 2022, 10, 1023340. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Nakada, D.; Morrison, S.J. Mechanisms of stem cell self-renewal. Annu. Rev. Cell Dev. Biol. 2009, 25, 377–406. [Google Scholar] [CrossRef] [PubMed]
- Biehl, J.K.; Russell, B. Introduction to stem cell therapy. J. Cardiovasc. Nurs. 2009, 24, 98–103. [Google Scholar] [CrossRef] [PubMed]
- Zakrzewski, W.; Dobrzynski, M.; Szymonowicz, M.; Rybak, Z. Stem cells: Past, present, and future. Stem Cell Res. Ther. 2019, 10, 68. [Google Scholar] [CrossRef] [PubMed]
- Chehelgerdi, M.; Behdarvand Dehkordi, F.; Chehelgerdi, M.; Kabiri, H.; Salehian-Dehkordi, H.; Abdolvand, M.; Salmanizadeh, S.; Rashidi, M.; Niazmand, A.; Ahmadi, S.; et al. Exploring the promising potential of induced pluripotent stem cells in cancer research and therapy. Mol. Cancer 2023, 22, 189. [Google Scholar] [CrossRef]
- Adhya, D.; Swarup, V.; Nagy, R.; Dutan, L.; Shum, C.; Valencia-Alarcon, E.P.; Jozwik, K.M.; Mendez, M.A.; Horder, J.; Loth, E.; et al. Atypical Neurogenesis in Induced Pluripotent Stem Cells From Autistic Individuals. Biol. Psychiatry 2021, 89, 486–496. [Google Scholar] [CrossRef]
- Liou, R.H.; Edwards, T.L.; Martin, K.R.; Wong, R.C. Neuronal Reprogramming for Tissue Repair and Neuroregeneration. Int. J. Mol. Sci. 2020, 21, 4273. [Google Scholar] [CrossRef] [PubMed]
- Paik, D.T.; Chandy, M.; Wu, J.C. Patient and Disease-Specific Induced Pluripotent Stem Cells for Discovery of Personalized Cardiovascular Drugs and Therapeutics. Pharmacol. Rev. 2020, 72, 320–342. [Google Scholar] [CrossRef] [PubMed]
- Thomson, J.A.; Itskovitz-Eldor, J.; Shapiro, S.S.; Waknitz, M.A.; Swiergiel, J.J.; Marshall, V.S.; Jones, J.M. Embryonic stem cell lines derived from human blastocysts. Science 1998, 282, 1145–1147. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, T. Morality and human embryo research. Introduction to the Talking Point on morality and human embryo research. EMBO Rep. 2009, 10, 299–300. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.; Yoo, J.E.; Lee, J.A.; Lee, D.R.; Kim, J.Y.; Huh, Y.J.; Kim, D.S.; Park, C.Y.; Hwang, D.Y.; Kim, H.S.; et al. Disease-specific induced pluripotent stem cells: A platform for human disease modeling and drug discovery. Exp. Mol. Med. 2012, 44, 202–213. [Google Scholar] [CrossRef] [PubMed]
- Elitt, M.S.; Barbar, L.; Tesar, P.J. Drug screening for human genetic diseases using iPSC models. Hum. Mol. Genet. 2018, 27, R89–R98. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Lee, S.; Mallard, W.; Clement, K.; Tagliazucchi, G.M.; Lim, H.; Choi, I.Y.; Ferrari, F.; Tsankov, A.M.; Pop, R.; et al. A comparison of genetically matched cell lines reveals the equivalence of human iPSCs and ESCs. Nat. Biotechnol. 2015, 33, 1173–1181. [Google Scholar] [CrossRef]
- Marei, H.E.; Althani, A.; Lashen, S.; Cenciarelli, C.; Hasan, A. Genetically unmatched human iPSC and ESC exhibit equivalent gene expression and neuronal differentiation potential. Sci. Rep. 2017, 7, 17504. [Google Scholar] [CrossRef]
- Kristiansen, C.K.; Chen, A.; Hoyland, L.E.; Ziegler, M.; Sullivan, G.J.; Bindoff, L.A.; Liang, K.X. Comparing the mitochondrial signatures in ESCs and iPSCs and their neural derivations. Cell Cycle 2022, 21, 2206–2221. [Google Scholar] [CrossRef]
- Chambers, S.M.; Fasano, C.A.; Papapetrou, E.P.; Tomishima, M.; Sadelain, M.; Studer, L. Highly efficient neural conversion of human ES and iPS cells by dual inhibition of SMAD signaling. Nat. Biotechnol. 2009, 27, 275–280. [Google Scholar] [CrossRef]
- Xu, R.H.; Sampsell-Barron, T.L.; Gu, F.; Root, S.; Peck, R.M.; Pan, G.; Yu, J.; Antosiewicz-Bourget, J.; Tian, S.; Stewart, R.; et al. NANOG is a direct target of TGFbeta/activin-mediated SMAD signaling in human ESCs. Cell Stem Cell 2008, 3, 196–206. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.H.; Chen, X.; Li, D.S.; Li, R.; Addicks, G.C.; Glennon, C.; Zwaka, T.P.; Thomson, J.A. BMP4 initiates human embryonic stem cell differentiation to trophoblast. Nat. Biotechnol. 2002, 20, 1261–1264. [Google Scholar] [CrossRef]
- D’Amour, K.A.; Agulnick, A.D.; Eliazer, S.; Kelly, O.G.; Kroon, E.; Baetge, E.E. Efficient differentiation of human embryonic stem cells to definitive endoderm. Nat. Biotechnol. 2005, 23, 1534–1541. [Google Scholar] [CrossRef]
- Laflamme, M.A.; Chen, K.Y.; Naumova, A.V.; Muskheli, V.; Fugate, J.A.; Dupras, S.K.; Reinecke, H.; Xu, C.; Hassanipour, M.; Police, S.; et al. Cardiomyocytes derived from human embryonic stem cells in pro-survival factors enhance function of infarcted rat hearts. Nat. Biotechnol. 2007, 25, 1015–1024. [Google Scholar] [CrossRef]
- Massague, J. The transforming growth factor-beta family. Annu. Rev. Cell Biol. 1990, 6, 597–641. [Google Scholar] [CrossRef] [PubMed]
- Massague, J.; Chen, Y.G. Controlling TGF-beta signaling. Genes Dev. 2000, 14, 627–644. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Xu, L. Mechanism and regulation of nucleocytoplasmic trafficking of smad. Cell Biosci. 2011, 1, 40. [Google Scholar] [CrossRef]
- Tang, L.Y.; Zhang, Y.E. Non-degradative ubiquitination in Smad-dependent TGF-beta signaling. Cell Biosci. 2011, 1, 43. [Google Scholar] [CrossRef]
- Schmierer, B.; Hill, C.S. TGFbeta-SMAD signal transduction: Molecular specificity and functional flexibility. Nat. Rev. Mol. Cell Biol. 2007, 8, 970–982. [Google Scholar] [CrossRef]
- Reddi, A.H.; Reddi, A. Bone morphogenetic proteins (BMPs): From morphogens to metabologens. Cytokine Growth Factor Rev. 2009, 20, 341–342. [Google Scholar] [CrossRef]
- Sieber, C.; Kopf, J.; Hiepen, C.; Knaus, P. Recent advances in BMP receptor signaling. Cytokine Growth Factor Rev. 2009, 20, 343–355. [Google Scholar] [CrossRef]
- Miyazono, K.; Maeda, S.; Imamura, T. Coordinate regulation of cell growth and differentiation by TGF-beta superfamily and Runx proteins. Oncogene 2004, 23, 4232–4237. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, E.E.; Skinner, M.K. Bone morphogenetic protein-4 acts as an ovarian follicle survival factor and promotes primordial follicle development. Biol. Reprod. 2003, 69, 1265–1272. [Google Scholar] [CrossRef]
- Winnier, G.; Blessing, M.; Labosky, P.A.; Hogan, B.L. Bone morphogenetic protein-4 is required for mesoderm formation and patterning in the mouse. Genes Dev. 1995, 9, 2105–2116. [Google Scholar] [CrossRef]
- Tan, B.T.; Wang, L.; Li, S.; Long, Z.Y.; Wu, Y.M.; Liu, Y. Retinoic acid induced the differentiation of neural stem cells from embryonic spinal cord into functional neurons in vitro. Int. J. Clin. Exp. Pathol. 2015, 8, 8129–8135. [Google Scholar]
- Kurokawa, R.; Soderstrom, M.; Horlein, A.; Halachmi, S.; Brown, M.; Rosenfeld, M.G.; Glass, C.K. Polarity-specific activities of retinoic acid receptors determined by a co-repressor. Nature 1995, 377, 451–454. [Google Scholar] [CrossRef]
- Mosher, K.I.; Schaffer, D.V. Proliferation versus Differentiation: Redefining Retinoic Acid’s Role. Stem Cell Rep. 2018, 10, 1673–1675. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Skromne, I. Retinoic acid regulates size, pattern and alignment of tissues at the head-trunk transition. Development 2014, 141, 4375–4384. [Google Scholar] [CrossRef] [PubMed]
- Sharpe, C.; Goldstone, K. The control of Xenopus embryonic primary neurogenesis is mediated by retinoid signalling in the neurectoderm. Mech. Dev. 2000, 91, 69–80. [Google Scholar] [CrossRef]
- Maden, M.; Holder, N. Retinoic acid and development of the central nervous system. Bioessays 1992, 14, 431–438. [Google Scholar] [CrossRef]
- Clagett-Dame, M.; DeLuca, H.F. The role of vitamin A in mammalian reproduction and embryonic development. Annu. Rev. Nutr. 2002, 22, 347–381. [Google Scholar] [CrossRef] [PubMed]
- Binder, D.K.; Scharfman, H.E. Brain-derived neurotrophic factor. Growth Factors 2004, 22, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Acheson, A.; Conover, J.C.; Fandl, J.P.; DeChiara, T.M.; Russell, M.; Thadani, A.; Squinto, S.P.; Yancopoulos, G.D.; Lindsay, R.M. A BDNF autocrine loop in adult sensory neurons prevents cell death. Nature 1995, 374, 450–453. [Google Scholar] [CrossRef] [PubMed]
- Huang, E.J.; Reichardt, L.F. Neurotrophins: Roles in neuronal development and function. Annu. Rev. Neurosci. 2001, 24, 677–736. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Reynolds, B.A.; Weiss, S. BDNF enhances the differentiation but not the survival of CNS stem cell-derived neuronal precursors. J. Neurosci. 1995, 15, 5765–5778. [Google Scholar] [CrossRef]
- Lim, J.Y.; Park, S.I.; Oh, J.H.; Kim, S.M.; Jeong, C.H.; Jun, J.A.; Lee, K.S.; Oh, W.; Lee, J.K.; Jeun, S.S. Brain-derived neurotrophic factor stimulates the neural differentiation of human umbilical cord blood-derived mesenchymal stem cells and survival of differentiated cells through MAPK/ERK and PI3K/Akt-dependent signaling pathways. J. Neurosci. Res. 2008, 86, 2168–2178. [Google Scholar] [CrossRef]
- Airaksinen, M.S.; Saarma, M. The GDNF family: Signalling, biological functions and therapeutic value. Nat. Rev. Neurosci. 2002, 3, 383–394. [Google Scholar] [CrossRef] [PubMed]
- Cik, M.; Masure, S.; Lesage, A.S.; Van Der Linden, I.; Van Gompel, P.; Pangalos, M.N.; Gordon, R.D.; Leysen, J.E. Binding of GDNF and neurturin to human GDNF family receptor alpha 1 and 2. Influence of cRET and cooperative interactions. J. Biol. Chem. 2000, 275, 27505–27512. [Google Scholar] [CrossRef] [PubMed]
- Matlik, K.; Garton, D.R.; Montano-Rodriguez, A.R.; Olfat, S.; Eren, F.; Casserly, L.; Damdimopoulos, A.; Panhelainen, A.; Porokuokka, L.L.; Kopra, J.J.; et al. Elevated endogenous GDNF induces altered dopamine signalling in mice and correlates with clinical severity in schizophrenia. Mol. Psychiatry 2022, 27, 3247–3261. [Google Scholar] [CrossRef]
- Aloe, L.; Rocco, M.L.; Balzamino, B.O.; Micera, A. Nerve Growth Factor: A Focus on Neuroscience and Therapy. Curr. Neuropharmacol. 2015, 13, 294–303. [Google Scholar] [CrossRef]
- Autar, K.; Guo, X.; Rumsey, J.W.; Long, C.J.; Akanda, N.; Jackson, M.; Narasimhan, N.S.; Caneus, J.; Morgan, D.; Hickman, J.J. A functional hiPSC-cortical neuron differentiation and maturation model and its application to neurological disorders. Stem Cell Rep. 2022, 17, 96–109. [Google Scholar] [CrossRef]
- Shi, Y.; Kirwan, P.; Livesey, F.J. Directed differentiation of human pluripotent stem cells to cerebral cortex neurons and neural networks. Nat. Protoc. 2012, 7, 1836–1846. [Google Scholar] [CrossRef]
- Ma, L.; Liu, Y.; Zhang, S.C. Directed differentiation of dopamine neurons from human pluripotent stem cells. Methods Mol. Biol. 2011, 767, 411–418. [Google Scholar] [CrossRef] [PubMed]
- Mahajani, S.; Raina, A.; Fokken, C.; Kugler, S.; Bahr, M. Homogenous generation of dopaminergic neurons from multiple hiPSC lines by transient expression of transcription factors. Cell Death Dis. 2019, 10, 898. [Google Scholar] [CrossRef] [PubMed]
- Karumbayaram, S.; Novitch, B.G.; Patterson, M.; Umbach, J.A.; Richter, L.; Lindgren, A.; Conway, A.E.; Clark, A.T.; Goldman, S.A.; Plath, K.; et al. Directed differentiation of human-induced pluripotent stem cells generates active motor neurons. Stem Cells 2009, 27, 806–811. [Google Scholar] [CrossRef] [PubMed]
- Shaltouki, A.; Peng, J.; Liu, Q.; Rao, M.S.; Zeng, X. Efficient generation of astrocytes from human pluripotent stem cells in defined conditions. Stem Cells 2013, 31, 941–952. [Google Scholar] [CrossRef]
- Douvaras, P.; Wang, J.; Zimmer, M.; Hanchuk, S.; O’Bara, M.A.; Sadiq, S.; Sim, F.J.; Goldman, J.; Fossati, V. Efficient generation of myelinating oligodendrocytes from primary progressive multiple sclerosis patients by induced pluripotent stem cells. Stem Cell Rep. 2014, 3, 250–259. [Google Scholar] [CrossRef]
- Pomeshchik, Y.; Klementieva, O.; Gil, J.; Martinsson, I.; Hansen, M.G.; de Vries, T.; Sancho-Balsells, A.; Russ, K.; Savchenko, E.; Collin, A.; et al. Human iPSC-Derived Hippocampal Spheroids: An Innovative Tool for Stratifying Alzheimer Disease Patient-Specific Cellular Phenotypes and Developing Therapies. Stem Cell Rep. 2020, 15, 256–273. [Google Scholar] [CrossRef]
- Valiulahi, P.; Vidyawan, V.; Puspita, L.; Oh, Y.; Juwono, V.B.; Sittipo, P.; Friedlander, G.; Yahalomi, D.; Sohn, J.W.; Lee, Y.K.; et al. Generation of caudal-type serotonin neurons and hindbrain-fate organoids from hPSCs. Stem Cell Rep. 2021, 16, 1938–1952. [Google Scholar] [CrossRef]
- Yang, D.; Zhang, Z.J.; Oldenburg, M.; Ayala, M.; Zhang, S.C. Human embryonic stem cell-derived dopaminergic neurons reverse functional deficit in parkinsonian rats. Stem Cells 2008, 26, 55–63. [Google Scholar] [CrossRef]
- Preman, P.; Tcw, J.; Calafate, S.; Snellinx, A.; Alfonso-Triguero, M.; Corthout, N.; Munck, S.; Thal, D.R.; Goate, A.M.; De Strooper, B.; et al. Human iPSC-derived astrocytes transplanted into the mouse brain undergo morphological changes in response to amyloid-beta plaques. Mol. Neurodegener. 2021, 16, 68. [Google Scholar] [CrossRef]
- Moradi, S.; Mahdizadeh, H.; Saric, T.; Kim, J.; Harati, J.; Shahsavarani, H.; Greber, B.; Moore, J.B.t. Research and therapy with induced pluripotent stem cells (iPSCs): Social, legal, and ethical considerations. Stem Cell Res. Ther. 2019, 10, 341. [Google Scholar] [CrossRef] [PubMed]
- Flahou, C.; Morishima, T.; Takizawa, H.; Sugimoto, N. Fit-For-All iPSC-Derived Cell Therapies and Their Evaluation in Humanized Mice with NK Cell Immunity. Front. Immunol. 2021, 12, 662360. [Google Scholar] [CrossRef] [PubMed]
- Otsuka, R.; Wada, H.; Murata, T.; Seino, K.I. Immune reaction and regulation in transplantation based on pluripotent stem cell technology. Inflamm. Regen. 2020, 40, 12. [Google Scholar] [CrossRef] [PubMed]
- Schweitzer, J.S.; Song, B.; Herrington, T.M.; Park, T.Y.; Lee, N.; Ko, S.; Jeon, J.; Cha, Y.; Kim, K.; Li, Q.; et al. Personalized iPSC-Derived Dopamine Progenitor Cells for Parkinson’s Disease. N. Engl. J. Med. 2020, 382, 1926–1932. [Google Scholar] [CrossRef]
- Sugai, K.; Sumida, M.; Shofuda, T.; Yamaguchi, R.; Tamura, T.; Kohzuki, T.; Abe, T.; Shibata, R.; Kamata, Y.; Ito, S.; et al. First-in-human clinical trial of transplantation of iPSC-derived NS/PCs in subacute complete spinal cord injury: Study protocol. Regen. Ther. 2021, 18, 321–333. [Google Scholar] [CrossRef]
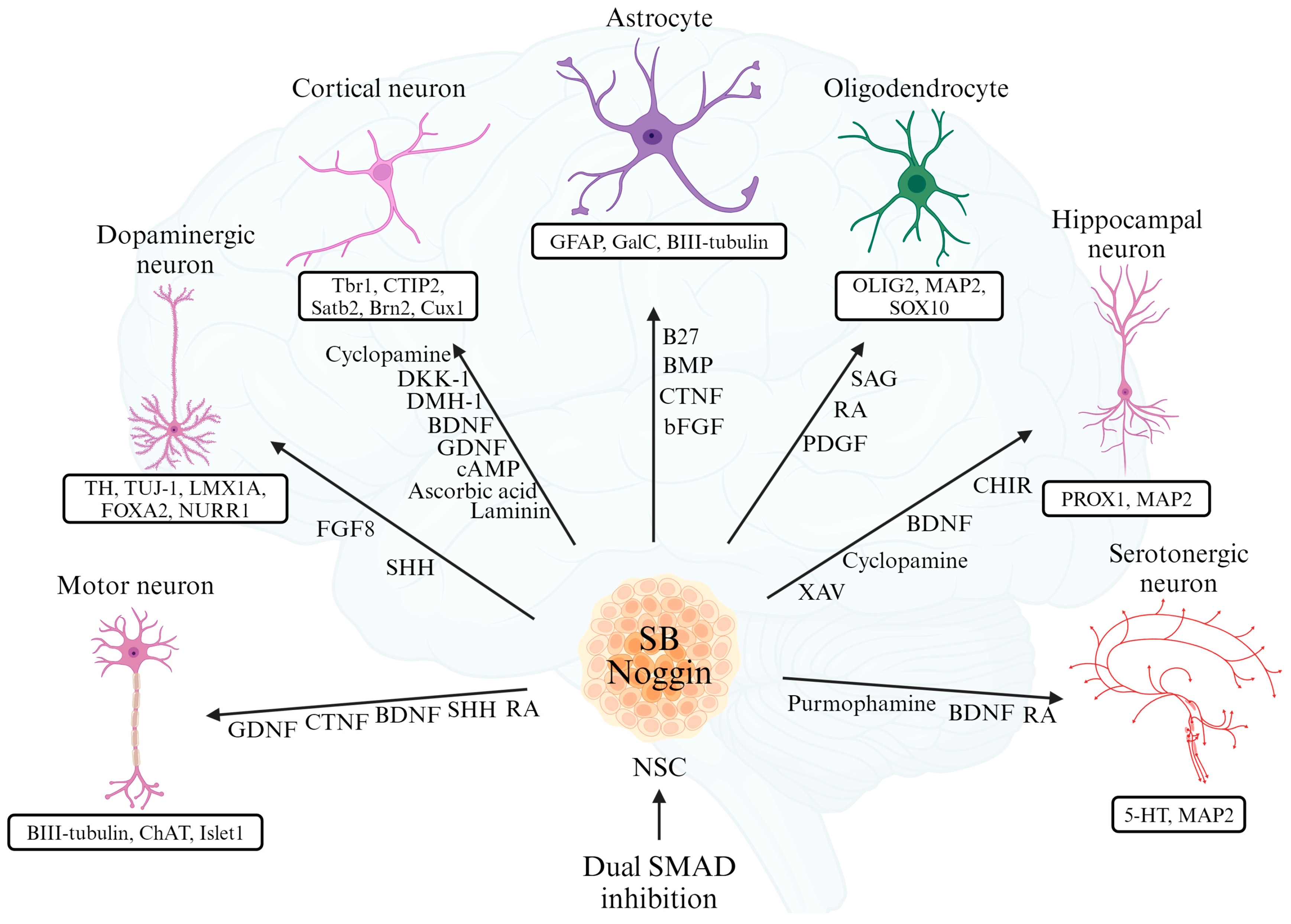
| References | Type of Neuron | Differentiation Inducers | Specific Markers |
|---|---|---|---|
| [51,52] | Cortical Neurons | Cyclopamine, DKK-1, DMH-1, BDNF, GDNF, cAMP, Ascorbic acid, Laminin | Tbr1, CTIP2, Satb2, Brn2, Cux1 |
| [53,54] | Dopaminergic Neurons | FGF8, SHH | TH, TUJ-1, LMX1A, FOXA2, NURR1 |
| [55] | Motor Neurons | GDNF, CTNF, BDNF, SHH, RA | BIII-tubulin, ChAT, Islet1 |
| [56] | Astrocytes | B27, BMP, CTNF, bFGF | GFAP, GalC, BIII-tubulin |
| [57] | Oligodendrocytes | PDGF, RA, SAG | OLIG2, MAP2, SOX10 |
| [58] | Hippocampal Neurons | CHIR, BDNF, Cyclopamine, XAV | PROX1, MAP2 |
| [59] | Serotonergic Neurons | Purmophamine, BDNF, RA | 5-HT, MAP2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, D.-H.; Lee, E.C.; Lee, J.y.; Lee, M.R.; Shim, J.-w.; Oh, J.S. Neuronal Cell Differentiation of iPSCs for the Clinical Treatment of Neurological Diseases. Biomedicines 2024, 12, 1350. https://doi.org/10.3390/biomedicines12061350
Lee D-H, Lee EC, Lee Jy, Lee MR, Shim J-w, Oh JS. Neuronal Cell Differentiation of iPSCs for the Clinical Treatment of Neurological Diseases. Biomedicines. 2024; 12(6):1350. https://doi.org/10.3390/biomedicines12061350
Chicago/Turabian StyleLee, Dong-Hun, Eun Chae Lee, Ji young Lee, Man Ryul Lee, Jae-won Shim, and Jae Sang Oh. 2024. "Neuronal Cell Differentiation of iPSCs for the Clinical Treatment of Neurological Diseases" Biomedicines 12, no. 6: 1350. https://doi.org/10.3390/biomedicines12061350
APA StyleLee, D.-H., Lee, E. C., Lee, J. y., Lee, M. R., Shim, J.-w., & Oh, J. S. (2024). Neuronal Cell Differentiation of iPSCs for the Clinical Treatment of Neurological Diseases. Biomedicines, 12(6), 1350. https://doi.org/10.3390/biomedicines12061350






