Longitudinal Genome-Wide Association Study of Cognitive Impairment after Subarachnoid Hemorrhage
Abstract
1. Introduction
2. Material and Methods
2.1. Study Population
2.2. Study Outcomes
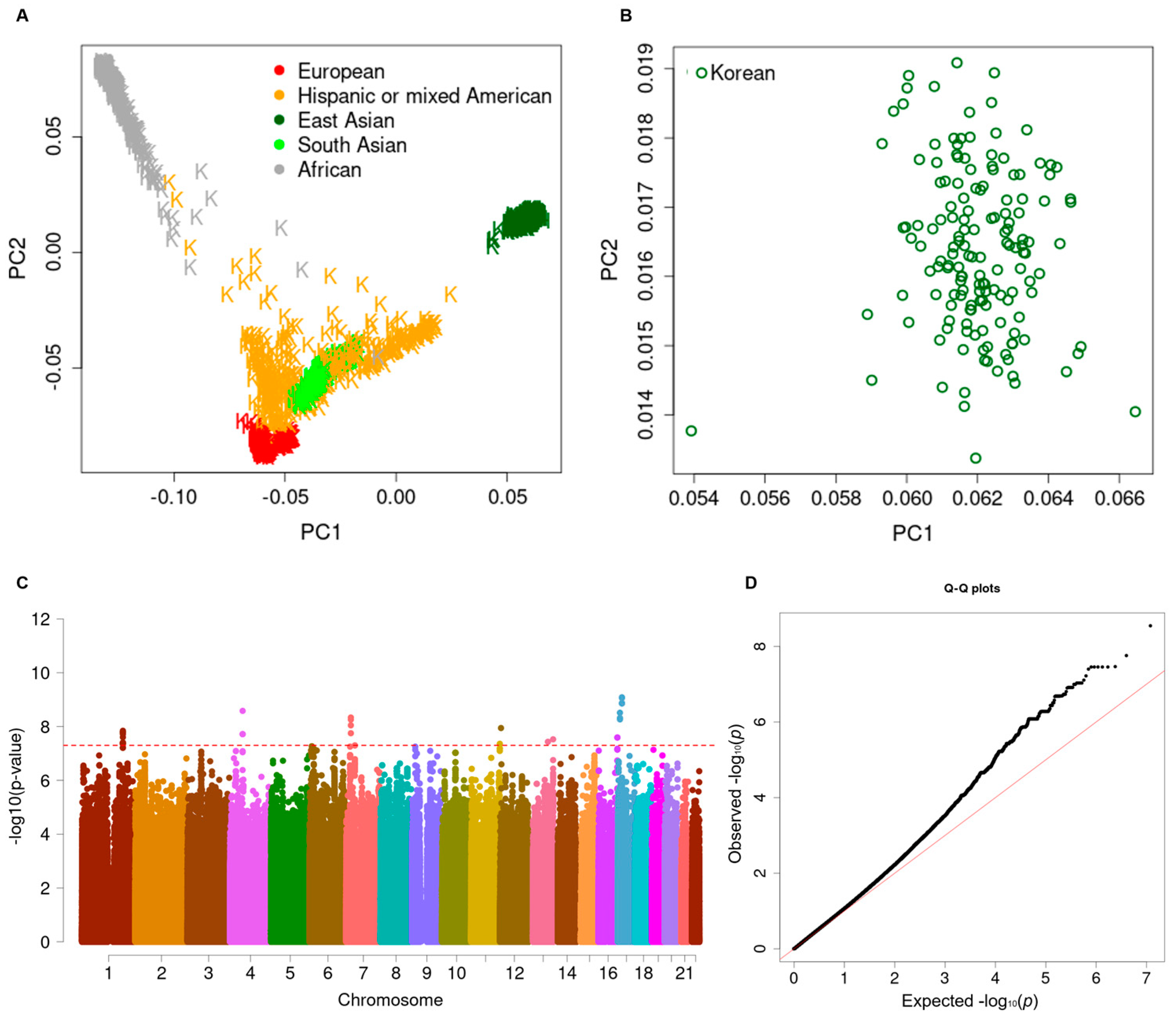
2.3. Genotyping and Quality Controls
2.4. High-Throughput Imputation and Quality Control
2.5. Statistical Analysis
3. Results
3.1. Baseline Characteristics of the Study Participants
3.2. Longitudinal Genome-Wide Association Study
3.3. Subgroup Analysis after Stratification into Hp Subtypes
3.4. Polygenic Risk Assessment of Cognitive Impairment
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ruigrok, Y.M.; Rinkel, G.J.; Wijmenga, C. Genetics of intracranial aneurysms. Lancet Neurol. 2005, 4, 179–189. [Google Scholar] [CrossRef] [PubMed]
- Horie, N.; Takahashi, N.; Furuichi, S.; Mori, K.; Onizuka, M.; Tsutsumi, K.; Shibata, S. Giant fusiform aneurysms in the middle cerebral artery presenting with hemorrhages of different origins. Report of three cases and review of the literature. J. Neurosurg. 2003, 99, 391–396. [Google Scholar] [CrossRef] [PubMed]
- Stegmayr, B.; Eriksson, M.; Asplund, K. Declining mortality from subarachnoid hemorrhage: Changes in incidence and case fatality from 1985 through 2000. Stroke 2004, 35, 2059–2063. [Google Scholar] [CrossRef] [PubMed]
- Shea, A.M.; Reed, S.D.; Curtis, L.H.; Alexander, M.J.; Villani, J.J.; Schulman, K.A. Characteristics of nontraumatic subarachnoid hemorrhage in the united states in 2003. Neurosurgery 2007, 61, 1131–1137, discussion 1137–1138. [Google Scholar] [CrossRef] [PubMed]
- Kreiter, K.T.; Copeland, D.; Bernardini, G.L.; Bates, J.E.; Peery, S.; Claassen, J.; Du, Y.E.; Stern, Y.; Connolly, E.S.; Mayer, S.A. Predictors of cognitive dysfunction after subarachnoid hemorrhage. Stroke 2002, 33, 200–208. [Google Scholar] [CrossRef] [PubMed]
- Haug Nordenmark, T.; Karic, T.; Sorteberg, W.; Sorteberg, A. Predictors of cognitive function in the acute phase after aneurysmal subarachnoid hemorrhage. Acta Neurochir. 2019, 161, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Louko, A.M.; Vilkki, J.; Niskakangas, T. Apoe genotype and cognition after subarachnoid haemorrhage: A longitudinal study. Acta Neurol. Scand. 2006, 114, 315–319. [Google Scholar] [CrossRef]
- Lanterna, L.A.; Rigoldi, M.; Tredici, G.; Biroli, F.; Cesana, C.; Gaini, S.M.; Dalprà, L. Apoe influences vasospasm and cognition of noncomatose patients with subarachnoid hemorrhage. Neurology 2005, 64, 1238–1244. [Google Scholar] [CrossRef] [PubMed]
- Han, S.W.; Kim, B.J.; Kim, T.Y.; Lim, S.H.; Youn, D.H.; Hong, E.P.; Rhim, J.K.; Park, J.J.; Lee, J.J.; Cho, Y.J.; et al. Association of haptoglobin phenotype with neurological and cognitive outcomes in patients with subarachnoid hemorrhage. Front. Aging Neurosci. 2022, 14, 819628. [Google Scholar] [CrossRef]
- Chatterjee, N.; Shi, J.; Garcia-Closas, M. Developing and evaluating polygenic risk prediction models for stratified disease prevention. Nat. Rev. Genet. 2016, 17, 392–406. [Google Scholar] [CrossRef]
- Park, J.J.; Kim, B.J.; Youn, D.H.; Choi, H.J.; Jeon, J.P. A Preliminary Study of the Association between SOX17 Gene Variants and Intracranial Aneurysms Using Exome Sequencing. J. Korean Neurosurg. Soc. 2020, 63, 559–565. [Google Scholar] [CrossRef] [PubMed]
- Hong, E.P.; Kim, B.J.; Cho, S.S.; Yang, J.S.; Choi, H.J.; Kang, S.H.; Jeon, J.P. Genomic variations in susceptibility to intracranial aneurysm in the Korean population. J. Clin. Med. 2019, 8, 275. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.J.; Hong, E.P.; Youn, D.H.; Jeon, J.P. Genome-wide association study of the relationship between matrix metalloproteinases and intracranial aneurysms. J. Clin. Neurol. 2022, 18, 163–170. [Google Scholar] [CrossRef] [PubMed]
- King, J.T., Jr.; DiLuna, M.L.; Cicchetti, D.V.; Tsevat, J.; Roberts, M.S. Cognitive functioning in patients with cerebral aneurysms measured with the mini mental state examination and the telephone interview for cognitive status. Neurosurgery 2006, 59, 803–810, discussion 810–811. [Google Scholar] [CrossRef] [PubMed]
- Gluhm, S.; Goldstein, J.; Loc, K.; Colt, A.B.; Van Liew, C.; Corey-Bloom, J. Cognitive performance on the mini-mental state examination and the montreal cognitive assessment across the healthy adult lifespan. Cogn. Behav. Neurol. 2013, 26, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kim, C.H.; Kang, H.S.; Park, C.K.; Chung, C.K. Cognitive function of korean neurosurgical patients: Cross-sectional study using the Korean version of the mini-mental status examination. J. Cerebrovasc. Endovasc. Neurosurg. 2012, 14, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.J.; Kim, Y.; Kim, S.E.; Jeon, J.P. Study of Correlation Between Hp α1 Expression of Haptoglobin 2-1 and Clinical Course in Aneurysmal Subarachnoid Hemorrhage. World Neurosurg. 2018, 117, e221–e227. [Google Scholar] [CrossRef] [PubMed]
- GenomeAsia100K Consortium. The GenomeAsia 100K Project enables genetic discoveries across Asia. Nature 2019, 576, 106–111. [Google Scholar] [CrossRef] [PubMed]
- Rowland, M.J.; Garry, P.; Ezra, M.; Corkill, R.; Baker, I.; Jezzard, P.; Westbrook, J.; Douaud, G.; Pattinson, K.T.S. Early brain injury and cognitive impairment after aneurysmal subarachnoid haemorrhage. Sci. Rep. 2021, 11, 23245. [Google Scholar] [CrossRef]
- Gallek, M.J.; Conley, Y.P.; Sherwood, P.R.; Horowitz, M.B.; Kassam, A.; Alexander, S.A. APOE genotype and functional outcome following aneurysmal subarachnoid hemorrhage. Biol. Res. Nurs. 2009, 10, 205–212. [Google Scholar] [CrossRef]
- Ravnik, J.; Starovasnik, B.; Sesok, S.; Pirtosek, Z.; Svigelj, V.; Bunc, G.; Bosnjak, R. Long-term cognitive deficits in patients with good outcomes after aneurysmal subarachnoid hemorrhage from anterior communicating artery. Croat. Med. J. 2006, 47, 253–263. [Google Scholar] [PubMed]
- Kim, H.; Crago, E.; Kim, M.; Sherwood, P.; Conley, Y.; Poloyac, S.; Kerr, M. Cerebral vasospasm after sub-arachnoid hemorrhage as a clinical predictor and phenotype for genetic association study. Int. J. Stroke 2013, 8, 620–625. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.J.; Wang, L.M.; Zhang, Y.; Yenush, L.; Myers, M.G., Jr.; Glasheen, E.; Lane, W.S.; Pierce, J.H.; White, M.F. Role of irs-2 in insulin and cytokine signalling. Nature 1995, 377, 173–177. [Google Scholar] [CrossRef] [PubMed]
- Martín, E.D.; Sánchez-Perez, A.; Trejo, J.L.; Martin-Aldana, J.A.; Jaimez, M.C.; Pons, S.; Umanzor, C.A.; Menes, L.; White, M.F.; Burks, D.J. IRS-2 Deficiency impairs NMDA receptor-dependent long-term potentiation. Cereb. Cortex 2012, 22, 1717–1727. [Google Scholar] [CrossRef] [PubMed]
- Ochiai, T.; Sano, T.; Nagayama, T.; Kubota, N.; Kadowaki, T.; Wakabayashi, T.; Iwatsubo, T. Differential involvement of insulin receptor substrate (IRS)-1 and IRS-2 in brain insulin signaling is associated with the effects on amyloid pathology in a mouse model of Alzheimer’s disease. Neurobiol. Dis. 2021, 159, 105510. [Google Scholar] [CrossRef] [PubMed]
- Niedowicz, D.M.; Reeves, V.L.; Platt, T.L.; Kohler, K.; Beckett, T.L.; Powell, D.K.; Lee, T.L.; Sexton, T.R.; Song, E.S.; Brewer, L.D.; et al. Obesity and diabetes cause cognitive dysfunction in the absence of accelerated β-amyloid deposition in a novel murine model of mixed or vascular dementia. Acta Neuropathol. Commun. 2014, 2, 64. [Google Scholar] [CrossRef] [PubMed]
- Cetinkalp, S.; Simsir, I.Y.; Ertek, S. Insulin resistance in brain and possible therapeutic approaches. Curr. Vasc. Pharmacol. 2014, 12, 553–564. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Jang, S.H. Hypothalamic injury in spontaneous subarachnoid hemorrhage: A diffusion tensor imaging study. Clin. Auton. Res. 2021, 31, 321–322. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zaitlen, N.A.; Goddard, M.E.; Visscher, P.M.; Price, A.L. Advantages and pitfalls in the application of mixed-model association methods. Nat. Genet. 2014, 46, 100–106. [Google Scholar] [CrossRef]
- Abraham, G.; Malik, R.; Yonova-Doing, E.; Salim, A.; Wang, T.; Danesh, J.; Butterworth, A.; Howson, J.M.M.; Inouye, M.; Dichgans, M. Genomic risk score offers predictive performance comparable to clinical risk factors for ischaemic stroke. Nat. Commun. 2019, 10, 5819. [Google Scholar] [CrossRef]
- Chatterjee, N.; Wheeler, B.; Sampson, J.; Hartge, P.; Chanock, S.J.; Park, J.-H. Projecting the performance of risk prediction based on polygenic analyses of genome-wide association studies. Nat. Genet. 2013, 45, 400–405. [Google Scholar] [CrossRef] [PubMed]
- Hay, M.; Barnes, C.; Huentelman, M.; Brinton, R.; Ryan, L. Hypertension and Age-Related Cognitive Impairment: Common Risk Factors and a Role for Precision Aging. Curr. Hypertens. Rep. 2020, 22, 80. [Google Scholar] [CrossRef]
- Khera, A.V.; Chaffin, M.; Aragam, K.G.; Haas, M.E.; Roselli, C.; Choi, S.H.; Natarajan, P.; Lander, E.S.; Lubitz, S.A.; Ellinor, P.T.; et al. Genome-wide polygenic scores for common diseases identify individuals with risk equivalent to monogenic mutations. Nat. Genet. 2018, 50, 1219–1224. [Google Scholar] [CrossRef]
- Mujwara, D.; Kintzle, J.; Di Domenico, P.; Busby, G.B.; Bottà, G. Cost-effectiveness analysis of implementing polygenic risk score in a workplace cardiovascular disease prevention program. Health Policy Plan. 2024, 39, 355–362. [Google Scholar] [CrossRef]
- Slunecka, J.L.; van der Zee, M.D.; Beck, J.J.; Johnson, B.N.; Finnicum, C.T.; Pool, R.; Hottenga, J.-J.; de Genus, E.J.C.; Ehli, E.A. Implementation and implications for polygenic risk scores in healthcare. Hum. Genom. 2021, 15, 46. [Google Scholar] [CrossRef]
- Eagles, M.E.; Tso, M.K.; Macdonald, R.L. Cognitive Impairment, Functional Outcome, and Delayed Cerebral Ischemia After Aneurysmal Subarachnoid Hemorrhage. World Neurosurg. 2019, 124, e558–e562. [Google Scholar] [CrossRef]
- Bakker, M.K.; Kanning, J.P.; Abraham, G.; Martinsen, A.E.; Winsvold, B.S.; Zwart, J.-A.; Bourcier, R.; Sawada, T.; Koido, M.; Kamatani, Y.; et al. Genetic Risk Score for Intracranial Aneurysms: Prediction of Subarachnoid Hemorrhage and Role in Clinical Heterogeneity. Stroke 2023, 54, 810–818. [Google Scholar] [CrossRef] [PubMed]
- Vahanian, A.; Beyersdorf, F.; Praz, F.; Milojevic, M.; Baldus, S.; Bauersachs, J.; Capodanno, D.; Conradi, L.; De Bonis, M.; De Paulis, R.; et al. 2021 ESC Guidelines on cardiovascular disease prevention in clinical practice. Eur. Heart J. 2022, 43, 561–632. [Google Scholar] [CrossRef] [PubMed]
- Park, H.-Y.; Jeon, S.-S.; Lee, J.-Y.; Cho, A.-R.; Park, J.H. Korean Version of the Mini-Mental State Examination Using Smartphone: A Validation Study. Telemed. J. E Health 2017, 23, 815–821. [Google Scholar] [CrossRef]
- O’Bryant, S.E.; Humphreys, J.D.; Smith, G.E.; Ivnik, R.J.; Graff-Radford, N.R.; Petersen, R.C.; Lucas, J.A. Detecting dementia with the mini-mental state examination in highly educated individuals. Arch. Neurol. 2008, 65, 963–967. [Google Scholar] [CrossRef]
- Franceschini, M.; Massimiani, M.P.; Paravati, S.; Franceschini, M.; Massimiani, M.P.; Paravati, S.; Agosti, M. Return to work: A cut-off of FIM gain with Montebello rehabilitation factor score in order to identify predictive factors in subjects with acquired brain injury. PLoS ONE 2016, 11, e0165165. [Google Scholar] [CrossRef] [PubMed]


| Variables a | Cognitive Impairment (N = 65) | No Cognitive Impairment (N = 88) | p |
|---|---|---|---|
| Clinical factors | |||
| Female | 44 (67.7%) | 53 (60.2%) | 0.698 |
| Age, years | 64.2 ± 12.3 | 57.5 ± 11.0 | <0.001 |
| Hypertension | 38 (58.5%) | 34 (38.6%) | 0.002 |
| Diabetes mellitus | 8 (12.3%) | 7 (8.0%) | 0.085 |
| Hyperlipidemia | 5 (7.7%) | 11 (12.5%) | 0.481 |
| Smoking | 8 (12.3%) | 12 (13.6%) | 0.873 |
| SAH-related variables | |||
| Aneurysm size (mm) | 4.8 ± 1.3 | 4.6 ± 1.2 | 0.589 |
| Anterior circulation aneurysm | 55 (84.6%) | 78 (88.6%) | 0.640 |
| Hunt and Hess grade IV and V | 25 (38.4%) | 26 (29.5%) | 0.247 |
| Fisher grade III and IV | 32 (49.2%) | 35 (39.7%) | 0.244 |
| Delayed cerebral ischemia | 17 (26.2%) | 21 (23.9%) | 0.746 |
| Hydrocephalus | 7 (10.8%) | 5 (5.7%) | 0.247 |
| Treatment methods | |||
| Coil embolization | 61 (93.8%) | 78 (88.6%) | 0.269 |
| Gene | Chr. | Region | SNP | BP | M/m a | MAF | HWE p | HR b | 95% CI b | p b |
|---|---|---|---|---|---|---|---|---|---|---|
| PDCD6IP-LOC101928135 | 3p22.3 | intergenic | rs138753053 | 33961435 | G/A | 0.013 | 1 | 28.33 | 8.64–92.92 | 3.4 × 10−8 |
| LINC00499 | 4q28.3 | ncRNA | rs56823384 | 139255670 | T/C | 0.026 | 1 | 12.47 | 5.42–28.67 | 2.8 × 10−9 |
| CASC15 | 6p22.3 | ncRNA | rs145397166 | 22067476 | C/G | 0.026 | 1 | 11.16 | 4.82–25.83 | 1.7 × 10−8 |
| LPL-SLC18A1 | 8p21.3 | intergenic | rs10503670 | 19985382 | G/A | 0.490 | 0.178 | 2.88 | 1.97–4.20 | 4.0 × 10−8 |
| IRS2 | 13q34 | near UTR-3 | rs76507772 | 110382882 | A/C | 0.062 | 1 | 5.99 | 3.17–11.32 | 3.5 × 10−8 |
| rs138753053 | rs56823384 | rs145397166 | rs10503670 | rs76507772 | |||||
|---|---|---|---|---|---|---|---|---|---|
| HR (95% CI) a | p a | HR (95% CI) a | p a | HR (95% CI) a | p a | HR (95% CI) a | p a | HR (95% CI) a | p a |
| Adjusted model 1: sex + age + targeted SNP | |||||||||
| 28.33 (8.64–92.92) | 3.4 × 10−8 | 12.47 (5.42–28.67) | 2.8 × 10−9 | 11.16 (4.82–25.83) | 1.7 × 10−8 | 2.88 (1.97–4.2) | 4.0 × 10−8 | 5.99 (3.17–11.32) | 3.5 × 10−8 |
| Adjusted model 2: sex + age + hypertension + diabetes + targeted SNP | |||||||||
| 23.48 (6.92–79.67) | 4.1×10−7 | 10.68 (4.5–25.38) | 8.1 × 10−8 | 10.16 (4.24–24.34) | 2.0 × 10−7 | 2.82 (1.93–4.13) | 8.3 × 10−8 | 5.69 (2.99–10.84) | 1.3 × 10−7 |
| Adjusted model 3: sex + age + hypertension + diabetes + hyperlipidemia + smoking + targeted SNP | |||||||||
| 19.10 (5.49–66.39) | 3.5×10−6 | 11.17 (4.52–27.61) | 1.7 × 10−7 | 9.60 (3.86–23.90) | 2.1 × 10−6 | 2.78 (1.87–4.12) | 3.8 × 10−7 | 5.35 (2.77–10.32) | 5.8 × 10−7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hong, E.P.; Lim, S.H.; Youn, D.H.; Han, S.W.; Jung, H.; Lee, J.J.; Jeon, J.P.; on behalf of the First Korean Stroke Genetics Association Research (The FirstKSGAR) Study. Longitudinal Genome-Wide Association Study of Cognitive Impairment after Subarachnoid Hemorrhage. Biomedicines 2024, 12, 1387. https://doi.org/10.3390/biomedicines12071387
Hong EP, Lim SH, Youn DH, Han SW, Jung H, Lee JJ, Jeon JP, on behalf of the First Korean Stroke Genetics Association Research (The FirstKSGAR) Study. Longitudinal Genome-Wide Association Study of Cognitive Impairment after Subarachnoid Hemorrhage. Biomedicines. 2024; 12(7):1387. https://doi.org/10.3390/biomedicines12071387
Chicago/Turabian StyleHong, Eun Pyo, Seung Hyuk Lim, Dong Hyuk Youn, Sung Woo Han, Harry Jung, Jae Jun Lee, Jin Pyeong Jeon, and on behalf of the First Korean Stroke Genetics Association Research (The FirstKSGAR) Study. 2024. "Longitudinal Genome-Wide Association Study of Cognitive Impairment after Subarachnoid Hemorrhage" Biomedicines 12, no. 7: 1387. https://doi.org/10.3390/biomedicines12071387
APA StyleHong, E. P., Lim, S. H., Youn, D. H., Han, S. W., Jung, H., Lee, J. J., Jeon, J. P., & on behalf of the First Korean Stroke Genetics Association Research (The FirstKSGAR) Study. (2024). Longitudinal Genome-Wide Association Study of Cognitive Impairment after Subarachnoid Hemorrhage. Biomedicines, 12(7), 1387. https://doi.org/10.3390/biomedicines12071387





