Multiple Sclerosis and Clostridium perfringens Epsilon Toxin: Is There a Relationship?
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Approval
2.2. Patients
2.3. Sample Collection
2.4. Microbiome Analysis
2.5. Epsilon Toxin Analysis: Quantification of and Immunoreactivity against Epsilon Toxin
3. Results
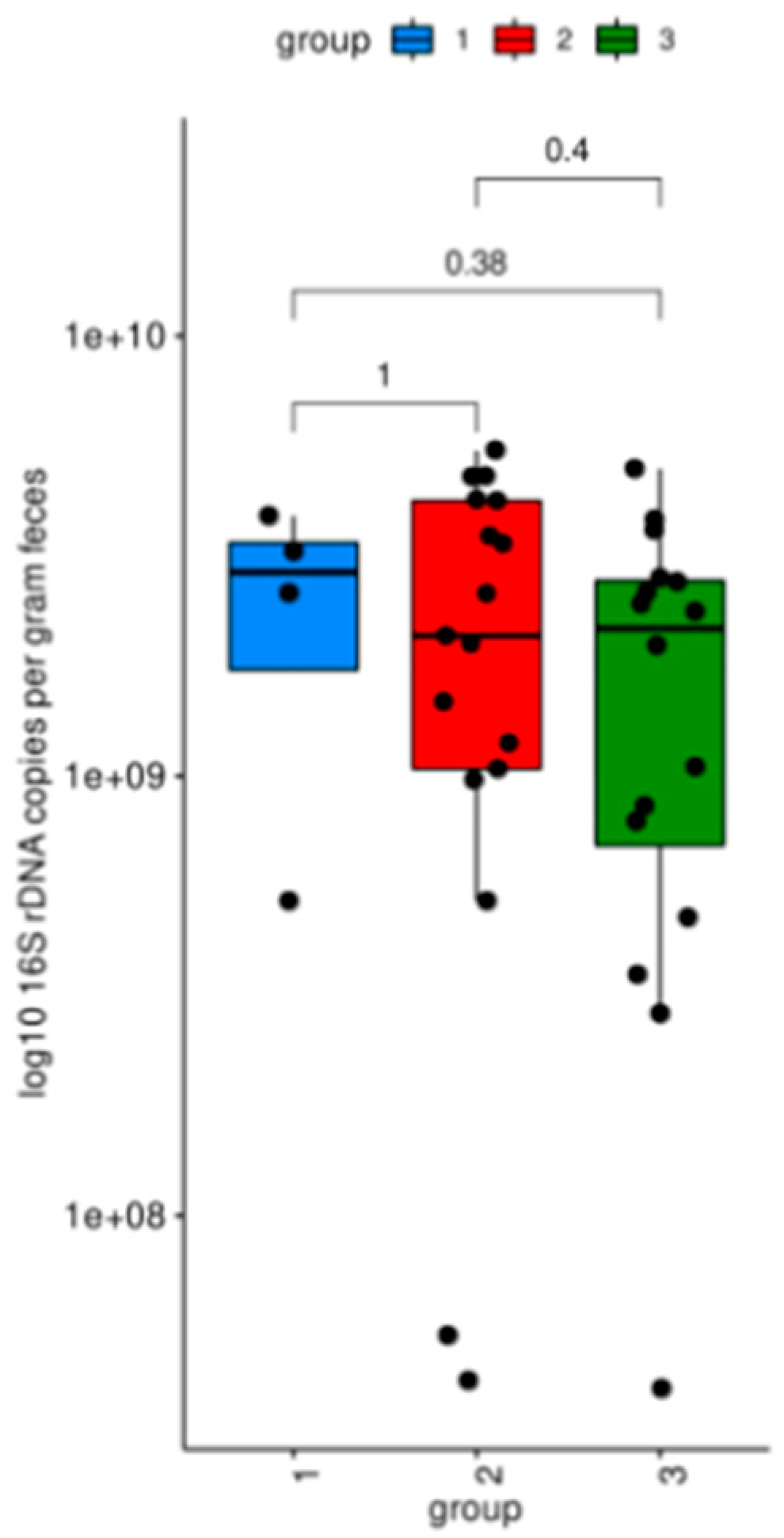
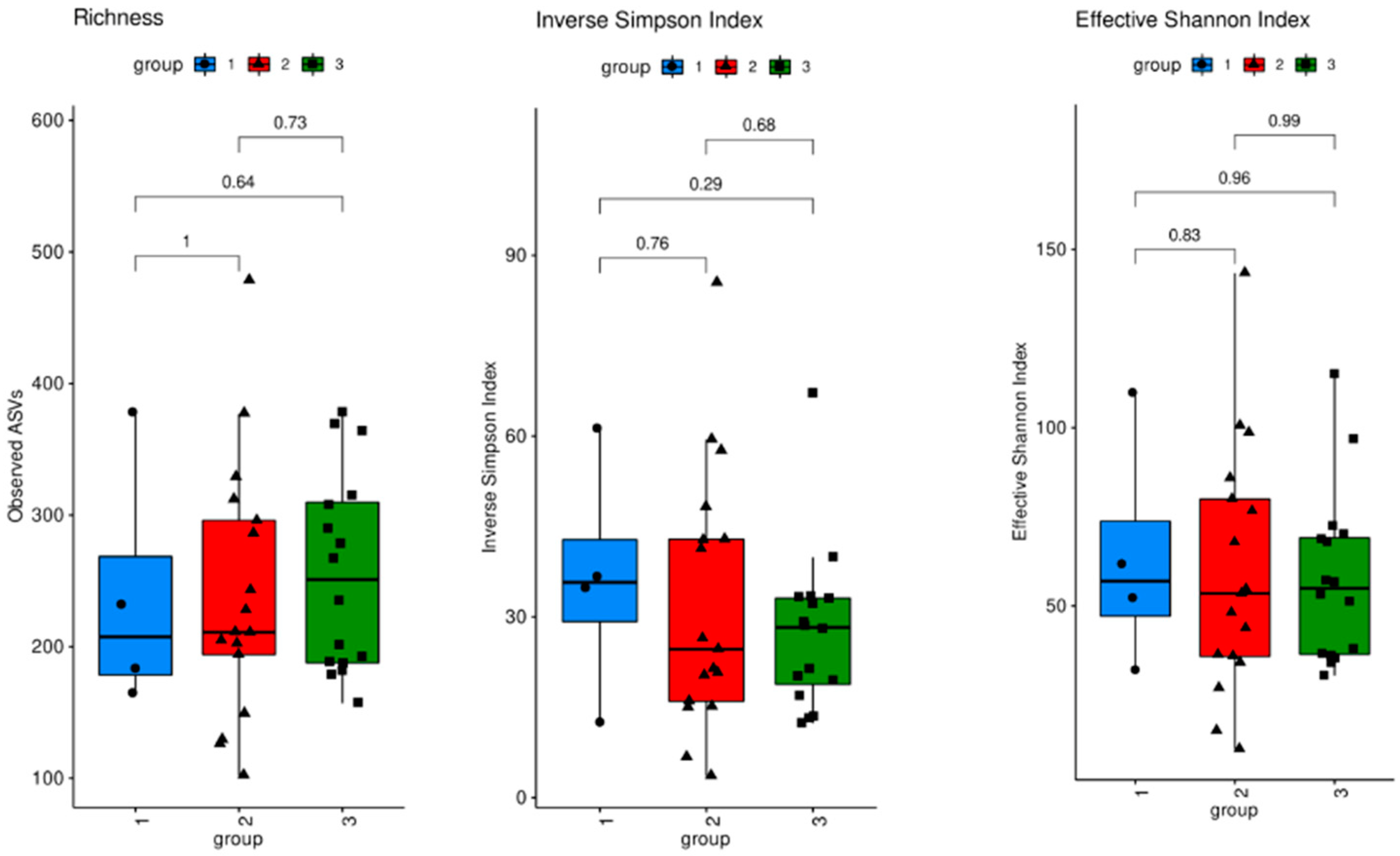
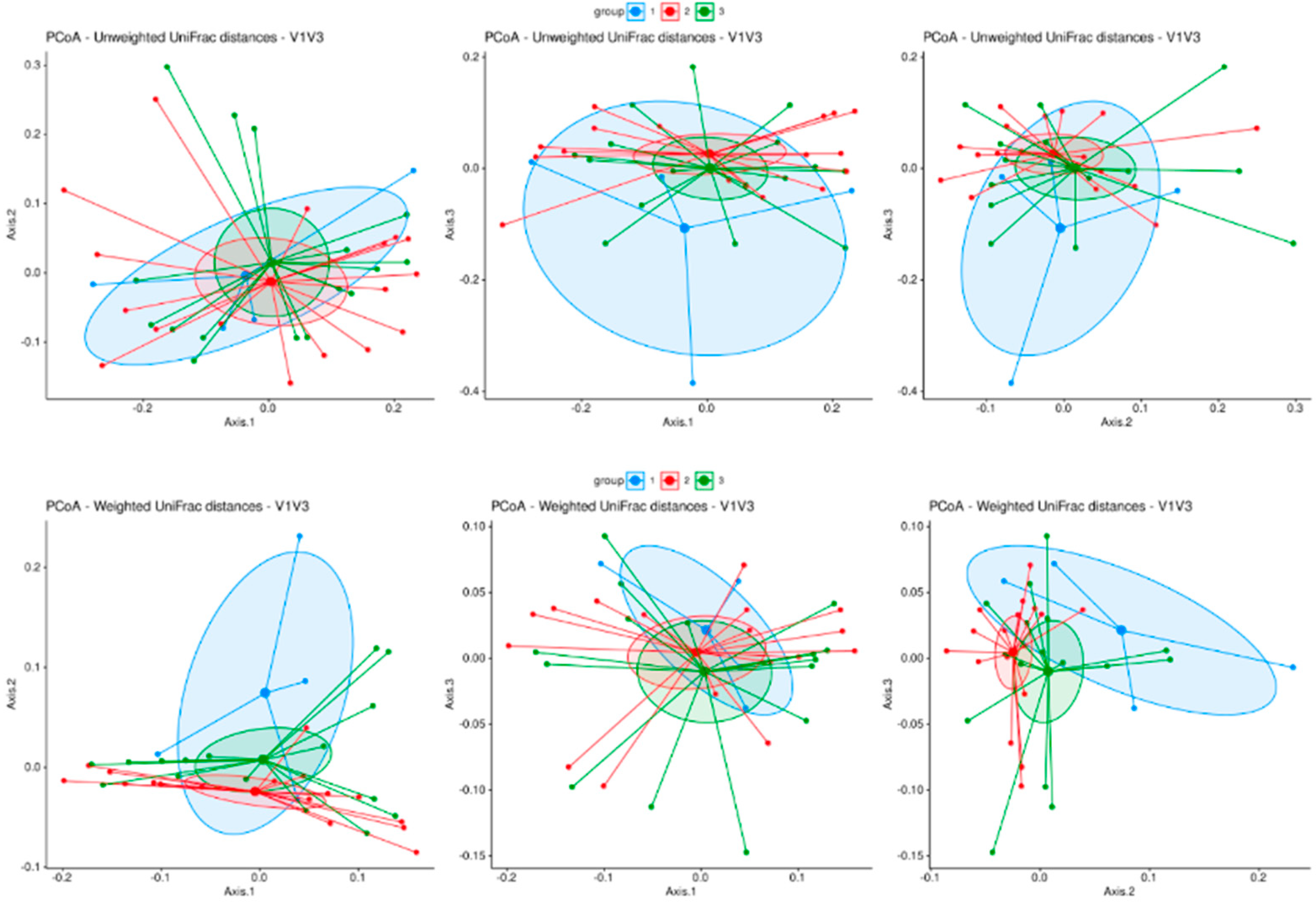
3.1. Microbiome Analysis
3.2. Epsilon Toxin in Serum
3.2.1. Direct Detection of Epsilon Toxin
3.2.2. Detection of Immunoglobulin/Epsilon Toxin Immunocomplexes
3.2.3. Detection of Anti-Epsilon-Toxin Antibodies
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Berg, G.; Rybakova, D.; Fischer, D.; Cernava, T.; Vergès, M.C.; Charles, T.; Chen, X.; Cocolin, L.; Eversole, K.; Corral, G.H.; et al. Microbiome definition re-visited: Old concepts and new challenges. Microbiome 2020, 8, 103. [Google Scholar]
- Sharon, G.; Sampson, T.R.; Geschwind, D.H.; Mazmanian, S.K. The Central Nervous System and the Gut Microbiome. Cell 2016, 167, 915–932. [Google Scholar] [CrossRef]
- Thirion, F.; Sellebjerg, F.; Fan, Y.; Lyu, L.; Hansen, T.H.; Pons, N.; Levenez, F.; Quinquis, B.; Stankevic, E.; Søndergaard, H.B.; et al. The gut microbiota in multiple sclerosis varies with disease activity. Genome Med. 2023, 15, 1. [Google Scholar] [CrossRef] [PubMed]
- Berer, K.; Mues, M.; Koutrolos, M.; Al Rasbi, Z.; Boziki, M.; Johner, C.; Wekerle, H.; Krishnamoorthy, G. Commensal microbiota and myelin autoantigen cooperate to trigger autoimmune demyelination. Nature 2011, 479, 538. [Google Scholar] [CrossRef] [PubMed]
- Berer, K.; Gerdes, L.A.; Cekanaviciute, E.; Jia, X.; Xiao, L.; Xia, Z.; Liu, C.; Klotz, L.; Stauffer, U.; Baranzini, S.E.; et al. Gut microbiota from multiple sclerosis patients enables spontaneous autoimmune encephalomyelitis in mice. Proc. Natl. Acad. Sci. USA 2017, 114, 10719–10724. [Google Scholar] [CrossRef] [PubMed]
- Smedley, J.G.; Fisher, D.J.; Sayeed, S.; Chakrabarti, G.; McClane, B.A. The enteric toxins of Clostridium perfringens. Rev. Physiol. Biochem. Pharmacol. 2004, 152, 183–204. [Google Scholar] [PubMed]
- Stiles, B.G.; Barth, G.; Barth, H.; Popoff, M.R. Clostridium perfringens Epsilon Toxin: A Malevolent Molecule for Animals and Man? Toxins 2013, 5, 2138–2160. [Google Scholar] [CrossRef]
- Wioland, L.; Dupont, J.L.; Doussau, F.; Gaillard, S.; Heid, F.; Isope, P.; Pauillac, S.; Popoff, M.R.; Bossu, J.L.; Poulain, B. Epsilon toxin from Clostridium perfringens acts on oligodendrocytes without forming pores, and causes demyelination. Cell. Microbiol. 2015, 17, 369–388. [Google Scholar] [CrossRef] [PubMed]
- Linden, J.R.; Ma, Y.; Zhao, B.; Harris, J.M.; Rumah, K.R.; Schaeren-Wiemers, N.; Vartanian, T. Clostridium perfringens Epsilon Toxin Causes Selective Death of Mature Oligodendrocytes and Central Nervous System Demyelination. mBio 2015, 6, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- Rumah, K.R.; Linden, J.; Fischetti, V.A.; Vartanian, T. Isolation of Clostridium perfringens type B in an individual at first clinical presentation of multiple sclerosis provides clues for environmental triggers of the disease. PLoS ONE 2013, 8, e76359. [Google Scholar] [CrossRef] [PubMed]
- Wagley, S.; Bokori-Brown, M.; Morcrette, H.; Malaspina, A.; D’arcy, C.; Gnanapavan, S.; Lewis, N.; Popoff, M.R.; Raciborska, D.; Nicholas, R.; et al. Evidence of Clostridium perfringens epsilon toxin associated with multiple sclerosis. Mult. Scler. J. 2019, 25, 653–660. [Google Scholar] [CrossRef] [PubMed]
- Gold, R.; Wolinsky, J.S. Pathophysiology of multiple sclerosis and the place of teriflunomide. Acta Neurol. Scand. 2011, 124, 75–84. [Google Scholar] [CrossRef]
- Rumah, K.R.; Vartanian, T.K.; Fischetti, V.A. Oral Multiple Sclerosis Drugs Inhibit the In vitro Growth of Epsilon Toxin Producing Gut Bacterium, Clostridium perfringens. Front. Cell. Infect. Microbiol. 2017, 7, 11. [Google Scholar] [CrossRef]
- Hiergeist, A.; Ruelle, J.; Emler, S.; Gessner, A. Reliability of species detection in 16S microbiome analysis: Comparison of five widely used pipelines and recommendations for a more standardized approach. PLoS ONE 2023, 18, e0280870. [Google Scholar] [CrossRef]
- Hiergeist, A.; Gläsner, J.; Reischl, U.; Gessner, A. Analyses of Intestinal Microbiota: Culture versus Sequencing. ILAR J. 2015, 56, 228–240. [Google Scholar] [CrossRef] [PubMed]
- Hiergeist, A.; Reischl, U.; Gessner, A. Multicenter quality assessment of 16S ribosomal DNA-sequencing for microbiome analyses reveals high inter-center variability. Int. J. Med. Microbiol. 2016, 306, 334–342. [Google Scholar] [CrossRef]
- Stämmler, F.; Gläsner, J.; Hiergeist, A.; Holler, E.; Weber, D.; Oefner, P.J.; Gessner, A.; Spang, R. Adjusting microbiome profiles for differences in microbial load by spike-in bacteria. Microbiome 2016, 4, 28. [Google Scholar] [CrossRef]
- Segata, N.; Izard, J.; Waldron, L.; Gevers, D.; Miropolsky, L.; Garrett, W.S.; Huttenhower, C. Metagenomic biomarker discovery and explanation. Genome Biol. 2011, 12, R60. [Google Scholar] [CrossRef] [PubMed]
- Féraudet-Tarisse, C.; Mazuet, C.; Pauillac, S.; Krüger, M.; Lacroux, C.; Popoff, M.R.; Dorner, B.G.; Andréoletti, O.; Plaisance, M.; Volland, H.; et al. Highly sensitive sandwich immunoassay and immunochromatographic test for the detection of Clostridial epsilon toxin in complex matrices. PLoS ONE 2017, 12, e0181013. [Google Scholar] [CrossRef]
- Grassi, J.; Frobert, Y.; Pradelles, P.; Chercuitte, F.; Gruaz, D.; Dayer, J.M.; Poubelle, P.E. Production of monoclonal antibodies against interleukin-1 alpha and -1 beta. Development of two enzyme immunometric assays (EIA) using acetylcholinesterase and their application to biological media. J. Immunol. Methods 1989, 123, 193–210. [Google Scholar] [CrossRef]
- Reiber, H.; Ungefehr, S.; Jacobi, C. The intrathecal, polyspecific and oligoclonal immune response in multiple sclerosis. Mult. Scler. J. 1998, 4, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Brändle, S.M.; Obermeier, B.; Senel, M.; Bruder, J.; Mentele, R.; Khademi, M.; Olsson, T.; Tumani, H.; Kristoferitsch, W.; Lottspeich, F.; et al. Distinct oligoclonal band antibodies in multiple sclerosis recognize ubiquitous self-proteins. Proc. Natl. Acad. Sci. USA 2016, 113, 7864–7869. [Google Scholar] [CrossRef] [PubMed]
- Rostasy, K.; Reiber, H.; Pohl, D.; Lange, P.; Ohlenbusch, A.; Eiffert, H.; Maass, M.; Hanefeld, F. Chlamydia pneumoniae in children with MS: Frequency and quantity of intrathecal antibodies. Neurology 2003, 61, 125–128. [Google Scholar] [CrossRef] [PubMed]
- Villar, L.M.; Sádaba, M.C.; Roldán, E.; Masjuan, J.; González-Porqué, P.; Villarrubia, N.; Espiño, M.; García-Trujillo, J.A.; Bootello, A.; Álvarez-Cermeño, J.C. Intrathecal synthesis of oligoclonal IgM against myelin lipids predicts an aggressive disease course in MS. J. Clin. Investig. 2005, 115, 187. [Google Scholar] [CrossRef] [PubMed]
- Felgenhauer, K.; Reiber, H. The diagnostic significance of antibody specificity indices in multiple sclerosis and herpes virus induced diseases of the nervous system. Clin. Investig. 1992, 70, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Bjornevik, K.; Cortese, M.; Healy, B.C.; Kuhle, J.; Mina, M.J.; Leng, Y.; Elledge, S.J.; Niebuhr, D.W.; Scher, A.I.; Munger, K.L.; et al. Longitudinal analysis reveals high prevalence of Epstein-Barr virus associated with multiple sclerosis. Science 2022, 375, 296–301. [Google Scholar] [CrossRef]
- Soldan, S.S.; Lieberman, P.M. Epstein–Barr virus and multiple sclerosis. Nat. Rev. Microbiol. 2022, 21, 51–64. [Google Scholar] [CrossRef]
- Tang, Q.; Jin, G.; Wang, G.; Liu, T.; Liu, X.; Wang, B.; Cao, H. Current Sampling Methods for Gut Microbiota: A Call for More Precise Devices. Front. Cell. Infect. Microbiol. 2020, 10, 151. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huss, A.; Bachhuber, F.; Feraudet-Tarisse, C.; Hiergeist, A.; Tumani, H. Multiple Sclerosis and Clostridium perfringens Epsilon Toxin: Is There a Relationship? Biomedicines 2024, 12, 1392. https://doi.org/10.3390/biomedicines12071392
Huss A, Bachhuber F, Feraudet-Tarisse C, Hiergeist A, Tumani H. Multiple Sclerosis and Clostridium perfringens Epsilon Toxin: Is There a Relationship? Biomedicines. 2024; 12(7):1392. https://doi.org/10.3390/biomedicines12071392
Chicago/Turabian StyleHuss, André, Franziska Bachhuber, Cécile Feraudet-Tarisse, Andreas Hiergeist, and Hayrettin Tumani. 2024. "Multiple Sclerosis and Clostridium perfringens Epsilon Toxin: Is There a Relationship?" Biomedicines 12, no. 7: 1392. https://doi.org/10.3390/biomedicines12071392
APA StyleHuss, A., Bachhuber, F., Feraudet-Tarisse, C., Hiergeist, A., & Tumani, H. (2024). Multiple Sclerosis and Clostridium perfringens Epsilon Toxin: Is There a Relationship? Biomedicines, 12(7), 1392. https://doi.org/10.3390/biomedicines12071392





