The Monocrotaline Rat Model of Right Heart Disease Induced by Pulmonary Artery Hypertension
Abstract
1. Introduction
2. The Monocrotaline Rat Model of Pulmonary Artery Hypertension
2.1. Historical Perspective
2.2. Development of MCT-Induced Toxicity
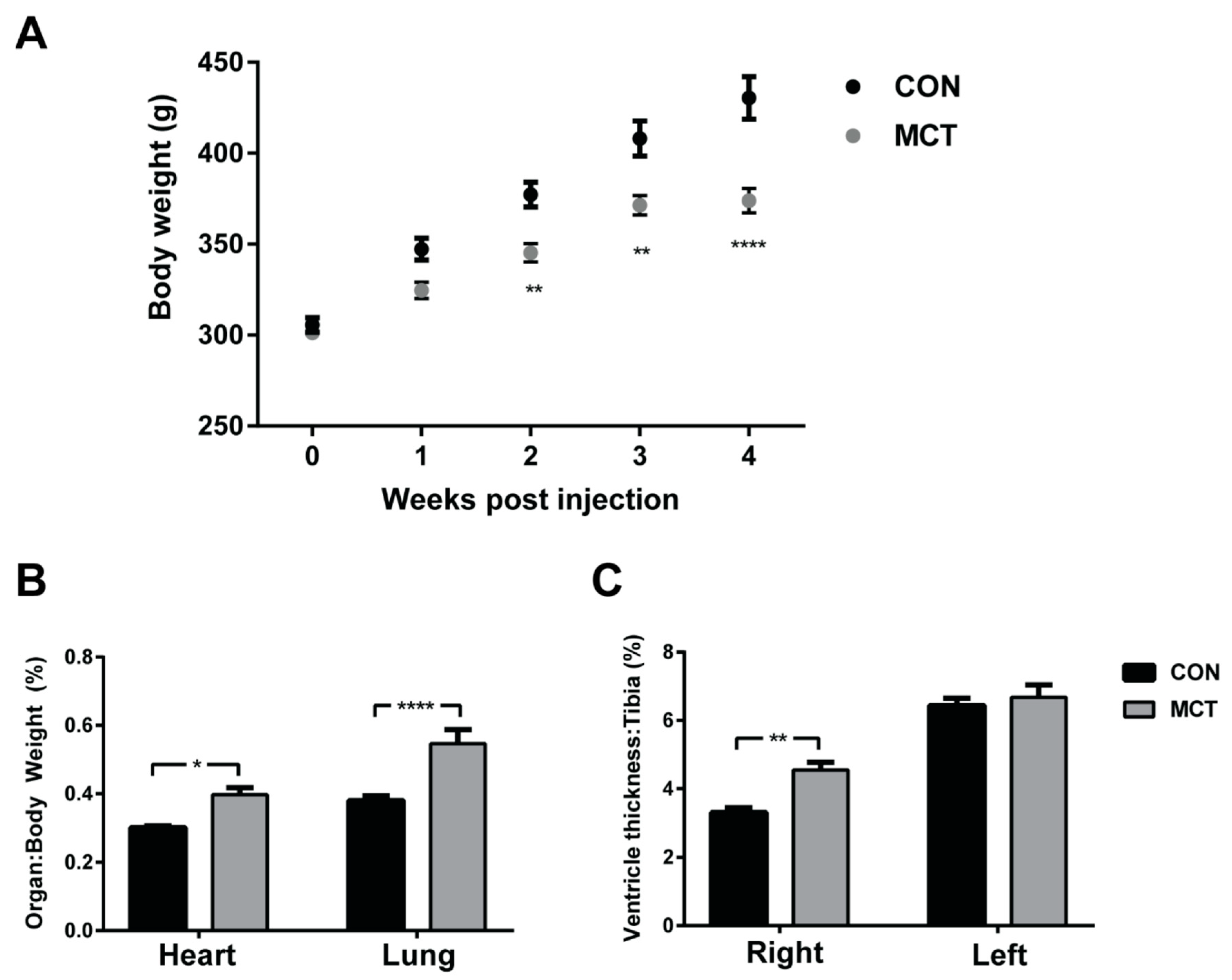
2.3. Response to MCT Administration in Rats
3. Myocardial Changes in Response to MCT
3.1. Compensated RV Hypertrophy
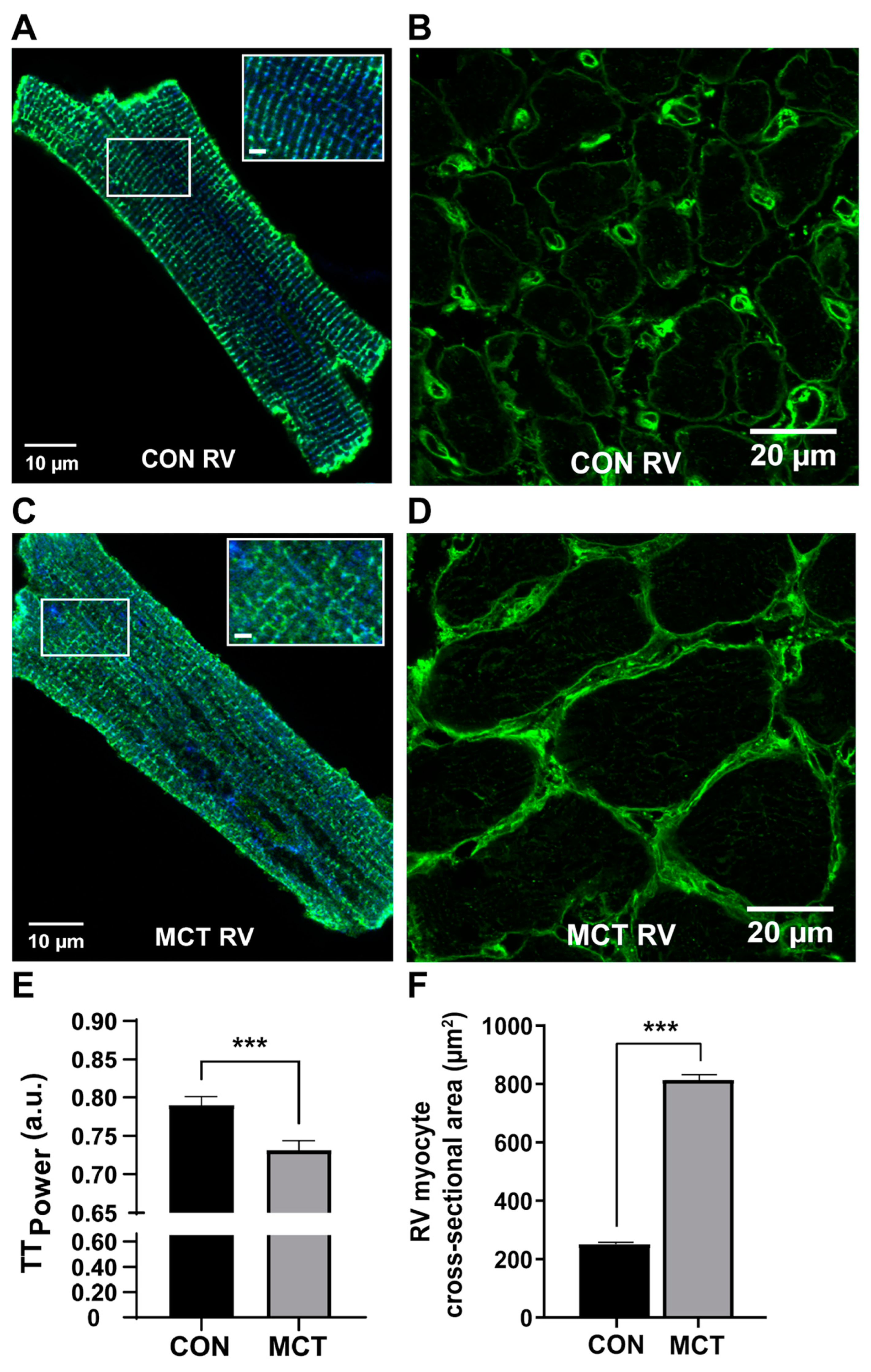
3.2. Cardiomyocyte Changes during Compensated RV Hypertrophy
4. Impact of MCT on Cardiomyocyte Excitation–Contraction Coupling
4.1. Changes in Excitation–Contraction Coupling during Compensated RV Hypertrophy
4.2. Beta-Adrenergic Response of MCT-Induced RV Hypertrophy
5. Limitations of the Model and Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Naeije, R.; Manes, A. The right ventricle in pulmonary arterial hypertension. Eur. Respir. Rev. 2014, 23, 476–487. [Google Scholar] [CrossRef] [PubMed]
- Ekman, I.; Cleland, J.G.; Andersson, B.; Swedberg, K. Exploring symptoms in chronic heart failure. Eur. J. Heart Fail. 2005, 7, 699–703. [Google Scholar] [CrossRef] [PubMed]
- Nieminen, M.S.; Brutsaert, D.; Dickstein, K.; Drexler, H.; Follath, F.; Harjola, V.-P.; Hochadel, M.; Komajda, M.; Lassus, J.; Lopez-Sendon, J.L.; et al. EuroHeart Failure Survey II (EHFS II): A survey on hospitalized acute heart failure patients: Description of population. Eur. Heart J. 2006, 27, 2725–2736. [Google Scholar] [CrossRef]
- Maeder, M.T.; Weber, L.; Pohle, S.; Chronis, J.; Baty, F.; Rigger, J.; Brutsche, M.; Haager, P.; Rickli, H.; Brenner, R. Impact of the 2022 pulmonary hypertension definition on haemodynamic classification and mortality in patients with aortic stenosis undergoing valve replacement. Eur. Heart J. Open 2024, 4, oeae037. [Google Scholar] [CrossRef]
- Deng, Z.; Morse, J.H.; Slager, S.L.; Cuervo, N.; Moore, K.J.; Venetos, G.; Kalachikov, S.; Cayanis, E.; Fischer, S.G.; Barst, R.J.; et al. Familial primary pulmonary hypertension (gene PPH1) is caused by mutations in the bone morphogenetic protein receptor-II gene. Am. J. Hum. Genet. 2000, 67, 737–744. [Google Scholar] [CrossRef]
- Olsson, K.M.; Corte, T.J.; Kamp, J.C.; Montani, D.; Nathan, S.D.; Neubert, L.; Price, L.C.; Kiely, D.G. Pulmonary hypertension associated with lung disease: New insights into pathomechanisms, diagnosis, and management. Lancet Respir. Med. 2023, 11, 820–835. [Google Scholar] [CrossRef] [PubMed]
- Sola, J.R.; Mena, M.S.-C.; Riera-Mestre, A. Update in the management of chronic thrombo-embolic pulmonary hypertension. Med. Clin. 2024, 162, 126–133. [Google Scholar]
- Galiè, N.; Humbert, M.; Vachiery, J.L.; Gibbs, S.; Lang, I.; Torbicki, A.; Simonneau, G.; Peacock, A.; Vonk Noordegraaf, A.; Beghetti, M.; et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur. Heart J. 2016, 37, 67–119. [Google Scholar]
- Olivari, M.T.; Fiorentini, C.; Polese, A.; Guazzi, M.D. Pulmonary hemodynamics and right ventricular function in hypertension. Circulation 1978, 57, 1185–1190. [Google Scholar] [CrossRef]
- Pfeffer, J.M.; Pfeffer, M.A.; Fishbein, M.C.; Frohlich, E.D. Cardiac function and morphology with aging in the spontaneously hypertensive rat. Am. J. Physiol. 1979, 237, H461–H468. [Google Scholar] [CrossRef]
- Mocumbi, A.; Humbert, M.; Saxena, A.; Jing, Z.C.; Sliwa, K.; Thienemann, F.; Archer, S.L.; Stewart, S. Pulmonary hypertension. Nat. Rev. Dis. Primers 2024, 10, 1. [Google Scholar] [CrossRef] [PubMed]
- Monteagudo-Vela, M.; Tindale, A.; Monguió-Santín, E.; Reyes-Copa, G.; Panoulas, V. Right ventricular failure: Current strategies and future development. Front. Cardiovasc. Med. 2023, 10, 998382. [Google Scholar] [CrossRef] [PubMed]
- Dignam, J.P.; Scott, T.E.; Kemp-Harper, B.K.; Hobbs, A.J. Animal models of pulmonary hypertension: Getting to the heart of the problem. Br. J. Pharmacol. 2022, 179, 811–837. [Google Scholar] [CrossRef] [PubMed]
- Borgdorff, M.A.; Dickinson, M.G.; Berger, R.M.; Bartelds, B. Right ventricular failure due to chronic pressure load: What have we learned in animal models since the NIH working group statement? Heart Fail. Rev. 2015, 20, 475–491. [Google Scholar] [CrossRef][Green Version]
- Crosby, A.; Jones, F.M.; Southwood, M.; Stewart, S.; Schermuly, R.; Butrous, G.; Dunne, D.W.; Morrell, N.W. Pulmonary vascular remodeling correlates with lung eggs and cytokines in murine schistosomiasis. Am. J. Respir. Crit. Care Med. 2010, 181, 279–288. [Google Scholar] [CrossRef]
- Tsikis, S.T.; Klouda, T.; Hirsch, T.I.; Fligor, S.C.; Liu, T.; Kim, Y.; Pan, A.; Quigley, M.; Mitchell, P.D.; Puder, M.; et al. A pneumonectomy model to study flow-induced pulmonary hypertension and compensatory lung growth. Cell Rep. Methods 2023, 3, 100613. [Google Scholar] [CrossRef]
- Sztuka, K.; Jasińska-Stroschein, M. Animal models of pulmonary arterial hypertension: A systematic review and meta-analysis of data from 6126 animals. Pharmacol. Res. 2017, 125 Pt B, 201–214. [Google Scholar] [CrossRef]
- Maarman, G.; Lecour, S.; Butrous, G.; Thienemann, F.; Sliwa, K. A comprehensive review: The evolution of animal models in pulmonary hypertension research; are we there yet? Pulm. Circ. 2013, 3, 739–756. [Google Scholar] [CrossRef]
- Greshoff, M. Mittheilungen aus dem chemisch-pharmakologischen Laboratorium des Botanischen Gartens zu Buitenzorg (Java). Ber. Dtsch. Chem. Ges. 1890, 23, 3537–3550. [Google Scholar] [CrossRef]
- Neal, W.M.; Rusoff, L.L.; Ahmann, C.F. The Isolation and Some Properties of an Alkaloid from Crotalaria spectabilis. J. Am. Chem. Soc. 1935, 57, 2560–2561. [Google Scholar] [CrossRef]
- Adams, R.; Rogers, E. The structure of monocrotaline, the alkaloid in Crotalaria spectabilis and Crotalaria retusa. J. Am. Chem. Soc. 1938, 61, 2815–2819. [Google Scholar] [CrossRef]
- Lalich, J.; Merkow, L. Pulmonary arteritis produced in rat by feeding Crotalaria spectabilis. Lab. Investig. 1961, 10, 744–750. [Google Scholar] [PubMed]
- Lalich, J.; Ehrhart, L. Monocrotaline-induced pulmonary arteritis in rats. J. Atheroscler. Res. 1962, 2, 482–492. [Google Scholar] [CrossRef]
- Kay, J.M.; Harris, P.; Heath, D. Pulmonary hypertension produced in rats by ingestion of Crotalaria spectabilis seeds. Thorax 1967, 22, 176–179. [Google Scholar] [CrossRef]
- D’Alonzo, G.E.; Barst, R.J.; Ayres, S.M.; Bergofsky, E.H.; Brundage, B.H.; Detre, K.M.; Fishman, A.P.; Goldring, R.M.; Groves, B.M.; Kernis, J.T.; et al. Survival in patients with primary pulmonary hypertension. Results from a national prospective registry. Ann. Intern. Med. 1991, 115, 343–349. [Google Scholar] [CrossRef]
- Wilson, D.W.; Segall, H.J.; Pan, L.C.; Lamé, M.W.; Estep, J.E.; Morin, D. Mechanisms and pathology of monocrotaline pulmonary toxicity. Crit. Rev. Toxicol. 1992, 22, 307–325. [Google Scholar] [CrossRef] [PubMed]
- Mattocks, A.; White, I. The conversion of pyrrolizidine alkaloids to N-oxides and to dihydropyrrolizine derivatives by rat-liver microsomes in vitro. Chem. Biol. Interact. 1971, 3, 383–396. [Google Scholar] [CrossRef]
- Kasahara, Y.; Kiyatake, K.; Tatsumi, K.; Sugito, K.; Kakusaka, I.; Yamagata, S.-I.; Ohmori, S.; Kitada, M.; Kuriyama, T. Bioactivation of monocrotaline by P-450 3A in rat liver. J. Cardiovasc. Pharmacol. 1997, 30, 124–129. [Google Scholar] [CrossRef]
- Mattocks, A.R. Toxicity of pyrrolizidine alkaloids. Nature 1968, 217, 723–728. [Google Scholar] [CrossRef]
- Mattocks, A.R.; White, I.N.H. Pyrrolic metabolites from non-toxic pyrrolizidine alkaloids. Nat. New Biol. 1971, 231, 114–115. [Google Scholar] [CrossRef]
- Bruner, L.H.; Carpenter, L.J.; Hamlow, P.; Roth, R.A. Effect of a mixed function oxidase inducer and inhibitor on monocrotaline pyrrole pneumotoxicity. Toxicol. Appl. Pharmacol. 1986, 85, 416–427. [Google Scholar] [CrossRef] [PubMed]
- Hanwell, A.; Linzell, J. Validation of the thermodilution technique for the estimation of cardiac output in the rat. Comp. Biochem. Physiol. A Comp. Physiol. 1972, 41, 647–657. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.C.; Lamé, M.W.; Morin, D.; Wilson, D.W.; Segall, H. Red blood cells augment transport of reactive metabolites of monocrotaline from liver to lung in isolated and tandem liver and lung preparations. Toxicol. Appl. Pharmacol. 1991, 110, 336–346. [Google Scholar] [CrossRef]
- E Estep, J.; Lamé, M.W.; Morin, D.; Jones, A.D.; Wilson, D.W.; Segall, H.J. [14C]monocrotaline kinetics and metabolism in the rat. Drug Metab. Dispos. 1991, 19, 135–139. [Google Scholar]
- Wilson, D.W.; Segall, H.; Pan, L.C.; Dunston, S.K. Progressive inflammatory and structural changes in the pulmonary vasculature of monocrotaline-treated rats. Microvasc. Res. 1989, 38, 57–80. [Google Scholar] [CrossRef]
- Meyrick, B.; Reid, L. Development of pulmonary arterial changes in rats fed Crotalaria spectabilis. Am. J. Pathol. 1979, 94, 37–50. [Google Scholar]
- Meyrick, B.; Gamble, W.; Reid, L. Development of Crotalaria pulmonary hypertension: Hemodynamic and structural study. Am. J. Physiol. 1980, 239, H692–H702. [Google Scholar] [CrossRef]
- Sardu, C.; Paolisso, G.; Marfella, R. Inflammatory Related Cardiovascular Diseases: From Molecular Mechanisms to Therapeutic Targets. Curr. Pharm. Des. 2020, 26, 2565–2573. [Google Scholar] [CrossRef] [PubMed]
- Hilliker, K.S.; Bell, T.G.; Roth, R.A. Pneumotoxicity and thrombocytopenia after single injection of monocrotaline. Am. J. Physiol. 1982, 242, H573–H579. [Google Scholar] [CrossRef]
- Buermans, H.P.J.; Redout, E.M.; Schiel, A.E.; Musters, R.J.P.; Zuidwijk, M.; Eijk, P.P.; van Hardeveld, C.; Kasanmoentalib, S.; Visser, F.C.; Ylstra, B.; et al. Microarray analysis reveals pivotal divergent mRNA expression profiles early in the development of either compensated ventricular hypertrophy or heart failure. Physiol. Genom. 2005, 21, 314–323. [Google Scholar] [CrossRef]
- Mathew, R.; Altura, B.; Altura, B. Strain differences in pulmonary hypertensive response to monocrotaline alkaloid and the beneficial effect of oral magnesium treatment. Magnesium 1989, 8, 110–116. [Google Scholar]
- Goldenthal, E.I.; D’Aguanno, W.; Lynch, J.F. Hormonal Modification of the Sex Differences Following Monocrotaline Administration. Toxicol. Appl. Pharmacol. 1964, 6, 434–441. [Google Scholar] [CrossRef]
- Tofovic, P.S.; Zhang, X.; Petrusevska, G. Progesterone inhibits vascular remodeling and attenuates monocrotaline-induced pulmonary hypertension in estrogen-deficient rats. Prilozi 2009, 30, 25–44. [Google Scholar]
- Lookin, O.; Kuznetsov, D.; Protsenko, Y. Sex differences in stretch-dependent effects on tension and Ca(2+) transient of rat trabeculae in monocrotaline pulmonary hypertension. J. Physiol. Sci. 2015, 65, 89–98. [Google Scholar] [CrossRef]
- Silveyra, P.; Fuentes, N.; Bauza, D.E.R. Sex and Gender Differences in Lung Disease. Adv. Exp. Med. Biol. 2021, 1304, 227–258. [Google Scholar]
- Nogueira-Ferreira, R.; Vitorino, R.; Ferreira, R.; Henriques-Coelho, T. Exploring the monocrotaline animal model for the study of pulmonary arterial hypertension: A network approach. Pulm. Pharmacol. Ther. 2015, 35, 8–16. [Google Scholar] [CrossRef]
- Lai, Y.L.; Olson, J.W.; Gillespie, M.N.; Gomez-Arroyo, J.G.; Farkas, L.; Alhussaini, A.A.; Farkas, D.; Kraskauskas, D.; Voelkel, N.F.; Bogaard, H.J.; et al. Ventilatory dysfunction precedes pulmonary vascular changes in monocrotaline-treated rats. J. Appl. Physiol. 1991, 70, 561–566. [Google Scholar] [CrossRef]
- Hardziyenka, M.; Campian, M.E.; de Bruin-Bon, H.R.; Michel, M.C.; Tan, H.L. Sequence of echocardiographic changes during development of right ventricular failure in rat. J. Am. Soc. Echocardiogr. 2006, 19, 1272–1279. [Google Scholar] [CrossRef]
- Power, A.S. Mitochondrial Energy Transfer in Animal Models of Left and Right Ventricular Hypertrophy: Investigating Mitochondrial Oxidative Phosphorylation and Calcium Handling in the Heart and Their Contribution to Contractile Dysfunction in Cardiac Hypertrophy. Ph.D. Thesis, University of Auckland, Auckland, New Zealand, 2017. [Google Scholar]
- Power, A.S.; Hickey, A.J.; Crossman, D.J.; Loiselle, D.S.; Ward, M.-L. Calcium mishandling impairs contraction in right ventricular hypertrophy prior to overt heart failure. Pflug. Arch. 2018, 470, 1115–1126. [Google Scholar] [CrossRef]
- Krstic, A.M.; Kaur, S.; Ward, M.-L. Response of non-failing hypertrophic rat hearts to prostaglandin F2α. Curr. Res. Physiol. 2020, 2, 1–11. [Google Scholar] [CrossRef]
- Krstic, A.M.; Power, A.S.; Ward, M.-L. Increased Mitochondrial Calcium Fluxes in Hypertrophic Right Ventricular Cardiomyocytes from a Rat Model of Pulmonary Artery Hypertension. Life 2023, 13, 540. [Google Scholar] [CrossRef]
- Lookin, O.; Kuznetsov, D.; Protsenko, Y. Omecamtiv mecarbil attenuates length-tension relationship in healthy rat myocardium and preserves it in monocrotaline-induced pulmonary heart failure. Clin. Exp. Pharmacol. Physiol. 2022, 49, 84–93. [Google Scholar] [CrossRef]
- Fowler, E.D.; Hauton, D.; Boyle, J.; Egginton, S.; Steele, D.S.; White, E. Energy Metabolism in the Failing Right Ventricle: Limitations of Oxygen Delivery and the Creatine Kinase System. Int. J. Mol. Sci. 2019, 20, 1805. [Google Scholar] [CrossRef]
- Han, J.-C.; Guild, S.-J.; Pham, T.; Nisbet, L.; Tran, K.; Taberner, A.J.; Loiselle, D.S. Left-Ventricular Energetics in Pulmonary Arterial Hypertension-Induced Right-Ventricular Hypertrophic Failure. Front. Physiol. 2017, 8, 1115. [Google Scholar] [CrossRef]
- Fowler, E.D.; Benoist, D.; Drinkhill, M.J.; Stones, R.; Helmes, M.; Wüst, R.C.; Stienen, G.J.; Steele, D.S.; White, E. Decreased creatine kinase is linked to diastolic dysfunction in rats with right heart failure induced by pulmonary artery hypertension. J. Mol. Cell. Cardiol. 2015, 86, 1–8. [Google Scholar] [CrossRef]
- Krstic, A.M.; Power, A.S.; Ward, M.-L. Visualization of Dynamic Mitochondrial Calcium Fluxes in Isolated Cardiomyocytes. Front. Physiol. 2022, 12, 808798. [Google Scholar] [CrossRef]
- Pham, T.; Loiselle, D.; Power, A.; Hickey, A.J.R. Mitochondrial inefficiencies and anoxic ATP hydrolysis capacities in diabetic rat heart. Am. J. Physiol. Cell Physiol. 2014, 307, C499–C507. [Google Scholar] [CrossRef]
- Power, A.S.; Norman, R.; Jones, T.L.M.; Hickey, A.J.; Ward, M.-L. Mitochondrial function remains impaired in the hypertrophied right ventricle of pulmonary hypertensive rats following short duration metoprolol treatment. PLoS ONE 2019, 14, e0214740. [Google Scholar] [CrossRef]
- Pham, T.; Nisbet, L.; Taberner, A.; Loiselle, D.; Han, J. Pulmonary arterial hypertension reduces energy efficiency of right, but not left, rat ventricular trabeculae. J. Physiol. 2018, 596, 1153–1166. [Google Scholar] [CrossRef] [PubMed]
- Korstjens, I.; Rouws, C.; Van Der Laarse, W.; Van Der Zee, L.; Stienen, G. Myocardial force development and structural changes associated with monocrotaline induced cardiac hypertrophy and heart failure. J. Muscle Res. Cell Motil. 2002, 23, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Kögler, H.; Hartmann, O.; Leineweber, K.; Nguyen van, P.; Schott, P.; Brodde, O.E.; Hasenfuss, G. Mechanical load-dependent regulation of gene expression in monocrotaline-induced right ventricular hypertrophy in the rat. Circ. Res. 2003, 93, 230–237. [Google Scholar] [CrossRef]
- Lamberts, R.R.; Hamdani, N.; Soekhoe, T.W.; Boontje, N.M.; Zaremba, R.; Walker, L.A.; De Tombe, P.P.; Van Der Velden, J.; Stienen, G.J. Frequency-dependent myofilament Ca2+ desensitization in failing rat myocardium. J. Physiol. 2007, 582 Pt 2, 695–709. [Google Scholar] [CrossRef]
- Miura, M.; Hirose, M.; Endoh, H.; Wakayama, Y.; Sugai, Y.; Nakano, M.; Fukuda, K.; Shindoh, C.; Shirato, K.; Shimokawa, H. Acceleration of Ca2+ waves in monocrotaline-induced right ventricular hypertrophy in the rat. Circ. J. Off. J. Jpn. Circ. Soc. 2011, 75, 1343–1349. [Google Scholar] [CrossRef] [PubMed]
- Wüst, R.C.; de Vries, H.J.; Wintjes, L.T.; Rodenburg, R.J.; Niessen, H.W.; Stienen, G.J. Mitochondrial complex I dysfunction and altered NAD(P)H kinetics in rat myocardium in cardiac right ventricular hypertrophy and failure. Cardiovasc. Res. 2016, 111, 362–372. [Google Scholar] [CrossRef] [PubMed]
- Werchan, P.M.; Summer, W.R.; Gerdes, A.M.; McDonough, K.H. Right ventricular performance after monocrotaline-induced pulmonary hypertension. Am. J. Physiol. 1989, 256 Pt 2, H1328–H1336. [Google Scholar] [CrossRef]
- Vescovo, G.; Harding, S.E.; Jones, M.; Libera, L.D.; Pessina, A.C.; Poole-Wilson, P.A. Contractile abnormalities of single right ventricular myocytes isolated from rats with right ventricular hypertrophy. J. Mol. Cell. Cardiol. 1989, 21 (Suppl. S5), 103–111. [Google Scholar] [CrossRef] [PubMed]
- Honda, M.; Kuramochi, T.; Ishinaga, Y.; Kuzuo, H.; Tanaka, K.; Morioka, S.; Enornoto, K.I.; Takabatake, T. Contrasting effects of isoproterenol and phosphodiesterase III inhibitor on intracellular calcium transients in cardiac myocytes from failing hearts. Clin. Exp. Pharmacol. Physiol. 1994, 21, 1001–1008. [Google Scholar] [CrossRef]
- Benoist, D.; Stones, R.; Drinkhill, M.J.; Benson, A.P.; Yang, Z.; Cassan, C.; Gilbert, S.H.; Saint, D.A.; Cazorla, O.; Steele, D.S.; et al. Cardiac arrhythmia mechanisms in rats with heart failure induced by pulmonary hypertension. Am. J. Physiol. Heart Circ. Physiol. 2012, 302, H2381–H2395. [Google Scholar] [CrossRef]
- Umar, S.; Lee, J.-H.; de Lange, E.; Iorga, A.; Partow-Navid, R.; Bapat, A.; van der Laarse, A.; Saggar, R.; Saggar, R.; Ypey, D.L.; et al. Spontaneous ventricular fibrillation in right ventricular failure secondary to chronic pulmonary hypertension. Circ. Arrhythm. Electrophysiol. 2012, 5, 181–190. [Google Scholar] [CrossRef]
- Benoist, D.; Stones, R.; Drinkhill, M.J.; Bernus, O.; White, E.; Natali, A.J.; Fowler, E.D.; Calaghan, S.C.; Benson, A.P.; Yang, Z.; et al. Arrhythmogenic substrate in hearts of rats with monocrotaline-induced pulmonary hypertension and right ventricular hypertrophy. Am. J. Physiol. Heart Circ. Physiol. 2011, 300, H2230–H2237. [Google Scholar] [CrossRef]
- Bers, D.M. Cardiac excitation-contraction coupling. Nature 2002, 415, 198–205. [Google Scholar] [CrossRef]
- Fabiato, A. Calcium-induced release of calcium from the cardiac sarcoplasmic reticulum. Am. J. Physiol. 1983, 245, C1–C14. [Google Scholar] [CrossRef]
- Lee, J.-K.; Kodama, I.; Honjo, H.; Anno, T.; Kamiya, K.; Toyama, J.; Benoist, D.; Stones, R.; Drinkhill, M.J.; Benson, A.P.; et al. Stage-dependent changes in membrane currents in rats with monocrotaline-induced right ventricular hypertrophy. Am. J. Physiol. 1997, 272 Pt 2, H2833–H2842. [Google Scholar] [CrossRef] [PubMed]
- Power, A.; Kaur, S.; Dyer, C.; Ward, M.-L. Disruption of Transverse-Tubules Eliminates the Slow Force Response to Stretch in Isolated Rat Trabeculae. Front. Physiol. 2020, 11, 193. [Google Scholar] [CrossRef]
- Bristow, M.R.; Minobe, W.; Rasmussen, R.; Larrabee, P.; Skerl, L.; Klein, J.W.; Anderson, F.L.; Murray, J.; Mestroni, L.; Karwande, S.V. Beta-adrenergic neuroeffector abnormalities in the failing human heart are produced by local rather than systemic mechanisms. J. Clin. Investig. 1992, 89, 803–815. [Google Scholar] [CrossRef] [PubMed]
- Krstic, A.; Ward, M.-L. Ca2+ Handling in Non-Failing Hypertrophic Cardiomyocytes Subjected to Inotropic Interventions. Biophys. J. 2021, 120, 110a–111a. [Google Scholar] [CrossRef]
- Jonigk, D.; Golpon, H.; Bockmeyer, C.L.; Maegel, L.; Hoeper, M.M.; Gottlieb, J.; Nickel, N.; Hussein, K.; Maus, U.; Lehmann, U.; et al. Plexiform lesions in pulmonary arterial hypertension composition, architecture, and microenvironment. Am. J. Pathol. 2011, 179, 167–179. [Google Scholar] [CrossRef]
- Ruiter, G.; de Man, F.S.; Schalij, I.; Sairras, S.; Grünberg, K.; Westerhof, N.; van der Laarse, W.J.; Vonk-Noordegraaf, A. Reversibility of the monocrotaline pulmonary hypertension rat model. Eur. Respir. J. 2013, 42, 553–556. [Google Scholar] [CrossRef]
- Faber, M.J.; Dalinghaus, M.; Lankhuizen, I.M.; Steendijk, P.; Hop, W.C.; Schoemaker, R.G.; Duncker, D.J.; Lamers, J.M.J.; Helbing, W.A. Right and left ventricular function after chronic pulmonary artery banding in rats assessed with biventricular pressure-volume loops. Am. J. Physiol. Heart Circ. Physiol. 2006, 291, H1580–H1586. [Google Scholar] [CrossRef]
- Hessel, M.H.M.; Steendijk, P.; Adel, B.D.; Schutte, C.I.; van der Laarse, A. Characterization of right ventricular function after monocrotaline-induced pulmonary hypertension in the intact rat. Am. J. Physiol. Heart Circ. Physiol. 2006, 291, H2424–H2430. [Google Scholar] [CrossRef]
| CON (n = 11) | MCT (n = 10) | |
|---|---|---|
| Body weight (g) | 444 ± 9 | 386 ± 5 *** |
| RV–BW (%) | 0.36 ± 0.03 | 0.61 ± 0.02 *** |
| LV–BW (%) | 0.72 ± 0.09 | 0.89 ± 0.09 |
| Tibial length (mm) | 54.51 ± 0.87 | 51.98 ± 0.54 * |
| RV–tibial length (%) | 2.95 ± 0.22 | 4.58 ± 0.15 *** |
| LV–tibial length (%) | 6.93 ± 0.30 | 7.12 ± 0.37 |
| Lung weight (g) | 1.80 ± 0.1 | 2.2 ± 0.1 ** |
| Liver weight (g) | 15.40 ± 0.48 | 14.5 ± 0.03 |
| Lung weight–BW (%) | 0.39 ± 0.02 | 0.56 ± 0.03 *** |
| Heart rate (min−1) | 367 ± 23 | 340 ± 13 ** |
| QTc interval (s) | 0.14 ± 0.01 | 0.15 ± 0.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krstic, A.M.; Jones, T.L.M.; Power, A.S.; Ward, M.-L. The Monocrotaline Rat Model of Right Heart Disease Induced by Pulmonary Artery Hypertension. Biomedicines 2024, 12, 1944. https://doi.org/10.3390/biomedicines12091944
Krstic AM, Jones TLM, Power AS, Ward M-L. The Monocrotaline Rat Model of Right Heart Disease Induced by Pulmonary Artery Hypertension. Biomedicines. 2024; 12(9):1944. https://doi.org/10.3390/biomedicines12091944
Chicago/Turabian StyleKrstic, Anna Maria, Timothy L. M. Jones, Amelia S. Power, and Marie-Louise Ward. 2024. "The Monocrotaline Rat Model of Right Heart Disease Induced by Pulmonary Artery Hypertension" Biomedicines 12, no. 9: 1944. https://doi.org/10.3390/biomedicines12091944
APA StyleKrstic, A. M., Jones, T. L. M., Power, A. S., & Ward, M.-L. (2024). The Monocrotaline Rat Model of Right Heart Disease Induced by Pulmonary Artery Hypertension. Biomedicines, 12(9), 1944. https://doi.org/10.3390/biomedicines12091944






