Results of the Phase 1 Open-Label Safety Study of Umbilical Cord Lining Mesenchymal Stromal/Stem Cells (Corlicyte®) to Heal Chronic Diabetic Foot Ulcers
Abstract
1. Introduction
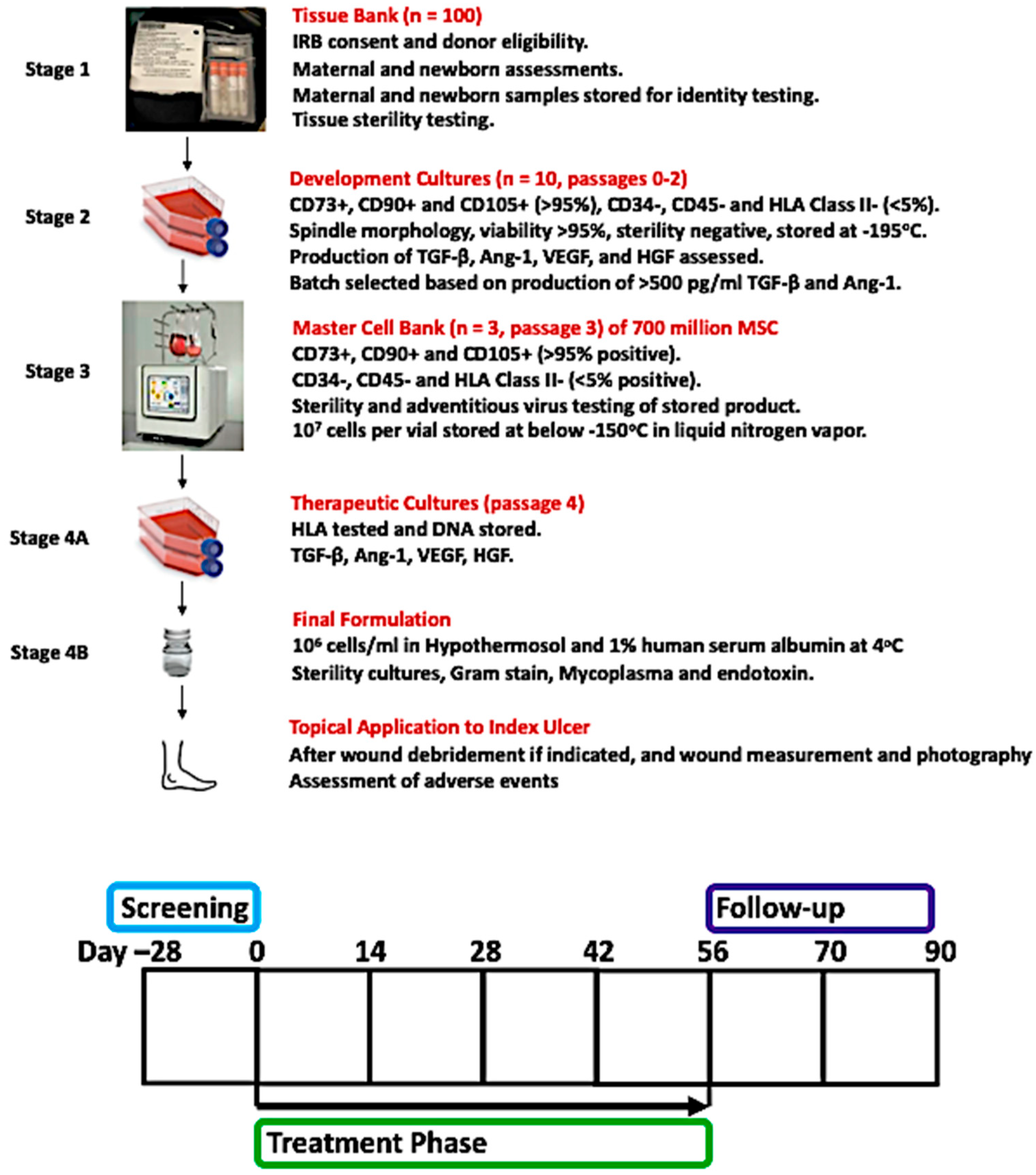
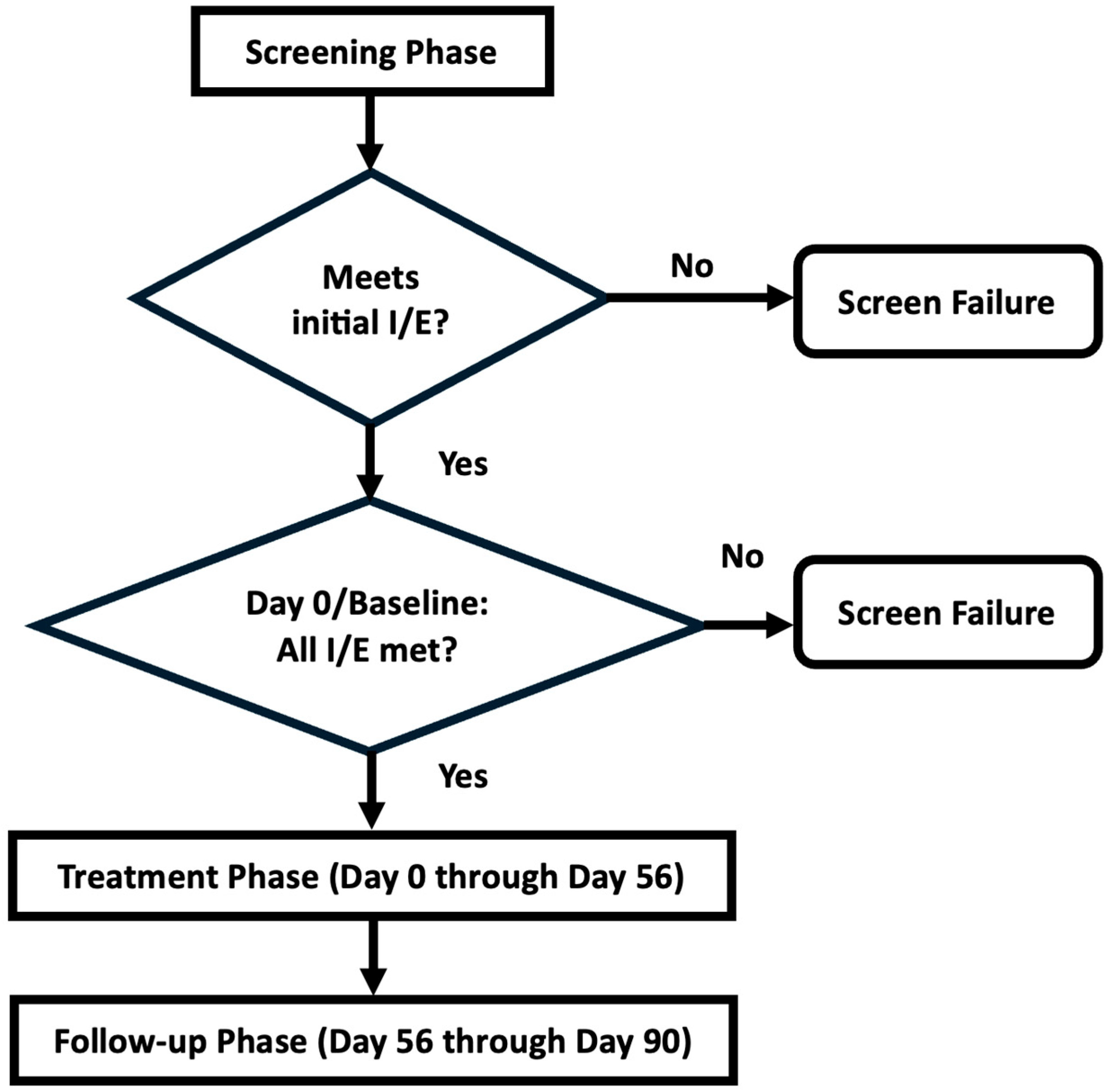
2. Materials and Methods
3. Results
3.1. Enrollment of Cohorts
3.2. Adverse Events
3.3. Endpoints and Safety Laboratory Results
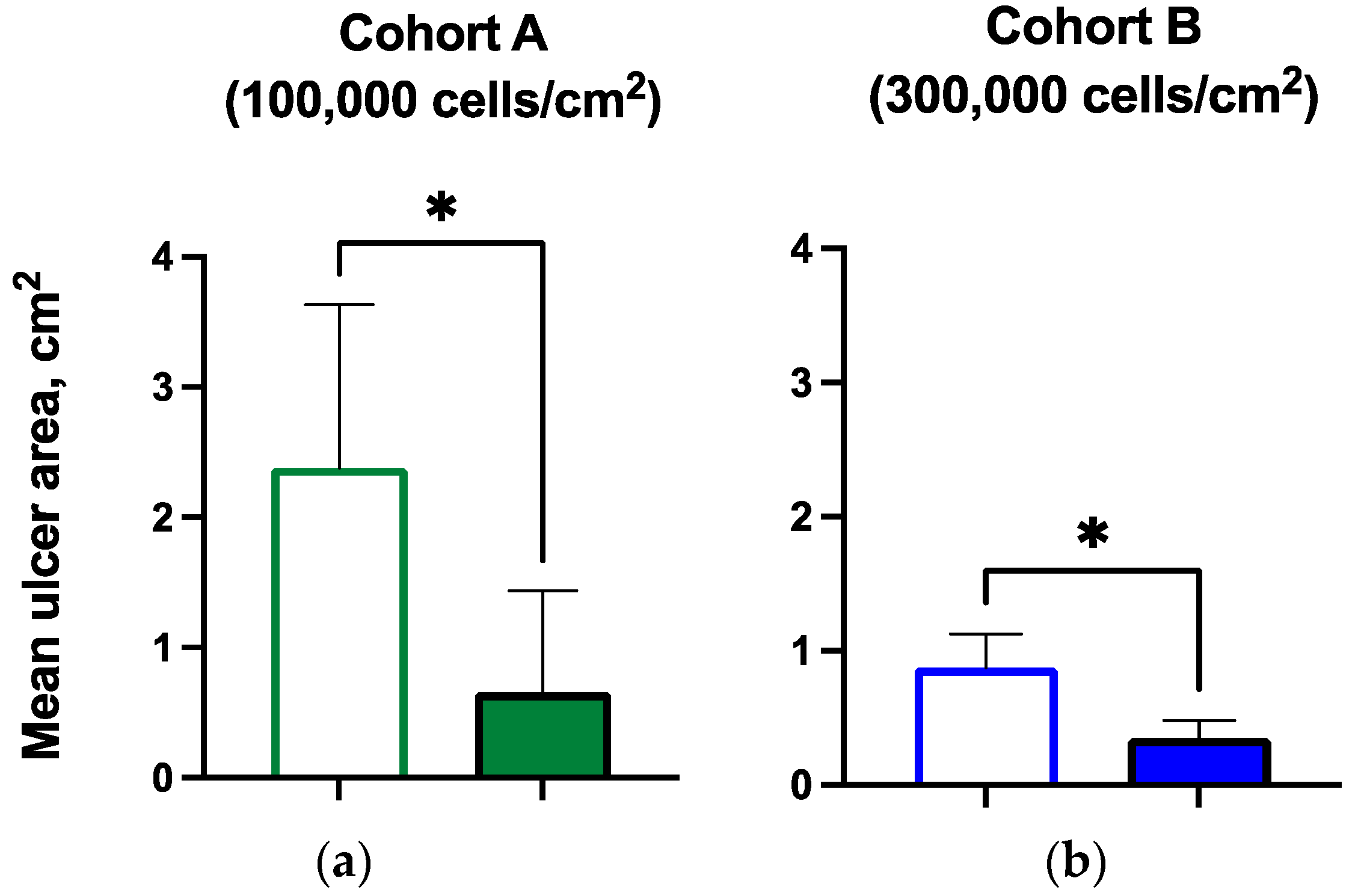
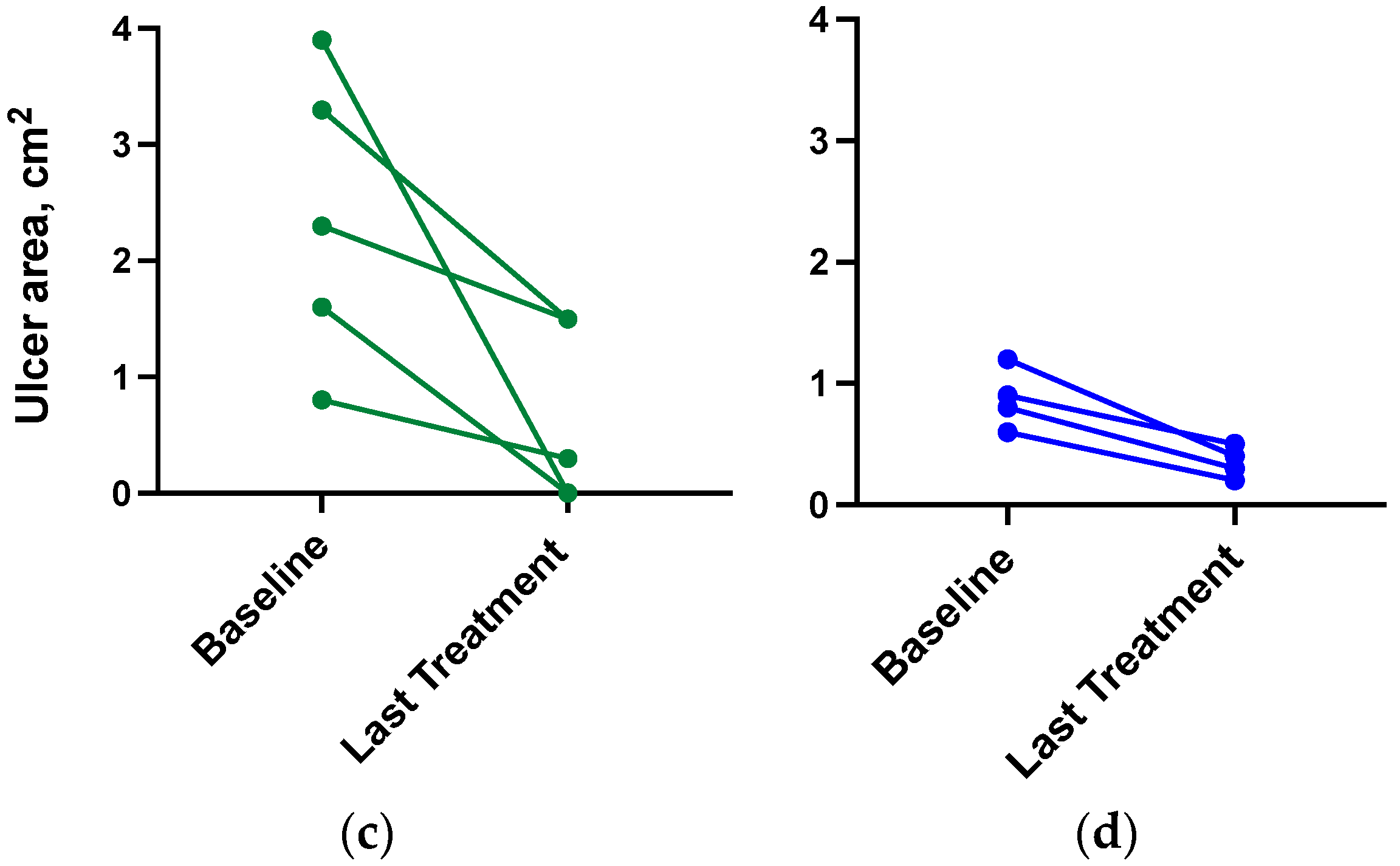
3.4. Ulcer Closure and Ulcer Size Reduction
3.5. Hemoglobin A1c
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Armstrong, D.G.; Boulton, A.J.; Bus, S.A. Diabetic foot ulcers and their recurrence. N. Engl. J. Med. 2017, 376, 2367–2375. [Google Scholar] [CrossRef]
- Schmidt, B.M.; Holmes, C.M.; Najarian, K.; Gallagher, K.; Haus, J.M.; Shadiow, J.; Ye, W.; Ang, L.; Burant, A.; Baker, N.; et al. On diabetic foot ulcer knowledge gaps, innovation, evaluation, prediction markers, and clinical needs. J. Diabetes Complicat. 2022, 36, 108317. [Google Scholar] [CrossRef]
- Armstrong, D.G.; Swerdlow, M.A.; Armstrong, A.A.; Conte, M.S.; Padula, W.V.; Bus, S.A. Five year mortality and direct costs of care for people with diabetic foot complications are comparable to cancer. J. Foot Ankle Res. 2020, 13, 16. [Google Scholar] [CrossRef] [PubMed]
- Hocking, A.M.; Gibran, N.S. Mesenchymal stem cells: Paracrine signaling and differentiation during cutaneous wound repair. Exp. Cell Res. 2010, 316, 2213–2219. [Google Scholar] [CrossRef]
- Lee, D.E.; Ayoub, N.; Agrawal, D. Mesenchymal stem cells and cutaneous wound healing: Novel methods to increase cell delivery and therapeutic efficacy. Stem Cell Res. Ther. 2016, 7, 37. [Google Scholar] [CrossRef] [PubMed]
- Walter, M.N.M.; Wright, K.T.; Fuller, H.R.; MacNeil, S.; Johnson, W.E.B. Mesenchymal stem cell-conditioned medium accelerates skin wound healing: An in vitro study of fibroblast and keratinocyte scratch assays. Exp. Cell Res. 2010, 316, 1271–1281. [Google Scholar] [CrossRef]
- Smith, A.N.; Willis, E.; Chan, V.T.; Muffley, L.A.; Isik, F.F.; Gibran, N.S.; Hocking, A.M. Mesenchymal stem cells induce dermal fibroblast responses to injury. Exp. Cell Res. 2010, 316, 48–54. [Google Scholar] [CrossRef]
- Jeon, Y.K.; Jang, Y.H.; Yoo, D.R.; Kim, S.N.; Lee, S.K.; Nam, M.J. Mesenchymal stem cells’ interaction with skin: Wound-healing effect on fibroblast cells and skin tissue. Wound Repair Regen. 2010, 18, 655–661. [Google Scholar] [CrossRef]
- Liu, L.; Yu, Y.; Hou, Y.; Chai, J.; Duan, H.; Chu, W.; Zhang, H.; Hu, Q.; Du, J. Human umbilical cord mesenchymal stem cells transplantation promotes cutaneous wound healing of severe burned rats. PLoS ONE 2014, 9, e88348. [Google Scholar] [CrossRef] [PubMed]
- Kato, J.; Kamiya, H.; Himeno, T.; Shibata, T.; Kondo, M.; Okawa, T.; Fujia, A.; Fukami, A.; Uenishi, E.; Seino, Y.; et al. Mesenchymal stem cells ameliorate impaired wound healing through enhancing keratinocyte functions in diabetic foot ulcerations on the plantar skin of rats. J. Diabetes Complicat. 2014, 28, 588–595. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Menocal, L.; Shareef, S.; Salgado, M.; Shabbir, A.; Van Badiavas, E. Role of whole bone marrow, whole bone marrow cultured cells, and mesenchymal stem cells in chronic wound healing. Stem Cell Res. Ther. 2015, 6, 25. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Zhang, H.; Guo, L.; Kim, J.; Kim, M.H. Amniotic mesenchymal stem cells enhance wound healing in diabetic NOD/SCID mice through high angiogenic and engraftment capabilities. PLoS ONE 2012, 7, e4110. [Google Scholar] [CrossRef]
- Shin, L.; Peterson, D.A. Human mesenchymal stem cell grafts enhance normal and impaired wound healing by recruiting existing endogenous tissue stem/progenitor cells. Stem Cells Transl. Med. 2013, 2, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.; Keating, A.; Prockop, D.J.; Horwitz, E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef] [PubMed]
- Cottle, C.; Paige Porter, A.; Lipat, A.; Turner-Lyles, C.; Nguyen, J.; Moll, G.; Chinnadurai, R. Impact of Cryopreservation and Freeze-Thawing on Therapeutic Properties of Mesenchymal Stromal/Stem Cells and Other Common Cellular Therapeutics. Curr. Stem Cell Rep. 2022, 8, 72–92. [Google Scholar] [CrossRef] [PubMed]
| Mean (Range), pg/mL | Cohort A, n = 5 | Cohort B, n = 4 |
|---|---|---|
| TGF-β | 1198 (549–2610) | 1793 (749–6237) |
| Ang-1 | 1128 (900–1357) | 677 (84–1733) |
| VEGF | 232 (211–254) | 790 (101–2280) |
| HGF | 357 (276–438) | 764 (54–2395) |
| Overall, n = 9 | Cohort A, n = 5 | Cohort B, n = 4 | |
|---|---|---|---|
| Age (yr): mean (range) | 55 (44–70) | 56 (45–70) | 54 (44–60) |
| Female/male (number) | 3/6 | 2/3 | 1/3 |
| White/Black (number) | 8/1 | 4/1 | 4/0 |
| Type 2 diabetes/Type 1 diabetes | 8/1 | 4/1 | 4/0 |
| Diabetes duration (yr): mean (range) | 24 (5–49) | 33 (21–49) | 12 (5–16) |
| Ulcer chronicity (months): mean (range) | 7.3 (3–13) | 7.7 (3–15) | 6.8 (1.7–13.5) |
| Diabetes medication use: n (%) | 9 (100%) | 5 (100%) | 4 (100%) |
| Insulin | 8 (89%) | 5 (100%) | 3 (75%) |
| Non-insulin | 4 (44%) | 2 (40%) | 2 (50%) |
| Metformin | 3 (33%) | 1 (20%) | 2 (50%) |
| GLP1RA | 0 | 0 | 0 |
| DPP4i | 4 (44%) | 2 (40%) | 2 (50%) |
| SGLT2i | 0 | 0 | 0 |
| Sulfonylurea | 2 (22%) | 1 (20%) | 1 (25%) |
| Thiazolidinedione | 1 (11%) | 1 (20%) | 0 |
| n = 9 | Baseline | Last Visit |
|---|---|---|
| Hemoglobin A1c: mean (range) | ||
| mmol/mol | 90.2 (58.5–107.7) | 76.0 (44.3–113.1) |
| % | 10.4 (7.5–12) | 9.1 (6.2–12.5) |
| WBC (×103/mm3): mean (range) | 8.8 (4–13.7) | 9.2 (6.4–12.9) |
| Creatinine (mg/dL): mean (range) | 0.9 (0.5–1.4) | 0.9 (0.6–1.5) |
| Adverse Event (AE) | Cohort A Corlicyte® 100,000 cells/cm2 n = 5 | Cohort B Corlicyte® 300,000 cells/cm2 n = 4 | Total Overall n = 9 |
|---|---|---|---|
| Number of AEs | 11 | 1 | 12 |
| Number (%) of patients with at least one AE | 5 (100.00%) | 1 (25.00%) | 6 (66.67%) |
| Number (%) of patients with at least one AE related to study treatment | 0 | 0 | 0 |
| Number (%) of patients with at least one AE leading to study drug withdrawal | 0 | 0 | 0 |
| Number (%) of patients with adverse reactions to study drug | 0 | 0 | 0 |
| Number (%) of patients with at least one SAE | 1 (20.00%) | 0 | 1 (11.11%) |
| Number (%) of patients with at least one SAE related to study treatment | 0 | 0 | 0 |
| Number of AEs by severity | |||
| Grade 1 Mild | 8 | 1 | 9 |
| Grade 2 Moderate | 1 | 0 | 1 |
| Grade 3 Severe | 2 | 0 | 2 |
| Grade 4 Life-threatening | 0 | 0 | 0 |
| Grade 5 Death | 0 | 0 | 0 |
| Cohort A n = 5 | Cohort B n = 4 | |||
|---|---|---|---|---|
| Baseline | Day 90 Visit | Baseline | Day 90 Visit | |
| Hemoglobin A1c (mean) | ||||
| mmol/mol | 86.9 | 81.4 | 93.4 | 71.6 |
| % | 10.1 | 9.6 | 10.7 | 8.7 |
| * p < 0.05 | Baseline | Last Treatment Visit | Baseline | Last Treatment Visit |
| Ulcer size (mean, cm2) | 2.38 | 0.66 * | 0.88 | 0.35 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Low Wang, C.C.; Chong, T.; Moore, G.; Echalier, B.; Haakonsen, N.; Carter, J.E., Jr.; Mathes, D.; Hsia, J.; Phan, T.T.; Lim, I.J.; et al. Results of the Phase 1 Open-Label Safety Study of Umbilical Cord Lining Mesenchymal Stromal/Stem Cells (Corlicyte®) to Heal Chronic Diabetic Foot Ulcers. Biomedicines 2024, 12, 1375. https://doi.org/10.3390/biomedicines12061375
Low Wang CC, Chong T, Moore G, Echalier B, Haakonsen N, Carter JE Jr., Mathes D, Hsia J, Phan TT, Lim IJ, et al. Results of the Phase 1 Open-Label Safety Study of Umbilical Cord Lining Mesenchymal Stromal/Stem Cells (Corlicyte®) to Heal Chronic Diabetic Foot Ulcers. Biomedicines. 2024; 12(6):1375. https://doi.org/10.3390/biomedicines12061375
Chicago/Turabian StyleLow Wang, Cecilia C., Tae Chong, Garrett Moore, Benjamin Echalier, Nicola Haakonsen, James E. Carter, Jr., David Mathes, Judith Hsia, Toan Thang Phan, Ivor J. Lim, and et al. 2024. "Results of the Phase 1 Open-Label Safety Study of Umbilical Cord Lining Mesenchymal Stromal/Stem Cells (Corlicyte®) to Heal Chronic Diabetic Foot Ulcers" Biomedicines 12, no. 6: 1375. https://doi.org/10.3390/biomedicines12061375
APA StyleLow Wang, C. C., Chong, T., Moore, G., Echalier, B., Haakonsen, N., Carter, J. E., Jr., Mathes, D., Hsia, J., Phan, T. T., Lim, I. J., & Freed, B. M. (2024). Results of the Phase 1 Open-Label Safety Study of Umbilical Cord Lining Mesenchymal Stromal/Stem Cells (Corlicyte®) to Heal Chronic Diabetic Foot Ulcers. Biomedicines, 12(6), 1375. https://doi.org/10.3390/biomedicines12061375






