Therapeutic Efficacy of Interferon-Gamma and Hypoxia-Primed Mesenchymal Stromal Cells and Their Extracellular Vesicles: Underlying Mechanisms and Potentials in Clinical Translation
Abstract
1. Introduction
2. MSCs Priming with IFN-γ
3. Phenotypic Characterization of Primed MSCs
4. IFN-γ Concentrations and Durations
5. The Therapeutic Effects of IFN-γ-Primed MSCs (The Relationship between In Vitro or Functional Markers with Therapeutic Effects)
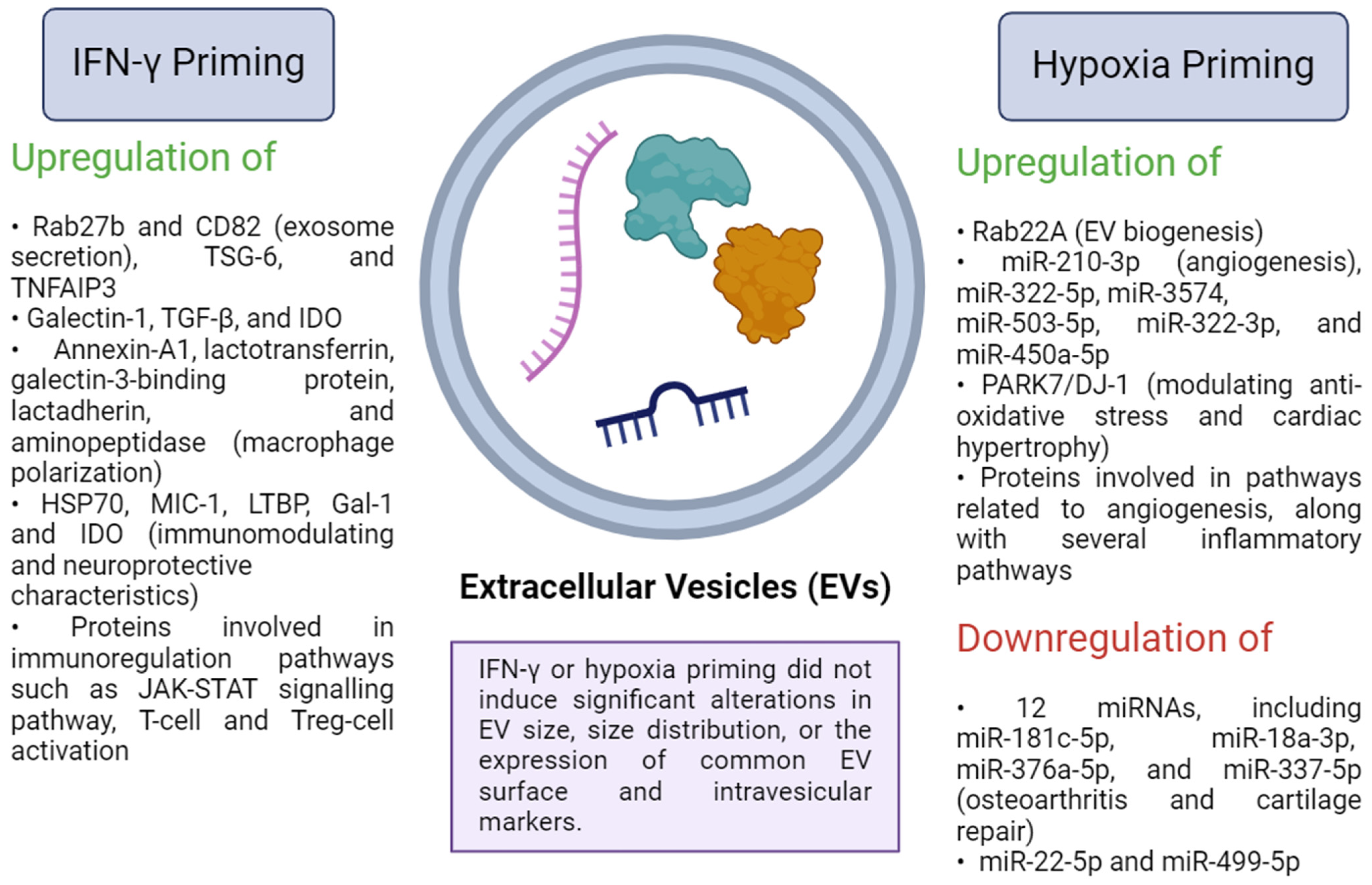
6. Extracellular Vesicles Derived from IFN-γ-Primed MSCs
7. Long-Term Safety and Efficacy of IFN-γ Priming
8. Signalling Pathways and Mechanisms of Action of IFN-γ Priming
9. MSCs Priming with Hypoxia
10. Phenotypic Characterization of Primed MSCs
11. Hypoxia Priming Methods (Oxygen Levels) and Durations
12. Therapeutic Effects of Hypoxia-Primed MSCs (The Relationship between In Vitro or Functional Markers with Therapeutic Effects)
13. Extracellular Vesicles Derived from Hypoxia-Primed MSCs
14. Long-Term Safety and Efficacy of Hypoxia Priming
15. Signalling Pathways and Mechanisms of Action of Hypoxia Priming
16. Primed MSC in Clinical Trials
17. Ethical and Regulatory Considerations
18. Conclusions and Future Recommendations
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MSCs | Mesenchymal stromal cells |
| IFN-γ | Interferon-gamma |
| EVs | Extracellular vesicles |
| JAK/STAT | Janus kinase/signal transducer and activator of transcription |
| PI3K/AKT | Phosphoinositide 3-kinase/Ak strain transforming |
| CD | Clusters of differentiation |
| TNF-α | Tumour necrosis factor-alpha |
| IL- | Interleukin- |
| NF-κB | Nuclear factor kappa light chain enhancer of activated B cells |
| IDO | Indoleamine 2,3-dioxygenase |
| PGE2 | Prostaglandin E2 |
| TGF-β | Transforming growth factor-beta |
| HLA | Histocompatibility leucocyte antigen |
| PBMCs | Peripheral blood mononuclear cells |
| DNA | Deoxyribonucleic acid |
| hWJ-MSCs | Human Wharton’s Jelly MSCs |
| HLA-DR | Human leukocyte antigen—DR isotype |
| BM-MSCs | Bone marrow-MSCs |
| U/mL | Units per millilitre |
| IU/mL | International units per millilitre |
| MI | Myocardial infarction |
| CXCL | C-X-C motif chemokine ligand |
| COX-2 | Cyclooxygenase-2 |
| LPS | Lipopolysaccharide |
| Poly I | C |
| Polyinosinic | polycytidylic acid |
| EAE | Experimental autoimmune encephalomyelitis |
| HGF | Hepatocyte growth factor |
| VEGF | Vascular endothelial growth factor |
| MMP-3 | Matrix metalloproteinase-3 |
| CCL | C-C motif chemokine ligand |
| MHC | Major histocompatibility complex |
| PD-L1 | Programmed death-ligand 1 |
| ECAR | Extracellular acidification rate |
| C-MSCs | MSC extracellular matrix (ECM) complex |
| TSG-6 | TNF-stimulated gene-6 |
| GVHD | Graft-versus-host disease |
| IEQ | Islet equivalent |
| PFO | Pericapsular fibrotic overgrowth |
| KYNA | Kynurenic acid |
| AT | Adipose tissue |
| Rab | Ras-associated binding protein |
| TNFAIP3 | Tumour necrosis factor-alpha-induced protein 3 |
| iPSC-MSC | Induced pluripotent stem cell-MSC |
| MCP-1 | Monocyte chemoattractant protein-1 |
| HSP70 | Heat shock protein 70 |
| MIC-1 | Macrophage inhibitory cytokine 1 |
| LTBP | Latent transforming growth factor-binding protein |
| Gal-1 | Galectin-1 |
| eNOS | Endothelial nitric oxide synthase |
| UC-MSCs | Umbilical cord-MSCs |
| IRI | Ischemia-reperfusion injury |
| HIF-1 | Hypoxia-inducible factor 1 |
| bFGF | Basic fibroblast growth factor |
| OCR | Oxygen consumption rate |
| PARK7/DJ-1 | Parkinson’s disease protein 7 |
| AT1R | Angiotensin II type 1 receptor |
| ATRAP | AT1R-associated protein |
| ATP | Adenosine triphosphate |
| ROS | Reactive oxygen species |
| ALB | Albumin |
| MAPK1 | Mitogen-activated protein kinase 1 |
| ERK | Extracellular signal-regulated kinase |
| SDF-1 | Stromal cell-derived factor 1 |
| CXCR4 | C-X-C chemokine receptor type 4 |
| Smad | Suppressor of mothers against decapentaplegic |
| α-SMA | Alpha-smooth muscle actin |
| TLR3 | Toll-like receptor 3 |
| Foxp3 | Forkhead box P3 |
| ROR-γt | Retinoic acid-related orphan receptor gamma t |
| RTK | Receptor tyrosine kinase |
| Th | Helper T cells |
| Tregs | Regulatory T cells |
| PSMB10 | Proteasome subunit beta type 10 |
| GMP | Good manufacturing practice |
References
- Hmadcha, A.; Martin-Montalvo, A.; Gauthier, B.R.; Soria, B.; Capilla-Gonzalez, V. Therapeutic Potential of Mesenchymal Stem Cells for Cancer Therapy. Front. Bioeng. Biotechnol. 2020, 8, 43. [Google Scholar] [CrossRef]
- Murray, I.R.; Péault, B. Q&A: Mesenchymal stem cells—Where do they come from and is it important? BMC Biol. 2015, 13, 99. [Google Scholar] [CrossRef]
- Miceli, V.; Bulati, M.; Iannolo, G.; Zito, G.; Gallo, A.; Conaldi, P.G. Therapeutic properties of mesenchymal stromal/stem cells: The need of cell priming for cell-free therapies in regenerative medicine. Int. J. Mol. Sci. 2021, 22, 763. [Google Scholar] [CrossRef]
- Hafez, P.; Chowdhury, S.R.; Jose, S.; Law, J.X.; Ruszymah, B.H.I.; Mohd Ramzisham, A.R.; Ng, M.H. Development of an In Vitro Cardiac Ischemic Model Using Primary Human Cardiomyocytes. Cardiovasc. Eng. Technol. 2018, 9, 529–538. [Google Scholar] [CrossRef]
- Lim, J.; Razi, Z.R.M.; Law, J.X.; Nawi, A.M.; Idrus, R.B.H.; Chin, T.G.; Mustangin, M.; Ng, M.H. Mesenchymal Stromal Cells from the Maternal Segment of Human Umbilical Cord is Ideal for Bone Regeneration in Allogenic Setting. Tissue Eng. Regen. Med. 2018, 15, 75–87. [Google Scholar] [CrossRef]
- Rashidbenam, Z.; Jasman, M.H.; Tan, G.H.; Goh, E.H.; Fam, X.I.; Ho, C.C.K.; Zainuddin, Z.M.; Rajan, R.; Rani, R.A.; Nor, F.M.; et al. Fabrication of adipose-derived stem cell-based self-assembled scaffold under hypoxia and mechanical stimulation for urethral tissue engineering. Int. J. Mol. Sci. 2021, 22, 3350. [Google Scholar] [CrossRef]
- Noronha, N.D.C.; Mizukami, A.; Caliári-Oliveira, C.; Cominal, J.G.; Rocha, J.L.M.; Covas, D.T.; Swiech, K.; Malmegrim, K.C.R. Correction to: Priming approaches to improve the efficacy of mesenchymal stromal cell-based therapies. Stem Cell Res. Ther. 2019, 10, 131. [Google Scholar] [CrossRef]
- Yao, M.; Chen, Z.; He, X.; Long, J.; Xia, X.; Li, Z.; Yang, Y.; Ao, L.; Xing, W.; Lian, Q.; et al. Cross talk between glucose metabolism and immunosuppression in IFN-γ–primed mesenchymal stem cells. Life Sci. Alliance 2022, 5, 1–13. [Google Scholar] [CrossRef]
- Fan, X.L.; Zhang, Y.; Li, X.; Fu, Q.L. Mechanisms underlying the protective effects of mesenchymal stem cell-based therapy. Cell. Mol. Life Sci. 2020, 77, 2771–2794. [Google Scholar] [CrossRef] [PubMed]
- Cheng, A.; Choi, D.; Lora, M.; Shum-Tim, D.; Rak, J.; Colmegna, I. Human multipotent mesenchymal stromal cells cytokine priming promotes RAB27B-regulated secretion of small extracellular vesicles with immunomodulatory cargo. Stem Cell Res. Ther. 2020, 11, 539. [Google Scholar] [CrossRef] [PubMed]
- Harting, M.T.; Srivastava, A.K.; Zhaorigetu, S.; Bair, H.; Prabhakara, K.S.; Toledano Furman, N.E.; Vykoukal, J.V.; Ruppert, K.A.; Cox, C.S.; Olson, S.D. Inflammation-Stimulated Mesenchymal Stromal Cell-Derived Extracellular Vesicles Attenuate Inflammation. Stem Cells 2018, 36, 79–90. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Yuan, Z.; Weng, J.; Pei, D.; Du, X.; He, C.; Lai, P. Challenges and advances in clinical applications of mesenchymal stromal cells. J. Hematol. Oncol. 2021, 14, 24. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Dai, J.; Zhang, X.A. Environmental physical cues determine the lineage specification of mesenchymal stem cells. Biochim. Biophys. Acta Gen. Subj. 2015, 1850, 1261–1266. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Li, L. Preconditioning influences mesenchymal stem cell properties in vitro and in vivo. J. Cell Mol. Med. 2018, 22, 1428–1442. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Tsai, T.L.; Li, W.J. Strategies to retain properties of bone marrow-derived mesenchymal stem cells ex vivo. Ann. N. Y. Acad. Sci. 2017, 1409, 3–17. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, X.; Cao, W.; Shi, Y. Plasticity of mesenchymal stem cells in immunomodulation: Pathological and therapeutic implications. Nat. Immunol. 2014, 15, 1009–1016. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.N.; Shin, J.Y.; Kim, D.Y.; Lee, J.E.; Lee, P.H. Priming mesenchymal stem cells with uric acid enhances neuroprotective properties in parkinsonian models. J. Tissue Eng. 2021, 12, 20417314211004816. [Google Scholar] [CrossRef] [PubMed]
- Uberti, B.; Plaza, A.; Henríquez, C. Pre-conditioning Strategies for Mesenchymal Stromal/Stem Cells in Inflammatory Conditions of Livestock Species. Front. Vet. Sci. 2022, 9, 806069. [Google Scholar] [CrossRef] [PubMed]
- Prasanna, S.J.; Gopalakrishnan, D.; Shankar, S.R.; Vasandan, A.B. Pro-Inflammatory Cytokines, IFNγ and TNFα, Influence Immune Properties of Human Bone Marrow and Wharton Jelly Mesenchymal Stem Cells Differentially. PLoS ONE 2010, 5, e9016. [Google Scholar] [CrossRef] [PubMed]
- de Witte, S.F.H.; Franquesa, M.; Baan, C.C.; Hoogduijn, M.J. Toward development of imesenchymal stem cells for immunomodulatory therapy. Front. Immunol. 2016, 6, 168716. [Google Scholar] [CrossRef][Green Version]
- Zhang, J.; Lu, Y.; Mao, Y.; Yu, Y.; Wu, T.; Zhao, W.; Zhu, Y.; Zhao, P.; Zhang, F. IFN-γ enhances the efficacy of mesenchymal stromal cell-derived exosomes via miR-21 in myocardial infarction rats. Stem Cell Res. Ther. 2022, 13, 333. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, C.; Zhou, L.; Wang, Z.; Gao, W.; Chen, W.; Zhang, H.; Jing, B.; Zhu, X.; Chen, L.; Zheng, C.; et al. Eradication of specific donor-dependent variations of mesenchymal stem cells in immunomodulation to enhance therapeutic values. Cell Death Dis. 2021, 12, 357. [Google Scholar] [CrossRef] [PubMed]
- Ng, C.Y.; Kee, L.T.; Al-Masawa, M.E.; Lee, Q.H.; Subramaniam, T.; Kok, D.; Ng, M.H.; Law, J.X. Scalable Production of Extracellular Vesicles and Its Therapeutic Values: A Review. Int. J. Mol. Sci. 2022, 23, 7986. [Google Scholar] [CrossRef] [PubMed]
- Bétous, R.; Renoud, M.L.; Hoede, C.; Gonzalez, I.; Jones, N.; Longy, M.; Sensebé, L.; Cazaux, C.; Hoffmann, J.-S. Human Adipose-Derived Stem Cells Expanded Under Ambient Oxygen Concentration Accumulate Oxidative DNA Lesions and Experience Procarcinogenic DNA Replication Stress. Stem Cells Transl. Med. 2017, 6, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Estrada, J.C.; Albo, C.; Benguría, A.; Dopazo, A.; López-Romero, P.; Carrera-Quintanar, L.; Roche, E.; Clemente, E.P.; Enríquez, J.A.; Bernad, A.; et al. Culture of human mesenchymal stem cells at low oxygen tension improves growth and genetic stability by activating glycolysis. Cell Death Differ. 2012, 19, 743–755. [Google Scholar] [CrossRef] [PubMed]
- Haque, N.; Rahman, M.T.; Abu Kasim, N.H.; Alabsi, A.M. Hypoxic culture conditions as a solution for mesenchymal stem cell based regenerative therapy. Sci. World J. 2013, 2013, 632972. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Xu, Y.; Zhong, Z.; Wu, Y.; Zhao, J.; Wang, Y.; Cheng, H.; Kong, M.; Zhang, F.; Chen, Q.; et al. A large-scale investigation of hypoxia-preconditioned allogeneic mesenchymal stem cells for myocardial repair in nonhuman primates: Paracrine activity without remuscularization. Circ. Res. 2016, 118, 970–983. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.C.; Jeong, H.J.; Lee, S.K.; Kim, S.J. Hypoxic Conditioned Medium From Human Adipose-Derived Stem Cells Promotes Mouse Liver Regeneration through JAK/STAT3 Signaling. Stem Cells Transl. Med. 2016, 5, 816–825. Available online: https://academic.oup.com/stcltm/article/5/6/816-825/6397762 (accessed on 30 January 2024). [CrossRef]
- Yu, J.; Yin, S.; Zhang, W.; Gao, F.; Liu, Y.; Chen, Z.; Zhang, M.; He, J.; Zheng, S. Hypoxia preconditioned bone marrow mesenchymal stem cells promote liver regeneration in a rat massive hepatectomy model. Stem Cell Res. Ther. 2013, 4, 83. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Huang, Q.M.; Liu, Y.; Zhou, J.; Tang, W.R.; Wang, X.Y.; Wang, L.F.; Zhang, Z.H.; Tan, H.L.; Guan, X.H.; et al. Long-term hypoxic hUCMSCs-derived extracellular vesicles alleviates allergic rhinitis through triggering immunotolerance of their VEGF-mediated inhibition of dendritic cells maturation. Int. Immunopharmacol. 2023, 124, 110875. [Google Scholar] [CrossRef]
- Farooqui, N.; Mohan, A.; Isik, B.; Goksu, B.B.; Thaler, R.; Zhu, X.Y.; Krier, J.D.; Saadiq, I.M.; Ferguson, C.M.; Jordan, K.L.; et al. Effect of Hypoxia Preconditioning on the Regenerative Capacity of Adipose Tissue Derived Mesenchymal Stem Cells in a Model of Renal Artery Stenosis. Stem Cells 2023, 41, 50–63. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Wang, Y.; Luo, X.; Gao, X.; Gu, W.; Ma, Y.; Xu, L.; Yu, M.; Liu, X.; Liu, J.; et al. A non-invasive strategy for suppressing asthmatic airway inflammation and remodeling: Inhalation of nebulized hypoxic hUCMSC-derived extracellular vesicles. Front. Immunol. 2023, 14, 1150971. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.H.; Jin, H.J.; Heo, J.; Ju, H.; Lee, H.Y.; Kim, S.; Lee, S.; Lim, J.; Jeong, S.Y.; Kwon, J.H.; et al. Small hypoxia-primed mesenchymal stem cells attenuate graft-versus-host disease. Leukemia 2018, 32, 2672–2684. [Google Scholar] [CrossRef] [PubMed]
- Miceli, V.; Zito, G.; Bulati, M.; Gallo, A.; Busà, R.; Iannolo, G.; Conaldi, P.G. Different priming strategies improve distinct therapeutic capabilities of mesenchymal stromal/stem cells: Potential implications for their clinical use. World J. Stem Cells 2023, 15, 400–420. Available online: https://www.wjgnet.com/1948-0210/full/v15/i5/400.htm (accessed on 18 December 2023). [CrossRef] [PubMed]
- Rozier, P.; Maumus, M.; Maria, A.T.J.; Toupet, K.; Jorgensen, C.; Guilpain, P.; Noël, D. Lung fibrosis is improved by extracellular vesicles from ifnγ-primed mesenchymal stromal cells in murine systemic sclerosis. Cells 2021, 10, 2727. [Google Scholar] [CrossRef] [PubMed]
- Barrachina, L.; Remacha, A.R.; Romero, A.; Vitoria, A.; Albareda, J.; Prades, M.; Roca, M.; Zaragoza, P.; Vázquez, F.J.; Rodellar, C. Assessment of effectiveness and safety of repeat administration of proinflammatory primed allogeneic mesenchymal stem cells in an equine model of chemically induced osteoarthritis. BMC Vet. Res. 2018, 14, 241. [Google Scholar] [CrossRef] [PubMed]
- Park, A.; Park, H.; Yoon, J.; Kang, D.; Kang, M.H.; Park, Y.Y.; Suh, N.; Yu, J. Priming with Toll-like receptor 3 agonist or interferon-gamma enhances the therapeutic effects of human mesenchymal stem cells in a murine model of atopic dermatitis. Stem Cell Res. Ther. 2019, 10, 66. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Feng, C.; Huang, P.; Li, Y.; Liu, R.; Liu, C.; Han, Y.; Chen, L.; Ding, Y.; Shao, C.; et al. TNFα and IFNγ rapidly activate PI3K-AKT signaling to drive glycolysis that confers mesenchymal stem cells enhanced anti-inflammatory property. Stem Cell Res. Ther. 2022, 13, 491. [Google Scholar] [CrossRef] [PubMed]
- Riazifar, M.; Mohammadi, M.R.; Pone, E.J.; Yeri, A.; Lasser, C.; Segaliny, A.I.; McIntyre, L.L.; Shelke, G.V.; Hutchins, E.; Hamamoto, A.; et al. Stem Cell-Derived Exosomes as Nanotherapeutics for Autoimmune and Neurodegenerative Disorders. ACS Nano 2019, 13, 6670–6688. [Google Scholar] [CrossRef]
- Ye, Y.; Zhang, X.; Su, D.; Ren, Y.; Cheng, F.; Yao, Y.; Shi, G.; Ji, Y.; Chen, S.; Shi, P.; et al. Therapeutic efficacy of human adipose mesenchymal stem cells in Crohn’s colon fibrosis is improved by IFN-γ and kynurenic acid priming through indoleamine 2,3-dioxygenase-1 signaling. Stem Cell Res. Ther. 2022, 13, 465. [Google Scholar] [CrossRef]
- Zhou, X.; Liu, X.; Liu, L.; Han, C.; Xie, Z.; Liu, X.; Xu, Y.; Li, F.; Bi, J.; Zheng, C. Transplantation of IFN-γ Primed hUCMSCs Significantly Improved Outcomes of Experimental Autoimmune Encephalomyelitis in a Mouse Model. Neurochem. Res. 2020, 45, 1510–1517. [Google Scholar] [CrossRef] [PubMed]
- Ling, X.; Wang, T.; Han, C.; Wang, P.; Liu, X.; Zheng, C.; Bi, J.; Zhou, X. IFN-γ-Primed hUCMSCs Significantly Reduced Inflammation via the Foxp3/ROR-γt/STAT3 Signaling Pathway in an Animal Model of Multiple Sclerosis. Front. Immunol. 2022, 13, 835345. [Google Scholar] [CrossRef]
- Baudry, N.; Starck, J.; Aussel, C.; Lund, K.; Aletti, M.; Duranteau, J.; Banzet, S.; Lataillade, J.J.; Vicaut, E.; Peltzer, J. Effect of Preconditioned Mesenchymal Stromal Cells on Early Microvascular Disturbance in a Mouse Sepsis Model. Stem Cells Dev. 2019, 28, 1595–1606. [Google Scholar] [CrossRef]
- Varkouhi, A.K.; Jerkic, M.; Ormesher, L.; Gagnon, S.; Goyal, S.; Rabani, R.; Masterson, C.; Spring, C.; Chen, P.Z.; Gu, F.X.; et al. Extracellular Vesicles from Interferon-γ–primed Human Umbilical Cord Mesenchymal Stromal Cells Reduce Escherichia coli –induced Acute Lung Injury in Rats. Anesthesiology 2019, 130, 778–790. Available online: https://pubs.asahq.org/anesthesiology/article/130/5/778/19042/Extracellular-Vesicles-from-Interferon-primed (accessed on 28 January 2024). [CrossRef]
- Takeshita, K.; Motoike, S.; Kajiya, M.; Komatsu, N.; Takewaki, M.; Ouhara, K.; Iwata, T.; Takeda, K.; Mizuno, N.; Fujita, T.; et al. Xenotransplantation of interferon-gamma-pretreated clumps of a human mesenchymal stem cell/extracellular matrix complex induces mouse calvarial bone regeneration. Stem Cell Res. Ther. 2017, 8, 101. [Google Scholar] [CrossRef] [PubMed]
- Khatab, S.; van Osch, G.J.V.M.; Kops, N.; Bastiaansen-Jenniskens, Y.M.; Bos, P.K.; Verhaar, J.A.N.; Bernsen, M.R.; Buul, G.M. Mesenchymal stem cell secretome reduces pain and prevents cartilage damage in a murine osteoarthritis model. Eur. Cell Mater. 2018, 36, 218–230. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Huang, H.; Cui, S.; Zhou, Y.; Zhang, T.; Zhou, Y. IFN-γ promoted exosomes from mesenchymal stem cells to attenuate colitis via miR-125a and miR-125b. Cell Death Dis. 2020, 11, 603. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, S.; Tsuchiya, A.; Iwasawa, T.; Nojiri, S.; Watanabe, T.; Ogawa, M.; Yoshida, T.; Fujiki, K.; Koui, Y.; Kido, T.; et al. Small extracellular vesicles derived from interferon-γ pre-conditioned mesenchymal stromal cells effectively treat liver fibrosis. NPJ Regen. Med. 2021, 6, 6–7. [Google Scholar] [CrossRef]
- Kim, D.S.; Jang, I.K.; Lee, M.W.; Ko, Y.J.; Lee, D.H.; Lee, J.W.; Sung, K.W.; Koo, H.H.; Yoo, K.H. Enhanced Immunosuppressive Properties of Human Mesenchymal Stem Cells Primed by Interferon-γ. EBioMedicine 2018, 28, 261–273. [Google Scholar] [CrossRef]
- Vaithilingam, V.; Evans, M.D.M.; Lewy, D.M.; Bean, P.A.; Bal, S.; Tuch, B.E. Co-encapsulation and co-transplantation of mesenchymal stem cells reduces pericapsular fibrosis and improves encapsulated islet survival and function when allografted. Sci. Rep. 2017, 7, 10059. [Google Scholar] [CrossRef]
- Tobin, L.M.; Healy, M.E.; English, K.; Mahon, B.P. Human mesenchymal stem cells suppress donor CD4+ T cell proliferation and reduce pathology in a humanized mouse model of acute graft-versus-host disease. Clin. Exp. Immunol. 2013, 172, 333–348. [Google Scholar] [CrossRef]
- Torkaman, M.; Ghollasi, M.; Mohammadnia-Afrouzi, M.; Salimi, A.; Amari, A. The effect of transplanted human Wharton’s jelly mesenchymal stem cells treated with IFN-γ on experimental autoimmune encephalomyelitis mice. Cell Immunol. 2017, 311, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Haan, M.C.D.; van Zuylen, V.L.; Pluijmert, N.J.; Schutte, C.I.; Fibbe, W.E.; Schalij, M.J.; Roelofs, H.; Atsma, D.E. Discrepant results of experimental human mesenchymal stromal cell therapy after myocardial infarction: Are animal models robust enough? PLoS ONE 2016, 11, e0152938. [Google Scholar]
- Grauss, R.W.; Winter, E.M.; van Tuyn, J.; Pijnappels, D.A.; Steijn, R.V.; Hogers, B.; van der Geest, R.J.; de Vries, A.A.F.; Steendijk, P.; van der Laarse, A.; et al. Mesenchymal stem cells from ischemic heart disease patients improve left ventricular function after acute myocardial infarction. Am. J. Physiol. Heart Circ. Physiol. 2007, 293, 2438–2447. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Boyt, D.T.; Boland, L.K.; Burand, A.J.; Brown, A.J.; Ankrum, J.A. Dose and duration of interferon γ pre-licensing interact with donor characteristics to influence the expression and function of indoleamine-2,3-dioxygenase in mesenchymal stromal cells. J. R. Soc. Interface 2020, 17, 20190815. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Wang, Y.; Li, Q.; Liu, K.; Hou, J.; Shao, C.; Wang, Y. Immunoregulatory mechanisms of mesenchymal stem and stromal cells in inflammatory diseases. Nat. Rev. Nephrol. 2018, 14, 493–507. [Google Scholar] [CrossRef] [PubMed]
- de Pedro, M.Á.; Gómez-Serrano, M.; Marinaro, F.; López, E.; Pulido, M.; Preußer, C.; von Strandmann, E.P.; Sánchez-Margallo, F.M.; Álvarez, V.; Casado, J.G. Ifn-gamma and tnf-alpha as a priming strategy to enhance the immunomodulatory capacity of secretomes from menstrual blood-derived stromal cells. Int. J. Mol. Sci. 2021, 22, 12177. [Google Scholar] [CrossRef] [PubMed]
- Ragni, E.; Perucca Orfei, C.; De Luca, P.; Mondadori, C.; Viganò, M.; Colombini, A.; de Girolamo, L. Inflammatory priming enhances mesenchymal stromal cell secretome potential as a clinical product for regenerative medicine approaches through secreted factors and EV-miRNAs: The example of joint disease. Stem Cell Res. Ther. 2020, 11, 165. Available online: https://stemcellres.biomedcentral.com/articles/10.1186/s13287-020-01677-9 (accessed on 28 January 2024). [CrossRef] [PubMed]
- Gornostaeva, A.; Andreeva, E.; Buravkova, L. Inflammatory priming of mesenchymal stem cells: Focus on growth factors enhancement. Biocell 2022, 46, 2049–2052. Available online: https://www.techscience.com/biocell/v46n9/47742 (accessed on 15 May 2024). [CrossRef]
- Li, C.; Li, G.; Liu, M.; Zhou, T.; Zhou, H. Paracrine effect of inflammatory cytokine-activated bone marrow mesenchymal stem cells and its role in osteoblast function. J. Biosci. Bioeng. 2016, 121, 213–219. Available online: https://linkinghub.elsevier.com/retrieve/pii/S1389172315002108 (accessed on 28 January 2024). [CrossRef]
- Liu, Y.; Han, Z.; Zhang, S.; Jing, Y.; Bu, X.; Wang, C.; Sun, K.; Jiang, G.; Zhao, X.; Li, R.; et al. Effects of Inflammatory Factors on Mesenchymal Stem Cells and Their Role in the Promotion of Tumor Angiogenesis in Colon Cancer. J. Biol. Chem. 2011, 286, 25007–25015. Available online: https://linkinghub.elsevier.com/retrieve/pii/S0021925819486626 (accessed on 20 February 2024). [CrossRef] [PubMed]
- Deuse, T.; Stubbendorff, M.; Tang-Quan, K.; Phillips, N.; Kay, M.A.; Eiermann, T.; Phan, T.T.; Volk, H.-D.; Reichenspurner, H.; Robbins, R.C.; et al. Immunogenicity and Immunomodulatory Properties of Umbilical Cord Lining Mesenchymal Stem Cells. Cell Transpl. 2011, 20, 655–667. Available online: http://journals.sagepub.com/doi/10.3727/096368910X536473 (accessed on 28 January 2024). [CrossRef] [PubMed]
- Jiang, W.; Xu, J. Immune modulation by mesenchymal stem cells. Cell Prolif. 2020, 53, e12712. Available online: https://onlinelibrary.wiley.com/doi/10.1111/cpr.12712 (accessed on 28 January 2024). [CrossRef] [PubMed]
- Weiss, M.L.; Anderson, C.; Medicetty, S.; Seshareddy, K.B.; Weiss, R.J.; VanderWerff, I.; Troyer, D.; McIntosh, K.R. Immune Properties of Human Umbilical Cord Wharton’s Jelly-Derived Cells. Stem Cells 2008, 26, 2865–2874. Available online: https://academic.oup.com/stmcls/article/26/11/2865-2874/6415549 (accessed on 20 February 2024). [CrossRef] [PubMed]
- Plas, D.R.; Rathmell, J.C.; Thompson, C.B. Homeostatic control of lymphocyte survival: Potential origins and implications. Nat. Immunol. 2002, 3, 515–521. [Google Scholar] [CrossRef] [PubMed]
- Frauwirth, K.A.; Thompson, C.B. Regulation of T Lymphocyte Metabolism. J. Immunol. 2004, 172, 4661–4665. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.G.; Thompson, C.B. Revving the Engine: Signal Transduction Fuels T Cell Activation. Immunity 2007, 27, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Mrahleh, M.A.; Matar, S.; Jafar, H.; Wehaibi, S.; Aslam, N.; Awidi, A. Human Wharton’s Jelly-Derived Mesenchymal Stromal Cells Primed by Tumor Necrosis Factor-α and Interferon-γ Modulate the Innate and Adaptive Immune Cells of Type 1 Diabetic Patients. Front. Immunol. 2021, 12, 732549. Available online: https://www.frontiersin.org/articles/10.3389/fimmu.2021.732549/full (accessed on 28 January 2024). [CrossRef] [PubMed]
- Wang, L.; Qi, C.; Cao, H.; Zhang, Y.; Liu, X.; Qiu, L.; Wang, H.; Xu, L.; Wu, Z.; Liu, J.; et al. Engineered Cytokine-Primed Extracellular Vesicles with High PD-L1 Expression Ameliorate Type 1 Diabetes. Small 2023, 19, 2301019. Available online: https://onlinelibrary.wiley.com/doi/10.1002/smll.202301019 (accessed on 30 May 2024). [CrossRef] [PubMed]
- Barachini, S.; Biso, L.; Kolachalam, S.; Petrini, I.; Maggio, R.; Scarselli, M.; Longoni, B. Mesenchymal Stem Cell in Pancreatic Islet Transplantation. Biomedicines 2023, 11, 1426. Available online: https://www.mdpi.com/2227-9059/11/5/1426 (accessed on 10 May 2024). [CrossRef]
- Gregory, G.A.; Robinson, T.I.G.; Linklater, S.E.; Wang, F.; Colagiuri, S.; de Beaufort, C.; Donaghue, K.C.; Magliano, D.J.; Maniam, J.; Orchard, T.J.; et al. Global incidence, prevalence, and mortality of type 1 diabetes in 2021 with projection to 2040: A modelling study. Lancet Diabetes Endocrinol. 2022, 10, 741–760. Available online: https://linkinghub.elsevier.com/retrieve/pii/S2213858722002182 (accessed on 10 May 2024). [CrossRef] [PubMed]
- Meisel, R.; Zibert, A.; Laryea, M.; Göbel, U.; Däubener, W.; Dilloo, D. Human bone marrow stromal cells inhibit allogeneic T-cell responses by indoleamine 2,3-dioxygenase–mediated tryptophan degradation. Blood 2004, 103, 4619–4621. Available online: https://ashpublications.org/blood/article/103/12/4619/131370/Human-bone-marrow-stromal-cells-inhibit-allogeneic (accessed on 2 February 2024). [CrossRef] [PubMed]
- Serejo, T.R.T.; Silva-Carvalho, A.É.; Braga, L.D.D.C.F.; Neves, F.D.A.R.; Pereira, R.W.; de Carvalho, J.L.; Saldanha-Araujo, F. Assessment of the immunosuppressive potential of INF-γ licensed adipose mesenchymal stem cells, their secretome and extracellular vesicles. Cells 2019, 8, 22. [Google Scholar] [CrossRef] [PubMed]
- di Trapani, M.; Bassi, G.; Midolo, M.; Gatti, A.; Kamga, P.T.; Cassaro, A.; Carusone, R.; Adamo, A.; Krampera, M. Differential and transferable modulatory effects of mesenchymal stromal cell-derived extracellular vesicles on T, B and NK cell functions. Sci. Rep. 2016, 6, 24120. [Google Scholar] [CrossRef] [PubMed]
- Jorgovanovic, D.; Song, M.; Wang, L.; Zhang, Y. Roles of IFN-γ in tumor progression and regression: A review. Biomark. Res. 2020, 8, 49. Available online: https://biomarkerres.biomedcentral.com/articles/10.1186/s40364-020-00228-x (accessed on 1 March 2024). [CrossRef] [PubMed]
- Ishiuchi, N.; Nakashima, A.; Doi, S.; Yoshida, K.; Maeda, S.; Kanai, R.; Yamada, Y.; Ike, T.; Doi, T.; Kato, Y.; et al. Hypoxia-preconditioned mesenchymal stem cells prevent renal fibrosis and inflammation in ischemia-reperfusion rats. Stem Cell Res. Ther. 2020, 11, 130. [Google Scholar] [CrossRef] [PubMed]
- Ishiuchi, N.; Nakashima, A.; Doi, S.; Kanai, R.; Maeda, S.; Takahashi, S.; Nagao, M.; Masaki, T. Serum-free medium and hypoxic preconditioning synergistically enhance the therapeutic effects of mesenchymal stem cells on experimental renal fibrosis. Stem Cell Res. Ther. 2021, 12, 472. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Liu, L.; Huo, Y.; Yang, Y.; Wang, Y. Hypoxia-Pretreated Human MSCs Attenuate Acute Kidney Injury through Enhanced Angiogenic and Antioxidative Capacities. Biomed. Res. Int. 2014, 2014, 333. [Google Scholar] [CrossRef] [PubMed]
- Soares, M.A.; Massie, J.P.; Rifkin, W.J.; Rao, N.; Duckworth, A.M.; Park, C.; Kadle, R.L.; David, J.A.; Rabbani, P.S.; Ceradini, D.J. Ex vivo allotransplantation engineering: Delivery of mesenchymal stem cells prolongs rejection-free allograft survival. Am. J. Transplant. 2018, 18, 1657–1667. [Google Scholar] [CrossRef]
- Xu, C.; Diao, Y.F.; Wang, J.; Liang, J.; Xu, H.H.; Zhao, M.L.; Zheng, B.; Luan, Z.; Wang, J.J.; Yang, X.P.; et al. Intravenously Infusing the Secretome of Adipose-Derived Mesenchymal Stem Cells Ameliorates Neuroinflammation and Neurological Functioning after Traumatic Brain Injury. Stem Cells Dev. 2020, 29, 222–234. [Google Scholar] [CrossRef]
- Kim, J.; Lee, S.K.; Jung, M.; Jeong, S.Y.; You, H.; Won, J.Y.; Han, S.D.; Cho, H.J.; Park, S.; Park, J.; et al. Extracellular vesicles from IFN-γ-primed mesenchymal stem cells repress atopic dermatitis in mice. J. Nanobiotechnol. 2022, 20, 526. [Google Scholar] [CrossRef]
- Peltzer, J.; Lund, K.; Goriot, M.E.; Grosbot, M.; Lataillade, J.J.; Mauduit, P.; Banzet, S. Interferon-γ and Hypoxia Priming Have Limited Effect on the miRNA Landscape of Human Mesenchymal Stromal Cells-Derived Extracellular Vesicles. Front. Cell Dev. Biol. 2020, 8, 581436. [Google Scholar] [CrossRef] [PubMed]
- Seif, F.; Khoshmirsafa, M.; Aazami, H.; Mohsenzadegan, M.; Sedighi, G.; Bahar, M. The role of JAK-STAT signaling pathway and its regulators in the fate of T helper cells. Cell Commun. Signal. 2017, 15, 23. [Google Scholar] [CrossRef] [PubMed]
- Ichiyama, K.; Yoshida, H.; Wakabayashi, Y.; Chinen, T.; Saeki, K.; Nakaya, M.; Takaesu, G.; Hori, S.; Yoshimura, A.; Kobayashi, T. Foxp3 inhibits RORγt-mediated IL-17A mRNA transcription through direct interaction with RORγt. J. Biol. Chem. 2008, 283, 17003–17008. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Wu, R.; Jiang, Z.; Wang, L.; Chen, P.; Zhang, L.; Yang, L.; Wu, Y.; Chen, H.; Chen, H.; et al. Leptin signaling is required for augmented therapeutic properties of mesenchymal stem cells conferred by hypoxia preconditioning. Stem Cells 2014, 32, 2702–2713. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Zhang, J.; Han, B.; Yu, Y.; Zhao, W.; Wu, T.; Mao, Y.; Zhang, F. Extracellular vesicles DJ-1 derived from hypoxia-conditioned hMSCs alleviate cardiac hypertrophy by suppressing mitochondria dysfunction and preventing ATRAP degradation. Pharmacol. Res. 2023, 187, 106607. [Google Scholar] [CrossRef] [PubMed]
- Hendrawan, S.; Kusnadi, Y.; Lagonda, C.A.; Fauza, D.; Lheman, J.; Budi, E.; Manurung, B.S.; Baer, H.U.; Tan, S.T. Wound healing potential of human umbilical cord mesenchymal stem cell conditioned medium: An in vitro and in vivo study in diabetes-induced rats. Vet. World 2021, 14, 2109–2117. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Lou, B.; Wu, X.; Wu, R.; Wang, H.; Gao, L.; Pi, J.; Xu, Y. Comparative study on in vitro culture of mouse bone marrow mesenchymal stem cells. Stem Cells Int. 2018, 2018, 6704583. [Google Scholar] [CrossRef] [PubMed]
- Chan, W.K.; Sik-Yin Lau, A.; Chun-Bong Li, J.; Ka-Wai Law, H.; Lau, Y.L.; Chi-Fung Chan, G. MHC expression kinetics and immunogenicity of mesenchymal stromal cells after short-term IFN-γ challenge. Exp. Hematol. 2008, 36, 1545–1555. [Google Scholar] [CrossRef]
- Ziello, J.E.; Jovin, I.S.; Huang, Y. Hypoxia-Inducible Factor (HIF)-1 regulatory pathway and its potential for therapeutic intervention in malignancy and ischemia. Yale J. Biol. Med. 2007, 80, 51–60. [Google Scholar]
- Kahrizi, M.S.; Mousavi, E.; Khosravi, A.; Rahnama, S.; Salehi, A.; Nasrabadi, N.; Ebrahimzadeh, F.; Jamali, S. Recent advances in pre-conditioned mesenchymal stem/stromal cell (MSCs) therapy in organ failure; a comprehensive review of preclinical studies. Stem Cell Res. Ther. 2023, 14, 155. Available online: https://stemcellres.biomedcentral.com/articles/10.1186/s13287-023-03374-9 (accessed on 28 January 2024). [CrossRef] [PubMed]
- Ge, L.; Xun, C.; Li, W.; Jin, S.; Liu, Z.; Zhuo, Y.; Duan, D.; Hu, Z.; Chen, P.; Lu, M. Extracellular vesicles derived from hypoxia-preconditioned olfactory mucosa mesenchymal stem cells enhance angiogenesis via miR-612. J. Nanobiotechnol. 2021, 19, 380. [Google Scholar] [CrossRef]
- Lavrentieva, A.; Majore, I.; Kasper, C.; Hass, R. Effects of hypoxic culture conditions on umbilical cord-derived human mesenchymal stem cells. Cell Commun. Signal. 2010, 8, 18. [Google Scholar] [CrossRef] [PubMed]
- Nowak-Stȩpniowska, A.; Osuchowska, P.N.; Fiedorowicz, H.; Trafny, E.A. Insight in Hypoxia-Mimetic Agents as Potential Tools for Mesenchymal Stem Cell Priming in Regenerative Medicine. Stem Cells Int. 2022, 2022, 8775591. [Google Scholar] [CrossRef]
- Wheaton, W.W.; Chandel, N.S. Hypoxia. 2. Hypoxia regulates cellular metabolism. Am. J. Physiol. Cell Physiol. 2011, 300, C385–C393. [Google Scholar] [CrossRef]
- Zhang, B.; Tian, X.; Qu, Z.; Hao, J.; Zhang, W. Hypoxia-Preconditioned Extracellular Vesicles from Mesenchymal Stem Cells Improve Cartilage Repair in Osteoarthritis. Membranes 2022, 12, 225. [Google Scholar] [CrossRef]
- Bister, N.; Pistono, C.; Huremagic, B.; Jolkkonen, J.; Giugno, R.; Malm, T. Hypoxia and extracellular vesicles: A review on methods, vesicular cargo and functions. J. Extracell. Vesicles 2020, 10, e12002. [Google Scholar] [CrossRef]
- Zhang, F.; Li, R.; Yang, Y.; Shi, C.; Shen, Y.; Lu, C.; Chen, Y.; Zhou, W.; Lin, A.; Yu, L.; et al. Specific Decrease in B-Cell-Derived Extracellular Vesicles Enhances Post-Chemotherapeutic CD8+ T Cell Responses. Immunity 2019, 50, 738–750.e7. [Google Scholar] [CrossRef] [PubMed]
- Andrews, S.; Maughon, T.; Marklein, R.; Stice, S. Priming of MSCs with inflammation-relevant signals affects extracellular vesicle biogenesis, surface markers, and modulation of T cell subsets. J. Immunol. Regen. Med. 2021, 13, 100036. [Google Scholar] [CrossRef]
- Zhuang, Y.; Cheng, M.; Li, M.; Cui, J.; Huang, J.; Zhang, C.; Si, J.; Lin, K.; Yu, H. Small extracellular vesicles derived from hypoxic mesenchymal stem cells promote vascularized bone regeneration through the miR-210-3p/EFNA3/PI3K pathway. Acta Biomater. 2022, 150, 413–426. [Google Scholar] [CrossRef]
- Xu, C.M.; Karbasiafshar, C.; Brinck Teixeira, R.; Ahsan, N.; Blume Corssac, G.; Sellke, F.W.; Abid, M.R. Proteomic Assessment of Hypoxia-Pre-Conditioned Human Bone Marrow Mesenchymal Stem Cell-Derived Extracellular Vesicles Demonstrates Promise in the Treatment of Cardiovascular Disease. Int. J. Mol. Sci. 2023, 24, 1674. [Google Scholar] [CrossRef] [PubMed]
- Maurya, R.; Sebastian, P.; Namdeo, M.; Devender, M.; Gertler, A. COVID-19 Severity in Obesity: Leptin and Inflammatory Cytokine Interplay in the Link between High Morbidity and Mortality. Front. Immunol. 2021, 12, 649359. Available online: https://www.frontiersin.org/articles/10.3389/fimmu.2021.649359/full (accessed on 5 April 2024). [CrossRef]
- Eguchi, M.; Liu, Y.; Shin, E.J.; Sweeney, G. Leptin protects H9c2 rat cardiomyocytes from H2O 2-induced apoptosis. FEBS J. 2008, 275, 3136–3144. [Google Scholar] [CrossRef]
- Gonzalez-Perez, R.R.; Xu, Y.; Guo, S.; Watters, A.; Zhou, W.; Leibovich, S.J. Leptin upregulates VEGF in breast cancer via canonic and non-canonical signalling pathways and NFκB/HIF-1α activation. Cell Signal 2010, 22, 1350–1362. [Google Scholar] [CrossRef] [PubMed]
- Mcgaffin, K.R.; Zou, B.; Mctiernan, C.F.; O’donnell, C.P. Leptin attenuates cardiac apoptosis after chronic ischaemic injury. Cardiovasc. Res. 2009, 83, 313–324. [Google Scholar] [CrossRef] [PubMed]
- McGaffin, K.R.; Witham, W.G.; Yester, K.A.; Romano, L.C.; Odoherty, R.M.; McTiernan, C.F.; Odonnell, C.P. Cardiac-specific leptin receptor deletion exacerbates ischaemic heart failure in mice. Cardiovasc. Res. 2011, 89, 60–71. [Google Scholar] [CrossRef]
- Saxena, N.K.; Sharma, D.; Ding, X.; Lin, S.; Marra, F.; Merlin, D.; Anania, F.A. Concomitant activation of the JAK/STAT, PI3K/AKT, and ERK signaling is involved in leptin-mediated promotion of invasion and migration of hepatocellular carcinoma cells. Cancer Res. 2007, 67, 2497–2507. [Google Scholar] [CrossRef]
- Wang, M.; Tan, J.; Coffey, A.; Fehrenbacher, J.; Weil, B.R.; Meldrum, D.R. Signal transducer and activator of transcription 3-stimulated hypoxia inducible factor-1α mediates estrogen receptor-α-induced mesenchymal stem cell vascular endothelial growth factor production. J. Thorac. Cardiovasc. Surg. 2009, 138, 163–171.e1. [Google Scholar] [CrossRef][Green Version]
- Tang, Y.L.; Zhu, W.; Cheng, M.; Chen, L.; Zhang, J.; Sun, T.; Kishore, R.; Phillips, M.I.; Losordo, D.W.; Qin, G. Hypoxic preconditioning enhances the benefit of cardiac progenitor cell therapy for treatment of myocardial infarction by inducing CXCR4 expression. Circ. Res. 2009, 104, 1209–1216. [Google Scholar] [CrossRef]
- Meng, X.M.; Tang, P.M.K.; Li, J.; Lan, H.Y. TGF-ß/Smad signaling in renal fibrosis. Front. Physiol. 2015, 6, 134394. [Google Scholar] [CrossRef]
- Chan, J.Y.H.; Chan, S.H.H. Activation of endogenous antioxidants as a common therapeutic strategy against cancer, neurodegeneration and cardiovascular diseases: A lesson learnt from DJ-1. Pharmacol. Ther. 2015, 156, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Cai, Z.; Tao, K.; Zeng, W.; Lu, F.; Yang, R.; Feng, D.; Gao, G.; Yang, Q. Essential control of mitochondrial morphology and function by chaperone-mediated autophagy through degradation of PARK7. Autophagy 2016, 12, 1215–1228. [Google Scholar] [CrossRef] [PubMed]
- Wobma, H.M.; Kanai, M.; Ma, S.P.; Shih, Y.; Li, H.W.; Duran-Struuck, R.; Winchester, R.; Goeta, S.; Brown, L.M.; Vunjak-Novakovic, G. Dual IFN-γ/hypoxia priming enhances immunosuppression of mesenchymal stromal cells through regulatory proteins and metabolic mechanisms. J. Immunol. Regen. Med. 2018, 1, 45–56. Available online: https://linkinghub.elsevier.com/retrieve/pii/S2468498817300197 (accessed on 1 February 2024). [CrossRef] [PubMed]
- Guess, A.J.; Daneault, B.; Wang, R.; Bradbury, H.; La Perle, K.M.D.; Fitch, J.; Hedrick, S.L.; Hamelberg, E.; Astbury, C.; White, P.; et al. Safety Profile of Good Manufacturing Practice Manufactured Interferon γ-Primed Mesenchymal Stem/Stromal Cells for Clinical Trials. Stem Cells Transl. Med. 2017, 6, 1868–1879. [Google Scholar] [CrossRef] [PubMed]
- Elabd, C.; Centeno, C.J.; Schultz, J.R.; Lutz, G.; Ichim, T.; Silva, F.J. Intra-discal injection of autologous, hypoxic cultured bone marrow-derived mesenchymal stem cells in five patients with chronic lower back pain: A long-term safety and feasibility study. J. Transl. Med. 2016, 14, 253. [Google Scholar] [CrossRef] [PubMed]
- Putra, A.; Pertiwi, D.; Milla, M.N.; Indrayani, U.D.; Jannah, D.; Sahariyani, M.; Trisnadi, S.; Wibowo, J.W. Hypoxia-preconditioned MSCs have superior effect in ameliorating renal function on acute renal failure animal model. Open Access Maced. J. Med. Sci. 2019, 7, 305–310. [Google Scholar] [CrossRef] [PubMed]
- Muhar, A.M.; Makharim, F.; Hermansyah, D.; Putra, A.; Hidayah, N.; Amalina, N.D.; Alif, I. Hypoxic mesenchymal stem cell-conditioned medium accelerates wound healing by regulating IL-10 and TGF-β levels in a full-thickness-wound rat model. Indones. J. Biotechnol. 2022, 27, 187–194. [Google Scholar] [CrossRef]
- Cherian, D.S.; Bhuvan, T.; Meagher, L.; Heng, T.S.P. Biological Considerations in Scaling Up Therapeutic Cell Manufacturing. Front. Pharmacol. 2020, 11, 654. Available online: https://www.frontiersin.org/article/10.3389/fphar.2020.00654/full (accessed on 8 May 2024). [CrossRef] [PubMed]
- Jankovic, M.G.; Stojkovic, M.; Bojic, S.; Jovicic, N.; Kovacevic, M.M.; Ivosevic, Z.; Juskovic, A.; Kovacevic, V.; Ljujic, B. Scaling up human mesenchymal stem cell manufacturing using bioreactors for clinical uses. Curr. Res. Transl. Med. 2023, 71, 103393. Available online: https://linkinghub.elsevier.com/retrieve/pii/S245231862300017X (accessed on 8 May 2024). [CrossRef]
- Lembong, J.; Kirian, R.; Takacs, J.D.; Olsen, T.R.; Lock, L.T.; Rowley, J.A.; Ahsan, T. Bioreactor Parameters for Microcarrier-Based Human MSC Expansion under Xeno-Free Conditions in a Vertical-Wheel System. Bioengineering 2020, 7, 73. Available online: https://www.mdpi.com/2306-5354/7/3/73 (accessed on 8 May 2024). [CrossRef]
- Busatto, S.; Vilanilam, G.; Ticer, T.; Lin, W.L.; Dickson, D.; Shapiro, S.; Bergese, P.; Wolfram, J. Tangential Flow Filtration for Highly Efficient Concentration of Extracellular Vesicles from Large Volumes of Fluid. Cells 2018, 7, 273. Available online: https://www.mdpi.com/2073-4409/7/12/273 (accessed on 8 May 2024). [CrossRef] [PubMed]
- Bari, E.; Perteghella, S.; Catenacci, L.; Sorlini, M.; Croce, S.; Mantelli, M.; Avanzini, M.A.; Sorrenti, M.; Torre, M.L. Freeze-Dried and GMP-Compliant Pharmaceuticals Containing Exosomes for Acellular Mesenchymal Stromal Cell Immunomodulant Therapy. Nanomedicine 2019, 14, 753–765. Available online: https://www.tandfonline.com/doi/full/10.2217/nnm-2018-0240 (accessed on 8 May 2024). [CrossRef] [PubMed]


| Priming Concentration and Duration | Cell Sources | Pathological Condition and Recipients | Dosage | Route of Administration | In Vitro Markers | Functional Tests | Therapeutic Effects with Priming | Mechanisms of Action | Remarks | References |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 or 20 ng/mL recombinant mouse IFN-γ, 48 h | Mouse BM-MSCs | Systemic sclerosis (SSc) mouse model; immunocompetent mice | 250 ng EVs; large-size EVs (lsEVs) or small-size EVs (ssEVs) | Intravenous injection | Cytokine level-HGF, IL1RA, PGE2 (ELISA), miRNA profile analysis (NGS) | Not stated | Improve remodelling and inflammatory mediators, reduce the expression of the fibrotic and inflammatory markers | Not stated (the upregulation of anti-inflammatory factors in MSCs via IFN-γ pre-activation was not observed in EVs, suggesting that other factors may be responsible for the improvement | Low-dose-primed EVs (derived from primed MSCs) were less efficient than naïve EVs (derived from naïve MSCs), while high-dose-primed EVs have similar efficacy to naïve EVs. Large-size EVs (lsEVs) were more efficient than small-size EVs (ssEVs), particularly in terms of improving remodelling and inflammatory markers in the skin. | Rozier et al., 2021 [35] |
| 5 ng/mL IFN-γ + 5 ng/mL TNF-α, 12 h | Equine BM-MSCs | Osteoarthritis equine model; immunocompetent ponies | 1 × 107 MSCs | Intra-arterial injection | Not stated | Not stated | Reduce clinical and synovial inflammatory signs, as well as synovial effusion, improve cartilage macroscopic appearance and synovium histopathology | Via increasing anti-inflammatory effect | Despite the lack of significant differences between naïve MSCs and primed MSCs, the overall outcome suggested that primed MSCs had enhanced anti-inflammatory capabilities, with most improvements noted during the earlier time points. | Barrachina et al., 2018 [36] |
| 10 ng/mL IFN-γ, 24 h | Human WJ-MSCs | Atopic dermatitis (AD) mouse model; immunocompetent mice | 2 × 106 MSCs | Subcutaneous injection | Gene expression profile (microarray analysis) | Not stated | Decrease immune cell infiltration, improve the features of AD, reduce epidermal and dermal thickness | Via the regulation of neutrophil-related Th17 immune responses | MSCs primed with IFN-γ elicited improved therapeutic effects in AD mice (better than non-primed MSCs). | Park et al., 2019 [37] |
| 10 ng/mL IFN-γ + 10 ng/mL TNF-α, 24 h | Human UC-MSCs | LPS-induced neuroinflammation mouse model; immunocompetent mice | 200 µL concentrated human UC-MSC-conditioned medium (injected twice on days 4 and 6) | Intravenous injection | Gene expression profile-IDO1, CXCL9, IL6 (qRT-PCR), transcriptomic analysis (two-dimensional PCA, GO enrichment analysis) | Mouse microglial cell line -BV2 cell inhibition assay | Enhance the anti-inflammatory capacity, minimize the impact of donor-specific variations in MSC immunomodulation | Via stimulating IFN-γ and NF-κB signalling pathways | Priming MSCs improves immune suppressive function and reduces donor-dependent variations in immunomodulation. However, it hinders MSC proliferation. Thus, avoid priming during the expansion phase of MSCs. | Zhang et al., 2021 [22] |
| 10 ng/mL IFN-γ + 10 ng/mL TNF-α, 24 h | Human UC-MSCs | Inflammatory bowel disease (IBD) mouse model; immunocompetent mice | 1 × 106 MSCs | Intravenous injection | Gene expression profile-β-actin, CXCL9, CXCL10, CXCL11, IDO1, TSG-6 (qRT-PCR), protein level-IDO1, HA, COX2, AKT, HKII, β-actin, GLUT1(Western blot), TSG-6 (ELISA) | ECAR (glycolysis stress test kit), glucose uptake assay (flow cytometry), hexokinase activity assay (Hexokinase activity kit) | In vitro, there is an increase in glucose consumption and a metabolic shift towards glycolysis, while in vivo, there is a reduction in inflammatory parameters. | Via enhanced IDO and TSG-6 expression by promoting glycolysis, increasing glucose uptake and HKII activity | There is no naïve MSC group so no comparison was made. TNF-α and IFN-γ rapidly activate PI3K-AKT signalling promotes glycolysis in human MSCs, which is responsible for MSC-based anti-inflammatory therapy. | Xu et al., 2022 [38] |
| 10 ng/mL IFN-γ, 48 h | Human BM-MSCs | Experimental autoimmune encephalomyelitis (EAE) mouse model; immunocompetent mice | 1 × 106 MSCs or 150 μg EVs derived from 5 to 7 × 106 MSCs | Intravenous injection | MHC II, PD-L1 expression (flow cytometry), cytokine secretion-IDO, IL-6, etc. (luminex assay, ELISA) | T-cell (PBMC) proliferation (flow cytometry), Treg induction assay (flow cytometry) | Reduce demyelination and neuroinflammation | Via suppressing pathological T cell subset activation, inducing Tregs, probably via the “hit and run” mechanism | Exosomes were found to have similar efficacy to their MSC counterparts. IFN-γ priming ameliorated the disease to a higher extent than native MSCs and exosomes. | Riazifar et al., 2019 [39] |
| 20 ng/mL IFN-γ + 50 μM KYNA, 24 h | Human AT-MSCs | 2,4,6-trinitrobenzen sulfonic acid (TNBS)-induced acute colitis and chronic colon fibrosis rat model; immunocompetent rats | 1.5 × 106 MSCs (injected twice on days 1 and 3) for acute colitis; 2 × 106 MSCs (injected three times for every two weeks) for chronic colon fibrosis | Intravenous injection | Transcriptome sequencing (GO enrichment analysis, KEGG enrichment analysis), IDO-1, iNOS, COX2 (qPCR, Western blot) | Not stated | Mitigate TNBS-induced colitis and colonic fibrosis, inhibit extracellular matrix (ECM) deposition and the EMT process, diminish the infiltration of inflammatory cells, suppress the inflammatory response, promote the polarization of M2 macrophages, and enhance homing to colon tissue | Via IFN-γ and KYNA-induced IDO-1, which facilitates cell homing, induces the polarization of intestinal macrophages to the anti-inflammatory M2 and elevates the expression of IL-10 to inhibit inflammation | MSCs primed with both IFN-γ and KYNA exhibit significant therapeutic efficacy in addressing acute colitis and chronic colon fibrosis in rats. | Ye et al., 2022 [40] |
| 20 ng/mL IFN-γ, 48 h | Human UC-MSCs | EAE mouse model; immunocompetent mice | 1 × 106 MSCs | Intravenous injection | IDO1 expression (RT-PCR, Western blot) | Not stated | Mitigate body weight loss and clinical symptoms, reduce inflammation and latency of motor evoked potentials (MEP) | Via suppression of IL-17A and TNF-α expression, upregulation of IDO1 | IFN-γ-MSCs showed more potent treatment efficacy than naïve MSCs. | Zhou et al., 2020 [41] |
| 20 ng/mL IFN-γ, 48 h | Human UC-MSCs | EAE mouse model; immunocompetent mice | 1 × 106 MSCs | Intravenous injection | Not stated | Not stated | Alleviate body weight loss and clinical scores, regulate inflammation response | Via regulating the Th17/Tregs balance via the regulation of inflammatory cytokines production (increased IL-10 and decreased IL-17) | IFN-γ-MSCs showed more potent treatment efficacy than naïve MSCs. | Ling et al., 2022 [42] |
| 25 ng/mL IFN-γ, 48 h | Human BM-MSCs | Sepsis mouse model; immunocompetent mice | 1 × 106 MSCs | Intravenous injection | Not stated | Not stated | Enhance microvascular hemodynamics during the initial stages of sepsis | Via increasing the red blood cell velocity, the rolling white blood cell flux and the number of venules with circulating white blood cells, the rate of soluble E-selectin (which may serve as a biomarker for monitoring endothelial damage in organs) was reduced | MSCs-IFN-γ appear to have a better beneficial effect on microvascular hemodynamics compared with naïve MSCs. However, the 6 h post-sepsis time point is too early to observe organ failures with this model. | Baudry et al., 2019 [43] |
| 40 ng/mL IFN-γ, 24 h | Human UC-MSCs | Acute pneumonia mouse model; immunocompetent mice | 1 × 106 MSCs | Intravenous injection | Transcriptome sequencing (Illumina HiSeq 2500 system), IDO, PD-L1, JAK2, STAT1-3, etc. (Western blot) | T-cell (PBMC) proliferation (CFSE-flow cytometry), metabolism analysis (real-time assays of ECAR and OCR), metabolite analysis (LC-MS) | Preserve the alveolar structure in lung tissues, decrease the infiltration of inflammatory cells in alveoli, and lower levels of IL-1β and TNF-α | By redirecting the energy metabolism of MSCs towards aerobic oxidation and thus activates JAK-STAT signalling as well as induces IDO and PD-L1 production | Compared to the sole administration of IFN-γ, the combined treatment involving ATP and IFN-γ demonstrated better therapeutic efficacy. ATP amplifies the immunosuppressive capabilities of IFN-γ–primed MSCs by triggering the JAK-STAT pathway. | Yao et al., 2022 [8] |
| 50 ng/mL IFN-γ, 8 h | Human UC-MSCs | Escherichia coli-induced pneumonia rat model; immunocompetent rats | 1 × 108 EVs, derived from 3.5 to 4 × 107 MSCs | Intravenous injection | Not stated | Bacterial phagocytosis and killing assays (immunofluorescent) | Enhance survival rate, alleviate the severity of lung injury, modulate inflammatory response, reduce structural damage, restore lung structure | Via enhancement of macrophage phagocytosis and bacteria killing as well as restoration of endothelial nitric oxide synthase | IFN-γ-primed MSC-EVs were more effective than naïve MSC-EVs in reducing E. coli-induced lung injury. | Varkouhi et al., 2019 [44] |
| 50 ng/mL IFN-γ, 24 h | Human BM-MSCs | Calvarial defect mouse model; both immunocompetent and immunodeficient mice | 1–1.5 mm diameter MSC extracellular matrix (ECM) complex (C-MSCs) | Graft without artificial scaffold | IDO expression (RT-PCR, immunoblotting), IDO activity (level of kynurenine as a product of IDO-catabolism), osteogenic markers expression-OPN, ALPase, BMP-2, OC (RT-PCR) | T-cell (PBMC) proliferation (ELISA) | Induce bone regeneration, attenuate xenoreactive T cell response | By reducing the activity of xenoreactive T cells in mice via increased IDO expression and T cell suppression capacity in vitro, mainly via indirect paracrine effects rather than direct osteogenic differentiation | The use of primed C-MSCs was effective in preventing an unwanted immune response and promoting successful bone regeneration in immunocompetent mice, whereas the use of C-MSCs alone was not able to induce bone regeneration. | Takeshita et al., 2017 [45] |
| 50 ng/mL IFN-γ + 50 ng/mL TNF-α, 24 h | Human BM-MSCs (aged human donors with end-stage OA) | Osteoarthritis (OA) mouse model; immunocompetent mice | 2 × 104 MSCs (without stimulation) or secretome from 2 × 104 MSCs (with stimulation) (injected three times every two days) | Intra-articular injection | IDO activity (level of kynurenine as a product of IDO-catabolism). However, the comparison was not clear | Not stated | Early pain reduction, protect against cartilage damage | Not stated (no significant results observed to support any conclusions) | Secretome from stimulated MSCs diminished pain and OA-related structural changes, and these effects were at least as effective as the injection of MSCs without stimulation. | Khatab et al., 2018 [46] |
| 50 ng/mL IFN-γ, not stated | Mouse BM-MSCs | Dextran sulphate sodium (DSS)- induced colitis mouse model; immunocompetent mice | 200 μg EVs | Intravenous injection | EVs number (EXOCET exosome quantitation kit), EVs protein-CD9, CD81 (Western blot) | Not stated | Restore body weight loss and impaired intestinal structure, decrease disease activity index (DAI) score and colon shortening, reduce inflammation cytokines | Via inhibiting Th17 cell differentiation via increased levels of miR-125a and miR-125b in EVs, promoting Treg cell differentiation | Priming MSCs with IFN-γ generated EVs with better anti-colitis therapeutic efficacy. | Yang et al., 2020 [47] |
| 100 ng/mL IFN-γ, 48 h | Human AT-MSCs | Carbon tetrachloride (CCl4)-induced liver cirrhosis mouse model; immunocompetent mice | 2 μg or 5 μg EVs | Intravenous injection | Proteomics (nano-LC-MS) and miRNA content analysis (DNAFORM) of EVs | Macrophage polarity-mRNA expression of genes encoding pro- and anti-inflammatory factors (qPCR), motility and phagocytosis (immunofluorescent) assays | Ameliorate fibrosis and inflammation, promote tissue repair | Via the induction of anti-inflammatory macrophages with higher motility and phagocytic ability, increasing regulatory T cell counts | IFN-γ priming resulted in enhanced efficacy of MSC-derived EVs. | Takeuchi et al., 2021 [48] |
| 200 IU/mL IFN-γ, 24 h | Human BM-MSCs, CB-MSCs, AT-MSCs, WJ-MSCs | Graft-versus-host disease (GVHD) mouse model; immunodeficient mice | 1 × 106 MSCs (injected twice on days 0 and 7) | Intravenous injection | Gene expression profile-CXCL9, CXCL10, CCL8, IDO, etc. (RT-PCR, qRT-PCR, microarray analysis) | T-cell (PBMC) proliferation (BrdU Incorporation Assay) | Improve survival rate, decrease clinical symptoms and immune cell infiltration | Via the suppression of antigen-driven proliferation of T-cells | IFN-γ-primed MSCs showed better therapeutic efficacy than naïve MSCs. The effect between different MSC sources was not compared. | Kim et al., 2018 [49] |
| 500 U/mL IFN-γ + 5000 U/mL TNF-α, 24 h | Mouse BM-MSCs | Diabetic mouse model; immunocompetent mice | 500 IEQ of encapsulated islets with MSCs at a 1:1 ratio | Intraperitoneal injection | Cytokine/chemokine secretion-CXCL9, CXCL10, IL-6, COX-2, IDO, etc. (RT-PCR, cytokine protein array panel), nitric oxide (NO) production (RT-PCR, nitrite/nitrate (NO2/NO3) colorimetric kit) | Not stated | Normalize blood glucose levels, reduce pericapsular fibrotic overgrowth (PFO) | By increasing the expression of anti-inflammatory cytokines such as IL-4, IL-6, IL-10, and G-CSF, as well as boosting NO production, which are known to regulate the immune response | IFN-γ, in combination with TNF-α, synergistically enhanced the immunosuppressive effects of murine MSCs. Neither IFN-γ nor TNF-α are sufficient on their own. | Vaithilingam et al., 2017 [50] |
| 500 U/mL recombinant human IFN-γ, 48 h | Human BM-MSCs | Humanized GVHD mouse model; Immunodeficient mice | 4.4 × 104 MSCs per gram | Intravenous injection | Not related to the in vitro functional tests that used naïve MSCs instead of primed MSCs | Not related | Extend lifespan, decrease liver and gut pathology but not lung pathology | By directly inhibiting the proliferation of donor T cells and decreasing the production of human TNF-α from T cells | Therapeutic efficacy of IFN-γ stimulated MSCs on day 0 is comparable to that of unstimulated MSCs on day 7, where MSCs require IFN-γ pre-stimulation for efficacy at the earliest time points. | Tobin et al., 2013 [51] |
| 500 U/mL recombinant human IFN-γ, 72 h | Human WJ-MSCs | EAE mouse model; immunocompetent mice | 1 × 106 MSCs (injected twice on days 3 and 11 post-immunization) | Intravenous injection | Cytokine level-VEGF, IL-10, TGF-β, HGF (ELISA) | PBMC proliferation (CFSE-flow cytometry) | Reduce T cell reactivity, enhance neurological functional recovery, reduce infiltration of inflammatory cells, delay the onset of clinical symptom | Via modulating immune differentiation from a Th1 towards a Th2 phenotype, increasing the frequency of CD4+CD25+CD127low/neg Foxp3+T regulatory cells | IFN-γ-primed MSCs have better immunomodulatory function than unprimed MSCs. | Torkaman et al., 2017 [52] |
| 500 U/mL IFN-γ, 7 days | Human BM-MSCs | Myocardial infarction (MI) mouse model; Immunodeficient mice | 2 × 105 MSCs | Intramyocardial injection | IDO expression (immunohistochemistry). However, no comparison was made between naïve MSC and primed MSC | PBMC proliferation (3H-thymidine uptake in counts per minute (CCPM)). However, no comparison was made | Neither unstimulated MSC therapy nor IFN-γ-stimulated MSC therapy shows any significant positive impact on cardiac function or remodelling | Not stated | Both MSCs engraft in infarct myocardium. The animal models used for cardiac MSC therapy seem to be less strong than originally anticipated. | Haan et al., 2016 [53] |
| Priming Method and Duration | Cell Sources | Pathological Condition and Recipients | Dosage | Route of Administration | In Vitro Markers | Functional Tests | Therapeutic Effects of Priming | Mechanisms of Action | Remarks | References |
|---|---|---|---|---|---|---|---|---|---|---|
| Hypoxic condition (0.5% O2), 24 h | Mouse BM-MSCs | MI mouse model; immunocompetent mice | 2 × 105 MSCs | Intramyocardial injection | hLeptin expression (qRT-PCR, ELISA), mCXCR4 (qRT-PCR, flow cytometry) | MSC migration assay (Trans-well system), MSC apoptosis assay (TUNEL staining), tube formation assay (HUVEC model), cardio-protection assay (TUNEL staining) | Enhance cell homing and survival rate, improve systolic function and cardiac function, promote angiogenesis, reduce apoptosis rate | Via enhanced leptin expression | Hypoxia-induced expression of leptin plays a crucial role in the protective effects of hypoxic MSCs. Hypoxic MSCs showed significantly better therapeutic efficacy than normoxic MSCs. | Hu et al., 2014 [85] |
| Hypoxic condition (1% O2), 24 h | Human AT-MSCs | Acute kidney injury (AKI/renal IRI) rat model; immunocompetent rats | 2 × 106 MSCs | Injected into the left kidney cortex | Growth factor assay-bFGF, VEGF, HGF (RT-PCR, ELISA) | Cell viability assay (flow cytometry-propidium iodide (PI) solution) | Enhance antioxidative capacity and angiogenic effects, decrease apoptosis rate, attenuate renal injury, improve renal function | Via improved activated paracrine effects (secretion of angiogenic factors) | Hypoxia preconditioning significantly enhanced the therapeutic effects of MSCs on AKI. | Zhang et al., 2014 [78] |
| Hypoxic condition (1% O2), 24 h | Rat or Human BM-MSCs | Renal ischemia-reperfusion injury (IRI) rat model; immunocompetent rats | 5 × 105 MSCs | Injected through the abdominal aorta clamped above and below the left renal artery bifurcation | Phosphorylated Smad2 (pSmad2) and α-SMA expression (Western blot), VEGF, HGF, PGE2 levels (ELISA), VEGF, HGF expression (qRT-PCR) | Not stated | Attenuate IRI-induced renal fibrosis, suppress the infiltration of inflammatory cells | Via inhibition of TGF-β/Smad signalling and upregulation of VEGF expression (renal fibrosis attenuation), augmentation of PGE2 secretion (immunomodulation) | Hypoxic MSCs significantly ameliorate renal fibrosis and inflammation in IRI rats compared to normoxic MSCs. Hypoxic preconditioning does not increase the engraftment capacity of MSCs. | Ishiuchi et al., 2020 [76] |
| Hypoxic condition (1% O2), 24 h | Human BM-MSCs | Renal IRI rat model; immunocompetent rats | 5 × 105 MSCs | Injected into the abdominal aorta clamped above and below the left renal artery bifurcation | Phosphorylated Smad2 (pSmad2) and α-SMA expression (Western blot), VEGF and HGF secretion (ELISA) | Cell proliferation assay (WST-1 assay), migration assay (Trans-well system), macrophage polarization assay (Western blot and qRT-PCR) | Attenuate IRI-induced renal fibrosis, suppress inflammatory cell infiltration | Via the stimulation of HGF secretion and subsequently enhancing the inhibition of the TGF-β/Smad signalling pathway, the enhancement of paracrine activity without conflicting interactions | Serum-free medium and hypoxia preconditioning synergistically enhanced MSCs’ proliferative capacity and efficacy in attenuating renal fibrosis injury, whereas the anti-inflammatory effect of normoxic MSCs is almost equal to hypoxic MSCs. | Ishiuchi et al., 2021 [77] |
| Hypoxic condition (1% O2), 48 h | Porcine AT-MSCs | Atherosclerotic renal artery stenosis (ARAS) porcine model | 1 × 107 MSCs | Intra-arterial injection (injected into renal arteries) | Not stated | Not stated | Reduce diastolic blood pressure, restore renal medullary oxygenation, decrease kidney fibrosis and interstitial T-cell infiltration | Not stated | Hypoxia preconditioning of AT-MSCs was comparable to normoxia and did not improve the effects of MSC on renal function despite inducing a reduction in DNA hydroxymethylation (5hmC levels) of inflammatory and profibrotic genes. | Farooqui et al., 2023 [31] |
| Hypoxic condition (1% O2), 48 h | Human MSC cell line (HUM-iCELL-e009) | Transverse aortic constriction (TAC) mouse model; immunocompetent mice | 200 µg EVs (injected twice on days 7 and 14) | Intravenous injection | PARK7/DJ-1 protein levels (quantitative proteomics analysis) | OCR (Seahorse Extracellular Flux Analyzer), mitochondrial membrane potential (JC-1 kits), ROS levels (MitoSOX™ Red reagent), ubiquitylation assays (Western blot), mtDNA copy number (qPCR) | Alleviate myocardial hypertrophy, demonstrate cardioprotective properties, improve mitochondrial function | Via the expression of DJ-1 in hypoxic ps leads to the alleviation of mitochondrial damage and suppression of ATRAP degradation. | Hypoxic EVs demonstrate a heightened inhibitory impact on cardiac hypertrophy in comparison to normoxic EVs. | Lu et al., 2023 [86] |
| Hypoxic condition (1% O2) throughout the whole culturation process | Human UC-MSCs | OVA-induced allergic rhinitis (AR) mouse model; immunocompetent mice | 100 µg CM or EVs (injected six times for every three days) | Intravenous injection | OCT4, SOX2, Nanog expression (qRT-PCR), Telomerase activity (PCR-ELISA), VEGF level (Western blot) | Evaluation of maturation status of dendritic cells (flow cytometry) | Reduce the frequency of sneezing and scratching, suppress allergic inflammation, and support remodelling of the nasal mucosa | By increasing VEGF levels in hypoxic EVs that suppressed the differentiation and maturation of dendritic cells | Prolonged exposure to hypoxia improves the proliferative capacity, enhances telomerase activities, reduces senescence, and preserves the multipotent status of UC-MSCs compared to normoxic conditions. Notably, both hypoxic EVs and CM exhibit better therapeutic effects compared to their normoxic counterparts, implying that the essential components in the CM likely originate from EVs. | Wu et al., 2023 [30] |
| Hypoxic condition (3% O2) + 1.8 mM calcium throughout the whole culturation process | Human UCB-MSCs | Humanized graft-versus-host disease (GVHD) mouse model; immunodeficient mice | 5 × 105 SHC-MSCs (Small MSCs primed with hypoxia and calcium ions) | Intravenous injection | Genome-wide gene expression and DNA methylation analyses (microarray analysis/gene set enrichment analysis) | Anti-inflammation assay secretion of TNF-α (ELISA), MLR assay (Human T-cell (PBMC) proliferation), tube formation assay (HUVEC model) | Increase survival rate, attenuate weight loss, decrease immune cell infiltration and characteristic tissue injuries, enhance tissue repair and cell homing, reduce inflammatory cytokines level | Via the overexpression of PLK1, ZNF143, FOG, and DHRS3 and subsequently enhance the proliferative, self-renewal, migratory, pro-angiogenic, anti-inflammatory, and immunomodulatory capacities of MSCs | SHC-MSCs have an enhanced potency for treating GVHD compared to the naïve MSCs. | Kim et al., 2018 [33] |
| Hypoxic condition (5% O2), 24 h | Human AT-MSCs | Traumatic brain injury (TBI) rat model; immunocompetent rats | 0.1 mL/250 g secretome (ST) (once daily for 7 days) | Intravenous injection | Proteome analysis (LC-MS/MS), VEGF, BDNF, GDNF, PDGF-BB, ICAM-1 concentration (ELISA) | Not stated | Mitigate neurological impairment and cognitive deficiency, alleviate neuroinflammatory edema, reduce nerve fibre damage, improve neuroinflammatory environment, limit the apoptosis of neural cells | Via the mediation of secondary neuroinflammation, it fosters the polarization of microglia into M2 phenotypes (anti-inflammatory) | There is no normoxic MSC group while serum-free basal media was injected as comparison. Hypoxic MSC-ST can improve neural functional outcomes after TBI in its early stages by mediating secondary neuroinflammation. | Xu et al., 2020 [80] |
| Hypoxic condition (5% O2), 24 h | Human UC-MSCs | OVA-induced chronic asthma mouse model; immunocompetent mice | 40 µg EVs (inhaled or injected four times for every seven days) | Inhalation or intravenous injection | Not stated | Not stated | Reduce chronic airway inflammation, inhibit the prevailing type-2 immune response, and deter airway remodelling | Not stated | The non-invasive approach of nebulized hypoxic EVs inhalation significantly decreases chronic airway inflammation and remodelling, with the EVs predominantly accumulating in the lungs and maintaining their presence for a period of 7 days. | Xu et al., 2023 [32] |
| Hypoxic condition (5% O2), 1000 units of IFN-γ, 72 h | Rat BM-MSCs | Rat hindlimb allotransplantation model; immunocompetent rats | 2 × 106 MSCs | Ex vivo allograft engineering (vasculature was perfused with BM-MSCs in cold media, divided between 3 separate perfusates over the course of 1 h) | IDO expression (qRT-PCR) | CD8+ T cell-mediated cytotoxicity assay (lactate dehydrogenase-based), CD4+ T cell proliferation assay (3H-thymidine-based), migration assay (Trans-well system), cell proliferation assay (MTT Cell Proliferation Assay Kit) | Postpones the onset of acute rejection while preserving the recipient’s adaptive immune response | By elevating the expression of IDO, it provides a shield for endothelial cells, hinders the proliferation of CD4+ T cells, and enhances cell motility and proliferative potential | Hypoxic priming is significantly better than IFN-γ priming in prolonging allograft rejection. Both primed MSCs were better than unprimed MSCs. | Soares et al., 2018 [79] |
| Hypoxic condition (5% O2), 72 h | Human UC-MSCs | Cutaneous-wound healing in a diabetic rat model; immunocompetent rats | 0.5 mL of undiluted CM | Intradermal injection on a peripheral wound | VEGF, bFGF, pro-collagen 1 secretion (ELISA) | Rat fibroblast cell growth (CCK-8 viability assay), collagen production analysis on rat fibroblasts (ELISA) | Facilitate wound closure and re-epithelialization | Not stated | Hypoxic MSC-CM treatment showed a distinct effect in facilitating wound repair in the early stage of the diabetic wound model compared to the topical antibiotic treatment (bactoderm mupirocin ointment 2%). | Hendrawan et al., 2021 [87] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tan, Y.L.; Al-Masawa, M.E.; Eng, S.P.; Shafiee, M.N.; Law, J.X.; Ng, M.H. Therapeutic Efficacy of Interferon-Gamma and Hypoxia-Primed Mesenchymal Stromal Cells and Their Extracellular Vesicles: Underlying Mechanisms and Potentials in Clinical Translation. Biomedicines 2024, 12, 1369. https://doi.org/10.3390/biomedicines12061369
Tan YL, Al-Masawa ME, Eng SP, Shafiee MN, Law JX, Ng MH. Therapeutic Efficacy of Interferon-Gamma and Hypoxia-Primed Mesenchymal Stromal Cells and Their Extracellular Vesicles: Underlying Mechanisms and Potentials in Clinical Translation. Biomedicines. 2024; 12(6):1369. https://doi.org/10.3390/biomedicines12061369
Chicago/Turabian StyleTan, Yu Ling, Maimonah Eissa Al-Masawa, Sue Ping Eng, Mohamad Nasir Shafiee, Jia Xian Law, and Min Hwei Ng. 2024. "Therapeutic Efficacy of Interferon-Gamma and Hypoxia-Primed Mesenchymal Stromal Cells and Their Extracellular Vesicles: Underlying Mechanisms and Potentials in Clinical Translation" Biomedicines 12, no. 6: 1369. https://doi.org/10.3390/biomedicines12061369
APA StyleTan, Y. L., Al-Masawa, M. E., Eng, S. P., Shafiee, M. N., Law, J. X., & Ng, M. H. (2024). Therapeutic Efficacy of Interferon-Gamma and Hypoxia-Primed Mesenchymal Stromal Cells and Their Extracellular Vesicles: Underlying Mechanisms and Potentials in Clinical Translation. Biomedicines, 12(6), 1369. https://doi.org/10.3390/biomedicines12061369







