Revolutionizing Brain Tumor Care: Emerging Technologies and Strategies
Abstract
:1. Introduction
2. Imaging
2.1. Intraoperative MRI (iMRI)
2.2. Functional MRI (fMRI)
2.3. Quantitative MRI (qMRI)
2.4. Radiomic
3. Surgery
3.1. Morphological Imaging Modalities
3.2. Fluorescence
3.3. Awake Brain Surgery
3.4. Robotic Surgery
4. Irradiation
4.1. External Beam Radiation Therapy (EBRT)
4.2. Proton Therapy
4.3. Focused Ultrasound (FUS)
| Advantage | Disadvantage | |
|---|---|---|
| IMRT | Tumor-targeting, sparing normal cells Reduced side effects | Prolonged treatment affects the patient’s comfort and positioning Costlier due to complex planning and delivery Needs special equipment, expertise [91,92] |
| VMAT | Faster treatment delivery Improved dose conformity Reduced radiation exposure to patients | Complex planning and quality assurance needed Higher treatment costs Limited in some healthcare settings [80] |
| Proton Therapy | Accurate dosing, minimal harm to healthy tissues Reduced risk of long-term side effects | Expensive to set up and maintain Limited in some healthcare settings [93] |
| FUS | Noninvasive, no incisions or radiation Tumor-targeting, tissue-safe Quick recovery time and minimal side effects | Restricted to specific tumor locations Effectiveness varies by tumor size and type Limited in some healthcare settings [85,87,94] |
5. Chemotherapy
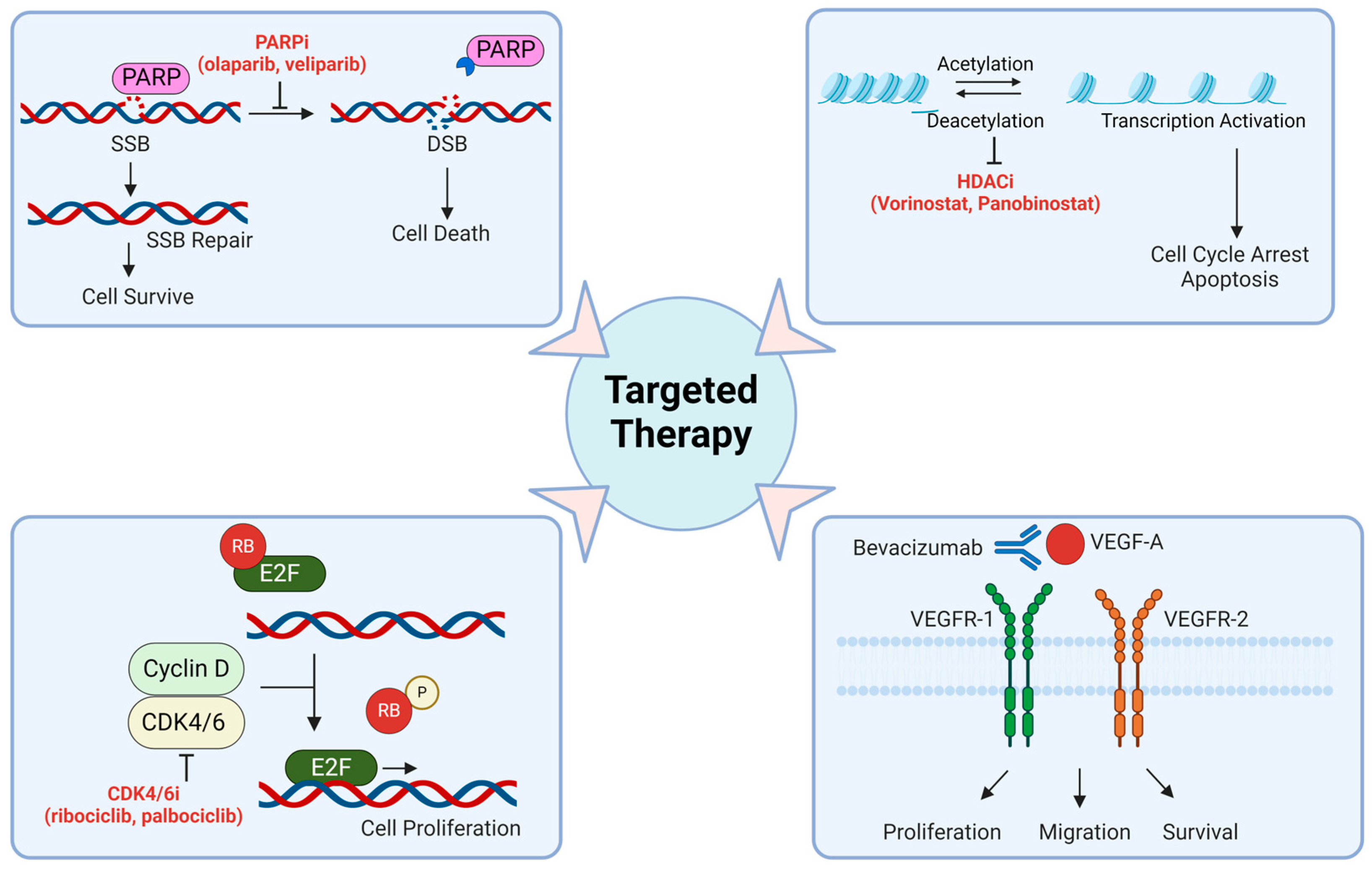
6. Targeted Therapy
6.1. DNA Damage Repair Targeting Drugs
6.2. Histone Deacetylase (HDAC) Inhibitors
6.3. Targeting Transcription Factors
6.4. Cell Cycle Checkpoint Inhibitors
6.5. Epidermal Growth Factor Receptor (EGFR) Inhibitors
6.6. Vascular Endothelial Growth Factor (VEGF) Inhibitors
6.7. Drug Metabolism Targets
6.8. Tumor Treating Fields (TTFields) Therapy
6.9. Convection-Enhanced Delivery (CED)
7. Gene and Cell Therapy
7.1. Immunotherapy
7.2. Oncolytic Virotherapy
7.3. CRISPR/Cas9
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Thakkar, J.P.; Dolecek, T.A.; Horbinski, C.; Ostrom, Q.T.; Lightner, D.D.; Barnholtz-Sloan, J.S.; Villano, J.L. Epidemiologic and molecular prognostic review of glioblastoma. Cancer Epidemiol. Biomark. Prev. 2014, 23, 1985–1996. [Google Scholar] [CrossRef]
- Teraiya, M.; Perreault, H.; Chen, V.C. An overview of glioblastoma multiforme and temozolomide resistance: Can LC-MS-based proteomics reveal the fundamental mechanism of temozolomide resistance? Front. Oncol. 2023, 13, 1166207. [Google Scholar] [CrossRef]
- Louis, D.N.; Perry, A.; Wesseling, P.; Brat, D.J.; Cree, I.A.; Figarella-Branger, D.; Hawkins, C.; Ng, H.K.; Pfister, S.M.; Reifenberger, G.; et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A summary. Neuro-Oncology 2021, 23, 1231–1251. [Google Scholar] [CrossRef] [PubMed]
- Obrador, E.; Moreno-Murciano, P.; Oriol-Caballo, M.; López-Blanch, R.; Pineda, B.; Gutiérrez-Arroyo, J.L.; Loras, A.; Gonzalez-Bonet, L.G.; Martinez-Cadenas, C.; Estrela, J.M.; et al. Glioblastoma Therapy: Past, Present and Future. Int. J. Mol. Sci. 2024, 25, 2529. [Google Scholar] [CrossRef]
- Lowe, S.; Bhat, K.P.; Olar, A. Current clinical management of patients with glioblastoma. Cancer Rep. 2019, 2, e1216. [Google Scholar] [CrossRef] [PubMed]
- Manzanares-Guzmán, A.; Lugo-Fabres, P.H.; Camacho-Villegas, T.A. vNARs as Neutralizing Intracellular Therapeutic Agents: Glioblastoma as a Target. Antibodies 2024, 13, 25. [Google Scholar] [CrossRef]
- Ageenko, A.; Vasileva, N.; Richter, V.; Kuligina, E. Combination of Oncolytic Virotherapy with Different Antitumor Approaches against Glioblastoma. Int. J. Mol. Sci. 2024, 25, 2042. [Google Scholar] [CrossRef]
- Salvato, I.; Marchini, A. Immunotherapeutic Strategies for the Treatment of Glioblastoma: Current Challenges and Future Perspectives. Cancers 2024, 16, 1276. [Google Scholar] [CrossRef] [PubMed]
- Matthias, H.; Chamberlain, M.C. Controversies in the Treatment of Elderly Patients with Newly Diagnosed Glioblastoma. J. Natl. Compr. Cancer Netw. 2013, 11, 1165–1173. [Google Scholar]
- Watson, S.S.; Duc, B.; Kang, Z.; de Tonnac, A.; Eling, N.; Font, L.; Whitmarsh, T.; Massara, M.; Joyce, J.A.; Bodenmiller, B.; et al. Microenvironmental reorganization in brain tumors following radiotherapy and recurrence revealed by hyperplexed immunofluorescence imaging. Nat. Commun. 2024, 15, 3226. [Google Scholar] [CrossRef]
- Phillips, M.C.L.; Thotathil, Z.; Dass, P.H.; Ziad, F.; Moon, B.G. Ketogenic metabolic therapy in conjunction with standard treatment for glioblastoma: A case report. Oncol. Lett. 2024, 27, 230. [Google Scholar] [CrossRef] [PubMed]
- Mo, F.; Pellerino, A.; Soffietti, R.; Rudà, R. Blood-Brain Barrier in Brain Tumors: Biology and Clinical Relevance. Int. J. Mol. Sci. 2021, 22, 12654. [Google Scholar] [CrossRef] [PubMed]
- Upton, D.H.; Ung, C.; George, S.M.; Tsoli, M.; Kavallaris, M.; Ziegler, D.S. Challenges and opportunities to penetrate the blood-brain barrier for brain cancer therapy. Theranostics 2022, 12, 4734–4752. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Thng, D.K.H.; Wong, A.L.A.; Toh, T.B. Mechanistic insights and the clinical prospects of targeted therapies for glioblastoma: A comprehensive review. Exp. Hematol. Oncol. 2024, 13, 40. [Google Scholar] [CrossRef] [PubMed]
- Breen, W.G.; Aryal, M.P.; Cao, Y.; Kim, M.M. Integrating multi-modal imaging in radiation treatments for glioblastoma. Neuro-Oncology 2024, 26 (Suppl. 2), S17–S25. [Google Scholar] [CrossRef] [PubMed]
- D’Amico, R.S.; Kennedy, B.C.; Bruce, J.N. Neurosurgical oncology: Advances in operative technologies and adjuncts. J. Neurooncol. 2014, 119, 451–463. [Google Scholar] [CrossRef]
- Kubben, P.L.; ter Meulen, K.J.; Schijns, O.E.; ter Laak-Poort, M.P.; van Overbeeke, J.J.; van Santbrink, H. Intraoperative MRI-guided resection of glioblastoma multiforme: A systematic review. Lancet Oncol. 2011, 12, 1062–1070. [Google Scholar] [CrossRef] [PubMed]
- Ram, Z.; Hadanl, M. Intraoperative Imaging—MRI; Acta Neurochirurgica; Springer: Vienna, Austria, 2003. [Google Scholar]
- Senft, C.; Bink, A.; Franz, K.; Vatter, H.; Gasser, T.; Seifert, V. Intraoperative MRI guidance and extent of resection in glioma surgery: A randomised, controlled trial. Lancet Oncol. 2011, 12, 997–1003. [Google Scholar] [CrossRef] [PubMed]
- De Simone, M.; Iaconetta, G.; Palermo, G.; Fiorindi, A.; Schaller, K.; De Maria, L. Clustering Functional Magnetic Resonance Imaging Time Series in Glioblastoma Characterization: A Review of the Evolution, Applications, and Potentials. Brain Sci. 2024, 14, 296. [Google Scholar] [CrossRef]
- Aghajani, M.; Jalilzadeh, N.; Aghebati-Maleki, A.; Yari, A.; Tabnak, P.; Mardi, A.; Saeedi, H.; Aghebati-Maleki, L.; Baradaran, B. Current approaches in glioblastoma multiforme immunotherapy. Clin. Transl. Oncol. 2024, 26, 1584–1612. [Google Scholar] [CrossRef]
- Hou, B.L.; Bradbury, M.; Peck, K.K.; Petrovich, N.M.; Gutin, P.H.; Holodny, A.I. Effect of brain tumor neovasculature defined by rCBV on BOLD fMRI activation volume in the primary motor cortex. Neuroimage 2006, 32, 489–497. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Chandra, P.S.; Sharma, B.S.; Garg, A.; Rath, G.K.; Bithal, P.K.; Tripathi, M. The role of neuronavigation-guided functional MRI and diffusion tensor tractography along with cortical stimulation in patients with eloquent cortex lesions. Br. J. Neurosurg. 2014, 28, 226–233. [Google Scholar] [CrossRef] [PubMed]
- Leuthardt, E.C.; Allen, M.; Kamran, M.; Hawasli, A.H.; Snyder, A.Z.; Hacker, C.D.; Mitchell, T.J.; Shimony, J.S. Resting-State Blood Oxygen Level-Dependent Functional MRI: A Paradigm Shift in Preoperative Brain Mapping. Stereotact. Funct. Neurosurg. 2015, 93, 427–439. [Google Scholar] [CrossRef] [PubMed]
- Calhoun, V.D.; Miller, R.; Pearlson, G.; Adalı, T. The chronnectome: Time-varying connectivity networks as the next frontier in fMRI data discovery. Neuron 2014, 84, 262–274. [Google Scholar] [CrossRef] [PubMed]
- Mazarakis, N.K.; Robinson, S.D.; Sinha, P.; Koutsarnakis, C.; Komaitis, S.; Stranjalis, G.; Short, S.C.; Chumas, P.; Giamas, G. Management of glioblastoma in elderly patients: A review of the literature. Clin. Transl. Radiat. Oncol. 2024, 46, 100761. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.H.; Smyser, C.D.; Shimony, J.S. Resting-state fMRI: A review of methods and clinical applications. AJNR Am. J. Neuroradiol. 2013, 34, 1866–1872. [Google Scholar] [CrossRef] [PubMed]
- De Luca, M.; Beckmann, C.; De Stefano, N.; Matthews, P.; Smith, S. fMRI resting state networks define distinct modes of long-distance interactions in the human brain. Neuroimage 2006, 29, 1359–1367. [Google Scholar] [CrossRef] [PubMed]
- Thorley, N.; Jones, A.; Ciurtin, C.; Castelino, M.; Bainbridge, A.; Abbasi, M.; Taylor, S.; Zhang, H.; Hall-Craggs, M.A.; Bray, T.J. Quantitative magnetic resonance imaging (qMRI) in axial spondyloarthritis. Br. J. Radiol. 2023, 96, 20220675. [Google Scholar] [CrossRef] [PubMed]
- Seiler, A.; Nöth, U.; Hok, P.; Reiländer, A.; Maiworm, M.; Baudrexel, S.; Meuth, S.; Rosenow, F.; Steinmetz, H.; Wagner, M.; et al. Multiparametric Quantitative MRI in Neurological Diseases. Front. Neurol. 2021, 12, 640239. [Google Scholar] [CrossRef]
- Cashmore, M.T.; McCann, A.J.; Wastling, S.J.; McGrath, C.; Thornton, J.; Hall, M.G. Clinical quantitative MRI and the need for metrology. Br. J. Radiol. 2021, 94, 20201215. [Google Scholar] [CrossRef]
- Cheng, H.L.; Stikov, N.; Ghugre, N.R.; Wright, G.A. Practical medical applications of quantitative MR relaxometry. J. Magn. Reson. Imaging 2012, 36, 805–824. [Google Scholar] [CrossRef]
- Keenan, K.E.; Biller, J.R.; Delfino, J.G.; Boss, M.A.; Does, M.D.; Evelhoch, J.L.; Griswold, M.A.; Gunter, J.L.; Hinks, R.S.; Hoffman, S.W.; et al. Recommendations towards standards for quantitative MRI (qMRI) and outstanding needs. J. Magn. Reson. Imaging 2019, 49, e26–e39. [Google Scholar] [CrossRef]
- Gulani, V.; Seiberlich, N. Quantitative MRI: Rationale and Challenges. In Quantitative Magnetic Resonance Imaging; Advances in Magnetic Resonance Technology and Applications Series; Academic Press: Cambridge, MA, USA, 2020; pp. xxxvii–li. [Google Scholar]
- Nöth, U.; Tichy, J.; Tritt, S.; Bähr, O.; Deichmann, R.; Hattingen, E. Quantitative T1 mapping indicates tumor infiltration beyond the enhancing part of glioblastomas. NMR Biomed. 2020, 33, e4242. [Google Scholar] [CrossRef]
- Blystad, I.; Warntjes, J.B.M.; Smedby, Ö.; Lundberg, P.; Larsson, E.-M.; Tisell, A. QuantitativeQuantitative MRI using relaxometry in malignant gliomas detects contrast enhancement in peritumoral oedema. Sci. Rep. 2020, 10, 17986. [Google Scholar] [CrossRef]
- Maurer, G.D.; Tichy, J.; Harter, P.N.; Nöth, U.; Weise, L.; Quick-Weller, J.; Deichmann, R.; Steinbach, J.P.; Bähr, O.; Hattingen, E. Matching Quantitative MRI Parameters with Histological Features of Treatment-Naive IDH Wild-Type Glioma. Cancers 2021, 13, 4060. [Google Scholar] [CrossRef] [PubMed]
- Karami, E.; Soliman, H.; Ruschin, M.; Sahgal, A.; Myrehaug, S.; Tseng, C.-L.; Czarnota, G.J.; Jabehdar-Maralani, P.; Chugh, B.; Lau, A.; et al. Quantitative MRI Biomarkers of Stereotactic Radiotherapy Outcome in Brain Metastasis. Sci. Rep. 2019, 9, 19830. [Google Scholar] [CrossRef]
- Ellingson, B.M.; Cloughesy, T.F.; Lai, A.; Nghiemphu, P.L.; Lalezari, S.; Zaw, T.; Motevalibashinaeini, K.; Mischel, P.S.; Pope, W.B. Quantification of edema reduction using differential quantitative T2 (DQT2) relaxometry mapping in recurrent glioblastoma treated with bevacizumab. J. Neurooncol. 2012, 106, 111–119. [Google Scholar] [CrossRef]
- Hattingen, E.; Jurcoane, A.; Daneshvar, K.; Pilatus, U.; Mittelbronn, M.; Steinbach, J.P.; Bähr, O. Quantitative T2 mapping of recurrent glioblastoma under bevacizumab improves monitoring for non-enhancing tumor progression and predicts overall survival. Neuro-Oncology 2013, 15, 1395–1404. [Google Scholar] [CrossRef]
- Ellingson, B.M.; Kim, H.J.; Woodworth, D.C.; Pope, W.B.; Cloughesy, J.N.; Harris, R.J.; Lai, A.; Nghiemphu, P.L.; Cloughesy, T.F. Recurrent Glioblastoma Treated with Bevacizumab: Contrast enhanced T1-weighted Subtraction Maps Improve Tumor Delineation and Aid Prediction of Survival in a Multicenter Clinical Trial. Radiology 2014, 271, 200–210. [Google Scholar] [CrossRef]
- Montagnon, E.; Cerny, M.; Cadrin-Chênevert, A.; Hamilton, V.; Derennes, T.; Ilinca, A.; Vandenbroucke-Menu, F.; Turcotte, S.; Kadoury, S.; Tang, A. Deep learning workflow in radiology: A primer. Insights Imaging 2020, 11, 22. [Google Scholar] [CrossRef]
- Martucci, M.; Russo, R.; Schimperna, F.; D’apolito, G.; Panfili, M.; Grimaldi, A.; Perna, A.; Ferranti, A.M.; Varcasia, G.; Giordano, C.; et al. Magnetic Resonance Imaging of Primary Adult Brain Tumors: State of the Art and Future Perspectives. Biomedicines 2023, 11, 364. [Google Scholar] [CrossRef]
- Yasaka, K.; Akai, H.; Kunimatsu, A.; Kiryu, S.; Abe, O. Deep learning with convolutional neural network in radiology. Jpn. J. Radiol. 2018, 36, 257–272. [Google Scholar] [CrossRef]
- Li, Q.; Bai, H.; Chen, Y.; Sun, Q.; Liu, L.; Zhou, S.; Wang, G.; Liang, C.; Li, Z.-C. A Fully-Automatic Multiparametric Radiomics Model: Towards Reproducible and Prognostic Imaging Signature for Prediction of Overall Survival in Glioblastoma Multiforme. Sci. Rep. 2017, 7, 14331. [Google Scholar] [CrossRef]
- Wagner, M.W.; Namdar, K.; Biswas, A.; Monah, S.; Khalvati, F.; Ertl-Wagner, B.B. Radiomics, machine learning, and artificial intelligence—What the neuroradiologist needs to know. Neuroradiology 2021, 63, 1957–1967. [Google Scholar] [CrossRef]
- Wei, J.; Yang, G.; Hao, X.; Gu, D.; Tan, Y.; Wang, X.; Dong, D.; Zhang, S.; Wang, L.; Zhang, H.; et al. A multi-sequence and habitat-based MRI radiomics signature for preoperative prediction of MGMT promoter methylation in astrocytomas with prognostic implication. Eur. Radiol. 2019, 29, 877–888. [Google Scholar] [CrossRef]
- Li, Y.; Liu, X.; Qian, Z.; Sun, Z.; Xu, K.; Wang, K.; Fan, X.; Zhang, Z.; Li, S.; Wang, Y.; et al. Genotype prediction of ATRX mutation in lower-grade gliomas using an MRI radiomics signature. Eur. Radiol. 2018, 28, 2960–2968. [Google Scholar] [CrossRef]
- Dastmalchian, S.; Kilinc, O.; Onyewadume, L.; Tippareddy, C.; McGivney, D.; Ma, D.; Griswold, M.; Sunshine, J.; Gulani, V.; Barnholtz-Sloan, J.S.; et al. Radiomic analysis of magnetic resonance fingerprinting in adult brain tumors. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 683–693. [Google Scholar] [CrossRef]
- Yi, Z.; Long, L.; Zeng, Y.; Liu, Z. Current Advances and Challenges in Radiomics of Brain Tumors. Front. Oncol. 2021, 11, 732196. [Google Scholar] [CrossRef]
- Apra, C.; Bemora, J.S.; Palfi, S. Achieving Gross Total Resection in Neurosurgery: A Review of Intraoperative Techniques and Their Influence on Surgical Goals. World Neurosurg. 2024, 185, 246–253. [Google Scholar] [CrossRef]
- Shim, K.W.; Park, E.K.; Kim, D.-S.; Choi, J.-U. Neuroendoscopy: Current and Future Perspectives. J. Korean Neurosurg. Soc. 2017, 60, 322–326. [Google Scholar] [CrossRef]
- Dixon, L.; Lim, A.; Grech-Sollars, M.; Nandi, D.; Camp, S. Intraoperative ultrasound in brain tumor surgery: A review and implementation guide. Neurosurg. Rev. 2022, 45, 2503–2515. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, K.; Stein, K.P.; Neyazi, B.; Sandalcioglu, I.E. Theranostic applications of optical coherence tomography in neurosurgery? Neurosurg. Rev. 2022, 45, 421–427. [Google Scholar] [CrossRef] [PubMed]
- Kut, C.; Chaichana, K.L.; Xi, J.; Raza, S.M.; Ye, X.; McVeigh, E.R.; Rodriguez, F.J.; Quiñones-Hinojosa, A.; Li, X. Detection of human brain cancer infiltration ex vivo and in vivo using quantitative optical coherence tomography. Sci. Transl. Med. 2015, 7, 292ra100. [Google Scholar] [CrossRef] [PubMed]
- Butte, P.V.; Fang, Q.; Jo, J.A.; Yong, W.H.; Pikul, B.K.; Black, K.L.; Marcu, L. Intraoperative delineation of primary brain tumors using time-resolved fluorescence spectroscopy. J. Biomed. Opt. 2010, 15, 027008. [Google Scholar] [CrossRef] [PubMed]
- Marcu, L.; Hartl, B.A. Fluorescence Lifetime Spectroscopy and Imaging in Neurosurgery. IEEE J. Sel. Top. Quantum Electron. 2012, 18, 1465–1477. [Google Scholar] [CrossRef] [PubMed]
- Stummer, W.; Reulen, H.J.; Novotny, A.; Steppe, H.; Tonn, J.C. Fluorescence-guided resections of malignant gliomas—An overview. In Local Therapies for Glioma Present Status and Future Developments. Acta Neurochirurgica Supplements; Springer: Vienna, Austria, 2003; Volume 88, pp. 9–12. [Google Scholar]
- Stummer, W.; Pichlmeier, U.; Meinel, T.; Wiestler, O.D.; Zanella, F.; Reulen, H.-J.; ALA-Glioma Study Group. Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: A randomised controlled multicentre phase III trial. Lancet Oncol. 2006, 7, 392–401. [Google Scholar] [CrossRef] [PubMed]
- Dupont, C.; Vermandel, M.; Leroy, H.-A.; Quidet, M.; Lecomte, F.; Delhem, N.; Mordon, S.; Reyns, N. INtraoperative photoDYnamic Therapy for GliOblastomas (INDYGO): Study Protocol for a Phase I Clinical Trial. Neurosurgery 2019, 84, E414–E419. [Google Scholar] [CrossRef] [PubMed]
- De Witt Hamer, P.C.; Robles, S.G.; Zwinderman, A.H.; Duffau, H.; Berger, M.S. Impact of intraoperative stimulation brain mapping on glioma surgery outcome: A meta-analysis. J. Clin. Oncol. 2012, 30, 2559–2565. [Google Scholar] [CrossRef] [PubMed]
- Papagno, C.; Pisoni, A.; Mattavelli, G.; Casarotti, A.; Comi, A.; Fumagalli, F.; Vernice, M.; Fava, E.; Riva, M.; Bello, L. Specific disgust processing in the left insula: New evidence from direct electrical stimulation. Neuropsychologia 2016, 84, 29–35. [Google Scholar] [CrossRef]
- Hugues, D.; Dominique, D.; Capelle, L. Absence of movement disorders after surgical resection of glioma invading the right striatum. J. Neurosurg. 2002, 97, 363–369. [Google Scholar]
- Gallet, C.; Clavreul, A.; Bernard, F.; Menei, P.; Lemée, J.-M. Frontal aslant tract in the non-dominant hemisphere: A systematic review of anatomy, functions, and surgical applications. Front. Neuroanat. 2022, 16, 1025866. [Google Scholar] [CrossRef] [PubMed]
- Gallet, C.; Clavreul, A.; Morandi, X.; Delion, M.; Madec, N.; Menei, P.; Lemée, J.-M. What surgical approach for left-sided eloquent glioblastoma: Biopsy, resection under general anesthesia or awake craniotomy? J. Neurooncol. 2022, 160, 445–454. [Google Scholar] [CrossRef] [PubMed]
- Obayashi, S. Cognitive and linguistic dysfunction after thalamic stroke and recovery process: Possible mechanism. AIMS Neurosci. 2022, 9, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Sanai, N.; Polley, M.-Y.; McDermott, M.W.; Parsa, A.T.; Berger, M.S. An extent of resection threshold for newly diagnosed glioblastomas. J. Neurosurg. 2011, 115, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishnan, P.K.; Saeed, F.; Thomson, S.; Corns, R.; Mathew, R.K.; Sivakumar, G. Awake craniotomy for high-grade gliomas—A prospective cohort study in a UK tertiary-centre. Surgeon 2024, 22, e3–e12. [Google Scholar] [CrossRef] [PubMed]
- Baron, R.B.; Lakomkin, N.; Schupper, A.J.; Nistal, D.; Nael, K.; Price, G.; Hadjipanayis, C.G. Postoperative outcomes following glioblastoma resection using a robot-assisted digital surgical exoscope: A case series. J. Neurooncol. 2020, 148, 519–527. [Google Scholar] [CrossRef]
- Rotim, K.; Splavski, B.; Vrban, F. The Safety and Efficacy of Robot-Assisted Stereotactic Biopsy for Brain Glioma: Earliest Institutional Experiences and Evaluation of Literature. Acta Clin. Croat. 2021, 60, 296–303. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Jiao, N.; Lin, D.; Li, N.; Ma, T.; Tung, S.; Cheng, W.; Wu, A.; Liu, L. Dual-Responsive Nanorobot-Based Marsupial Robotic System for Intracranial Cross-Scale Targeting Drug Delivery. Adv. Mater. 2024, 36, e2306876. [Google Scholar] [CrossRef] [PubMed]
- Maddahi, Y.; Zareinia, K.; Gan, L.S.; Sutherland, C.; Lama, S.; Sutherland, G.R. Treatment of Glioma Using neuroArm Surgical System. Biomed. Res. Int. 2016, 2016, 9734512. [Google Scholar] [CrossRef]
- Legnani, F.G.; Franzini, A.; Mattei, L.; Saladino, A.; Casali, C.; Prada, F.; Perin, A.; Cojazzi, V.; Saini, M.; Kronreif, G.; et al. Image-Guided Biopsy of Intracranial Lesions with a Small Robotic Device (iSYS1): A Prospective, Exploratory Pilot Study. Oper. Neurosurg. 2019, 17, 403–412. [Google Scholar] [CrossRef]
- Hermanto, U.; Frija, E.K.; Lii, M.J.; Chang, E.L.; Mahajan, A.; Woo, S.Y. Intensity-modulated radiotherapy (IMRT) and conventional three-dimensional conformal radiotherapy for high-grade gliomas: Does IMRT increase the integral dose to normal brain? Int. J. Radiat. Oncol. Biol. Phys. 2007, 67, 1135–1144. [Google Scholar] [CrossRef] [PubMed]
- Haque, W.; Butler, E.B.; Teh, B.S. Personalized radiation therapy for glioblastoma. Chin. Clin. Oncol. 2024, 13, 11. [Google Scholar] [CrossRef] [PubMed]
- Stupp, R.; Hegi, M.E.; Mason, W.P.; van den Bent, M.J.; Taphoorn, M.J.B.; Janzer, R.C.; Ludwin, S.K.; Allgeier, A.; Fisher, B.; Belanger, K.; et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009, 10, 459–466. [Google Scholar] [CrossRef] [PubMed]
- Amelio, D.; Lorentini, S.; Schwarz, M.; Amichetti, M. Intensity-modulated radiation therapy in newly diagnosed glioblastoma: A systematic review on clinical and technical issues. Radiother. Oncol. 2010, 97, 361–369. [Google Scholar] [CrossRef] [PubMed]
- Shaffer, R.; Nichol, A.M.; Vollans, E.; Fong, M.; Nakano, S.; Moiseenko, V.; Schmuland, M.; Ma, R.; McKenzie, M.; Otto, K. A comparison of volumetric modulated arc therapy and conventional intensity-modulated radiotherapy for frontal and temporal high-grade gliomas. Int. J. Radiat. Oncol. Biol. Phys. 2010, 76, 1177–1184. [Google Scholar] [CrossRef] [PubMed]
- Sheu, T.; Briere, T.M.; Olanrewaju, A.M.; McAleer, M.F. Intensity Modulated Radiation Therapy Versus Volumetric Arc Radiation Therapy in the Treatment of Glioblastoma—Does Clinical Benefit Follow Dosimetric Advantage? Adv. Radiat. Oncol. 2019, 4, 50–56. [Google Scholar] [CrossRef]
- Navarria, P.; Pessina, F.; Cozzi, L.; Ascolese, A.M.; Lobefalo, F.; Stravato, A.; D’agostino, G.; Franzese, C.; Caroli, M.; Bello, L.; et al. Can advanced new radiation therapy technologies improve outcome of high-grade glioma (HGG) patients? analysis of 3D-conformal radiotherapy (3DCRT) versus volumetric-modulated arc therapy (VMAT) in patients treated with surgery, concomitant and adjuvant chemo-radiotherapy. BMC Cancer 2016, 16, 362. [Google Scholar]
- Davidson, M.T.M.; Masucci, G.L.; Follwell, M.; Blake, S.J.; Xu, W.; Moseley, D.J.; Sanghera, P.; Wong, C.S.; Perry, J.; Tsao, M.; et al. Single Arc Volumetric Modulated Arc Therapy for Complex Brain Gliomas: Is There an Advantage as Compared to Intensity Modulated Radiotherapy or by Adding a Partial Arc? Technol. Cancer Res. Treat. 2012, 11, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Brown, P.D.; Chung, C.; Liu, D.D.; McAvoy, S.; Grosshans, D.; Al Feghali, K.; Mahajan, A.; Li, J.; McGovern, S.L.; McAleer, M.-F.; et al. A prospective phase II randomized trial of proton radiotherapy vs intensity-modulated radiotherapy for patients with newly diagnosed glioblastoma. Neuro-Oncology 2021, 23, 1337–1347. [Google Scholar] [CrossRef]
- Mori, T.; Mizumoto, M.; Maebayashi, K.; Nishioka, K.; Arakawa, Y.; Kurozumi, K.; Yasuda, K.; Sumiya, T.; Tamamura, H.; Sato, Y.; et al. Proton beam therapy for gliomas: A multicenter prospective registry study from all proton beam facilities in Japan. J. Radiat. Res. 2023, 64 (Suppl. 1), i59–i68. [Google Scholar] [CrossRef]
- Chambrelant, I.; Eber, J.; Antoni, D.; Burckel, H.; Noël, G.; Auvergne, R. Proton Therapy and Gliomas: A Systematic Review. Radiation 2021, 1, 218–233. [Google Scholar] [CrossRef]
- Bonosi, L.; Marino, S.; Benigno, U.E.; Musso, S.; Buscemi, F.; Giardina, K.; Gerardi, R.; Brunasso, L.; Costanzo, R.; Iacopino, D.G.; et al. Sonodynamic therapy and magnetic resonance-guided focused ultrasound: New therapeutic strategy in glioblastoma. J. Neuro-Oncol. 2023, 163, 219–238. [Google Scholar] [CrossRef] [PubMed]
- Mainprize, T.; Lipsman, N.; Huang, Y.; Meng, Y.; Bethune, A.; Ironside, S.; Heyn, C.; Alkins, R.; Trudeau, M.; Sahgal, A.; et al. Blood-Brain Barrier Opening in Primary Brain Tumors with Non-invasive MR-Guided Focused Ultrasound: A Clinical Safety and Feasibility Study. Sci. Rep. 2019, 9, 321. [Google Scholar] [CrossRef] [PubMed]
- Roberts, J.W.; Powlovich, L.; Sheybani, N.; LeBlang, S. Focused ultrasound for the treatment of glioblastoma. J. Neuro-Oncol. 2022, 157, 237–247. [Google Scholar] [CrossRef] [PubMed]
- Koutsarnakis, C.; Neromyliotis, E.; Komaitis, S.; Mazarakis, N.; O'Hara, D.J.; Stranjalis, G.; Chumas, P. Effects of brain radiotherapy on cognitive performance in adult low-grade glioma patients: A systematic review. Radiother. Oncol. 2021, 160, 202–211. [Google Scholar] [CrossRef] [PubMed]
- Tos, S.M.; Mantziaris, G.; Shaaban, A.; Sheehan, J.P. Stereotactic radiosurgery for intracranial cavernous malformations of the deep-seated locations: Systematic review and meta-analysis. Neurosurg. Rev. 2024, 47, 186. [Google Scholar] [CrossRef] [PubMed]
- Douw, L.; Klein, M.; Fagel, S.S.; van den Heuvel, J.; Taphoorn, M.J.; Aaronson, N.K.; Postma, T.J.; Vandertop, W.P.; Mooij, J.J.; Boerman, R.H.; et al. Cognitive and radiological effects of radiotherapy in patients with low-grade glioma: Long-term follow-up. Lancet Neurol. 2009, 8, 810–818. [Google Scholar] [CrossRef] [PubMed]
- Rehman, J.U.; Zahra; Ahmad, N.; Khalid, M.; Asghar, H.N.U.H.K.; Gilani, Z.A.; Ullah, I.; Nasar, G.; Akhtar, M.M.; Usmani, M.N. Intensity modulated radiation therapy: A review of current practice and future outlooks. J. Radiat. Res. Appl. Sci. 2018, 11, 361–367. [Google Scholar]
- Cheung, K. Intensity modulated radiotherapy: Advantages, limitations and future developments. Biomed. Imaging Interv. J. 2006, 2, e19. [Google Scholar] [CrossRef]
- Pierre, F. The Advantages and Drawbacks of Proton Cancer Therapy. J. Cancer Epidemiol. Prev. 2023, 8, 14. [Google Scholar]
- Schneider, C.S.; Woodworth, G.F.; Vujaskovic, Z.; Mishra, M.V. Radiosensitization of high-grade gliomas through induced hyperthermia: Review of clinical experience and the potential role of MR-guided focused ultrasound. Radiother. Oncol. 2020, 142, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Kinslow, C.J.; Rae, A.I.; Taparra, K.; Kumar, P.; Siegelin, M.D.; Grinband, J.; Gill, B.J.; McKhann, G.M.; Sisti, M.B.; Bruce, J.N.; et al. MGMT Promoter Methylation Predicts Overall Survival after Chemotherapy for 1p/19q-Codeleted Gliomas. Clin. Cancer Res. 2023, 29, 4399–4407. [Google Scholar] [CrossRef] [PubMed]
- Kinslow, C.J.; Mercurio, A.; Kumar, P.; Rae, A.I.; Siegelin, M.D.; Grinband, J.; Taparra, K.; Upadhyayula, P.S.; McKhann, G.M.; Sisti, M.B.; et al. Association of MGMT Promoter Methylation with Survival in Low-grade and Anaplastic Gliomas After Alkylating Chemotherapy. JAMA Oncol. 2023, 9, 919–927. [Google Scholar] [CrossRef]
- Hegi, M.E.; Diserens, A.C.; Godard, S.; Dietrich, P.Y.; Regli, L.; Ostermann, S.; Otten, P.; Van Melle, G.; De Tribolet, N.; Stupp, R. Clinical Trial Substantiates the Predictive Value of O-6-Methylguanine-DNA Methyltransferase Promoter Methylation in Glioblastoma Patients Treated with Temozolomide. Clin. Cancer Res. 2004, 10, 1871–1874. [Google Scholar] [CrossRef] [PubMed]
- Carrano, A.; Juarez, J.J.; Incontri, D.; Ibarra, A.; Cazares, H.G. Sex-Specific Differences in Glioblastoma. Cells 2021, 10, 1783. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Xia, Q.; Liu, L.; Li, S.; Dong, L. Current Opinion on Molecular Characterization for GBM Classification in Guiding Clinical Diagnosis, Prognosis, and Therapy. Front. Mol. Biosci. 2020, 7, 562798. [Google Scholar] [CrossRef] [PubMed]
- Cioffi, G.; Waite, K.; Dmukauskas, M.; Glantz, M.; Aulakh, S.; Nicolaides, T.; Sengupta, S.; Xiu, J.; Barnholtz-Sloan, J.S. Sex differences in glioblastoma response to treatment: Impact of MGMT methylation. Neurooncol. Adv. 2024, 6, vdae031. [Google Scholar] [CrossRef]
- Shen, D.; Guo, C.C.; Wang, J.; Qiu, Z.K.; Sai, K.; Yang, Q.Y.; Chen, Y.S.; Chen, F.R.; Wang, J.; Panasci, L.; et al. Interferon-α/β enhances temozolomide activity against MGMT-positive glioma stem-like cells. Oncol. Rep. 2015, 34, 2715–2721. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Yang, Q.; Xu, P.; Deng, M.; Jiang, T.; Cai, L.; Li, J.; Sai, K.; Xi, S.; Ouyang, H.; et al. Adjuvant Temozolomide Chemotherapy With or Without Interferon Alfa Among Patients With Newly Diagnosed High-grade Gliomas: A Randomized Clinical Trial. JAMA Netw. Open 2023, 6, e2253285. [Google Scholar] [CrossRef] [PubMed]
- Valerius, A.R.; Webb, L.M.; Sener, U. Novel Clinical Trials and Approaches in the Management of Glioblastoma. Curr. Oncol. Rep. 2024, 26, 439–465. [Google Scholar] [CrossRef]
- Kitange, G.J.; Mladek, A.C.; Carlson, B.L.; Schroeder, M.A.; Pokorny, J.L.; Cen, L.; Decker, P.A.; Wu, W.; Lomberk, G.A.; Gupta, S.K.; et al. Inhibition of histone deacetylation potentiates the evolution of acquired temozolomide resistance linked to MGMT upregulation in glioblastoma xenografts. Clin. Cancer Res. 2012, 18, 4070–4079. [Google Scholar] [CrossRef] [PubMed]
- Galanis, E.; Anderson, S.K.; Miller, C.R.; Sarkaria, J.N.; Jaeckle, K.; Buckner, J.C.; Ligon, K.L.; Ballman, K.V.; Moore, D.F., Jr.; Nebozhyn, M.; et al. Phase I/II trial of vorinostat combined with temozolomide and radiation therapy for newly diagnosed glioblastoma: Results of Alliance N0874/ABTC 02. Neuro-Oncology 2018, 20, 546–556. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Liu, B.; Feng, L.; Sun, B.; He, S.; Yang, Y.; Wu, G.; E, G.; Liu, C.; Gao, Y.; et al. Targeting JUN, CEBPB, and HDAC3: A Novel Strategy to Overcome Drug Resistance in Hypoxic Glioblastoma. Front. Oncol. 2019, 9, 33. [Google Scholar] [CrossRef] [PubMed]
- Scicchitano, B.M.; Sorrentino, S.; Proietti, G.; Lama, G.; Dobrowolny, G.; Catizone, A.; Binda, E.; Larocca, L.M.; Sica, G. Levetiracetam enhances the temozolomide effect on glioblastoma stem cell proliferation and apoptosis. Cancer Cell Int. 2018, 18, 136. [Google Scholar] [CrossRef] [PubMed]
- Pallud, J.; Huberfeld, G.; Dezamis, E.; Peeters, S.; Moiraghi, A.; Gavaret, M.; Guinard, E.; Dhermain, F.; Varlet, P.; Oppenheim, C.; et al. Effect of Levetiracetam Use Duration on Overall Survival of Isocitrate Dehydrogenase Wild-Type Glioblastoma in Adults: An Observational Study. Neurology 2022, 98, e125–e140. [Google Scholar] [CrossRef] [PubMed]
- Greene, L.A.; Zhou, Q.; Siegelin, M.D.; Angelastro, J.M. Targeting Transcription Factors ATF5, CEBPB and CEBPD with Cell-Penetrating Peptides to Treat Brain and Other Cancers. Cells 2023, 12, 581. [Google Scholar] [CrossRef] [PubMed]
- Vinson, C.R.; Hai, T.; Boyd, S.M. Dimerization specificity of the leucine zipper-containing bZIP motif on DNA binding: Prediction and rational design. Gene Dev. 1993, 7, 1047–1058. [Google Scholar] [CrossRef] [PubMed]
- Angelastro, J.M.; Canoll, P.D.; Kuo, J.; Weicker, M.; Costa, A.; Bruce, J.N.; A Greene, L. Selective destruction of glioblastoma cells by interference with the activity or expression of ATF5. Oncogene 2006, 25, 907–916. [Google Scholar] [CrossRef]
- Dupont, E.; Prochiantz, A.; Joliot, A. Penetratin Story: An Overview. Methods Mol. Biol. 2015, 1324, 21–29. [Google Scholar]
- Karpel-Massler, G.; Horst, B.A.; Shu, C.; Chau, L.; Tsujiuchi, T.; Bruce, J.N.; Canoll, P.; Greene, L.A.; Angelastro, J.M.; Siegelin, M.D. A Synthetic Cell-Penetrating Dominant-Negative ATF5 Peptide Exerts Anticancer Activity against a Broad Spectrum of Treatment-Resistant Cancers. Clin. Cancer Res. 2016, 22, 4698–4711. [Google Scholar] [CrossRef]
- Cates, C.C.; Arias, A.D.; Wong, L.S.N.; Lamé, M.W.; Sidorov, M.; Cayanan, G.; Rowland, D.J.; Fung, J.; Karpel-Massler, G.; Siegelin, M.D.; et al. Regression/Eradication of gliomas in mice by a systemically deliverable ATF5 dominant-negative peptide. Oncotarget 2016, 7, 12718. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Jefferson, P.; Zhou, Q.; Angelastro, J.M.; Greene, L.A. Dominant-Negative ATF5 Compromises Cancer Cell Survival by Targeting CEBPB and CEBPD. Mol. Cancer Res. 2020, 18, 216–228. [Google Scholar] [CrossRef] [PubMed]
- Darvishi, E.; Ghamsari, L.; Leong, S.F.; Ramirez, R.; Koester, M.; Gallagher, E.; Yu, M.; Mason, J.M.; Merutka, G.; Kappel, B.J.; et al. Anticancer Activity of ST101, A Novel Antagonist of CCAAT/Enhancer Binding Protein β. Mol. Cancer Ther. 2022, 21, 1632–1644. [Google Scholar] [CrossRef]
- Zhou, Q.; Sun, X.; Pasquier, N.; Jefferson, P.; Nguyen, T.T.T.; Siegelin, M.D.; Angelastro, J.M.; Greene, L.A. Cell-Penetrating CEBPB and CEBPD Leucine Zipper Decoys as Broadly Acting Anti-Cancer Agents. Cancers 2021, 13, 2504. [Google Scholar] [CrossRef] [PubMed]
- Gousias, K.; Theocharous, T.; Simon, M. Mechanisms of Cell Cycle Arrest and Apoptosis in Glioblastoma. Biomedicines 2022, 10, 564. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Jiang, J.; Wu, J.; Bao, X.; Sanai, N. Physiologically Based Pharmacokinetic Modeling of Central Nervous System Pharmacokinetics of CDK4/6 Inhibitors to Guide Selection of Drug and Dosing Regimen for Brain Cancer Treatment. Clin. Pharmacol. Ther. 2021, 109, 494–506. [Google Scholar] [CrossRef] [PubMed]
- Braal, C.L.; Jongbloed, E.M.; Wilting, S.M.; Mathijssen, R.H.; Koolen, S.L.; Jager, A. Inhibiting CDK4/6 in Breast Cancer with Palbociclib, Ribociclib, and Abemaciclib: Similarities and Differences. Drugs 2021, 81, 317–331. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Liang, J.; Fan, J.; Hou, P.; Li, X.; Zhang, H.; Li, K.; Bu, L.; Li, P.; He, M.; et al. CDK4/6 Inhibition Enhances Oncolytic Virus Efficacy by Potentiating Tumor-Selective Cell Killing and T-cell Activation in Refractory Glioblastoma. Cancer Res. 2022, 82, 3359–3374. [Google Scholar] [CrossRef] [PubMed]
- Taylor, J.W.; Parikh, M.; Phillips, J.J.; James, C.D.; Molinaro, A.M.; Butowski, N.A.; Clarke, J.L.; Oberheim-Bush, N.A.; Chang, S.M.; Berger, M.S.; et al. Phase-2 trial of palbociclib in adult patients with recurrent RB1-positive glioblastoma. J. Neurooncol. 2018, 140, 477–483. [Google Scholar] [CrossRef]
- Tien, A.C.; Li, J.; Bao, X.; Derogatis, A.; Kim, S.; Mehta, S.; Sanai, N. A Phase 0 Trial of Ribociclib in Recurrent Glioblastoma Patients Incorporating a Tumor Pharmacodynamic- and Pharmacokinetic-Guided Expansion Cohort. Clin. Cancer Res. 2019, 25, 5777–5786. [Google Scholar] [CrossRef]
- Thorne, A.H.; Zanca, C.; Furnari, F. Epidermal growth factor receptor targeting and challenges in glioblastoma. Neuro-Oncology 2016, 18, 914–918. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.W.; Jung, H.A.; Cho, H.J.; Kim, T.M.; Park, C.K.; Nam, D.H.; Lee, S.H. A multicenter, phase II trial of GC1118, a novel anti-EGFR antibody, for recurrent glioblastoma patients with EGFR amplification. Cancer Med. 2023, 12, 15788–15796. [Google Scholar] [CrossRef] [PubMed]
- Le Rhun, E.; Preusser, M.; Roth, P.; Reardon, D.A.; van den Bent, M.V.; Wen, P.; Reifenberger, G.; Weller, M. Molecular targeted therapy of glioblastoma. Cancer Treat. Rev. 2019, 80, 101896. [Google Scholar] [CrossRef] [PubMed]
- Kreisl, T.N.; Kim, L.; Moore, K.; Duic, P.; Royce, C.; Stroud, I.; Garren, N.; Mackey, M.; Butman, J.A.; Camphausen, K.; et al. Phase II trial of single-agent bevacizumab followed by bevacizumab plus irinotecan at tumor progression in recurrent glioblastoma. J. Clin. Oncol. 2009, 27, 740–745. [Google Scholar] [CrossRef] [PubMed]
- Cohen, M.H.; Shen, Y.L.; Keegan, P.; Pazdur, R. FDA drug approval summary: Bevacizumab (Avastin) as treatment of recurrent glioblastoma multiforme. Oncologist 2009, 14, 1131–1138. [Google Scholar] [CrossRef] [PubMed]
- Lombardi, G.; De Salvo, G.L.; Brandes, A.A.; Eoli, M.; Rudà, R.; Faedi, M.; Lolli, I.; Pace, A.; Daniele, B.; Pasqualetti, F.; et al. Regorafenib compared with lomustine in patients with relapsed glioblastoma (REGOMA): A multicenter, open label, randomized, controlled, phase 2 trial. Lancet Oncol. 2019, 20, 110–119. [Google Scholar] [CrossRef] [PubMed]
- Caragher, S.; Miska, J.; Shireman, J.; Park, C.H.; Muroski, M.; Lesniak, M.S.; Ahmed, A.U. Temozolomide Treatment Increases Fatty Acid Uptake in Glioblastoma Stem Cells. Cancers 2020, 12, 3126. [Google Scholar] [CrossRef] [PubMed]
- Shim, J.K.; Choi, S.; Yoon, S.-J.; Choi, R.J.; Park, J.; Lee, E.H.; Cho, H.J.; Lee, S.; Teo, W.-Y.; Moon, J.H.; et al. Etomoxir, a carnitine palmitoyltransferase 1 inhibitor, combined with temozolomide reduces stemness and invasiveness in patient-derived glioblastoma tumorspheres. Cancer Cell Int. 2022, 22, 309. [Google Scholar] [CrossRef] [PubMed]
- Eyme, K.M.; Sammarco, A.; Jha, R.; Mnatsakanyan, H.; Pechdimaljian, C.; Carvalho, L.; Neustadt, R.; Moses, C.; Alnasser, A.; Tardiff, D.F.; et al. Targeting de novo lipid synthesis induces lipotoxicity and impairs DNA damage repair in glioblastoma mouse models. Sci. Transl. Med. 2023, 15, eabq6288. [Google Scholar] [CrossRef]
- Badr, C.E.; Silver, D.J.; Siebzehnrubl, F.A.; Deleyrolle, L.P. Metabolic heterogeneity and adaptability in brain tumors. Cell. Mol. Life Sci. 2020, 77, 5101–5119. [Google Scholar] [CrossRef]
- Nguyen, T.T.T.; Shang, E.; Westhoff, M.A.; Karpel-Massler, G.; Siegelin, M.D. Methodological Approaches for Assessing Metabolomic Changes in Glioblastomas. Methods Mol. Biol. 2022, 2445, 305–328. [Google Scholar] [PubMed]
- Nguyen, T.T.T.; Shang, E.; Westhoff, M.-A.; Karpel-Massler, G.; Siegelin, M.D. Therapeutic Drug-Induced Metabolic Reprogramming in Glioblastoma. Cells 2022, 11, 2956. [Google Scholar] [CrossRef] [PubMed]
- Galanis, E.; Jaeckle, K.A.; Maurer, M.J.; Reid, J.M.; Ames, M.M.; Hardwick, J.S.; Reilly, J.F.; Loboda, A.; Nebozhyn, M.; Fantin, V.R.; et al. Phase II trial of vorinostat in recurrent glioblastoma multiforme: A north central cancer treatment group study. J. Clin. Oncol. 2009, 27, 2052–2058. [Google Scholar] [CrossRef]
- Nguyen, T.T.T.; Zhang, Y.; Shang, E.; Shu, C.; Torrini, C.; Zhao, J.; Bianchetti, E.; Mela, A.; Humala, N.; Mahajan, A.; et al. HDAC inhibitors elicit metabolic reprogramming by targeting super-enhancers in glioblastoma models. J. Clin. Investig. 2020, 130, 3699–3716. [Google Scholar] [CrossRef]
- Nguyen, T.T.T.; Shang, E.; Schiffgens, S.; Torrini, C.; Shu, C.; Akman, H.O.; Prabhu, V.V.; Allen, J.E.; Westhoff, M.A.; Karpel-Massler, G.; et al. Induction of Synthetic Lethality by Activation of Mitochondrial ClpP and Inhibition of HDAC1/2 in Glioblastoma. Clin. Cancer Res. 2022, 28, 1881–1895. [Google Scholar] [CrossRef]
- Yu, A.; Zeng, J.; Yu, J.; Cao, S.; Li, A. Theory and application of TTFields in newly diagnosed glioblastoma. CNS Neurosci. Ther. 2024, 30, e14563. [Google Scholar] [CrossRef] [PubMed]
- Onciul, R.; Brehar, F.-M.; Toader, C.; Covache-Busuioc, R.-A.; Glavan, L.-A.; Bratu, B.-G.; Costin, H.P.; Dumitrascu, D.-I.; Serban, M.; Ciurea, A.V. Deciphering Glioblastoma: Fundamental and Novel Insights into the Biology and Therapeutic Strategies of Gliomas. Curr. Issues Mol. Biol. 2024, 46, 2402–2443. [Google Scholar] [CrossRef]
- Mehta, M.; Wen, P.; Nishikawa, R.; Reardon, D.; Peters, K. Critical review of the addition of tumor treating fields (TTFields) to the existing standard of care for newly diagnosed glioblastoma patients. Crit. Rev. Oncol. Hematol. 2017, 111, 60–65. [Google Scholar] [CrossRef]
- Cao, Q.; Hajosch, A.; Kast, R.E.; Loehmann, C.; Hlavac, M.; Fischer-Posovszky, P.; Strobel, H.; Westhoff, M.-A.; Siegelin, M.D.; Wirtz, C.R.; et al. Tumor Treating Fields (TTFields) combined with the drug repurposing approach CUSP9v3 induce metabolic reprogramming and synergistic anti-glioblastoma activity in vitro. Br. J. Cancer 2024, 130, 1365–1376. [Google Scholar] [CrossRef]
- Stepanenko, A.A.; Chekhonin, V.P. Recent Advances in Oncolytic Virotherapy and Immunotherapy for Glioblastoma: A Glimmer of Hope in the Search for an Effective Therapy? Cancers 2018, 10, 492. [Google Scholar] [CrossRef]
- Kirson, E.D.; Giladi, M.; Gurvich, Z.; Itzhaki, A.; Mordechovich, D.; Schneiderman, R.S.; Wasserman, Y.; Ryffel, B.; Goldsher, D.; Palti, Y. Alternating electric fields (TTFields) inhibit metastatic spread of solid tumors to the lungs. Clin. Exp. Metastasis 2009, 26, 633–640. [Google Scholar] [CrossRef] [PubMed]
- Bobo, R.H.; Laske, D.W.; Akbasak, A.; Morrison, P.F.; Dedrick, R.L.; Oldfield, E.H. Convection-enhanced delivery of macromolecules in the brain. Proc. Nat. Acad. Sci. USA 1994, 91, 2076–2080. [Google Scholar] [CrossRef] [PubMed]
- D’Amico, R.S.; Aghi, M.K.; Vogelbaum, M.A.; Bruce, J.N. Convection-enhanced drug delivery for glioblastoma: A review. J. Neuro-Oncol. 2021, 151, 415–427. [Google Scholar] [CrossRef] [PubMed]
- Spinazzi, E.F.; Argenziano, M.G.; Upadhyayula, P.S.; A Banu, M.; A Neira, J.; O Higgins, D.M.; Wu, P.B.; Pereira, B.; Mahajan, A.; Humala, N.; et al. Chronic convection-enhanced delivery of topotecan for patients with recurrent glioblastoma: A first-in-patient, single-center, single-arm, phase 1b trial. Lancet Oncol. 2022, 23, 1409–1418. [Google Scholar] [CrossRef]
- Lidar, Z.; Mardor, Y.; Jonas, T.; Pfeffer, R.; Faibel, M.; Nass, D.; Hadani, M.; Ram, Z. Convection-enhanced delivery of paclitaxel for the treatment of recurrent malignant glioma: A Phase I/II clinical study. J. Neurosurg. 2004, 100, 472–479. [Google Scholar] [CrossRef] [PubMed]
- Jahangiri, A.; Chin, A.T.; Flanigan, P.M.; Chen, R.; Bankiewicz, K.; Aghi, M.K. Convection-enhanced delivery in glioblastoma: A review of preclinical and clinical studies. J. Neurosurg. 2017, 126, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Sperring, C.P.; Argenziano, M.G.; Savage, W.M.; E Teasley, D.; Upadhyayula, P.S.; Winans, N.J.; Canoll, P.; Bruce, J.N. Convection-enhanced delivery of immunomodulatory therapy for high-grade glioma. Neuro-Oncol. Adv. 2023, 5, vdad044. [Google Scholar] [CrossRef] [PubMed]
- Rayati, M.; Mansouri, V.; Ahmadbeigi, N. Gene therapy in glioblastoma multiforme: Can it be a role changer? Heliyon 2024, 10, e27087. [Google Scholar] [CrossRef] [PubMed]
- Walther, W. Gene Therapy of Cancer: Methods and Protocols. Methods Mol. Biol. 2022, 35, 2521. [Google Scholar]
- Romanishin, A.; Vasilev, A.; Khasanshin, E.; Evtekhov, A.; Pusynin, E.; Rubina, K.; Kakotkin, V.; Agapov, M.; Semina, E. Oncolytic viral therapy for gliomas: Advances in the mechanisms and approaches to delivery. Virology 2024, 593, 110033. [Google Scholar] [CrossRef]
- Yang, T.; Kong, Z.; Ma, W. PD-1/PD-L1 immune checkpoint inhibitors in glioblastoma: Clinical studies, challenges and potential. Hum. Vaccin. Immunother. 2021, 17, 546–553. [Google Scholar] [CrossRef] [PubMed]
- Caccese, M.; Indraccolo, S.; Zagonel, V.; Lombardi, G. PD-1/PD-L1 immune-checkpoint inhibitors in glioblastoma: A concise review. Crit. Rev. Oncol. Hematol. 2019, 135, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Woroniecka, K.; Chongsathidkiet, P.; Rhodin, K.; Kemeny, H.; Dechant, C.; Farber, S.H.; Elsamadicy, A.A.; Cui, X.; Koyama, S.; Jackson, C.; et al. T-Cell Exhaustion Signatures Vary with Tumor Type and Are Severe in Glioblastoma. Clin. Cancer Res. 2018, 24, 4175–4186. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Li, X.; Zhou, W.-L.; Huang, Y.; Liang, X.; Jiang, L.; Yang, X.; Sun, J.; Li, Z.; Han, W.-D.; et al. Genetically engineered T cells for cancer immunotherapy. Signal Transduct. Target. Ther. 2019, 4, 35. [Google Scholar] [CrossRef] [PubMed]
- Maude, S.L.; Laetsch, T.W.; Buechner, J.; Rives, S.; Boyer, M.; Bittencourt, H.; Bader, P.; Verneris, M.R.; Stefanski, H.E.; Myers, G.D.; et al. Tisagenlecleucel in Children and Young Adults with B-Cell Lymphoblastic Leukemia. N. Engl. J. Med. 2018, 378, 439–448. [Google Scholar] [CrossRef] [PubMed]
- Locke, F.L.; Ghobadi, A.; Jacobson, C.A.; Miklos, D.B.; Lekakis, L.J.; Oluwole, O.O.; Lin, Y.; Braunschweig, I.; Hill, B.T.; Timmerman, J.M.; et al. Long-term safety and activity of axicabtagene ciloleucel in refractory large B-cell lymphoma (ZUMA-1): A single-arm, multicentre, phase 1-2 trial. Lancet Oncol. 2019, 20, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Munoz, J.; Goy, A.; Locke, F.L.; Jacobson, C.A.; Hill, B.T.; Timmerman, J.M.; Holmes, H.; Jaglowski, S.; Flinn, I.W.; et al. KTE-X19 CAR T-Cell Therapy in Relapsed or Refractory Mantle-Cell Lymphoma. N. Engl. J. Med. 2020, 382, 1331–1342. [Google Scholar] [CrossRef] [PubMed]
- Abramson, J.S.; Palomba, M.L.; Gordon, L.I.; Lunning, M.A.; Wang, M.; Arnason, J.; Mehta, A.; Purev, E.; Maloney, D.G.; Andreadis, C.; et al. Lisocabtagene maraleucel for patients with relapsed or refractory large B-cell lymphomas (TRANSCEND NHL 001): A multicentre seamless design study. Lancet 2020, 396, 839–852. [Google Scholar] [CrossRef] [PubMed]
- Kochenderfer, J.N.; Wilson, W.H.; Janik, J.E.; Dudley, M.E.; Stetler-Stevenson, M.; Feldman, S.A.; Maric, I.; Raffeld, M.; Nathan, D.-A.N.; Lanier, B.J.; et al. Eradication of B-lineage cells and regression of lymphoma in a patient treated with autologous T cells genetically engineered to recognize CD19. Blood 2010, 116, 4099–4102. [Google Scholar] [CrossRef]
- Mitra, A.; Barua, A.; Huang, L.; Ganguly, S.; Feng, Q.; He, B. From bench to bedside: The history and progress of CAR T cell therapy. Front. Immunol. 2023, 14, 1188049. [Google Scholar] [CrossRef]
- Munshi, N.C.; Anderson, L.D., Jr.; Shah, N.; Madduri, D.; Berdeja, J.; Lonial, S.; Raje, N.; Lin, Y.; Siegel, D.; Oriol, A.; et al. Idecabtagene Vicleucel in Relapsed and Refractory Multiple Myeloma. N. Engl. J. Med. 2021, 384, 705–716. [Google Scholar] [CrossRef] [PubMed]
- Martin, T.; Usmani, S.Z.; Berdeja, J.G.; Agha, M.; Cohen, A.D.; Hari, P.; Avigan, D.; Deol, A.; Htut, M.; Lesokhin, A.; et al. Ciltacabtagene Autoleucel, an Anti-B-cell Maturation Antigen Chimeric Antigen Receptor T-Cell Therapy, for Relapsed/Refractory Multiple Myeloma: CARTITUDE-1 2-Year Follow-Up. J. Clin. Oncol. 2023, 41, 1265–1274. [Google Scholar] [CrossRef] [PubMed]
- O’Rourke, D.M.; Nasrallah, M.P.; Desai, A.; Melenhorst, J.J.; Mansfield, K.; Morrissette, J.J.; Martinez-Lage, M.; Brem, S.; Maloney, E.; Shen, A.; et al. A single dose of peripherally infused EGFRvIII-directed CAR T cells mediates antigen loss and induces adaptive resistance in patients with recurrent glioblastoma. Sci. Transl. Med. 2017, 9, eaaa0984. [Google Scholar] [CrossRef] [PubMed]
- Choi, B.D.; Gerstner, E.R.; Frigault, M.J.; Leick, M.B.; Mount, C.W.; Balaj, L.; Nikiforow, S.; Carter, B.S.; Curry, W.T.; Gallagher, K.; et al. Intraventricular CARv3-TEAM-E T Cells in Recurrent Glioblastoma. N. Engl. J. Med. 2024, 390, 1290–1298. [Google Scholar] [CrossRef] [PubMed]
- Hobbs, J.; Nikiforova, M.N.; Fardo, D.W.; Bortoluzzi, S.; Cieply, K.; Hamilton, R.L.; Horbinski, C. Paradoxical Relationship Between the Degree of EGFR Amplification and Outcome in Glioblastomas. Am. J. Surg. Pathol. 2012, 36, 1186–1193. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, N.; Brawley, V.; Hegde, M.; Bielamowicz, K.; Kalra, M.; Landi, D.; Robertson, C.; Gray, T.L.; Diouf, O.; Wakefield, A.; et al. HER2-Specific Chimeric Antigen Receptor-Modified Virus-Specific T Cells for Progressive Glioblastoma: A Phase 1 Dose-Escalation Trial. JAMA Oncol. 2017, 3, 1094–1101. [Google Scholar] [CrossRef] [PubMed]
- Mineo, J.F.; Bordron, A.; Baroncini, M.; Maurage, C.-A.; Ramirez, C.; Siminski, R.-M.; Berthou, C.; Hieu, P.D. Low HER2-expressing glioblastomas are more often secondary to anaplastic transformation of low-grade glioma. J. Neuro-Oncol. 2007, 85, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Shalhout, S.Z.; Miller, D.M.; Emerick, K.S.; Kaufman, H.L. Therapy with oncolytic viruses: Progress and challenges. Nat. Rev. Clin. Oncol. 2023, 20, 160–177. [Google Scholar] [CrossRef] [PubMed]
- Fukuhara, H.; Ino, Y.; Todo, T. Oncolytic virus therapy: A new era of cancer treatment at dawn. Cancer Sci. 2016, 107, 1373–1379. [Google Scholar] [CrossRef]
- Mowforth, O.D.; Brannigan, J.; El Khoury, M.; Sarathi, C.I.P.; Bestwick, H.; Bhatti, F.; Mair, R. Personalised therapeutic approaches to glioblastoma: A systematic review. Front. Med. 2023, 10, 1166104. [Google Scholar] [CrossRef]
- Bommareddy, P.K.; Wakimoto, H.; Martuza, R.L.; Kaufman, H.L.; Rabkin, S.D.; Saha, D. Oncolytic herpes simplex virus expressing IL-2 controls glioblastoma growth and improves survival. J. Immunother. Cancer 2024, 12, e008880. [Google Scholar] [CrossRef] [PubMed]
- Nassiri, F.; Patil, V.; Yefet, L.S.; Singh, O.; Liu, J.; Dang, R.M.; Yamaguchi, T.N.; Daras, M.; Cloughesy, T.F.; Colman, H.; et al. Oncolytic DNX-2401 virotherapy plus pembrolizumab in recurrent glioblastoma: A phase 1/2 trial. Nat. Med. 2023, 29, 1370–1378. [Google Scholar] [CrossRef] [PubMed]
- Lang, F.F.; Conrad, C.; Gomez-Manzano, C.; Yung, W.K.A.; Sawaya, R.; Weinberg, J.S.; Prabhu, S.S.; Rao, G.; Fuller, G.N.; Aldape, K.D.; et al. Phase I Study of DNX-2401 (Delta-24-RGD) Oncolytic Adenovirus: Replication and Immunotherapeutic Effects in Recurrent Malignant Glioma. J. Clin. Oncol. 2018, 36, 1419–1427. [Google Scholar] [CrossRef] [PubMed]
- Harrington, K.; Freeman, D.J.; Kelly, B.; Harper, J.; Soria, J.-C. Optimizing oncolytic virotherapy in cancer treatment. Nat. Rev. Drug Discov. 2019, 18, 689–706. [Google Scholar] [CrossRef] [PubMed]
- Todo, T.; Ito, H.; Ino, Y.; Ohtsu, H.; Ota, Y.; Shibahara, J.; Tanaka, M. Intratumoral oncolytic herpes virus G47∆ for residual or recurrent glioblastoma: A phase 2 trial. Nat. Med. 2022, 28, 1630–1639. [Google Scholar] [CrossRef] [PubMed]
- Friedman, G.K.; Johnston, J.M.; Bag, A.K.; Bernstock, J.D.; Li, R.; Aban, I.; Kachurak, K.; Nan, L.; Kang, K.-D.; Totsch, S.; et al. Oncolytic HSV-1 G207 Immunovirotherapy for Pediatric High-Grade Gliomas. N. Engl. J. Med. 2021, 384, 1613–1622. [Google Scholar] [CrossRef] [PubMed]
- Markert, J.M.; Razdan, S.N.; Kuo, H.C.; Cantor, A.; Knoll, A.; Karrasch, M.; Nabors, L.B.; Markiewicz, M.; Agee, B.S.; Coleman, J.M.; et al. A phase 1 trial of oncolytic HSV-1, G207, given in combination with radiation for recurrent GBM demonstrates safety and radiographic responses. Mol. Ther. 2014, 22, 1048–1055. [Google Scholar] [CrossRef] [PubMed]
- Ling, A.L.; Solomon, I.H.; Landivar, A.M.; Nakashima, H.; Woods, J.K.; Santos, A.; Masud, N.; Fell, G.; Mo, X.; Yilmaz, A.S.; et al. Clinical trial links oncolytic immunoactivation to survival in glioblastoma. Nature 2023, 623, 157–166. [Google Scholar] [CrossRef]
- Chiocca, E.A.; Nakashima, H.; Kasai, K.; Fernandez, S.A.; Oglesbee, M. Preclinical Toxicology of rQNestin34.5v.2: An Oncolytic Herpes Virus with Transcriptional Regulation of the ICP34.5 Neurovirulence Gene. Mol. Ther. Methods Clin. Dev. 2020, 17, 871–893. [Google Scholar] [CrossRef]
- Desjardins, A.; Gromeier, M.; Herndon, J.E.; Beaubier, N.; Bolognesi, D.P.; Friedman, A.H.; Friedman, H.S.; McSherry, F.; Muscat, A.M.; Nair, S.; et al. Recurrent Glioblastoma Treated with Recombinant Poliovirus. N. Engl. J. Med. 2018, 379, 150–161. [Google Scholar] [CrossRef]
- Chandramohan, V.; Bryant, J.D.; Piao, H.; Keir, S.T.; Lipp, E.S.; Lefaivre, M.; Perkinson, K.; Bigner, D.D.; Gromeier, M.; McLendon, R.E. Validation of an Immunohistochemistry Assay for Detection of CD155, the Poliovirus Receptor, in Malignant Gliomas. Arch. Pathol. Lab. Med. 2017, 141, 1697–1704. [Google Scholar] [CrossRef] [PubMed]
- Ji, N.; Weng, D.; Liu, C.; Gu, Z.; Chen, S.; Guo, Y.; Fan, Z.; Wang, X.; Chen, J.; Zhao, Y.; et al. Adenovirus-mediated delivery of herpes simplex virus thymidine kinase administration improves outcome of recurrent high-grade glioma. Oncotarget 2016, 7, 4369–4378. [Google Scholar] [CrossRef] [PubMed]
- Kang, X.; Wang, Y.; Liu, P.; Huang, B.; Zhou, B.; Lu, S.; Geng, W.; Tang, H. Progresses, Challenges, and Prospects of CRISPR/Cas9 Gene-Editing in Glioma Studies. Cancers 2023, 15, 396. [Google Scholar] [CrossRef] [PubMed]
- Al-Sammarraie, N.; Ray, S.K. Applications of CRISPR-Cas9 Technology to Genome Editing in Glioblastoma Multiforme. Cells 2021, 10, 2342. [Google Scholar] [CrossRef] [PubMed]
- Begagić, E.; Bečulić, H.; Đuzić, N.; Džidić-Krivić, A.; Pugonja, R.; Muharemović, A.; Jaganjac, B.; Salković, N.; Sefo, H.; Pojskić, M. CRISPR/Cas9-Mediated Gene Therapy for Glioblastoma: A Scoping Review. Biomedicines 2024, 12, 238. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; Du, B.; Jiang, H.; Gao, J. Over-expression of CHAF1A promotes cell proliferation and apoptosis resistance in glioblastoma cells via AKT/FOXO3a/Bim pathway. Biochem. Biophys. Res. Commun. 2016, 469, 1111–1116. [Google Scholar] [CrossRef] [PubMed]
- Ali, R.; Alabdullah, M.; Miligy, I.; Normatova, M.; Babaei-Jadidi, R.; Nateri, A.S.; Rakha, E.A.; Madhusudan, S. ATM Regulated PTEN Degradation Is XIAP E3 Ubiquitin Ligase Mediated in p85α Deficient Cancer Cells and Influence Platinum Sensitivity. Cells 2019, 8, 1271. [Google Scholar] [CrossRef] [PubMed]
- Lu, F.I.; Wang, Y.-S.; Wu, C.-Y.; Li, C.-C. Involvement of BIG1 and BIG2 in regulating VEGF expression and angiogenesis. FASEB J. 2019, 33, 9959–9973. [Google Scholar] [CrossRef]
- Wu, W.; Wu, Y.; Mayer, K.; von Rosenstiel, C.; Schecker, J.; Baur, S.; Würstle, S.; Liesche-Starnecker, F.; Gempt, J.; Schlegel, J. Lipid Peroxidation Plays an Important Role in Chemotherapeutic Effects of Temozolomide and the Development of Therapy Resistance in Human Glioblastoma. Transl. Oncol. 2020, 13, 100748. [Google Scholar] [CrossRef]
- Tong, F.; Zhao, J.-X.; Fang, Z.-Y.; Cui, X.-T.; Su, D.-Y.; Liu, X.; Zhou, J.-H.; Wang, G.-X.; Qiu, Z.-J.; Liu, S.-Z.; et al. MUC1 promotes glioblastoma progression and TMZ resistance by stabilizing EGFRvIII. Pharmacol. Res. 2023, 187, 106606. [Google Scholar] [CrossRef] [PubMed]
- Janik, E.; Niemcewicz, M.; Ceremuga, M.; Krzowski, L.; Saluk-Bijak, J.; Bijak, M. Various Aspects of a Gene Editing System-CRISPR-Cas9. Int. J. Mol. Sci. 2020, 21, 9604. [Google Scholar] [CrossRef] [PubMed]
- Tsuchida, C.A.; Brandes, N.; Bueno, R.; Trinidad, M.; Mazumder, T.; Yu, B.; Hwang, B.; Chang, C.; Liu, J.; Sun, Y.; et al. Mitigation of chromosome loss in clinical CRISPR-Cas9-engineered T cells. Cell 2023, 186, 4567–4582.e20. [Google Scholar] [CrossRef] [PubMed]
- Mehta, A.; Merkel, O.M. Immunogenicity of Cas9 Protein. J. Pharm. Sci. 2020, 109, 62–67. [Google Scholar] [CrossRef]
- Crudele, J.M.; Chamberlain, J.S. Cas9 immunity creates challenges for CRISPR gene editing therapies. Nat. Commun. 2018, 9, 3497. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, T.T.T.; Greene, L.A.; Mnatsakanyan, H.; Badr, C.E. Revolutionizing Brain Tumor Care: Emerging Technologies and Strategies. Biomedicines 2024, 12, 1376. https://doi.org/10.3390/biomedicines12061376
Nguyen TTT, Greene LA, Mnatsakanyan H, Badr CE. Revolutionizing Brain Tumor Care: Emerging Technologies and Strategies. Biomedicines. 2024; 12(6):1376. https://doi.org/10.3390/biomedicines12061376
Chicago/Turabian StyleNguyen, Trang T. T., Lloyd A. Greene, Hayk Mnatsakanyan, and Christian E. Badr. 2024. "Revolutionizing Brain Tumor Care: Emerging Technologies and Strategies" Biomedicines 12, no. 6: 1376. https://doi.org/10.3390/biomedicines12061376






