Hyperbaric Oxygen Therapy as a Novel Approach to Modulating Macrophage Polarization for the Treatment of Glioblastoma
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Cultures, Macrophage Polarization, and Reagents
2.2. Cell Proliferation Assay
2.3. Immunohistochemical Staining
2.4. Immunohistochemical Staining
2.5. Real-Time PCR
2.6. Western Blot Assay
2.7. Apoptosis Assay
2.8. Animal Model
2.9. Statistical Analysis
3. Results
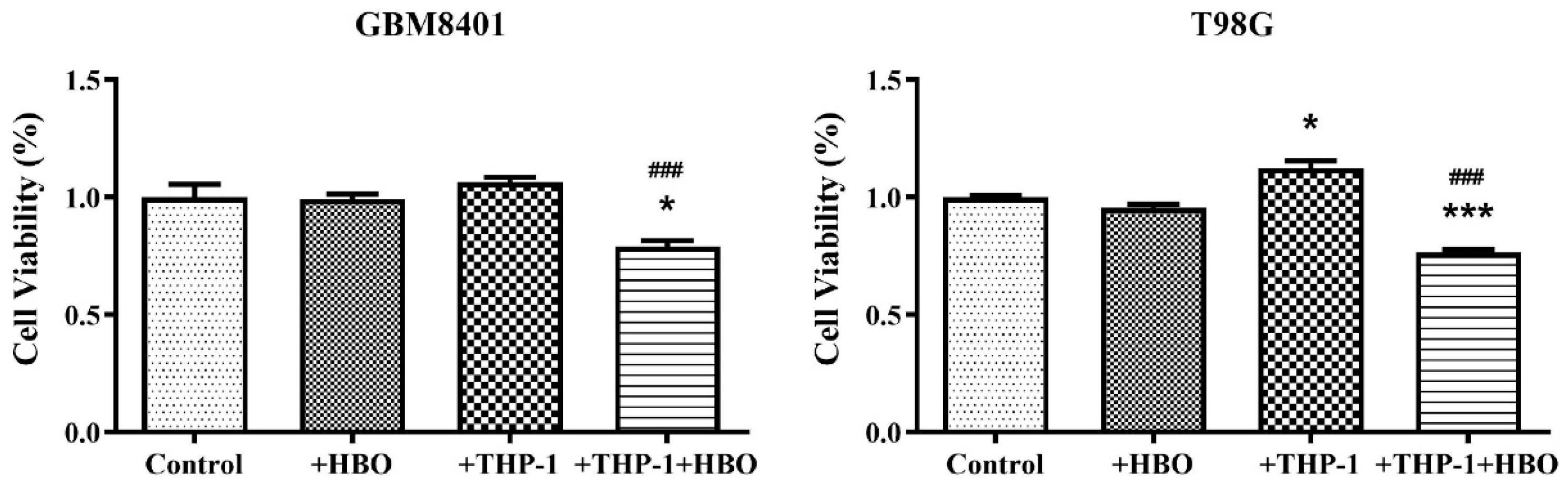
3.1. Differential Impact of Macrophage Polarization on Glioblastoma Cell Viability
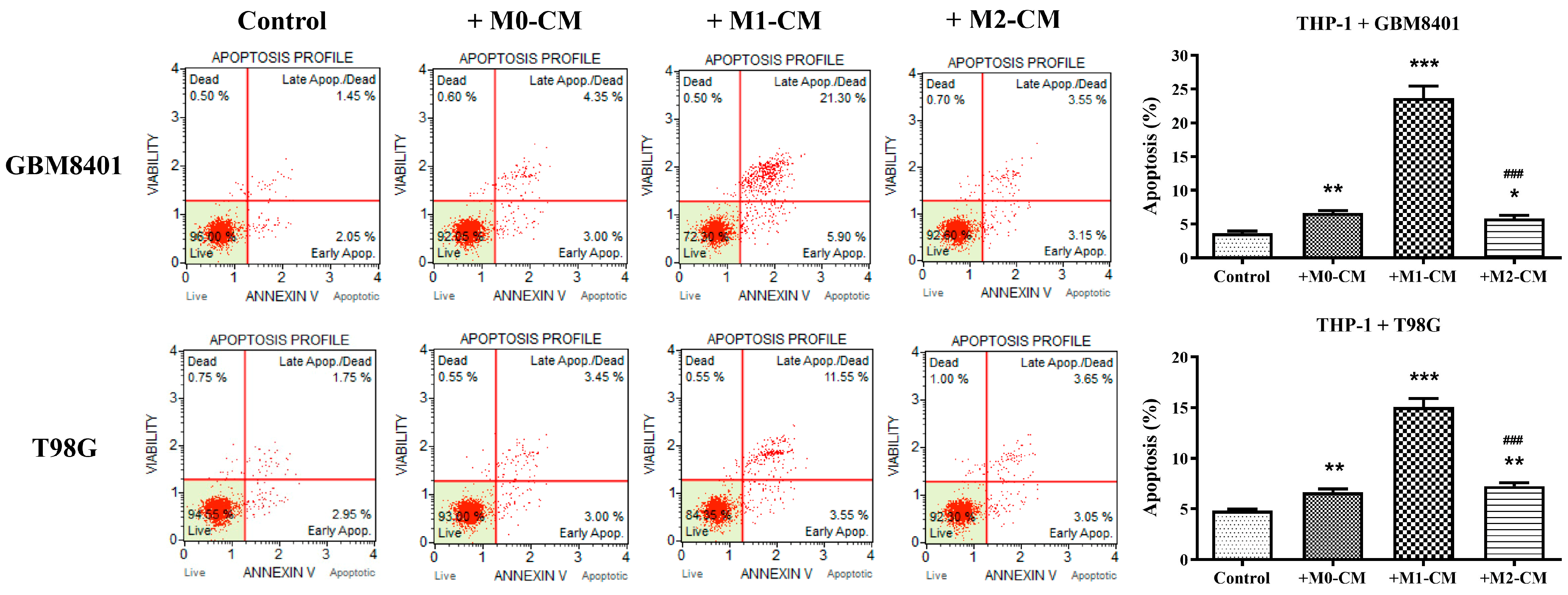
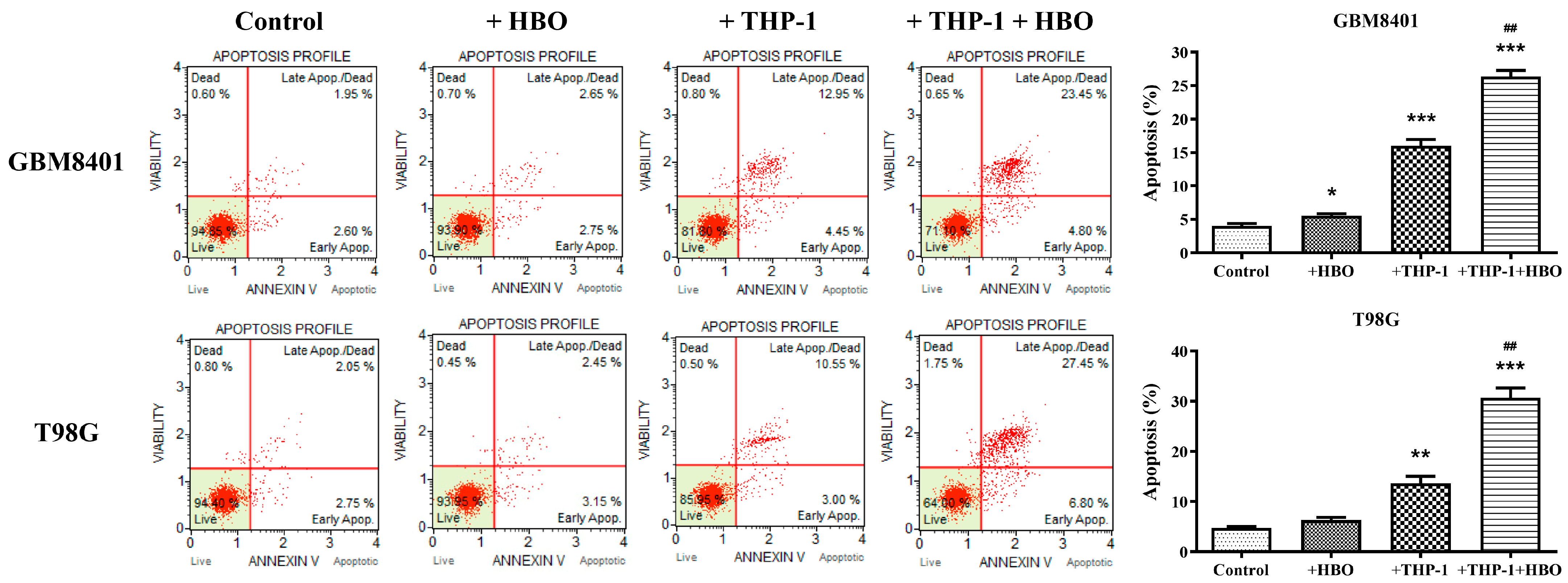
3.2. Macrophage Polarization Modulates Apoptosis in Glioblastoma Cells
3.3. Hyperbaric Oxygen Treatment Induces Macrophage Polarization towards Tumoricidal M1 Phenotype in Glioblastoma Co-Culture Systems
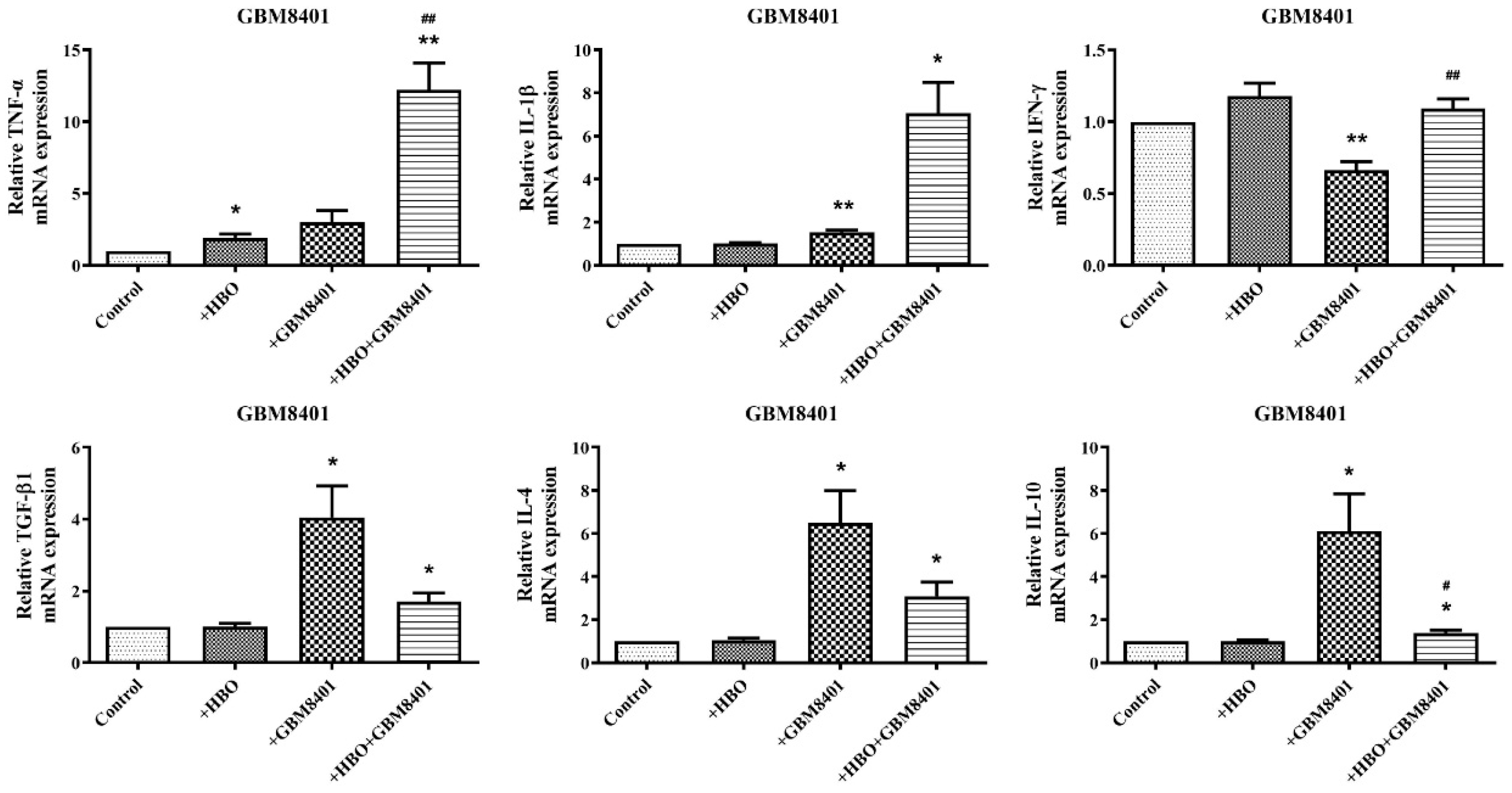
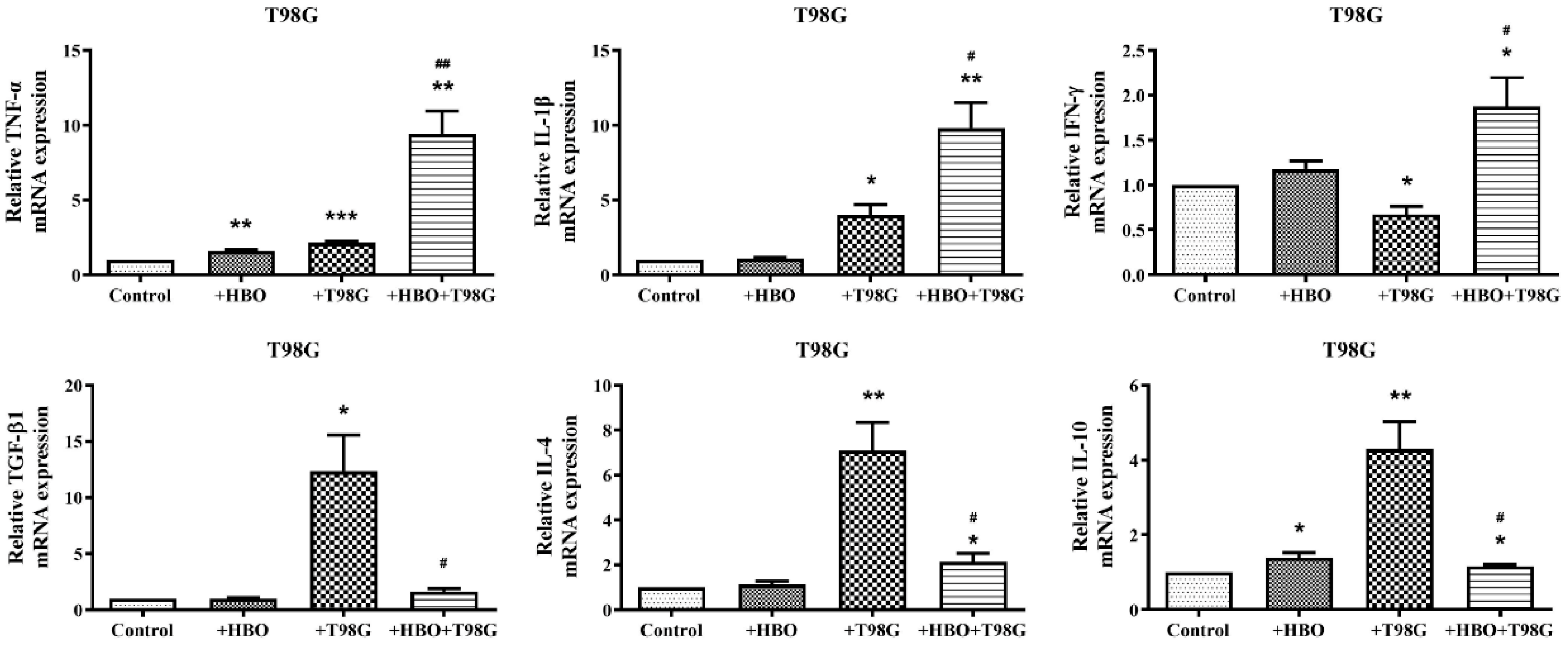
3.4. HBO Induces Dual Modulation of Pro- and Anti-Inflammatory Cytokine mRNA Expression in Macrophages Co-Cultured with GBM Cells
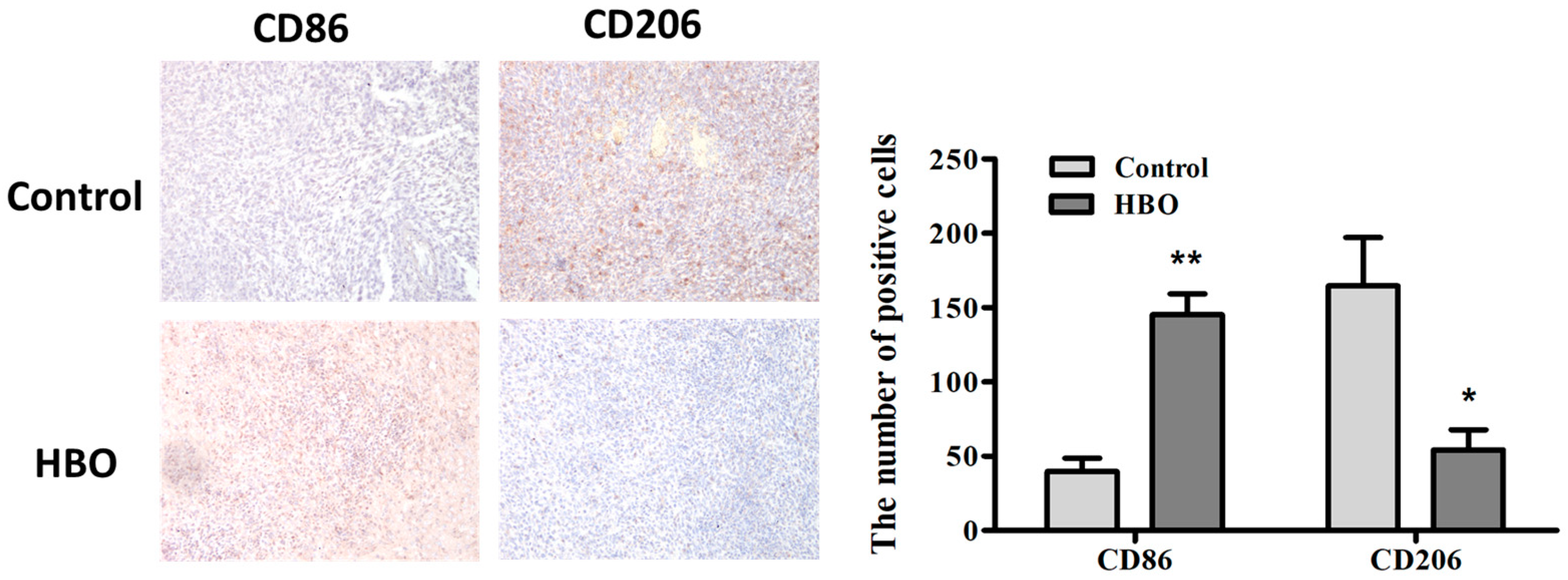
3.5. Modulation of Macrophage Polarization by Hyperbaric Oxygen Therapy in a Mouse Glioma Model
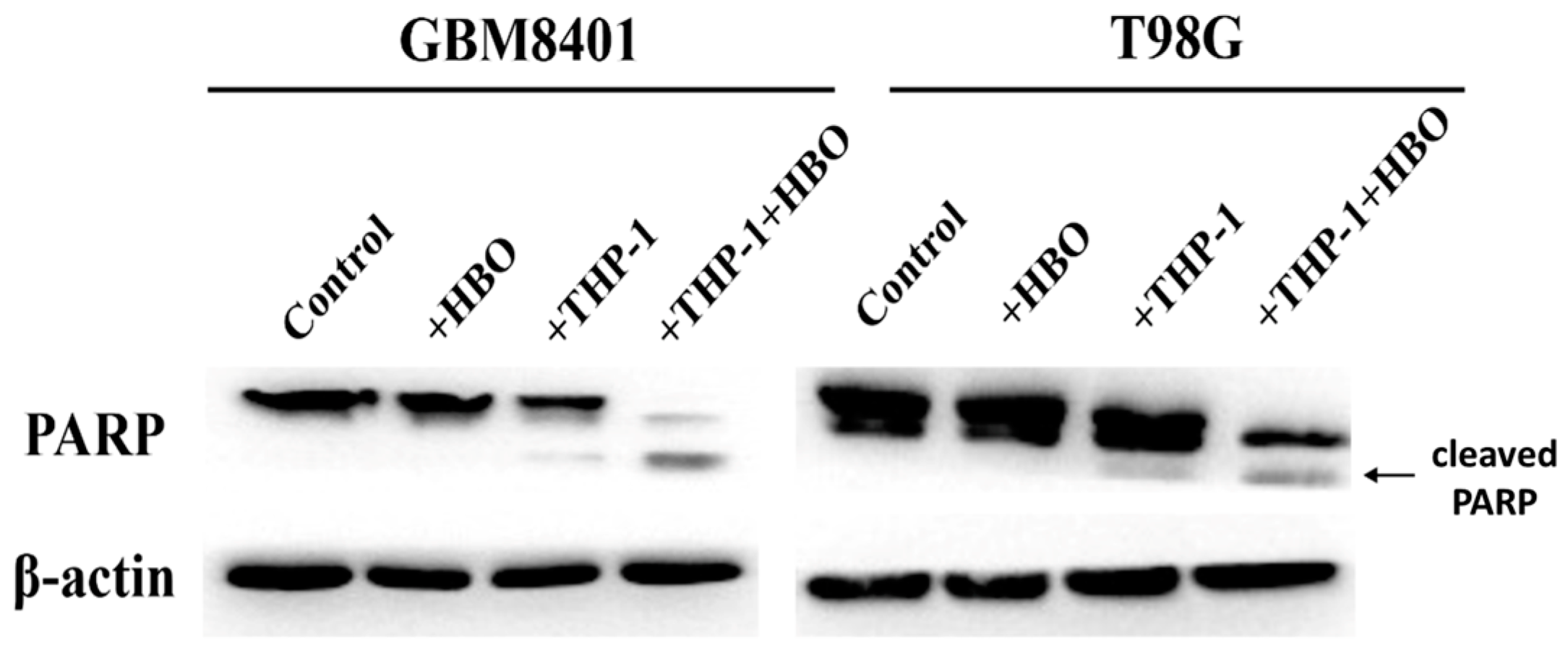
3.6. Synergistic Enhancement of Apoptosis in Glioblastoma Cells by THP-1 Macrophages and Hyperbaric Oxygen Therapy
3.7. Interplay between Hyperbaric Oxygen Therapy and Macrophage Interaction in Modulating Glioblastoma Cell Viability
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yalamarty, S.S.K.; Filipczak, N.; Li, X.; Subhan, M.A.; Parveen, F.; Ataide, J.A.; Rajmalani, B.A.; Torchilin, V.P. Mechanisms of Resistance and Current Treatment Options for Glioblastoma Multiforme (GBM). Cancers 2023, 15, 2116. [Google Scholar] [CrossRef] [PubMed]
- Marenco-Hillembrand, L.; Wijesekera, O.; Suarez-Meade, P.; Mampre, D.; Jackson, C.; Peterson, J.; Trifiletti, D.; Hammack, J.; Ortiz, K.; Lesser, E.; et al. Trends in glioblastoma: Outcomes over time and type of intervention: A systematic evidence based analysis. J. Neuro-Oncol. 2020, 147, 297–307. [Google Scholar] [CrossRef] [PubMed]
- Patwardhan, R.V.; Shorter, C.; Willis, B.K.; Reddy, P.; Smith, D.; Caldito, G.C.; Nanda, A. Survival trends in elderly patients with glioblastoma multiforme: Resective surgery, radiation, and chemotherapy. Surg. Neurol. 2004, 62, 207–213; discussion 205–214. [Google Scholar] [CrossRef] [PubMed]
- Reis-Sobreiro, M.; Teixeira da Mota, A.; Jardim, C.; Serre, K. Bringing Macrophages to the Frontline against Cancer: Current Immunotherapies Targeting Macrophages. Cells 2021, 10, 2364. [Google Scholar] [CrossRef] [PubMed]
- Morisse, M.C.; Jouannet, S.; Dominguez-Villar, M.; Sanson, M.; Idbaih, A. Interactions between tumor-associated macrophages and tumor cells in glioblastoma: Unraveling promising targeted therapies. Expert. Rev. Neurother. 2018, 18, 729–737. [Google Scholar] [CrossRef] [PubMed]
- Biswas, S.K.; Allavena, P.; Mantovani, A. Tumor-associated macrophages: Functional diversity, clinical significance, and open questions. Semin. Immunopathol. 2013, 35, 585–600. [Google Scholar] [CrossRef] [PubMed]
- Kumari, N.; Choi, S.H. Tumor-associated macrophages in cancer: Recent advancements in cancer nanoimmunotherapies. J. Exp. Clin. Cancer Res. 2022, 41, 68. [Google Scholar] [CrossRef] [PubMed]
- Andersen, J.K.; Miletic, H.; Hossain, J.A. Tumor-Associated Macrophages in Gliomas-Basic Insights and Treatment Opportunities. Cancers 2022, 14, 1319. [Google Scholar] [CrossRef] [PubMed]
- Sedighzadeh, S.S.; Khoshbin, A.P.; Razi, S.; Keshavarz-Fathi, M.; Rezaei, N. A narrative review of tumor-associated macrophages in lung cancer: Regulation of macrophage polarization and therapeutic implications. Transl. Lung Cancer Res. 2021, 10, 1889–1916. [Google Scholar] [CrossRef]
- Ricketts, T.D.; Prieto-Dominguez, N.; Gowda, P.S.; Ubil, E. Mechanisms of Macrophage Plasticity in the Tumor Environment: Manipulating Activation State to Improve Outcomes. Front. Immunol. 2021, 12, 642285. [Google Scholar] [CrossRef]
- Gregoire, H.; Roncali, L.; Rousseau, A.; Chérel, M.; Delneste, Y.; Jeannin, P.; Hindré, F.; Garcion, E. Targeting Tumor Associated Macrophages to Overcome Conventional Treatment Resistance in Glioblastoma. Front. Pharmacol. 2020, 11, 368. [Google Scholar] [CrossRef] [PubMed]
- Sahni, T.; Singh, P.; John, M.J. Hyperbaric oxygen therapy: Current trends and applications. J. Assoc. Physicians India 2003, 51, 280–284. [Google Scholar] [PubMed]
- Gill, A.L.; Bell, C.N. Hyperbaric oxygen: Its uses, mechanisms of action and outcomes. QJM 2004, 97, 385–395. [Google Scholar] [CrossRef] [PubMed]
- Sen, S.; Sen, S. Therapeutic effects of hyperbaric oxygen: Integrated review. Med. Gas. Res. 2021, 11, 30–33. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Ye, N.B.; Xiao, C.; Wang, X.X.; Li, S.; Deng, Y.; Yang, X.; Li, Z.; Yang, X. Hyperbaric oxygen regulates tumor microenvironment and boosts commercialized nanomedicine delivery for potent eradication of cancer stem-like cells. Nano Today 2021, 40, 101248. [Google Scholar] [CrossRef]
- Wang, P.; Wang, X.Y.; Man, C.F.; Gong, D.D.; Fan, Y. Advances in hyperbaric oxygen to promote immunotherapy through modulation of the tumor microenvironment. Front. Oncol. 2023, 13, 1200619. [Google Scholar] [CrossRef] [PubMed]
- Zou, Z.Z.; Chang, H.C.; Li, H.L.; Wang, S.M. Induction of reactive oxygen species: An emerging approach for cancer therapy. Apoptosis 2017, 22, 1321–1335. [Google Scholar] [CrossRef] [PubMed]
- Mates, J.M.; Sanchez-Jimenez, F.M. Role of reactive oxygen species in apoptosis: Implications for cancer therapy. Int. J. Biochem. Cell Biol. 2000, 32, 157–170. [Google Scholar] [CrossRef] [PubMed]
- Yuen, C.M.; Tsai, H.P.; Tseng, T.T.; Tseng, Y.L.; Lieu, A.S.; Kwan, A.L.; Chang, A.Y. Hyperbaric Oxygen Therapy Adjuvant Chemotherapy and Radiotherapy through Inhibiting Stemness in Glioblastoma. Curr. Issues Mol. Biol. 2023, 45, 8309–8320. [Google Scholar] [CrossRef]
- Ye, X.J.; Zhang, J.; Lu, R.; Zhou, G. HBO: A possible supplementary therapy for oral potentially malignant disorders. Med. Hypotheses 2014, 83, 131–136. [Google Scholar] [CrossRef]
- Daruwalla, J.; Christophi, C. Hyperbaric oxygen therapy for malignancy: A review. World J. Surg. 2006, 30, 2112–2131. [Google Scholar] [CrossRef] [PubMed]
- Genin, M.; Clement, F.; Fattaccioli, A.; Raes, M.; Michiels, C. M1 and M2 macrophages derived from THP-1 cells differentially modulate the response of cancer cells to etoposide. BMC Cancer 2015, 8, 577. [Google Scholar] [CrossRef]
- Kennedy, B.C.; Showers, C.R.; Anderson, D.E.; Anderson, L.; Canoll, P.; Bruce, J.N.; Anderson, R.C. Tumor-associated macrophages in glioma: Friend or foe? J. Oncol. 2013, 2013, 486912. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Liang, H.W.; Zen, K. Molecular mechanisms that influence the macrophage M1-M2 polarization balance. Front. Immunol. 2014, 5, 614. [Google Scholar] [CrossRef] [PubMed]
- Shapouri-Moghaddam, A.; Mohammadian, S.; Vazini, H.; Taghadosi, M.; Esmaeili, S.A.; Mardani, F.; Seifi, B.; Mohammadi, A.; Afshari, J.T.; Sahebkar, A. Macrophage plasticity, polarization, and function in health and disease. J. Cell. Physiol. 2018, 233, 6425–6440. [Google Scholar] [CrossRef] [PubMed]
- Esche, C.; Stellato, C.; Beck, L.A. Chemokines: Key players in innate and adaptive immunity. J. Investig. Dermatol. 2005, 125, 615–628. [Google Scholar] [CrossRef] [PubMed]
- Struyf, S.; Proost, P.; Van Damme, J. Regulation of the immune response by the interaction of chemokines and proteases. Adv. Immunol. 2003, 81, 1–44. [Google Scholar] [PubMed]
- Medzhitov, R. Recognition of microorganisms and activation of the immune response. Nature 2007, 449, 819–826. [Google Scholar] [CrossRef]
- Kim, R.; Emi, M.; Tanabe, K. Cancer cell immune escape and tumor progression by exploitation of anti-inflammatory and pro-inflammatory responses. Cancer Biol. Ther. 2005, 4, 924–933. [Google Scholar] [CrossRef]
- Allavena, P.; Sica, A.; Solinas, G.; Porta, C.; Mantovani, A. The inflammatory micro-environment in tumor progression: The role of tumor-associated macrophages. Crit. Rev. Oncol. Hemat. 2008, 66, 1–9. [Google Scholar] [CrossRef]
- Tardito, S.; Martinelli, G.; Soldano, S.; Paolino, S.; Pacini, G.; Patane, M.; Alessandri, E.; Smith, V.; Cutolo, M. Macrophage M1/M2 polarization and rheumatoid arthritis: A systematic review. Autoimmun. Rev. 2019, 18, 102397. [Google Scholar] [CrossRef] [PubMed]
- Arabpour, M.; Saghazadeh, A.; Rezaei, N. Anti-inflammatory and M2 macrophage polarization-promoting effect of mesenchymal stem cell-derived exosomes. Int. Immunopharmacol. 2021, 97, 107823. [Google Scholar] [CrossRef]
- Lorenzo-Sanz, L.; Muñoz, P. Tumor-Infiltrating Immunosuppressive Cells in Cancer-Cell Plasticity, Tumor Progression and Therapy Response. Cancer Microenviron. 2019, 12, 119–132. [Google Scholar] [CrossRef] [PubMed]
- El Sayed, R.; Haibe, Y.; Amhaz, G.; Bouferraa, Y.; Shamseddine, A. Metabolic Factors Affecting Tumor Immunogenicity: What Is Happening at the Cellular Level? Int. J. Mol. Sci. 2021, 22, 2142. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Chen, C.C.; Li, M. Macrophages/Microglia in the Glioblastoma Tumor Microenvironment. Int. J. Mol. Sci. 2021, 22, 5775. [Google Scholar] [CrossRef] [PubMed]
- Montemurro, N.; Pahwa, B.; Tayal, A.; Shukla, A.; De Jesus Encarnacion, M.; Ramirez, I.; Nurmukhametov, R.; Chavda, V.; De Carlo, A. Macrophages in Recurrent Glioblastoma as a Prognostic Factor in the Synergistic System of the Tumor Microenvironment. Neurol. Int. 2023, 15, 595–608. [Google Scholar] [CrossRef]
- Motwani, M.P.; Gilroy, D.W. Macrophage development and polarization in chronic inflammation. Semin. Immunol. 2015, 27, 257–266. [Google Scholar] [CrossRef] [PubMed]
- Sica, A.; Erreni, M.; Allavena, P.; Porta, C. Macrophage polarization in pathology. Cell. Mol. Life Sci. 2015, 72, 4111–4126. [Google Scholar] [CrossRef] [PubMed]
- Cutolo, M.; Campitiello, R.; Gotelli, E.; Soldano, S. The Role of M1/M2 Macrophage Polarization in Rheumatoid Arthritis Synovitis. Front. Immunol. 2022, 13, 867260. [Google Scholar] [CrossRef]
- Lotze, M.T.; Zeh, H.J.; Rubartelli, A.; Sparvero, L.J.; Amoscato, A.A.; Washburn, N.R.; DeVera, M.E.; Liang, X.; Tör, M.; Billiar, T. The grateful dead: Damage-associated molecular pattern molecules and reduction/oxidation regulate immunity. Immunol. Rev. 2007, 220, 60–81. [Google Scholar] [CrossRef]
- Jones, S.A.; Jenkins, B.J. Recent insights into targeting the IL-6 cytokine family in inflammatory diseases and cancer. Nat. Rev. Immunol. 2018, 18, 773–789. [Google Scholar] [CrossRef] [PubMed]
- Letterio, J.J.; Roberts, A.B. Regulation of immune responses by TGF-beta. Annu. Rev. Immunol. 1998, 16, 137–161. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuen, C.-M.; Tsai, H.-P.; Tseng, T.-T.; Tseng, Y.-L.; Lieu, A.-S.; Kwan, A.-L.; Chang, A.Y.W. Hyperbaric Oxygen Therapy as a Novel Approach to Modulating Macrophage Polarization for the Treatment of Glioblastoma. Biomedicines 2024, 12, 1383. https://doi.org/10.3390/biomedicines12071383
Yuen C-M, Tsai H-P, Tseng T-T, Tseng Y-L, Lieu A-S, Kwan A-L, Chang AYW. Hyperbaric Oxygen Therapy as a Novel Approach to Modulating Macrophage Polarization for the Treatment of Glioblastoma. Biomedicines. 2024; 12(7):1383. https://doi.org/10.3390/biomedicines12071383
Chicago/Turabian StyleYuen, Chun-Man, Hung-Pei Tsai, Tzu-Ting Tseng, Yu-Lung Tseng, Ann-Shung Lieu, Aij-Lie Kwan, and Alice Y. W. Chang. 2024. "Hyperbaric Oxygen Therapy as a Novel Approach to Modulating Macrophage Polarization for the Treatment of Glioblastoma" Biomedicines 12, no. 7: 1383. https://doi.org/10.3390/biomedicines12071383




