Loss of c-Kit in Endothelial Cells Protects against Hindlimb Ischemia
Abstract
1. Introduction
2. Methods
2.1. Animals
2.2. Hindlimb Ischemia
2.3. Laser Doppler Imaging (LDI)
2.4. Immunohistochemistry (IHC)
2.5. Endothelial Barrier Injury (Aortic Crush Injury)
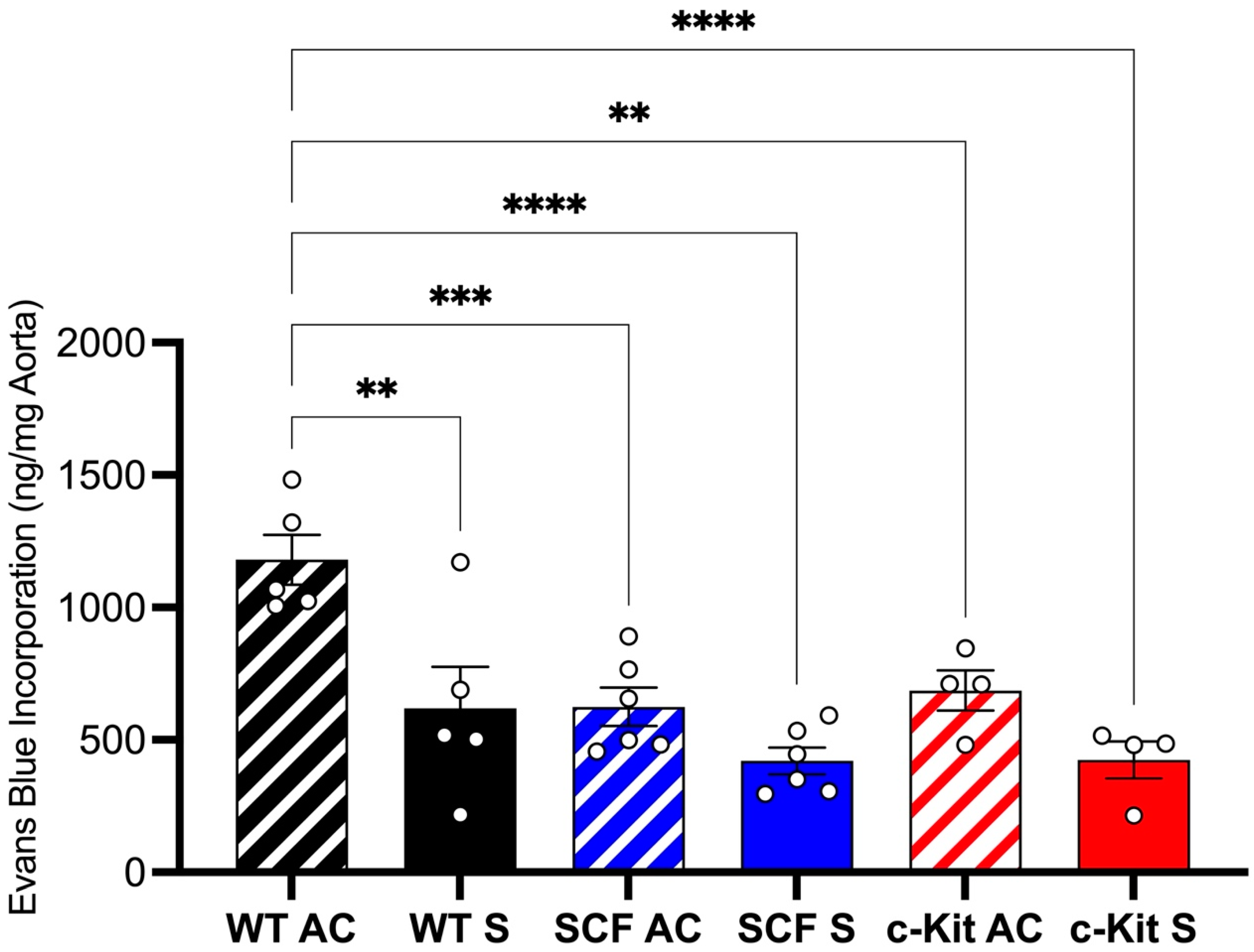
2.6. Evans Blue Quantification for Endothelial Barrier Integrity
2.7. Isolation of Lung ECs
2.8. qRT-PCR
2.9. Flow Cytometry
2.10. Data Analysis
3. Results
3.1. Confirmation of Animal Models
3.2. Endothelial Barrier Function
Loss of Endothelial c-Kit Signaling Improves Endothelial Integrity after Aortic Crush Injury
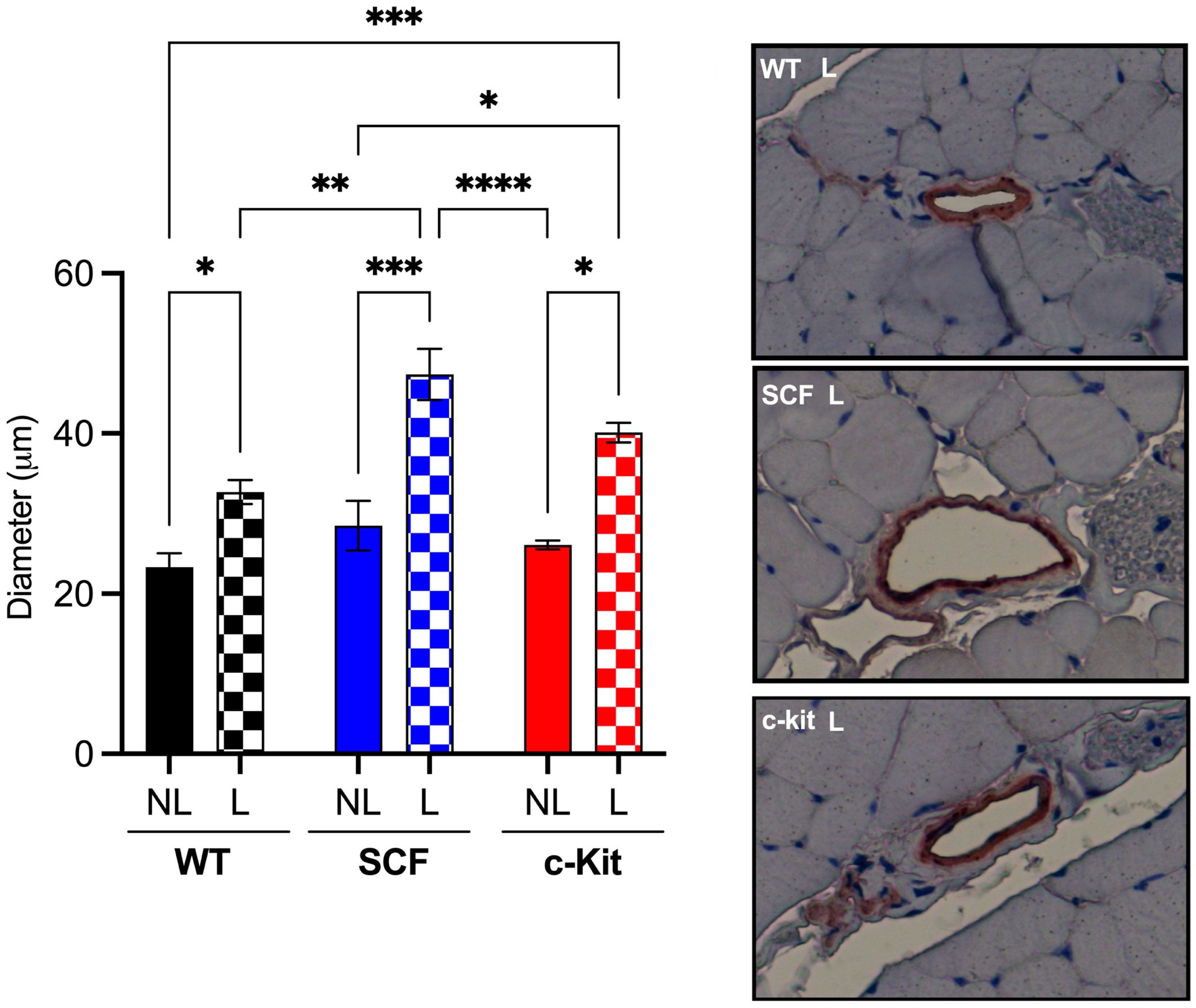
3.3. Perfusion and Neovascularization
3.3.1. Loss of Endothelial c-Kit Signaling Improves Blood Flow Recovery after Hindlimb Ischemia
3.3.2. Loss of Endothelial c-Kit Signaling Improves Arteriogenesis after Hindlimb Ischemia
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Criqui, M.H.; Aboyans, V. Epidemiology of Peripheral Artery Disease. Circ. Res. 2015, 116, 1509–1526. [Google Scholar] [CrossRef]
- Ziegler-Graham, K.; MacKenzie, E.J.; Ephraim, P.L.; Travison, T.G.; Brookmeyer, R. Estimating the prevalence of limb loss in the United States: 2005 to 2050. Arch. Phys. Med. Rehabil. 2008, 89, 422–429. [Google Scholar] [CrossRef]
- Song, P.; Rudan, D.; Zhu, Y.; Fowkes, F.J.I.; Rahimi, K.; Fowkes, F.G.R.; Rudan, I. Global, regional, and national prevalence and risk factors for peripheral artery disease in 2015: An updated systematic review and analysis. Lancet Glob. Health 2019, 7, e1020–e1030. [Google Scholar] [CrossRef]
- Fereydooni, A.; Gorecka, J.; Dardik, A. Using the epidemiology of critical limb ischemia to estimate the number of patients amenable to endovascular therapy. Vasc. Med. 2020, 25, 78–87. [Google Scholar] [CrossRef]
- Lawall, H.; Zemmrich, C.; Bramlage, P.; Amann, B. Health related quality of life in patients with critical limb ischemia. VASA Z. Gefasskrankh. 2012, 41, 78–88. [Google Scholar] [CrossRef]
- Hirsch, A.T.; Haskal, Z.J.; Hertzer, N.R.; Bakal, C.W.; Creager, M.A.; Halperin, J.L.; Hiratzka, L.F.; Murphy, W.R.; Olin, J.W.; Puschett, J.B.; et al. ACC/AHA 2005 Practice Guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): A collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery. Circulation 2006, 113, e463–e654. [Google Scholar] [CrossRef]
- Steg, P.G.; Bhatt, D.L.; Wilson, P.W.; D’Agostino, R.; Ohman, E.M.; Röther, J.; Liau, C.S.; Hirsch, A.T.; Mas, J.L.; Ikeda, Y.; et al. One-year cardiovascular event rates in outpatients with atherothrombosis. JAMA 2007, 297, 1197–1206. [Google Scholar] [CrossRef]
- Caro, J.; Migliaccio-Walle, K.; Ishak, K.J.; Proskorovsky, I. The morbidity and mortality following a diagnosis of peripheral arterial disease: Long-term follow-up of a large database. BMC Cardiovasc. Disord. 2005, 5, 14. [Google Scholar] [CrossRef]
- Rooke, T.W.; Hirsch, A.T.; Misra, S.; Sidawy, A.N.; Beckman, J.A.; Findeiss, L.K.; Golzarian, J.; Gornik, H.L.; Halperin, J.L.; Jaff, M.R.; et al. 2011 ACCF/AHA Focused Update of the Guideline for the Management of Patients with Peripheral Artery Disease (updating the 2005 guideline): A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J. Am. Coll. Cardiol. 2011, 58, 2020–2045. [Google Scholar] [CrossRef]
- Tendera, M.; Aboyans, V.; Bartelink, M.; Baumgartner, I.; Clement, D.L.; Collet, J.; Cremonesi, A.; De Carlo, M.; Erbel, R.; Fowkes, F.G.R.; et al. ESC Guidelines on the diagnosis and treatment of peripheral artery diseases: Document covering atherosclerotic disease of extracranial carotid and vertebral, mesenteric, renal, upper and lower extremity arteries: The Task Force on the Diagnosis and Treatment of Peripheral Artery Diseases of the European Society of Cardiology (ESC). Eur. Heart J. 2011, 32, 2851–2906. [Google Scholar] [CrossRef]
- Lawall, H.; Bramlage, P.; Amann, B. Treatment of peripheral arterial disease using stem and progenitor cell therapy. J. Vasc. Surg. 2011, 53, 445–453. [Google Scholar] [CrossRef]
- Dormandy, J.; Heeck, L.; Vig, S. The fate of patients with critical leg ischemia. Semin. Vasc. Surg. 1999, 12, 142–147. [Google Scholar]
- Grundmann, S.; Piek, J.J.; Pasterkamp, G.; Hoefer, I.E. Arteriogenesis: Basic mechanisms and therapeutic stimulation. Eur. J. Clin. Investig. 2007, 37, 755–766. [Google Scholar] [CrossRef]
- Schirmer, S.H.; van Royen, N. Stimulation of collateral artery growth: A potential treatment for peripheral artery disease. Expert Rev. Cardiovasc. Ther. 2004, 2, 581–588. [Google Scholar] [CrossRef]
- Krock, B.L.; Skuli, N.; Simon, M.C. Hypoxia-induced angiogenesis: Good and evil. Genes Cancer 2011, 2, 1117–1133. [Google Scholar] [CrossRef]
- Murdoch, C.; Giannoudis, A.; Lewis, C.E. Mechanisms regulating the recruitment of macrophages into hypoxic areas of tumors and other ischemic tissues. Blood 2004, 104, 2224–2234. [Google Scholar] [CrossRef]
- Samura, M.; Hosoyama, T.; Takeuchi, Y.; Ueno, K.; Morikage, N.; Hamano, K. Therapeutic strategies for cell-based neovascularization in critical limb ischemia. J. Transl. Med. 2017, 15, 49. [Google Scholar] [CrossRef]
- Lennartsson, J.; Jelacic, T.; Linnekin, D.; Shivakrupa, R. Normal and oncogenic forms of the receptor tyrosine kinase kit. Stem Cells 2005, 23, 16–43. [Google Scholar] [CrossRef]
- Li, W.; Xu, H.; Qian, C. c-Kit-Positive Adipose Tissue-Derived Mesenchymal Stem Cells Promote the Growth and Angiogenesis of Breast Cancer. BioMed Res. Int. 2017, 2017, 7407168. [Google Scholar] [CrossRef]
- Sheikh, E.; Tran, T.; Vranic, S.; Levy, A.; Bonfil, R.D. Role and significance of c-KIT receptor tyrosine kinase in cancer: A review. Bosn. J. Basic Med. Sci. 2022, 22, 683–698. [Google Scholar] [CrossRef]
- Hernandez, D.R.; Artiles, A.; Duque, J.C.; Martinez, L.; Pinto, M.T.; Webster, K.A.; Velazquez, O.C.; Vazquez-Padron, R.I.; Lassance-Soares, R.M. Loss of c-Kit function impairs arteriogenesis in a mouse model of hindlimb ischemia. Surgeey 2018, 163, 877–882. [Google Scholar] [CrossRef]
- Li, J.; Song, F.; Chen, R.; Yang, J.; Liu, J.; Huang, L.; Duan, F.; Kou, M.; Lian, B.X.; Zhou, X.; et al. Bradykinin-pretreated Human cardiac-specific c-kit+ Cells Enhance Exosomal miR-3059-5p and Promote Angiogenesis Against Hindlimb Ischemia in mice. Stem Cell Rev. Rep. 2023, 19, 2481–2496. [Google Scholar] [CrossRef]
- Yue, X.; Jiang, H.; Xu, Y.; Xia, M.; Cheng, X.W. Cathepsin K Deficiency Impaired Ischemia-Induced Neovascularization in Aged Mice. Stem Cells Int. 2020, 2020, 6938620. [Google Scholar] [CrossRef]
- Kim, J.Y.; Choi, J.-S.; Song, S.-H.; Im, J.-E.; Kim, J.-M.; Kim, K.; Kwon, S.; Shin, H.K.; Joo, C.-K.; Lee, B.H.; et al. Stem cell factor is a potent endothelial permeability factor. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 1459–1467. [Google Scholar] [CrossRef]
- Im, J.E.; Song, S.H.; Suh, W. Src tyrosine kinase regulates the stem cell factor–induced breakdown of the blood–retinal barrier. Mol. Vis. 2016, 22, 1213. [Google Scholar]
- Pathania, S.; Pentikäinen, O.T.; Singh, P.K. A holistic view on c-Kit in cancer: Structure, signaling, pathophysiology and its inhibitors. Biochim. Biophys. Acta Rev. Cancer 2021, 1876, 188631. [Google Scholar] [CrossRef]
- Zigmond, Z.M.; Song, L.; Martinez, L.; Lassance-Soares, R.M.; Velazquez, O.C.; Vazquez-Padron, R.I. c-Kit expression in smooth muscle cells reduces atherosclerosis burden in hyperlipidemic mice. Atherosclerosis 2021, 324, 133–140. [Google Scholar] [CrossRef]
- Kim, K.L.; Meng, Y.; Kim, J.Y.; Baek, E.J.; Suh, W. Direct and differential effects of stem cell factor on the neovascularization activity of endothelial progenitor cells. Cardiovasc. Res. 2011, 92, 132–140. [Google Scholar] [CrossRef]
- Kimura, Y.; Ding, B.; Imai, N.; Nolan, D.J.; Butler, J.M.; Rafii, S. c-Kit-mediated functional positioning of stem cells to their niches is essential for maintenance and regeneration of adult hematopoiesis. PLoS ONE 2011, 6, e26918. [Google Scholar] [CrossRef]
- Wang, Y.; Nakayama, M.; Pitulescu, M.E.; Schmidt, T.S.; Bochenek, M.L.; Sakakibara, A.; Adams, S.; Davy, A.; Deutsch, U.; Lüthi, U.; et al. Ephrin-B2 controls VEGF-induced angiogenesis and lymphangiogenesis. Nature 2010, 465, 483–486. [Google Scholar] [CrossRef]
- Yu, D.; Makkar, G.; Sarkar, R.; Strickland, D.K.; Monahan, T.S. Murine Aortic Crush Injury: An Efficient In Vivo Model of Smooth Muscle Cell Proliferation and Endothelial Function. J. Vis. Exp. 2017, 124, e55201. [Google Scholar] [CrossRef]
- Mustapha, J.A.; Katzen, B.T.; Neville, R.F.; Lookstein, R.A.; Zeller, T.; Miller, L.E.; Jaff, M.R. Determinants of Long-Term Outcomes and Costs in the Management of Critical Limb Ischemia: A Population-Based Cohort Study. J. Am. Heart Assoc. 2018, 7, e009724. [Google Scholar] [CrossRef]
- Spoorendonk, J.A.; Krol, M.; Alleman, C. The burden of amputation in patients with peripheral arterial disease in the Netherlands. J. Cardiovasc. Surg. 2020, 61, 435–444. [Google Scholar] [CrossRef]
- Vadia, R.; Malyar, N.; Stargardt, T. Cost-utility analysis of early versus delayed endovascular intervention in critical limb-threatening ischemia patients with rest pain. J. Vasc. Surg. 2023, 77, 299–308.e2. [Google Scholar] [CrossRef]
- Csore, J.; Drake, M.; Roy, T.L. Peripheral arterial disease treatment planning using noninvasive and invasive imaging methods. J. Vasc. Surg. Cases Innov. Tech. 2023, 9, 101263. [Google Scholar] [CrossRef]
- Bosch-Marce, M.; Okuyama, H.; Wesley, J.B.; Sarkar, K.; Kimura, H.; Liu, Y.V.; Zhang, H.; Strazza, M.; Rey, S.; Savino, L.; et al. Effects of aging and hypoxia-inducible factor-1 activity on angiogenic cell mobilization and recovery of perfusion after limb ischemia. Circ. Res. 2007, 101, 1310–1318. [Google Scholar] [CrossRef]
- Perrucci, G.L.; Straino, S.; Corlianò, M.; Scopece, A.; Napolitano, M.; Berk, B.C.; Lombardi, F.; Pompilio, G.; Capogrossi, M.C.; Nigro, P. Cyclophilin A modulates bone marrow-derived CD117(+) cells and enhances ischemia-induced angiogenesis via the SDF-1/CXCR4 axis. Int. J. Cardiol. 2016, 212, 324–335. [Google Scholar] [CrossRef]
- Takematsu, E.; Massidda, M.; Howe, G.; Goldman, J.; Felli, P.; Mei, L.; Callahan, G.; Sligar, A.D.; Smalling, R.; Baker, A.B. Transmembrane Stem Factor Nanodiscs Enhanced Revascularization in a Hind Limb Ischemia Model in Diabetic, Hyperlipidemic Rabbits. Sci. Rep. 2024, 14, 2352. [Google Scholar] [CrossRef]
- Lutz, M.; Rosenberg, M.; Kiessling, F.; Eckstein, V.; Heger, T.; Krebs, J.; Ho, A.D.; Katus, H.A.; Frey, N. Local injection of stem cell factor (SCF) improves myocardial homing of systemically delivered c-kit + bone marrow-derived stem cells. Cardiovasc. Res. 2008, 77, 143–150. [Google Scholar] [CrossRef]
- Reboll, M.R.; Klede, S.; Taft, M.H.; Cai, C.-L.; Field, L.J.; Lavine, K.J.; Koenig, A.L.; Fleischauer, J.; Meyer, J.; Schambach, A.; et al. Meteorin-like promotes heart repair through endothelial KIT receptor tyrosine kinase. Science 2022, 376, 1343–1347. [Google Scholar] [CrossRef]
- Wang, J.; Zheng, B.; Yang, S.; Fan, J.; Sun, H.; Wang, J. Mesoporous Silica Nanoparticles Carrying MicroRNA-124 to Target P2Y12 Facilitates Cerebral Angiogenesis in Lacunar Cerebral Infarction through Stem Cell Factor/c-Kit Signaling Pathway. J. Biomed. Nanotechnol. 2022, 18, 218–224. [Google Scholar] [CrossRef]
- Jin, K.; Mao, X.O.; Sun, Y.; Xie, L.; Greenberg, D.A. Stem cell factor stimulates neurogenesis in vitro and in vivo. J. Clin. Investig. 2002, 110, 311–319. [Google Scholar] [CrossRef][Green Version]
- Hu, J.G.; Yuan, R. Decreased expression of c-kit and telomerase in a rat model of chronic endometrial ischemia. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2011, 17, 103–109. [Google Scholar] [CrossRef]
- Stankov, K.; Popovic, S.; Mikov, M. C-KIT Signaling in Cancer Treatment. Curr. Pharm. Design 2014, 20, 2849–2880. [Google Scholar] [CrossRef]
- Schaper, W.; Scholz, D. Factors regulating arteriogenesis. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 1143–1151. [Google Scholar] [CrossRef]
- Shan, H.-J.; Jiang, K.; Zhao, M.-Z.; Deng, W.-J.; Cao, W.-H.; Li, J.-J.; Li, K.-R.; She, C.; Luo, W.-F.; Yao, J.; et al. SCF/c-Kit-activated signaling and angiogenesis require Gαi1 and Gαi3. Int. J. Biol. Sci. 2023, 19, 1910–1924. [Google Scholar] [CrossRef]
- Suzuki, T.; Suzuki, S.; Fujino, N.; Ota, C.; Yamada, M.; Suzuki, T.; Yamaya, M.; Kondo, T.; Kubo, H. c-Kit immunoexpression delineates a putative endothelial progenitor cell population in developing human lungs. Am. J. Physiol. Cell. Mol. Physiol. 2014, 306, L855–L865. [Google Scholar] [CrossRef]
- König, A.; Corbacioglu, S.; Ballmaier, M.; Welte, K. Downregulation of c-kit Expression in Human Endothelial Cells by Inflammatory Stimuli. Blood 1997, 90, 148–155. [Google Scholar] [CrossRef]
- Broudy, V.; Kovach, N.; Bennett, L.; Lin, N.; Jacobsen, F.; Kidd, P. Human Umbilical Vein Endothelial Cells Display High-Affinity c-kit Receptors and Produce a Soluble Form of the c-kit Receptor. Blood 1994, 83, 2145–2152. [Google Scholar] [CrossRef]
- Carpenter, S.K.; Rahman, S.; Lund, T.J.S.; Armstrong, P.I.; Lamm, M.H.; Reason, R.D.; Coffman, C.R. Students’ Use of Optional Online Reviews and Its Relationship to Summative Assessment Outcomes in Introductory Biology. CBE Life Sci. Educ. 2017, 16, ar23. [Google Scholar] [CrossRef]
- Hasegawa, T.; McLeod, D.S.; Prow, T.; Merges, C.; Grebe, R.; Lutty, G.A. Vascular precursors in developing human retina. Investig. Ophthalmol. Vis. Sci. 2008, 49, 2178–2192. [Google Scholar] [CrossRef]
- Takahashi, T.; Friedmacher, F.; Zimmer, J.; Puri, P. Increased c-kit and stem cell factor expression in the pulmonary vasculature of nitrofen-induced congenital diaphragmatic hernia. J. Pediatr. Surg. 2016, 51, 706–709. [Google Scholar] [CrossRef]
- Sheng, X.; Huang, D.; Guo, H.; Liu, X.; Qin, Z. [Prokaryotic expression and transmembrane transfer of fusion protein TAT-RIG-I-GFP]. Sheng Wu Gong Cheng Xue Bao = Chin. J. Biotechnol. 2019, 35, 1463–1468. [Google Scholar] [CrossRef]
- Hernandez, D.R.; Rojas, M.G.; Martinez, L.; Rodriguez, B.L.; Zigmond, Z.M.; Vazquez-Padron, R.I.; Lassance-Soares, R.M. c-Kit deficiency impairs nitric oxide signaling in smooth muscle cells. Biochem. Biophys. Res. Commun. 2019, 518, 227–232. [Google Scholar] [CrossRef]
- Song, L.; Martinez, L.; Zigmond, Z.M.; Hernandez, D.R.; Lassance-Soares, R.M.; Selman, G.; Vazquez-Padron, R.I. c-Kit modifies the inflammatory status of smooth muscle cells. PeerJ. 2017, 5, e3418. [Google Scholar] [CrossRef]
- Kim, K.L.; Seo, S.; Kim, J.T.; Kim, J.; Kim, W.; Yeo, Y.; Sung, J.-H.; Park, S.G.; Suh, W. SCF (Stem Cell Factor) and cKIT Modulate Pathological Ocular Neovascularization. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 2120–2131. [Google Scholar] [CrossRef]
- Huang, J.-C.; Chen, S.-C.; Chang, W.-A.; Hung, W.-W.; Wu, P.-H.; Wu, L.-Y.; Chang, J.-M.; Hsu, Y.-L.; Tsai, Y.-C. KITLG Promotes Glomerular Endothelial Cell Injury in Diabetic Nephropathy by an Autocrine Effect. Int. J. Mol. Sci. 2022, 23, 11723. [Google Scholar] [CrossRef]
- Shang, J.; Li, W.; Zhang, H.; Wang, W.; Liu, N.; Gao, D.; Wang, F.; Yan, X.; Gao, C.; Sun, R.; et al. C-kit controls blood-brain barrier permeability by regulating caveolae-mediated transcytosis after chronic cerebral hypoperfusion. Biomed. Pharmacother. 2024, 170, 115778. [Google Scholar] [CrossRef]
- Mehta, D.; Malik, A.B. Signaling mechanisms regulating endothelial permeability. Physiol. Rev. 2006, 86, 279–367. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Falero-Diaz, G.; Barboza, C.d.A.; Vazquez-Padron, R.I.; Velazquez, O.C.; Lassance-Soares, R.M. Loss of c-Kit in Endothelial Cells Protects against Hindlimb Ischemia. Biomedicines 2024, 12, 1358. https://doi.org/10.3390/biomedicines12061358
Falero-Diaz G, Barboza CdA, Vazquez-Padron RI, Velazquez OC, Lassance-Soares RM. Loss of c-Kit in Endothelial Cells Protects against Hindlimb Ischemia. Biomedicines. 2024; 12(6):1358. https://doi.org/10.3390/biomedicines12061358
Chicago/Turabian StyleFalero-Diaz, Gustavo, Catarina de A. Barboza, Roberto I. Vazquez-Padron, Omaida C. Velazquez, and Roberta M. Lassance-Soares. 2024. "Loss of c-Kit in Endothelial Cells Protects against Hindlimb Ischemia" Biomedicines 12, no. 6: 1358. https://doi.org/10.3390/biomedicines12061358
APA StyleFalero-Diaz, G., Barboza, C. d. A., Vazquez-Padron, R. I., Velazquez, O. C., & Lassance-Soares, R. M. (2024). Loss of c-Kit in Endothelial Cells Protects against Hindlimb Ischemia. Biomedicines, 12(6), 1358. https://doi.org/10.3390/biomedicines12061358





