Clinical and Metabolic Particularities of a Roma Population with Diabetes—Considering Ethnic Disparities in Approaching Healthcare Management
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Setting
2.2. Study Population
2.3. Data Collection
2.4. Clinical Measurements
2.5. Paraclinical Assessment
2.6. Definitions
2.7. Study Outcome
2.8. Statistical Analysis
3. Results
3.1. General Characteristics of the Patients
3.2. Prevalence of Comorbidities
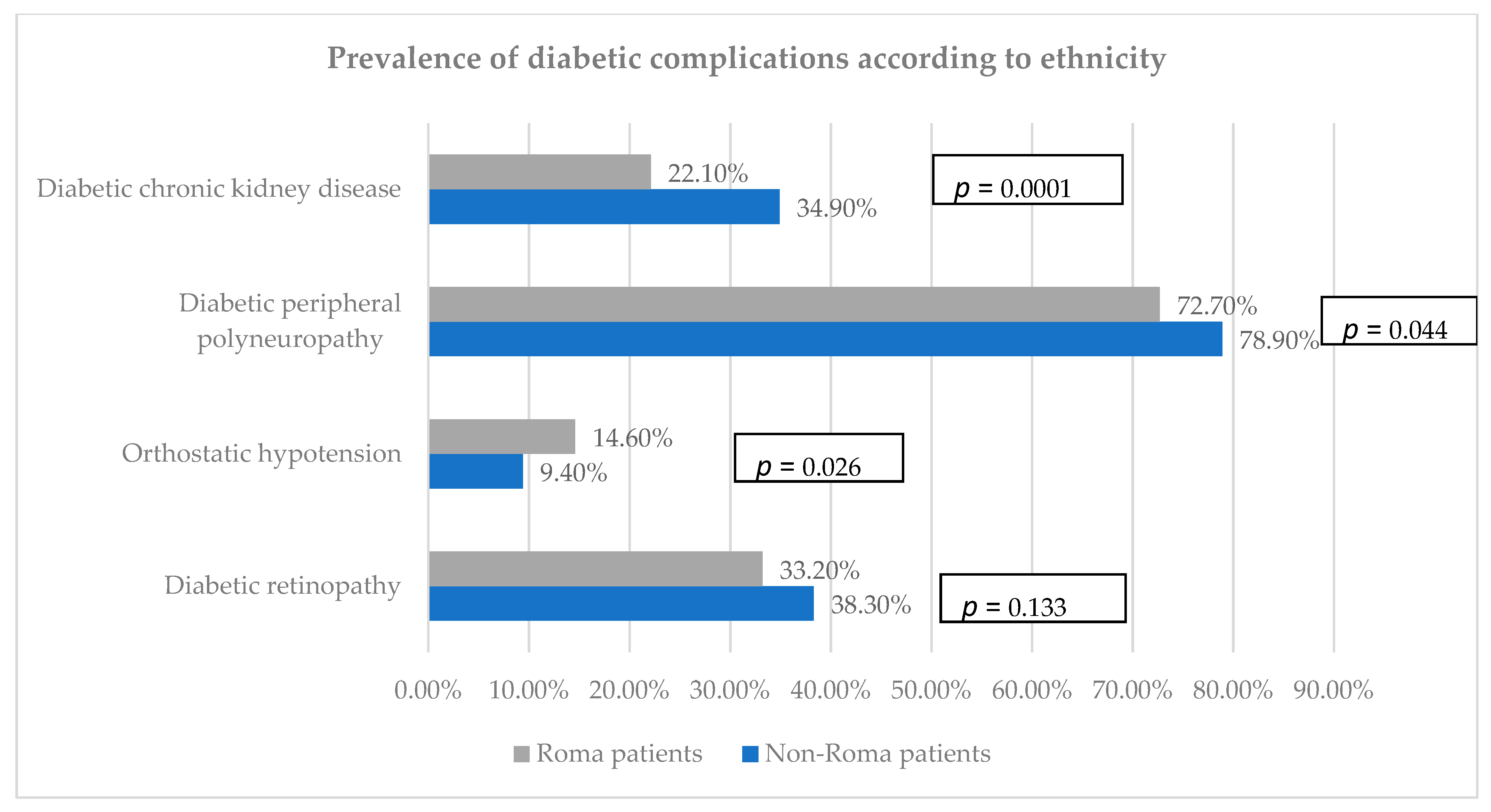
3.3. Prevalence of Diabetic Complications
3.4. Clinical and Paraclinical Assessments
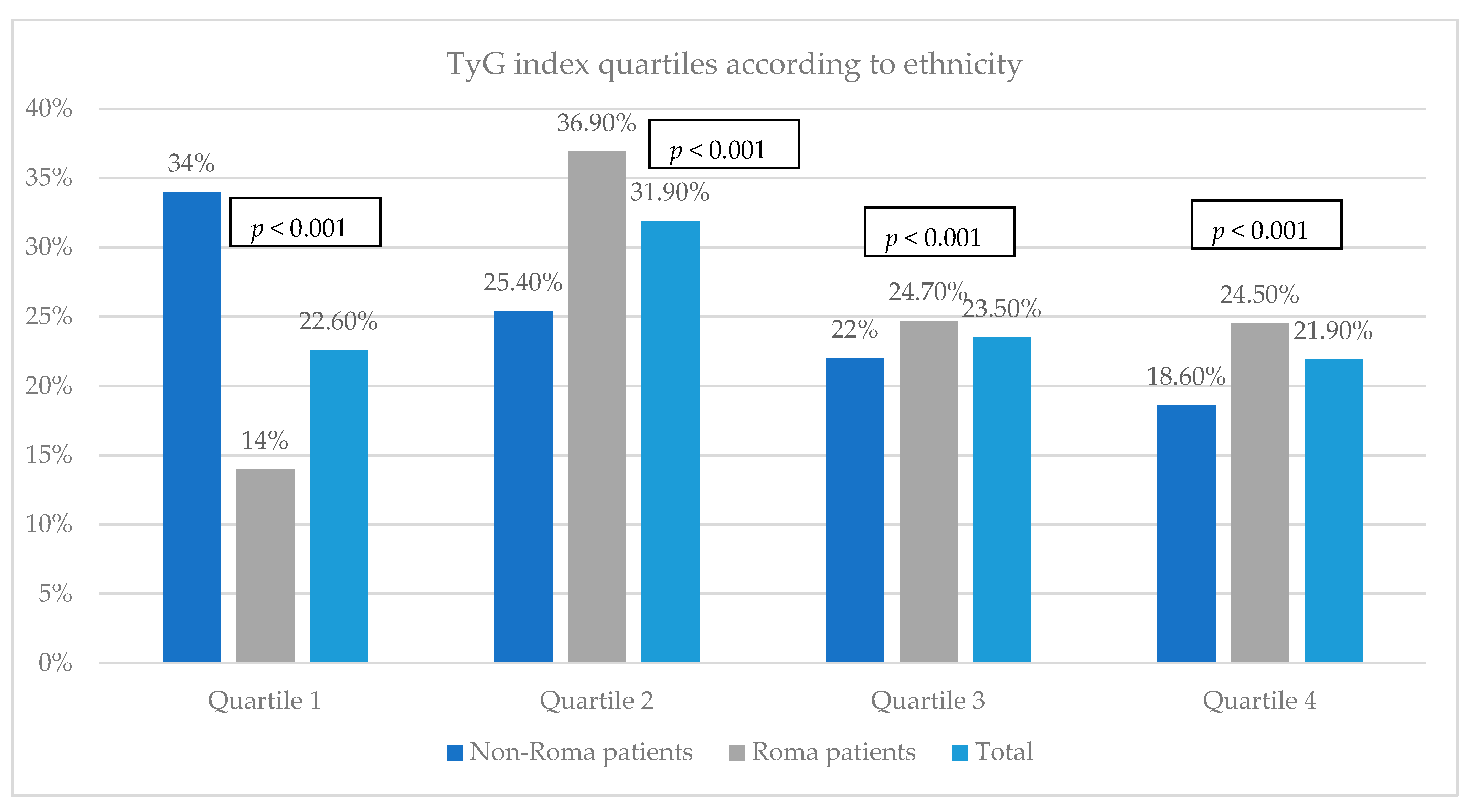
3.5. Evaluation of the Insulin Resistance among the Study Groups
3.6. Factors Associated with TyG Index
4. Discussion
5. Limitations of the Study
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kósa, Z.; Moravcsik-Kornyicki, Á.; Diószegi, J.; Roberts, B.; Szabó, Z.; Sándor, J.; Ádány, R. Prevalence of metabolic syndrome among Roma: A comparative health examination survey in Hungary. Eur. J. Public Health 2015, 25, 299–304. [Google Scholar] [CrossRef]
- Kanapeckiene, V.; Valinteliene, R.; Berzanskyte, A.; Kevalas, R.; Supranowicz, P. Health of Roma children in Vilnius and Ventspils. Medicina 2009, 45, 153–161. [Google Scholar] [CrossRef]
- Nunes, M.A.; Kučerová, K.; Lukáč, O.; Kvapil, M.; Brož, J. Prevalence of diabetes mellitus among Roma populations—A systematic review. Int. J. Environ. Res. Public Health 2018, 15, 2607. [Google Scholar] [CrossRef] [PubMed]
- Simental-Mendía, L.E.; Rodríguez-Morán, M.; Guerrero-Romero, F. The product of fasting glucose and triglycerides as surrogate for identifying insulin resistance in apparently healthy subjects. Metab. Syndr. Relat. Disord. 2008, 6, 299–304. [Google Scholar] [CrossRef]
- Cuschieri, S. The STROBE guidelines. Saudi J. Anaesth. 2019, 13, S31–S34. [Google Scholar] [CrossRef]
- Thomas, D.; Seeman, T.; Potter, A.; Hu, P.; Crimmins, E.; Herningtyas, E.H.; Sumantri, C.; Frankenberg, E. HPLC-based measurement of glycated hemoglobin using dried blood spots collected under adverse field conditions. Biodemography Soc. Biol. 2018, 64, 43–62. [Google Scholar] [CrossRef] [PubMed]
- Spectrophotometry|NIST. Available online: https://www.nist.gov/programs-projects/spectrophotometry (accessed on 14 June 2024).
- Chen, R.Y.; Shi, J. Evaluation of the CKD-EPI 2021 creatinine equation using laboratory data: Considerations for practice changes among clinical laboratories in British Columbia, Canada. Clin. Biochem. 2024, 123, 110686. [Google Scholar] [CrossRef] [PubMed]
- Mach, F.; Baigent, C.; Catapano, A.L.; Koskinas, K.C.; Casula, M.; Badimon, L.; Chapman, M.J.; De Backer, G.G.; Delgado, V.; Ference, B.A.; et al. 2019 ESC/EAS guidelines for the management of dyslipidaemias: Lipid modification to reduce cardiovascular risk. Eur. Heart J. 2019, 41, 111–188. [Google Scholar] [CrossRef]
- Selvi, N.M.K.; Nandhini, S.; Sakthivadivel, V.; Lokesh, S.; Srinivasan, A.R.; Sumathi, S. Association of triglyceride- glucose index (TyG index) with HbA1c and insulin resistance in type 2 diabetes mellitus. Maedica 2021, 16, 375–381. [Google Scholar]
- American Diabetes Association Professional Practice Committee. Standards of care in diabetes-2024. Diabetes Care 2024, 47, S5–S10. [Google Scholar] [CrossRef]
- Unger, T.; Borghi, C.; Charchar, F.; Khan, N.A.; Poulter, N.R.; Prabhakaran, D.; Ramirez, A.; Schlaich, M.; Stergiou, G.S.; Tomaszewski, M.; et al. 2020 International Society of Hypertension Global Hypertension Practice Guidelines. Hypertension 2020, 75, 1334–1357. [Google Scholar] [CrossRef]
- Body Mass Index (BMI). Available online: https://www.who.int/data/gho/data/themes/topics/topic-details/GHO/body-mass-index (accessed on 2 April 2024).
- Alberti, K.G.; Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z.; Cleeman, J.I.; Donato, K.A.; Fruchart, J.C.; James, W.P.T.; Loria, C.M.; Smith, S.C., Jr. Harmonizing the metabolic syndrome: A joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 2009, 120, 1640–1645. [Google Scholar] [PubMed]
- Stevens, P.E.; Ahmed, S.B.; Carrero, J.J.; Foster, B.; Francis, A.; Hall, R.K.; Herrington, W.G.; Hill, G.; Inker, L.A.; Kazancıoğlu, R.; et al. KDIGO 2024 Clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int. 2024, 105, S117–S314. [Google Scholar]
- Relhan, N.; Flynn, H.W. The early treatment diabetic retinopathy study historical review and relevance to today’s management of diabetic macular edema. Curr. Opin. Ophthalmol. 2017, 28, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Massey, F.J. The Kolmogorov-Smirnov Test for Goodness of Fit. J. Am. Stat. Assoc. 1951, 46, 68–78. [Google Scholar] [CrossRef]
- Shapiro, S.S.; Wilk, M.B. An Analysis of Variance Test for Normality (Complete Samples). Biometrika 1965, 52, 591. [Google Scholar] [CrossRef]
- Kolarčik, P.; Geckova, A.M.; Orosova, O.; van Dijk, J.P.; Reijneveld, S.A. Predictors of health-endangering behaviour among Roma and non-Roma adolescents in Slovakia by gender. J. Epidemiol. Community Health 2010, 64, 1043–1048. [Google Scholar] [CrossRef] [PubMed]
- Enache, G.; Rusu, E.; Ilinca, A.; Rusu, F.; Costache, A.; Jinga, M.; Pănuş, C.; Radulian, G. Prevalence of overweight and obesity in a Roma population from Southern Romania—Calarasi county. Acta Endocrinol. 2018, 14, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Rusu, E.; Enache, G.; Rusu, F.; Cosoreanu, A.; Cirstea, C.; Baleanu, M.; Radulian, G. 1466-P: Prevalence of glucose intolerance in the Roma population from a rural area in the south part of Romania: Călărași county. Diabetes 2020, 69 (Suppl. S1), 1466-P. [Google Scholar] [CrossRef]
- Weiss, E.; Japie, C.; Balahura, A.M.; Bartos, D.; Badila, E. Cardiovascular risk factors in a Roma sample population from Romania. Rom. J. Intern. Med. 2018, 56, 193–202. [Google Scholar] [CrossRef]
- Mota, M.; Popa, S.G.; Mota, E.; Mitrea, A.; Catrinoiu, D.; Cheta, D.M.; Guja, C.; Hancu, N.; Ionescu-Tirgoviste, C.; Lichiardopol, R.; et al. Prevalence of diabetes mellitus and prediabetes in the adult Romanian population: PREDATORR study. J. Diabetes. 2016, 8, 336–344. [Google Scholar] [CrossRef] [PubMed]
- Beljić Zivković, T.; Marjanović, M.; Prgomelja, S.; Soldatovic, I.; Koprivica, B.; Acković, D.; Zivković, R. Screening for diabetes among Roma people living in Serbia. Croat. Med. J. 2010, 51, 144. [Google Scholar] [CrossRef] [PubMed]
- Fedacko, J.; Pella, D.; Jarcuska, P.; Siegfried, L.; Janicko, M.; Veselíny, E.; Pella, J.; Sabol, F.; Jarcuska, P.; Mareková, M.; et al. Prevalence of cardiovascular risk factors in relation to metabolic syndrome in the Roma population compared with the non-Roma population in the eastern part of Slovakia. Cent. Eur. J. Public Health 2014, 22, S69–S74. [Google Scholar] [CrossRef] [PubMed]
- Health and the Roma Community, Analysis of the Situation in Europe. Available online: www.gitanos.org (accessed on 16 April 2024).
- Ginter, E.; Krajcovicova-Kudlackova, M.; Kacala, O.; Kovacic, V.; Valachovicova, M. Health status of Romanies (Gypsies) in the Slovak Republic and in the neighbouring countries. Bratisl. Lek. Listy 2001, 102, 479–484. [Google Scholar] [PubMed]
- Fedacko, J.; Pella, D.; Jarcuska, P. Clinical and biochemical determinants of metabolic syndrome among Roma and non-Roma subjects in the eastern part of Slovakia. Cent. Eur. J. Public Health 2014, 22, S75–S80. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Piko, P.; Dioszegi, J.; Kosa, Z.; Sandor, J.; Moizs, M.; Adany, R. Changes in the prevalence of metabolic syndrome, its components, and relevant preventive medication between 2011 and 2018 in the northeast hungarian roma population. J. Pers. Med. 2021, 11, 595. [Google Scholar] [CrossRef] [PubMed]
- Cook, B.; Wayne, G.F.; Valentine, A.; Lessios, A.; Yeh, E. Revisiting the evidence on health and health care disparities among the Roma: A systematic review 2003–2012. Int. J. Public Health. 2013, 58, 885–911. [Google Scholar] [CrossRef] [PubMed]
- Piko, P.; Werissa, N.A.; Adany, R. Genetic susceptibility to insulin resistance and its association with estimated longevity in the Hungarian general and Roma populations. Biomedicines 2022, 10, 1703. [Google Scholar] [CrossRef] [PubMed]
- Ádány, R.; Pikó, P.; Fiatal, S.; Kósa, Z.; Sándor, J.; Bíró, É.; Kósa, K.; Paragh, G.; Bácsné Bába, É.; Veres-Balajti, I.; et al. Prevalence of insulin resistance in the Hungarian general and Roma populations as defined by using Data generated in a complex health (Interview and Examination) Survey. Int. J. Environ. Res. Public Health 2020, 17, 4833. [Google Scholar] [CrossRef]
- Araújo, S.P.; Juvanhol, L.L.; Bressan, J.; Hermsdorff, H.H.M. Triglyceride glucose index: A new biomarker in predicting cardiovascular risk. Prev. Med. Rep. 2022, 29, 101941. [Google Scholar] [CrossRef]
- Delcheva, G.; Stankova, T.; Stefanova, K.; Bivolarska, A. Assessment of health status and cardiovascular risk factors in a Roma population sample from South Bulgaria. Cent. Eur. J. Public Health 2023, 31, 115–119. [Google Scholar] [CrossRef]
- Faselis, C.; Katsimardou, A.; Imprialos, K.; Deligkaris, P.; Kallistratos, M.; Dimitriadis, K. Microvascular complications of type 2 diabetes mellitus. Curr. Vasc. Pharmacol. 2020, 18, 117–124. [Google Scholar] [CrossRef]
- Rusu, E.; Coman, H.; Coșoreanu, A.; Militaru, A.M.; Popescu-Vâlceanu, H.C.; Teodoru, I.; Mihai, D.A.; Elian, V.; Gavan, N.A.; Radulian, G. Incidence of Lower extremity amputation in Romania: A nationwide 5-year cohort study, 2015–2019. Medicina 2023, 59, 1199. [Google Scholar] [CrossRef]
- Ghid de Management al Diabetului Zaharat. 2021. Available online: https://societate-diabet.ro/wp-content/uploads/2021/07/Ghidul-SRDNBM-2021.pdf (accessed on 16 April 2024).
- Coşoreanu, A.; Rusu, E.; Baleanu, M.; Marinescu, M.; Iordache, S.; Vlad, A.M.; Rusu, F.; Enache, G.; Radulian, G. Prevalence of limb amputations in a Roma population compared to Caucasians, in patients with diabetes mellitus. Rom. J. Orthop. Surg. Traumatol. 2020, 3, 41–48. [Google Scholar] [CrossRef]
- Cosoreanu, A.; Rusu, E.; Mihai, D.A.; Rusu, F.; Pantea, I.; Paunica, I.; Ungureanu, I.; Radulian, G. Diabetes distress among the Roma population from a tertiary care center in Romania. Cureus 2024, 16, e60348. [Google Scholar] [CrossRef] [PubMed]
- Werissa, N.A.; Piko, P.; Fiatal, S.; Kosa, Z.; Sandor, J.; Adany, R. SNP-based genetic risk score modeling suggests no increased genetic susceptibility of the Roma population to type 2 diabetes mellitus. Genes 2019, 10, 942. [Google Scholar] [CrossRef] [PubMed]
- EU Roma Strategic Framework for Equality, Inclusion and Participation for 2020–2030 Justice and Consumers. Available online: https://commission.europa.eu/publications/new-eu-roma-strategic-framework-equality-inclusion-and-participation-full-package_en (accessed on 14 June 2024).

| Variables | Non-Roma Patients (n = 350) | Roma Patients (n = 458) | p-Value | |
|---|---|---|---|---|
| Gender | Men | 54.6% (n = 191) | 51.5% (n = 236) | 0.391 |
| Women | 45.4% (n = 159) | 48.5% (n = 222) | ||
| Place of residence | Urban area | 65.1% (n = 228) | 52.2% (n = 239) | 0.0001 |
| Rural area | 34.9% (n = 122) | 47.8% (n = 219) | ||
| Type of diabetes | T1DM | 4.9% (n = 17) | 12.2% (n = 56) | 0.0001 |
| T2DM | 95.1% (n = 333) | 87.8% (n = 402) | ||
| Family history of diabetes | Yes | 47.1% (n = 165) | 45.4% (n = 208) | 0.625 |
| Smoking (former or active smokers) | Yes | 26.3% (n = 92) | 50.4% (n = 231) | 0.0001 |
| Alcohol consumption | Yes | 25.1% (n = 88) | 26.9% (n = 123) | 0.583 |
| Parameters | Non-Roma Patients (n = 350) | Roma Patients (n = 458) | Total (n = 808) | p-Value |
|---|---|---|---|---|
| Mean ± SD | Mean ± SD | Mean ± SD | ||
| Age (years) | 62.06 ± 10.6 | 55.62 ± 11.55 | 58.41 ± 11.59 | 0.0001 |
| Duration of diabetes (years) | 11.00 ± 8.18 * | 6.00 ± 6.89 * | 9.00 ± 7.77 * | 0.0001 |
| Height (cm) | 166.83 ± 9.68 | 164.54 ± 9.27 | 165.67 ± 9.54 | 0.001 |
| Weight (kg) | 84.81 ± 17.82 | 87.51 ± 20.12 | 86.19 ± 19.06 | 0.059 |
| WC (cm) | 103.73 ± 14.70 | 110.00 ± 15.87 | 107.08 ± 15.64 | 0.0001 |
| HC (cm) | 104.82 ± 14.02 | 108.61 ± 14.40 | 107.20 ± 14.36 | 0.011 |
| BMI (kg/m2) | 30.41 ± 5.60 | 32.28 ± 7.03 | 31.36 ± 6.43 | 0.0001 |
| HbA1c (%) | 9.07 ± 2.09 | 9.91 ± 2.45 | 9.53 ± 2.33 | 0.0001 |
| FPG (mg/dL) | 226.35 ± 87.96 | 232.00 ± 117.42 * | 243.59 ± 106.46 | 0.0001 |
| TC (mg/dL) | 192.66 ± 65.29 | 217.12 ± 63.41 | 205.67 ± 65.40 | 0.0001 |
| HDL-c (mg/dL) | 49.40 ± 14.09 | 45.57 ± 9.91 | 47.38 ± 12.20 | 0.0001 |
| TG (mg/dL) | 192.06 ± 138.62 | 234.39 ± 123.45 | 214.54 ± 132.39 | 0.0001 |
| LDL-c (mg/dL) | 103.64 ± 48.67 | 123.11 ± 52.59 | 113.79 ± 51.64 | 0.0001 |
| TyG index | 9.71 ± 0.82 | 10.07 ± 0.71 | 9.90 ± 0.78 | 0.36 |
| Creatinine (mg/dL) | 0.96 ± 0.37 | 1.04 ± 0.43 | 1.00 ± 0.40 | 0.010 |
| eGFR (mL/min/1.73 m2) | 83.00 ± 45.00 * | 80.00 ± 41.00 * | 83.00 ± 41.00 * | 0.255 |
| Urea (mg/dL) | 44.56 ± 20.08 | 44.34 ± 18.49 | 44.43 ± 19.16 | 0.904 |
| Uric acid (mg/dL) | 5.99 ± 1.98 | 6.16 ± 2.36 | 6.06 ± 2.15 | 0.578 |
| UACR (mg/g) | 25.13 ± 104.27 * | 25.00 ± 31.28 * | 24.14 ± 65.7 * | 0.003 |
| AST (UI/L) | 20.00 ± 22.66 * | 23.00 ± 25.88 * | 22.00 ± 13.18 * | 0.086 |
| ALT (UI/L) | 24.00 ± 27.31 * | 29.00 ± 28.75 * | 27.00 ± 23.00 * | 0.002 |
| GGT (UI/L) | 33.35 ± 102.97 * | 44.00 ± 47.58 * | 42.00 ± 38.00 * | 0.081 |
| Parameters | Forth Quartile of the TyG index | p-Value | |
|---|---|---|---|
| Non-Roma Patients (n = 65) | Roma Patients (n= 112) | ||
| Age (years) | 62.05 ± 11.67 | 55.26 ± 11.73 | <0.001 |
| Duration of diabetes (years) | 12.00 ± 4.50 * | 6.00 ± 9.30 * | <0.001 |
| Height (cm) | 167.03 ± 9.27 | 164.88 ± 8.27 | 0.123 |
| Weight (kg) | 85.55 ± 17.83 | 90.64 ± 20.30 | 0.102 |
| WC (cm) | 104.44 ± 15.26 | 112.65 ± 15.48 | 0.006 |
| HC (cm) | 106.74 ± 8.82 | 109.07 ± 13.49 | 0.422 |
| BMI (kg/m2) | 31.33 ± 4.23 | 31.83 ± 6.98 | 0.159 |
| HbA1c (%) | 9.55 ± 2.36 | 11.66 ± 2.05 | <0.001 |
| FPG (mg/dL) | 255.50 ± 100.50 * | 337.50 ± 108.50 * | 0.010 |
| TC (mg/dL) | 244.28 ± 93.54 | 247.82 ± 70.12 | 0.775 |
| HDL-c (mg/dl) | 45.44 ± 13.72 | 42.52 ± 9.71 | 0.103 |
| TG (mg/dL) | 401.25 ± 178.75 | 363.50 ± 125.93 | 0.102 |
| LDL-c (mg/dL) | 104.35 ± 40.20 | 127.42 ± 32.80 | 0.006 |
| Creatinine (mg/dL) | 1.02 ± 0.43 | 1.09 ± 0.43 | 0.661 |
| eGFR (mL/min/1.73 m2) | 76.63 ± 29.26 | 78.16 ± 24.09 | 0.716 |
| Urea (mg/dL) | 42.26 ± 17.36 | 49.56 ± 19.82 | 0.500 |
| Uric acid (mg/dL) | 5.90 ± 2.13 | 6.80 ± 2.80 | 0.363 |
| UACR (mg/g) | 45.80 ± 150.08 * | 36.00 ± 60.20 * | 0.047 |
| AST (UI/L) | 40.88 ± 20.00 | 23.00 ± 15.5 * | 0.320 |
| ALT (UI/L) | 27.83 ± 20.09 * | 31.00 ± 26.25 * | 0.904 |
| GGT (UI/L) | 94.13 ± 95.00 * | 44.00 ± 27.00 * | 0.076 |
| Variables | B | SE | p-Value | 95% CI | |
|---|---|---|---|---|---|
| Lower | Upper | ||||
| Non-Roma patients | |||||
| Weight (kg) | −0.001 | 0.002 | 0.706 | 7.215 | 8.420 |
| HbA1c (%) | 0.148 | 0.018 | <0.001 | 0.112 | 0.183 |
| TC (mg/dL) | 0.015 | 0.001 | <0.001 | 0.012 | 0.017 |
| HDL-c (mg/dL) | −0.022 | 0.003 | <0.001 | 0.028 | −0.015 |
| LDL-c (mg/dL) | −0.012 | 0.002 | <0.001 | −0.015 | −0.009 |
| ALT (UI/L) | 0.0007 | 0.001 | 0.945 | −0.002 | 0.002 |
| UACR (mg/g) | 0.0001 | 0.0001 | 0.059 | 0.0001 | 0.0001 |
| Roma patients | |||||
| HbA1c (%) | 0.150 | 0.027 | <0.001 | 0.096 | 0.204 |
| TC (mg/dL) | 0.004 | 0.003 | 0.232 | −0.002 | 0.009 |
| HDL-c (mg/dL) | −0.017 | 0.008 | 0.030 | −0.032 | −0.002 |
| LDL-c (mg/dL) | 0.0001 | 0.004 | 0.911 | −0.008 | 0.007 |
| Creatinine (mg/dL) | −0.071 | 0.170 | 0.678 | −0.411 | 0.269 |
| UACR (mg/g) | 0.001 | 0.001 | 0.344 | −0.001 | 0.002 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cosoreanu, A.; Rusu, E.; Rusu, F.; Stanciu, S.; Ungureanu, I.; Donici, M.; Visinescu, A.; Enache, G.; Radulian, G. Clinical and Metabolic Particularities of a Roma Population with Diabetes—Considering Ethnic Disparities in Approaching Healthcare Management. Biomedicines 2024, 12, 1422. https://doi.org/10.3390/biomedicines12071422
Cosoreanu A, Rusu E, Rusu F, Stanciu S, Ungureanu I, Donici M, Visinescu A, Enache G, Radulian G. Clinical and Metabolic Particularities of a Roma Population with Diabetes—Considering Ethnic Disparities in Approaching Healthcare Management. Biomedicines. 2024; 12(7):1422. https://doi.org/10.3390/biomedicines12071422
Chicago/Turabian StyleCosoreanu, Andrada, Emilia Rusu, Florin Rusu, Silviu Stanciu, Ioana Ungureanu, Marius Donici, Alexandra Visinescu, Georgiana Enache, and Gabriela Radulian. 2024. "Clinical and Metabolic Particularities of a Roma Population with Diabetes—Considering Ethnic Disparities in Approaching Healthcare Management" Biomedicines 12, no. 7: 1422. https://doi.org/10.3390/biomedicines12071422
APA StyleCosoreanu, A., Rusu, E., Rusu, F., Stanciu, S., Ungureanu, I., Donici, M., Visinescu, A., Enache, G., & Radulian, G. (2024). Clinical and Metabolic Particularities of a Roma Population with Diabetes—Considering Ethnic Disparities in Approaching Healthcare Management. Biomedicines, 12(7), 1422. https://doi.org/10.3390/biomedicines12071422






