Abstract
The primary mechanism of traumatic spinal cord injury (SCI) comprises the initial mechanical trauma due to the transmission of energy to the spinal cord, subsequent deformity, and persistent compression. The secondary mechanism of injury, which involves structures that remained undamaged after the initial trauma, triggers alterations in microvascular perfusion, the liberation of free radicals and neurotransmitters, lipid peroxidation, alteration in ionic concentrations, and the consequent cell death by necrosis and apoptosis. Research in the treatment of SCI has sought to develop early therapeutic interventions that mitigate the effects of these pathophysiological mechanisms. Clinical and experimental evidence has demonstrated the therapeutic benefits of sex-steroid hormone administration after traumatic brain injury and SCI. The administration of estradiol, progesterone, and testosterone has been associated with neuroprotective effects, better neurological recovery, and decreased mortality after SCI. This review evaluated evidence supporting hormone-related neuroprotection over SCI and the possible underlying mechanisms in animal models. As neuroprotection has been associated with signaling pathways, the effects of these hormones are observed on astrocytes and microglia, modulating the inflammatory response, cerebral blood flow, and metabolism, mediating glutamate excitotoxicity, and their antioxidant effects. Based on the current evidence, it is essential to analyze the benefit of sex steroid hormone therapy in the clinical management of patients with SCI.
1. Introduction
Spinal cord injury (SCI) causes loss of motor, sensory, and autonomic functions and produces severe functional alterations (urinary tract and kidney infections, bowel problems, and cardiac and respiratory dysfunctions). Depending on the severity of the injury, complications often result in the death of SCI patients [1,2,3,4].
To date, consistently effective clinical treatments for SCI are not widely available. Most surgical procedures target the stabilization and decompression of the spinal cord in combination with high doses of methylprednisolone [5]. However, the effects of surgery are limited. Moreover, there is no consensus on the real benefit of methylprednisolone administration, as significant side effects are frequently observed [6]. Therefore, developing reliable repair strategies and treatments for SCI is essential.
The beneficial modulation of synaptic plasticity in spared but reactive neural tissue will significantly differ from the requirements to bridge new axon growth across non-neural lesion cores of anatomically complete lesions. Consequently, different cellular and molecular targets are under investigation to improve the outcome after SCI by providing tissue protection, modulating circuit reorganization, and regulating neural bridging connectivity through the injury site [7].
Increasing experimental and clinical evidence has demonstrated the therapeutic benefits of sex steroid hormones after traumatic brain injury and SCI [8,9,10,11,12,13]. The administration of estradiol (E2) and progesterone (P4) has been associated with a decrease in mortality, better neurological outcomes, and neuroprotective effects after such injuries [10,14,15,16,17,18]. This review aims to analyze the evidence in studies that support hormone-related neuroprotection in SCI and the possible underlying mechanisms in animal models.
2. SCI Pathophysiology
SCI undergoes primary and secondary mechanisms of injury [19,20]. The primary mechanisms of injury result from physical forces, including compression, shearing, laceration, and acute stretch/distraction of the spinal cord in the initial traumatic event [21]. A cascade of secondary mechanisms of injury is then initiated, which expands the injured nerve tissue area and exacerbates neurological deficits accordingly [22,23]. Secondary mechanisms in SCI generate delayed and progressive tissue injuries due to the initial injury. Secondary damage can be subdivided into the immediate phase (approximately 2 h post-injury), acute phase (48 h post-injury), subacute phase (2–13 days post-injury), intermediate phase (2–6 weeks post-injury), and chronic phase (>6 months post-injury). Once the chronic phase is established, neurological abnormalities may occur due to axonal damage in orthograde and retrograde directions days to years after SCI [24,25].
During the secondary phase of injury, vascular changes such as hemorrhage, vasospasms, thrombosis, loss of autoregulation, breakdown of the blood-brain barrier, and inflammatory cell infiltrate at the injury site may be observed. Inflammatory cells trigger the release of proinflammatory cytokines, including tumor necrosis factor-α (TNF-α), interleukin (IL)-1α, IL-1β, and IL-6, which reach their maximum levels 6 to 12 h after SCI and remain elevated up to 4 days after injury [26]. In addition, the loss of ionic homeostasis after SCI generates intracellular hypercalcemia, activating calcium-dependent proteases, producing mitochondrial dysfunction, and leading to edema, ischemia, and cell death by apoptosis [27,28,29].
Furthermore, phagocytic inflammatory cells release reactive oxygen species (ROS). Therefore, free radicals react with the polyunsaturated fatty acids of the cell membrane, leading to peroxidation and disruption of the normal phospholipid architecture of cellular and subcellular membranes. Moreover, lipid peroxidation generates aldehyde products that impair the function of critical metabolic enzymes, such as Na+,K+-ATPase [30]. This enzyme’s activity is essential for maintaining neuronal excitability, so its failure leads to the loss of neuronal function and, ultimately, tissue disruption [31].
Following SCI, an increase in excitatory amino acid release (glutamate and aspartate) from disrupted cells is detected [32,33]. The excessive activation of excitatory amino acid receptors produces excitotoxicity and further loss of neurons and glia by necrotic and apoptotic cell death [34]. Oligodendrocytes are particularly susceptible to loss via apoptotic death at the injury site and in distant regions, leading to the demyelination of the preserved axons [35,36]. The death of oligodendrocytes in white matter tracts continues for several weeks after the injury and may contribute to post-injury demyelination [35]. One of the cellular mediators of apoptosis after SCI is the relationship between microglia and dying oligodendrocytes, suggesting the involvement of microglial activation [37].
The last phase of secondary injury, the chronic phase, comprises events such as white matter demyelination and gray matter dissolution, connective tissue deposition, and reactive gliosis, leading to the formation of a glial scar. The glial scar, composed predominantly of reactive astrocytes, microglia/macrophages, and extracellular matrix molecules, mainly chondroitin sulfate proteoglycans—molecules capable of inhibiting axonal growth—prevents axonal growth by acting as a physical barrier [38,39,40].
3. Sex Steroid Hormones
Under physiological conditions, sex steroid hormones (SSHs) mediate various functions such as reproduction, sexual behavior, bone mineralization, and respiration. In the brain, SSHs are involved in neuronal plasticity, memory, and learning processes [41]. Furthermore, SSHs have shown a neuroprotective effect in different models of neuronal damage, including traumatic brain injury (TBI), SCI, ischemic stroke, excitotoxic damage of hippocampal neurons, and neurodegenerative diseases [42,43,44,45].
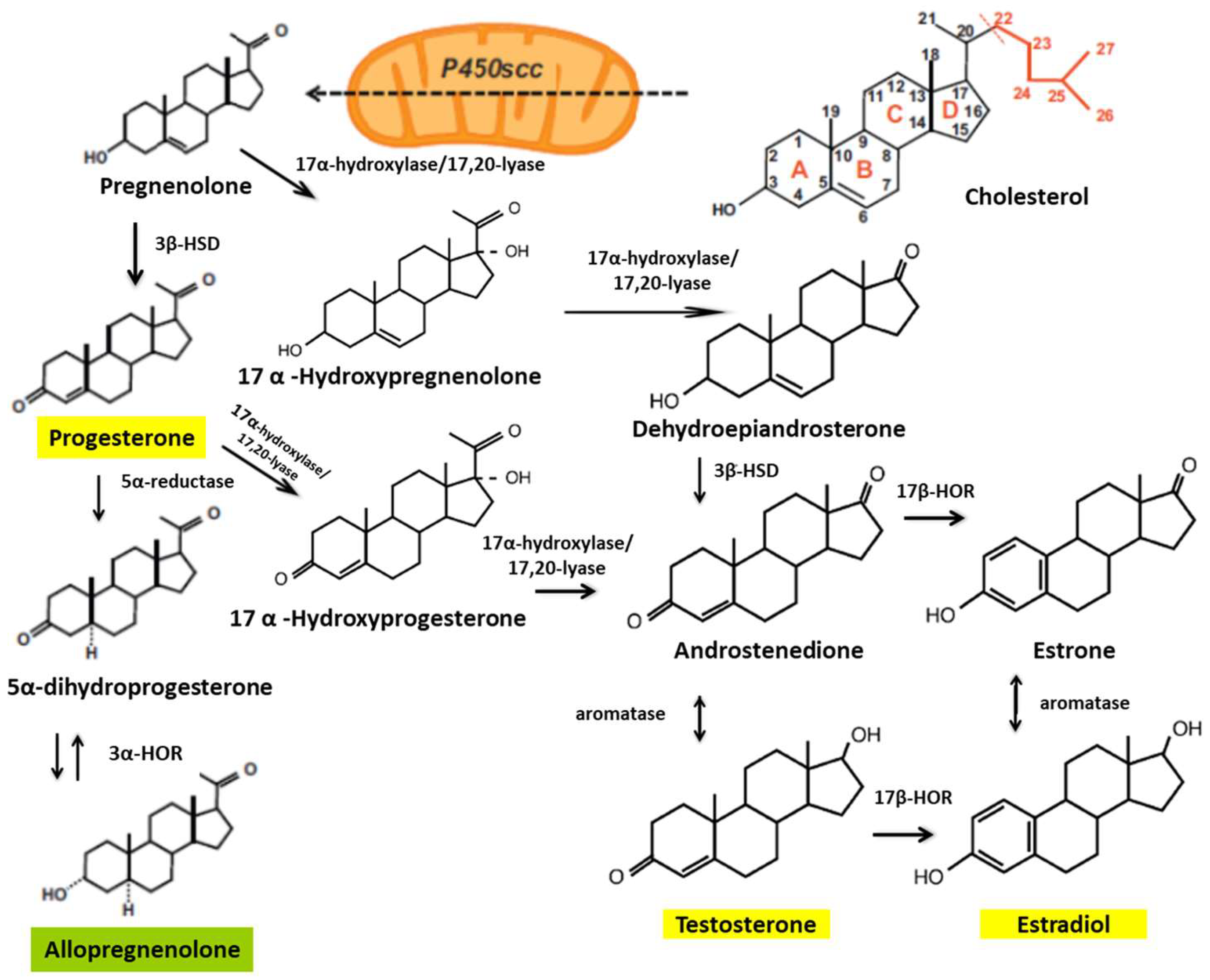
Some studies have shown that treatments with E2, P4, and testosterone—synthesized from cholesterol—reduce the damage caused by SCI (Figure 1). In animal models, researchers have found gender differences in functional outcomes of SCI, with a markedly better locomotor recovery in female rodents [46,47].
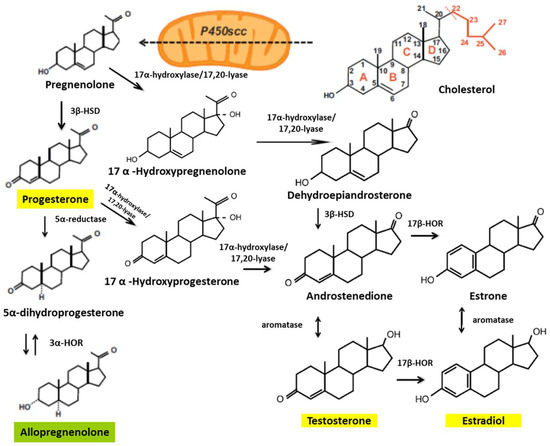
Figure 1.
Biosynthetic pathways of steroid hormones. The cytochrome mitochondrial cytochrome P450scc catalyzes the conversion of cholesterol to pregnenolone. Pregnenolone is then converted to progesterone by 3β-hydroxysteroid dehydrogenase (3β-HSD). Pregnenolone can also be converted to hydroxypregnenolone and progesterone to androstenedione by the cytochrome P450c17 (17-hydroxylase/17,20-lyase). The 17β-hydroxysteroid oxidoreductases (17β-HORs, also called 17β hydroxysteroid dehydrogenase) catalyze the final steps of androgen and estrogen biosynthesis (androstenedione ↔ testosterone and estrone ↔ estradiol). 5α-reductase is involved in the metabolism of progesterone, generating 5α-dihydro-progesterone, and 3α-hydroxysteroid oxidoreductase (3α-HOR) metabolizes 5α-dihydro-progesterone to allopregnanolone (modified from Schumacher et al., 2003 [41]).
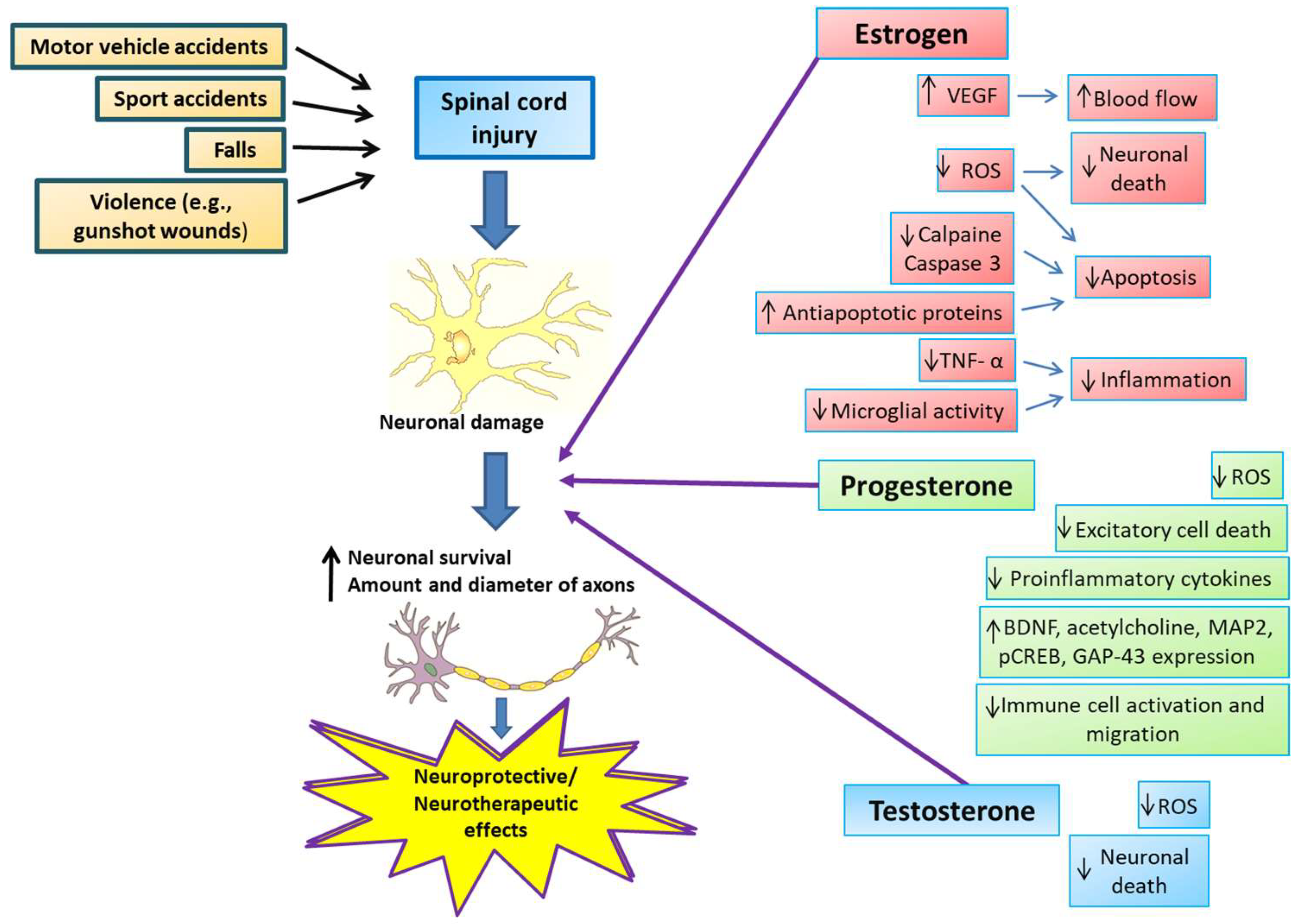
SSHs reduce excitotoxicity, free radical formation, edema, and apoptosis, inhibit inflammatory cytokines, and induce increased angiogenesis, mitochondrial recoupling, and remyelination [48,49] (Figure 2). Treatment with SSHs produces better results in SCI and has limited or more easily anticipated side effects than other treatments. In addition, SSH treatments are relatively accessible and inexpensive, which enhances their potential for widespread use [50].
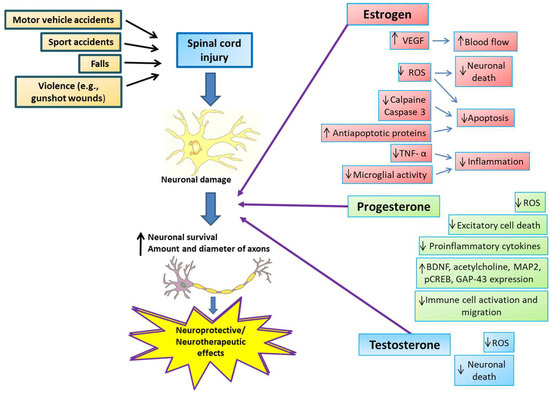
Figure 2.
Neuroprotective and neurotherapeutic effects of estrogen, progesterone, and testosterone on spinal cord injury. SCI causes include motor vehicle and sports accidents, falls, and violent encounters, producing neuronal damage directly from the injury and a subsequent secondary cascade of events in the spinal cord. After SCI, estrogen administration reduces tumor necrosis factor-α (TNF-α), microglial activity, and calpain and caspase-3 levels, increases anti-apoptotic protein levels, and increases vascular endothelial growth factor (VEGF) expression, consequently attenuating inflammation, decreasing apoptosis, and increasing blood flow to the site of injury, resulting in a more significant number of axons of larger diameter. Progesterone exerts its neuroprotective actions by increasing brain-derived neurotrophic factor (BDNF), acetylcholine, and other proteins necessary to restore motor function, such as microtubule-associated protein 2 (MAP2), cyclic-AMP response element-binding protein (CREB), and growth-associated protein 43 (GAP-43). Also, progesterone inhibits pro-inflammatory cytokine release and immune cell activation or migration. In addition, progesterone shows antioxidant properties and inhibits excitotoxic cell death. Testosterone administration reduces oxidative stress and cell death, promoting neurological recovery and neural regeneration.
Several signaling pathways that regulate SSHs are essential for functional recovery. SSH effects are carried out through at least two signaling pathways (previously classified as “genomic” and “non-genomic”). A more appropriate terminology suggested by Hammes and Levin is “nucleus-initiated signaling” for genomic signaling pathways and “membrane-initiated signaling” for fast and non-genomic pathways [51].
On the one hand, one mechanism of action of E2 and P4 involves nucleus-initiated signaling, which is conducted by interacting with specific receptors (ERs α/β and PRs A/B). On the other hand, membrane-initiated signaling is conducted through membrane receptors activating signaling pathways via second messenger cascades, such as those dependent upon nitric oxide (NO) [52,53,54,55]. The rate of protein biosynthesis limits SSH nucleus-initiated signaling and requires more time (minutes to hours) than membrane-initiated signaling modulation, which is faster (milliseconds to seconds) [56,57,58]. Thus, the mechanism of action of SSHs should be considered when timing treatment and counteracting the pathophysiological events that occur in SCI.
Different receptors for SSHs are found in the central and peripheral nervous systems. The classical estrogen receptors (ERs α and β) [59,60], progesterone receptors (PRs A and B) [61,62], and androgen receptors [63] are highly expressed in these systems.
In the spinal cord, ERα expression is higher in ependymal cells and neurons of the dorsal root ganglia and the dorsal horns [64]. Moreover, ERβ expression is lower than ERα in the outer dorsal horn neurons but higher in the deeper layers of the spinal cord [65]. PRs are expressed in motoneurons, glial cells, and ependymal cells of the central canal of the spinal cord [66]. Conversely, progesterone receptor membrane component 1 (PGRMC1) is expressed in neurons of the dorsal horn and ependymal cells lining the central canal [67]. The observation that P4 up-regulates PGRMC1 mRNA and protein levels in the dorsal horn of the injured spinal cord supports the role of PGRMC1 in mediating the protective effects of P4 in this pathology [68]. Androgen receptors are expressed in the motor neurons of the spinal cord [69].
3.1. Neuroprotective Effects of Estradiol on Spinal Cord Injury in Animal Models
Although the effect of SSHs as an alternative therapy in SCI patients has not been investigated in depth, several studies in animal models have provided information on the role of these hormones as a treatment for this injury.
Without treatment, the pathophysiological process in acute SCI progresses to chronic neurodegeneration of the spinal cord due to damage caused by the activation of cysteine proteases, such as calpain [70]. Several experiments in animal models of SCI have been conducted with E2 or 17β estradiol (17β-E2). These studies reported that E2 treatment decreases inflammation, reduces apoptosis, improves locomotor function, and decreases neuronal death after SCI in animal models [4,8,9,10,11,14,15] (Table 1).
Methylprednisolone treatment has been widely studied in SCI but has shown adverse side effects and limited efficacy [71,72]. In contrast, low doses of 17β-E2 (5–10 µg) showed no significant side effects or toxicity in rats with acute SCI treated 48 h after the injury. Moreover, E2 reduced pro-inflammatory and proteolytic activities and protected neurons from the penumbra zone in the caudal region of the spinal cord [4].
In addition to reducing some of the parameters produced after SCI, E2 showed a significant improvement in the treated group compared to the control group. Sribnick et al. (2010) administered E2 (4 mg/kg) intravenously 15 min and 24 h after the injury and continued with five daily intraperitoneal doses of E2 (2 mg/kg). These authors reported that moderately severe lesions (40 g*cm) were reduced. The Basso, Beattie, and Bresnahan (BBB) scale evaluation showed significant improvement in E2-treated animals compared to vehicle-treated animals. This difference was observed as early as 3 days after the injury. After 42 days, the mean final scores for E2-treated rats were four points higher (score 13) than vehicle-treated rats (score 9). These scores indicated that E2-treated rats, on average, supported their body weight, performed hindlimb stepping, and coordinated hindlimb/forelimb stepping more often than vehicle-treated rats, which were able, on average, to use the plantar surface of the hindlimb to support weight, but not to perform normal plantar stepping [14].
The immediacy of treatment administration after SCI is essential for the protective effect of E2. Letaif et al. administered 17β-E2 (4 mg/kg) to rats immediately after moderate SCI at T10 (10 g impact rod from a standardized height of 12.5 mm). These authors observed functional motor recovery and neuroprotection in the 17β-E2-treated group from the fourth week after the injury [11].
Other studies demonstrated that administration of E2 (4 mg/kg) in one-day (15 min and 24 h post-injury) and six-day (15 min and 24 h post-injury plus a 2 mg/kg dose for five days) schemes reduced the percentage of spinal cord damage and edema (water filtration) induced in T12 by a 40 g*cm force injury. E2 significantly reduced inflammation as assessed by infiltration of activated (ED2+ and OX-42+ cells) and non-activated (OX-42+ cells) microglia, prevented astroglia reactivity in the gray and white matter of the spinal cord, reduced cyclooxygenase 2 (COX-2) activity, and attenuated neuronal death (probably by inhibition of calpain and caspase-3 activity). In addition, E2 decreased axonal damage and myelin breakdown in the injury region compared to vehicle-treated rats [8,14]. Unfortunately, these authors did not evaluate functional recovery or how E2 regulates various pathophysiological processes in SCI. Therefore, further research is needed to determine whether the tested E2 treatment scheme positively affects functional recovery and not only pathophysiologic events.
Regarding the E2 mechanism, no significant changes were observed in the concentration and expression of the nuclear factor κB (NFκB) and its inhibitor (IκBα), neither in the cytosolic nor the pellet fraction of the lesion segment when analyzing the one-day scheme between the E2- and vehicle-treated groups. However, statistical differences were identified in NFκB and IκBα concentration and expression in the caudal penumbra region between both groups [8]. Conversely, the E2 six-day scheme decreased NF-κB translocation by diminishing its cytosolic decline. Thus, NF-κB levels were not increased in this region, as observed in vehicle-treated animals. Changes in NF-κB were correlated with the reduction of cytosolic IκB-α in SCI animals treated with E2 [14]. Therefore, the mechanism of E2 also depends on the site of the lesion (the caudal or cephalic region).
Another protective mechanism of E2 involves interacting with its receptor to prevent blood-brain barrier breakdown (BSCB). Lee et al. (2015) reported that E2 (300 g/kg) intravenous administration immediately after SCI and 6 and 24 h after the injury at the T9 level prevented BSCB through the inhibition of matrix metalloprotease-9 and reduction of sulfonylurea receptor 1/melastatin transient receptor potential 4 (SUR1/TrpM4) expression [73].
Endogenous hormones could modify the effects of hormonal treatments. The protective effects of E2 on SCI have been reported in intact and gonadectomized male rats. To eliminate endogenous testis-derived androgens, Kachadroka et al. conducted a bilateral gonadectomy in adult Sprague-Dawley rats one week before mid-thoracic crush injury (with forceps). After 30 min post-SCI, animals received different doses of 17β-E2 by implanting a subcutaneous pellet designed to release 0.05, 0.5, or 5.0 mg of 17β-E2 for 21 days. In animals treated with 0.5 or 5.0 mg/kg 17β-E2, they observed improved hindlimb locomotion associated with reduced apoptosis and increased neuronal survival produced and enhanced by the Bax/Bcl-xL protein ratio. Notably, the absence of androgens produced more severe damage than that observed in intact animals, but 17β-E2 treatment reduced SCI-induced apoptosis in gonadectomized and intact rats. These data suggest that E2 may reduce secondary damage after SCI in males [9].
The effects of E2 are concentration-dependent. The administration of high doses of E2 may have some disadvantages, such as gynecomastia, an increased risk of some types of cancer and infertility in females [74], and the development of other types of cancer in males [75]. Therefore, some authors studied the use of nanoparticles for administering low doses of E2 (2.5–25 µg/kg) focally delivered in Sprague-Dawley adult male rats with moderate to severe SCI. E2 nanoparticles diminished the levels of some inflammation factors in plasma, cerebrospinal fluid, and spinal cord tissue, such as leptin, TNF-α, macrophage inflammatory protein-1alpha (MIP-1α), IL-6, IL-4, IL-2, IL-10, interferón γ (IFNγ), and other interleukins [76].
Samantaray et al. performed two studies to evaluate the effect of low doses of acute E2 treatment in a severe injury model of SCI at T10. In the first study, animals received E2 injections (10 or 100 µg/kg) 15 min and 24 h post-injury and were sacrificed 48 h later. Both doses of E2 reduced reactive gliosis, calpain activity, caspase-3 activity, and the Bax/Bcl2 ratio, consequently reducing neuronal death produced by SCI in the penumbra zone in the caudal region and at the lesion site. In the second study, animals were treated for seven days with the same doses of E2 following injury. Protective effects were reported 42 days post-injury: tissue integrity preservation, edema decrease, and inflammation control, significantly reducing glial reactivity, axonal degeneration, and increasing angiogenic markers compared to vehicle-treated rats. Notably, both doses of E2 were equally effective in alleviating the acute damage in these studies [4,77]. As the E2 dose of 10 μg/kg is not physiological, it should be noted that it may cause significant side effects.
Another effort to achieve the beneficial effects of E2 without its side effects is treatment with estrogen receptor modulators such as tamoxifen. Tian et al. (2009) conducted a study with tamoxifen in a SCI model for the first time. They found that the administration of tamoxifen (5 mg/kg) 30 min post-injury improved locomotor activity, attenuated edema and myelin loss by decreasing the production of myelin-associated axonal growth inhibitors, and diminished the number of apoptotic neurons and IL-1β levels by microglial activation when compared with vehicle-treated rats [78].
Furthermore, Mosquera et al. observed that chronic treatment with E2 and tamoxifen improved locomotor recovery. Seven days after ovariectomy, Sprague-Dawley adult rats underwent a moderate spinal cord contusion. Pellets with E2 (3 mg) and tamoxifen (15 mg) implanted for 28 days post-injury improved locomotor recovery as assessed by the BBB test, where the locomotion score of the control group remained below that of the experimental group (10–11 vs. 14). When evaluating the antioxidant effect of E2 and tamoxifen 2 days post-injury, the ROS concentration in the lesioned epicenter of the animals treated with E2 was lower than in the control group. Similarly, tamoxifen reduced oxidative stress in the rostral and epicenter segments of the injured spinal cord evaluated 28 days post-injury. The long-term effect of tamoxifen on locomotor recovery, tissue preservation, and ROS formation suggests that tamoxifen administration is effective for the chronic stages of SCI and could be used as a long-term alternative treatment [16].
Recently, Sánchez-Torres et al. observed that tibolone, a selective tissue estrogen activity regulator (STEAR), significantly increased the amount of preserved tissue and improved the recovery of motor function [79]. Thus, estrogens and compounds with estrogenic properties could be a therapeutic alternative for recovering motor function after SCI.

Table 1.
Neuroprotective effects of estradiol on spinal cord injury in animal models.
Table 1.
Neuroprotective effects of estradiol on spinal cord injury in animal models.
| SCI Animal Model | Treatment | Evaluated Parameters | Outcome vs. Controls | Conclusions | Author (Year) [Ref] |
|---|---|---|---|---|---|
| Male rats with severe SCI at T12 | 17β-estradiol i.v. injection (4.0 mg/kg weight) 15 min and 24 h post-injury | Inflammation (tissue edema, infiltration of macrophages/microglia and NFkB levels, and myelin integrity) |
| Estrogen’s multi-active nature, acting as an anti-inflammatory, antiapoptotic, and antioxidant, suggests its potential as a therapeutic agent | Sribnick et al. (2005) [8] |
| C57/BL/6 mice (males and females) with moderate SCI at T10 | No treatment | Injury severity and locomotor function |
| Gender considerably influences the initial injury severity and the ultimate recovery of motor function after SCI. Recovery is remarkably better in females | Farooque et al. (2006) [47] |
| Male rats with severe SCI at T12 | Tamoxifen i.p. injection (5.0 mg/kg weight) 30 min post-injury | BSCB permeability, tissue edema formation, microglial activation, neuronal cell death, myelin loss, and locomotor testing |
| Tamoxifen provides neuroprotective effects for SCI-related pathology and disability, making it a potential neuroprotectant for human SCI therapy | Tian et al. (2009) [78] |
| Male rats with moderately severe SCI at T10 | Estrogen i.v. injection (4 mg/kg weight) 15 min and 24 h post-injury, followed by a daily dose (2 mg/kg weight) for 5 days | Inflammation, glial reactivity, neuron death, myelin loss, and locomotor function |
| Estrogen may help prevent damage and improve locomotor function in chronic SCI | Sribnick et al. (2010) [14] |
| Male rats with midthoracic crush SCI injury | 17β-estradiol s.c. pellet-release (0.05, 0.5, or 5.0 mg) over 21 days | Cell death, expression of Bcl-family proteins, white-matter sparing, and hindlimb locomotion |
| 17β-estradiol is an effective therapeutic intervention for reducing secondary damage after SCI in males | Kachadroka et al. (2010) [9] |
| Male rats with acute SCI at T10 | 17β-estradiol i.v. injection (1–10 μg/kg) 15 min–4 h post-SCI | Microgliosis and neuronal death |
| Low or physiologic doses of 17-β estradiol reverse secondary pathophysiology in an animal model of SCI through anti-inflammatory and anti-apoptotic actions | Samantaray et al. (2011) [10] |
| Male rats with acute SCI at T9 | 17β-estradiol i.v. injection (100 μg/kg weight) 15 min and 24 h after SCI | Neuronal death and functional recovery |
| GPER1 may mediate estrogenic neuroprotection in SCI | Hu et al. (2012) [15] |
| Female rats with moderate contusion SCI at T9–T10 | Estradiol (3 mg) or estradiol + tamoxifen (15 mg) silastic implants | Locomotor functional recovery, lesion area, estrogen receptor alpha (ER-α) expression |
| Estradiol improves functional outcomes mediated by ER-α dependent and independent mechanisms | Mosquera et al. (2014) [16] |
| Male rats with moderate SCI at T10 | 17β-estradiol i.p. injection (4 mg/kg weight) immediately after SCI | Functional recovery and motor-evoked potential |
| 17β-estradiol improved neurological and functional motor recovery in rats with SCI | Letaif et al. (2015) [11] |
| Male rats with moderate contusion SCI at T9 | Estradiol i.v. injection (300 g/kg weight) immediately after SCI | BSCB disruption, progressive bleeding, and inflammation |
| Estradiol’s neuroprotective effect after SCI is partially mediated by inhibiting BSCB disruption and hemorrhage | Lee et al. (2015) [73] |
| Male rats with moderate to severe SCI at T9 and T10 | Estradiol nanoparticles (25 µg or 2.5 µg) placed directly on the dural surface of the SCI | Inflammation |
| Nanoparticle-delivered estrogen may provide a safe and effective treatment option for patients with acute SCI | Cox et al. (2015) [76] |
| Male rats with moderately severe SCI at T10 | 17β-estradiol i.v. injection (10 μg/kg weight) 15 min and 24 h post-SCI | Inflammation, neural death, reactive gliosis |
| Acute treatment (48-h) with low doses (5–10 μg) of 17β-estradiol attenuates several destructive pathways and brings neuroprotection | Samantaray et al. (2016) [4] |
| Male rats with moderately severe SCI at T10 | 17β-estradiol i.v. injection (10 or 100 μg) 7 days post-SCI | Inflammation, cells and axons, and improved locomotor function |
| Very low doses of 17β-estradiol show significant therapeutic implications for improving locomotor function in chronic SCI | Samantaray et al. (2016) [77] |
Bax, protein X associated with Bcl-2; Bcl, B-cell CLL/lymphoma; Bcl-xL, B-cell CLL/lymphoma-extra-large; BSCB, blood-spinal cord barrier; ER, estrogen receptor; ERα, estrogen receptor alpha; GERP1, membrane-bound G-protein coupled estrogen receptor 1; i.p., intraperitoneal; i.v., intravenous; NF κB, nuclear factor κB; s.c., subcutaneous; SCI, spinal cord injury; T, thoracic vertebrae.
3.2. Neuroprotective Effects of Progesterone on Spinal Cord Injury in Animal Models
Progesterone can modify some biochemical and molecular processes triggered after SCI or TBI due to its neuroprotective effects [80]. In a SCI transection model, Sprague-Dawley males received daily subcutaneous injections of P4 (16 mg/kg/day for 3 or 21 days). P4 treatment inhibited astrocyte and microglia/macrophage proliferation and activation but stimulated oligodendrocyte precursor cell proliferation and differentiation after SCI. Under these conditions, P4 preserved motor neuron structure, reduced the proliferation and activation of astrocytes and microglia, and increased the production of oligodendrocyte progenitor cells, brain-derived neurotrophic factor (BDNF), and acetylcholine levels, among other processes necessary to restore motor function [81,82,83] (Table 2).
Remyelination is essential for functional recovery after SCI. Several studies have reported P4-promoted remyelination in rats with SCI [18,82,83]. In Schwann cells—the myelinating glia of the peripheral nervous system—P4 activates genes encoding the myelin proteins P0 and peripheral myelin protein 22 (PMP22), induces the expression of Krox 20 (a transcription factor related to myelinogenesis), and enhances the initiation and rate of myelin formation [84]. In the spinal cord, P4 differentiates oligodendrocyte precursor cells (OPC), which proliferate into mature oligodendrocytes 3 days after the lesion and produce myelin proteins such as the myelin basic protein (MBP) and proteolipid protein (PLP) [13,82]. Daily administration of P4 (16 mg/kg) also increases OPC survival, preventing apoptosis by a PR-dependent mechanism 48 h after SCI by transection at T10 [18].
Acting as a differentiation factor, P4 enhanced the expression of the oligodendrocyte lineage transcription factor 2 (Olig2), NK2 homeobox 2 (Nkx2.2), Sry-box 10 (Sox10), and achaete-scute complex homolog-1 (ASCL1, also known as Mash1), determined 3 days after the spinal cord transection at the T9 level [82,85]. Olig2 is a transcription factor involved in oligodendrocyte linage specification and differentiation, which is up-regulated during the differentiation program [86]. OPC differentiation requires up-regulation and co-expression of Olig2 and Nkx2.2 [87]. In turn, Mash 1 induces the expression of Nkx2.2 and, along with Olig2, promotes OPC differentiation in the spinal cord [88]. Simultaneously, Sox10 stimulates the transformation of premyelinating oligodendrocytes into myelinating cells by inducing the expression of MBP and the myelin regulatory factor (MRF), which participates in internodes and myelin formation [89,90].
In addition to increasing the expression of several myelination-related molecules, P4 increased the number of total mature oligodendrocytes, myelin basic protein immunoreactivity, and axonal profiles at the epicenter of the lesion. In 2014, male Wistar rats with moderate-severe contusion SCI at the T8 level received daily subcutaneous injections of P4 (16 mg/kg/day) for 60 days until sacrifice. P4 reduced the volume and rostrocaudal extent of the lesion 60 days after SCI. P4 treatment also significantly improved motor function recovery as assessed by the BBB functional scale and gait recovery as assessed by the Cat-Walk analysis [91].
In 2011, another study highlighted the effect of P4 on myelination. In a model of spinal cord demyelination by intraspinal injection of lysophosphatidylcholine, male mice received a P4 implant (100 mg), which increased circulating levels of steroids comparable to those observed during pregnancy. Seven days later, mice received a single dose of 1% lysophosphatidylcholine in the dorsal funiculus of the spinal cord. One week later, P4 treatment reduced the area of demyelination after injury and inhibited microglia/macrophage activation [92].
In addition to promoting myelination, P4 may exert other neuroprotective effects. The P4 treatment protected cultured spinal cord neurons against glutamate toxicity. In rats with moderately severe SCI, intraperitoneal treatment with P4 (4 mg/kg) 30 min after injury showed a better clinical and histological outcome than controls [93,94]. Furthermore, in rats with complete spinal cord transection at T10, treatment with P4 (4 mg/kg) at one hour (intraperitoneal) and 24, 48, and 72 h (subcutaneous) post-injury showed a significant neuroprotective effect [95].
P4 may exert its neuroprotective effects through several mechanisms. For example, P4 administration also restored the reduced levels of the sodium pump mRNA and choline acetyltransferase (ChAT), whereas the growth-associated protein (GAP-43) mRNA levels were further enhanced [95]. These results are significant because ChAT catalyzes acetylcholine synthesis—essential in muscle contraction, Na+,K+-ATPase maintains the membrane potential, neuronal excitability, and entry of metabolites and ions into the soma, whereas GAP-43 is involved in axonal regeneration [12,17,81]. The expression of BDNF mRNA and protein levels increased in ventral horn motoneurons, where P4 prevented the lesion-induced chromatolysis degeneration of spinal cord motoneurons as determined by Nissl staining [17]. P4 also enhanced the tropomyosin receptor kinase B (TrkB) neurotrophin receptor and phosphorylated cAMP-responsive element-binding protein (pCREB) immunoreactivity in motoneurons [12,96], and partly normalized the ultrastructural abnormalities, and preserved the microtubule-associated protein 2 (MAP2) immunostaining of deafferented motoneurons. As P4 treatment up-regulates MAP2 staining in dendrites and perikaryon, it is suggested to act on the cytoskeleton [97].
The neuroprotective effect of P4 has also been demonstrated with organotypic cultures of spinal cord slices from 3-week-old mice. Using a weight drop model, a decrease in the number of motoneurons was induced by in vitro SCI, which correlated with an increase in the number of dying cells and lactate dehydrogenase (LDH) release. When 10 µM of P4, 5α- dihydro-progesterone (5α-DHP), or allopregnanolone (3α, 5α-tetrahydro-progesterone) were added at the time of injury, the spinal cord slices were rescued from the effects of damage. These authors demonstrated that the neuroprotective effects of P4 are PR-dependent, as these neuroprotective effects were not observed in slices of homozygous knockout PR−/−mice [98].
Interestingly, Yang et al. (2017) conducted a study evaluating the mechanism by which P4 significantly reduced axonal dieback and neuronal death in mice following SCI. These authors demonstrated that this effect of P4 was mediated through the down-regulation of pro-inflammatory cytokines, including inducible nitric oxide synthase (iNOS), monocyte chemoattractant protein-1 (MCP-1), and IL-1β, and activation of caspase-3 and glial fibrillary acidic protein (GFAP). Repeated imaging showed that axonal dieback distance was significantly reduced in mice treated with P4 (16 mg/kg) after SCI by hemisection at the T11 level. The densities of astrocytes and microglia were significantly higher in the vehicle-treated group than in the P4-treated group. Moreover, P4 also improved behavioral performance post-injury. These findings suggest that P4 exerts a neuroprotective effect by attenuating axonal dieback, reducing the activation and proliferation of astrocytes and microglia, and inhibiting the release of pro-inflammatory cytokines [99].
Regarding neuroinflammation, P4 decreases the activation and proliferation of astrocytes and microglial cells in the acute and chronic phases of SCI. Moreover, P4 therapy regulates astrocytes and microglial cells by suppressing neuroinflammation and creating a pro-differentiating environment, which results in neurological recovery after SCI. Thus, P4 is a glioactive factor that favors remyelination and inhibits reactive gliosis [18,83].
In 2015, Labombarda et al. showed that P4 down-regulated pro-inflammatory cytokines, such as IL1β1, IL6, and TNFα, and enzymes involved in ROS production, such as COX-2 and iNOS, through a PR-dependent mechanism via NFkB inhibition [18]. Sprague-Dawley male rats received daily subcutaneous injections of P4 (16 mg/kg) after SCI by transection at the T10 level and were sacrificed 6 h, 24 h, or 48 h, and 3 or 21 days after surgery. P4 significantly attenuated the injury-induced hyperexpression of IL1β1, IL6, TNFα, iNOS, and COX-2 mRNAs, all involved in oligodendrocyte damage. Consequently, P4 down-regulated reactive gliosis and exerted potent anti-inflammatory effects in rats with SCI [18].
Furthermore, SCI is frequently associated with the development of chronic pain. Therefore, different studies have investigated the effects of P4 on the development of allodynia after SCI. In Sprague-Dawley male rats (200–220 g), the spinal cord was unilaterally hemisected at the T13 level, and natural P4 was administered (16 mg/kg/day) immediately after the lesion and once a day until the animals were euthanized (either at 1, 14, or 28 days post-injury). P4 administration prevents neuropathic pain-associated behaviors [100,101,102], modulates the expression of NMDAR subunits and the upregulation of protein kinase C γ (PKC γ) [100], and attenuates the proinflammatory cascade induced by SCI, which involves pro-inflammatory enzymes such as COX-2 and iNOS [101], and different cytokines [102], all key players in neuropathic pain generation, probably by reducing NFĸB transactivation potential [101]. In addition, early and sustained P4 treatment prevents mechanical and thermal allodynia in animals subjected to SCI [103].
Tissue preservation in the spinal cord may be related to functional recovery. After a moderate-intensity SCI in mature Sprague-Dawley male rats, the intraperitoneal administration of P4 (4 mg/kg) demonstrated neuroprotective effects over 5 days post-injury. The treatment was initiated 30 min after injury and repeated at 6, 24, 48, 72, 96, and 120 h intervals. Thomas et al. observed a significant reduction in the central cavitary process that follows SCI, accompanied by locomotor score improvement [94]. Therefore, P4 administered a few minutes after SCI promotes spinal cord tissue preservation and motor function recovery.
Interestingly, some studies have reported that the beneficial effects of P4 on SCI are inconclusive. For example, based on functional and histological analyses, Calvacante et al. observed that P4 acute administration (4 mg/kg) 30 min before transient occlusion of the proximal descending thoracic aorta of male rats failed to prevent or attenuate ischemia SCI, as no significant differences were found among the studied groups (17β-E2, P4, or vehicle). An initial significant impairment of hind limb motor function was observed in all the groups, with partial improvement over time but no significant differences among groups during the observation period. Similarly, gray matter analysis showed a lack of viable neurons, and the number of viable neurons per section showed no differences among these groups [104].
Furthermore, Fee et al. found no improvement with P4 injection following an SCI by contusion. Both the short-term (5 days of either 4 or 8 mg/kg) and long-term (14 days of either 8 or 16 mg/kg) therapies failed to show any significant alteration in locomotor functioning and injury morphometrics after 21 days [105]. Part of this discrepancy could be the duration of the treatment; i.e., treatments in the study of Fee et al. [105] were limited to 5 and 14 days, while treatments in studies with positive results lasted for up to 60 days [91,102,103].

Table 2.
Neuroprotective effects of progesterone on spinal cord injury in animal models.
Table 2.
Neuroprotective effects of progesterone on spinal cord injury in animal models.
| SCI Animal Model | Treatment | Evaluated Parameters | Outcome vs. Controls | Conclusions | Author (Year) [Ref] |
|---|---|---|---|---|---|
| Male rats with moderate SCI | P4 i.p. injection (4 mg/kg weight) 30 min after SCI and repeated at 6 h, 24 h, 48 h, 72 h, 96 h, and 120 h intervals | Injury severity and locomotor function |
| P4 showed potential therapeutic properties in managing acute SCI | Thomas et al. (1999) [94] |
| Male rats with complete spinal cord transection at T10 | P4 i.p. injection (4 mg/kg) 1 h after SCI, and s.c. administration 24, 48, and 72 h post-injury | Neuronal function under negative regulation (ChAT and Na,K-ATPase) and stimulated neuronal function (GAP-43) |
| P4 appears to replenish acetylcholine, restore membrane potential, ion transport, and nutrient uptake, and accelerate reparative responses to injury | Labombarda et al. (2002) [95] |
| Male rats with complete spinal cord transection at T10 | P4 i.p. injection (4 mg/kg) 1 h after SCI, and s.c. administration 24, 48, and 72 h post-injury | Expression of PR and 25-Dx binding proteins for P4 |
| Distinct membrane-binding sites may mediate P4 neuroprotective effects | Labombarda et al. (2003) [68] |
| Male rats with complete spinal cord transection at T10 | P4 s.c. administration (4 mg/kg) 1 h and again at 24, 48, and 72 h post-injury | Expression of BDNF at both the mRNA and protein levels Chromatolysis analysis |
| P4 increased neuronal BDNF, which could provide a trophic environment and might be part of the P4-activated pathways to provide neuroprotection | González et al. (2004) [17] |
| Male rats with SCI at T10 | P4 s.c. administration (4 mg/kg) 1 h and again at 24, 48, and 72 h post-injury | Expression of the BDNF mRNA and BDNF immunoreactivity receptor TrkB Chromatolysis analysis MBP expression at the mRNA and protein levels PR expression |
| P4-induced BDNF expression might regulate the function of neurons and glial cells in a paracrine or autocrine fashion and prevent the generation of SCI damage | De Nicola et al. (2006) [12] |
| Male rats with SCI at T10 | P4 s.c. administration (4 mg/kg) 1 h and again at 24, 48 and 72 h post-injury | Expression of MBP at the mRNA and protein levels NG2-immunopositivity as markers for OPCs RIP-immunopositivity as mature oligodendrocytes identifier |
| P4 effects on MBP expression and NG2 immunopositivity may contribute to neuroprotection | Labombarda et al. (2006) [13] |
| Male and female rats with moderate spinal cord contusion at T10 | Short-term (5 days) P4 (4 or 8 mg/kg) and long-term (14 days) P4 (8 or 16 mg/kg) | Locomotor recovery Morphologic assessment of white and grey matter |
| This study does not support P4 therapy as a potential therapeutic agent in SCI | Fee et al. (2007) [105] |
| Male rats with complete spinal cord transection at T10 | P4 s.c. administration (16 mg/kg/day) for 3 or 21 days after injury | OPC parameters (NG2 immunostaining), mature oligodendrocytes, and central myelin proteins (MBP, PLP) Oligodendrocyte transcription factors (Olig1, Olig2, and Nkx2.2) Myelin proteins (MBP and PLP-immunoreactivity) | Short treatment (3 days)
| Short P4 treatment influenced the proliferation and differentiation of OPC into mature oligodendrocytes. Prolonged P4 treatment favored remyelination and oligodendrocyte maturation. Thus, P4 effects on oligodendrogenesis and myelin proteins may constitute fundamental steps for repairing traumatic injuries to the spinal cord | Labombarda et al. (2009) [82] |
| Male rats unilaterally hemisected at T13 | P4 s.c. administration (16 mg/kg/day) | Behavioral evaluation of mechanical and cold allodynia Expression of NMDAR subunits (NR1, NR2A, NR2B), PKCγ, ppD, and KOR |
| P4 modulates neuropathic pain after SCI, creating a favorable molecular environment that may decrease spinal nociceptive signaling | Coronel et al. (2011) [100] |
| Primary demyelination model in male mice | Single implant of P4 (100 mg). | Determination of total myelin and MBP Determination of OX-42+ microglia/macrophages Staining of oligodendrocyte precursors (NG2+ cells) and mature oligodendrocytes (CC1+ cells) Analysis of the microglial marker CD11b mRNA |
| P4 exerts promyelinating and anti-inflammatory effects at the spinal cord level | Garay et al. (2011) [92] |
| Male rats with complete spinal cord transection at T10 | P4 s.c. administration (16 mg/kg/day) for 3 or 21 days after injury | Immunodetection of S100β, GFAP, NG2+ oligodendrocyte precursors, CC1+ oligodendrocytes, and OX-42+ microglia/macrophages | Acute treatment (3 days)
| P4 emerges as a glioactive factor, favoring remyelination and inhibiting reactive astro- and microgliosis | Labombarda et al. (2011) [83] |
| Male rats unilaterally hemisected at T13 | P4 s.c. administration (16 mg/kg/day) | Expression and activity of spinal COX-2 and iNOS IκB-α mRNA levels Profile of glial cell activation Pain-associated behaviors |
| P4 may represent a valuable strategy to prevent the development of central chronic pain by modulating early neuroinflammatory events after SCI | Coronel et al. (2014) [101] |
| Male rats with moderately severe SCI at T8 | P4 s.c. administration (16 mg/kg/day) for 60 days | Tissue preservation using magnetic resonance imaging and quantification of tissue-sparing Optical density of MBP staining Total number of APC+ cells Quantification of axonal profiles Functional outcome evaluated with the BBB scale Sensory function (mechanical and thermal sensitivity) |
| P4 beneficial actions on locomotor outcome could be related to the reduction of secondary damage and the preservation or regeneration of axons and myelin of the descending pathways | García-Ovejero et al. (2014) [91] |
| Male rats with complete spinal cord transection at T10 | P4 s.c. administration (16 mg/kg/day). Animals were euthanized 6 h, 24 h, 48 h, 3 days, or 21 days following surgery | Expression of proinflammatory factors and enzymes |
| PR participates in the anti-inflammatory effects of P4, the modulation of astrocyte and microglial responses, and the prevention of OPC apoptosis | Labombarda et al. (2015) [18] |
| Male PRKO mice (inactivated PRA and PRB isoforms) with complete spinal cord transection at T10 | Immunohistochemistry to assess astrocytes, microglia, and OPC Detection of OPC apoptosis |
| |||
| Male rats unilaterally hemisected at T13 | P4 s.c. administration (16 mg/kg/day) immediately after SCI and during 1 or 28 days after injury | Behavioral evaluation of mechanical and cold allodynia Expression of IL-1β, IL-1RI and IL-1RII, IL-1ra, IL-6, and TNFα IL-1β protein levels Immunofluorescence to detect NR1 subunit of NMDAR, IL-1β, NeuN |
| By modulating the expression of pro-inflammatory cytokines and neuronal IL-1RI/NR1 colocalization, P4 emerges as a promising agent for preventing chronic pain after SCI | Coronel et al. (2016) [102] |
| Male rats unilaterally hemisected at T13 | P4 s.c. administration (16 mg/kg/day) immediately after SCI and during 1 or 28 days after injury | Behavioral evaluation of mechanical and cold allodynia Expression of galanin, GalR1, GalR2, NPY, Y1R, Y2R CyCB | Early phase (1 day)
| Early and sustained P4 administration prevents temporal changes in the spinal expression of galanin and NPY and their associated receptors, which could potentially prevent and treat chronic pain after central injuries | Coronel et al. (2017) [103] |
| Male YFP-H and male CX3CR1GFP/+ transgenic mice with hemisected spinal cords at T11 | P4 i.p. injection (16 mg/kg) one-hour post-injury and s.c. injection at 3 h, 24 h, and 48 h after SCI | Axonal dynamics and survival neurons Identification of neurons, microglia, and astrocytes Protein levels of caspase-3, GFAP, and MBP mRNA expression of IL-1β, iNOS, and MCP-1 Behavioral function |
| P4 exerted a neuroprotective effect by attenuating axonal dieback, reducing the accumulation of astrocytes and microglia, and inhibiting the release of pro-inflammatory cytokines | Yang et al. (2017) [99] |
| Male rats with transitory occlusion of the proximal descending thoracic aorta | P4 (4 mg/kg) intra-arterial administration | Motor function Neuronal cell death in grey matter Apoptosis (Bcl-2 and annexin V) Necrosis (propidium iodide) |
| Acute P4 administration could not prevent or attenuate spinal cord ischemic injury based on functional and histological outcomes | Cavalcante et al. (2018) [104] |
| Male rats with complete spinal cord transection at T9 | P4 s.c. administration (16 mg/kg/day) for 3 days. The first injection was given immediately after SCI | Expression of transcriptional inhibitors (Id2, Id4, hes5) and activators (Olig2, Nkx2.2, Sox10, and Mash1) Immunostaining of OPC, astrocytes, and microglial cells, and double labeling of TGFβ1 and Olig2 |
| P4 differentiating effects might involve TGFβ1, indirectly mediating these actions by releasing microglial and astrocytic TGFβ1 | Jure et al. (2019) [85] |
APC, adenomatus polyposis coli; BBB, Basso, Beattie, and Bresnahan scale; BDNF, brain-derived neurotrophic factor; BMS, Basso Mouse scale; ChAT, choline acetyltransferase; COX-2, cyclooxygenase 2; CST, corticospinal tract; DAT, dorsal ascending tract; 25-Dx, progesterone binding protein 25-Dx; EC, eriochrome cyanine; GAP-43, growth-associated protein 43; GFAP, glial fibrillary acidic protein; GFP, green fluorescent protein; iNOS, inducible nitric oxide synthase; i.p., intraperitoneal; KOR, kappa opioid receptor; LFB, Luxol Fast Blue; LPC, lysophospatidylcholine; MBP, myelin basic protein; MCP-1, monocyte chemoattractant protein-1; MOG, myelin oligodendrocyte glycoprotein; NMDAR, N-methyl-D-aspartate receptor; OPC, oligodendrocyte-precursor cells; P4, progesterone; pCREB, phosphorylated cAMP-responsive element binding protein; PKCγ, protein kinase C gamma; PLP, proteolipid protein; ppD, preprodynorphin; PR, progesterone receptor; PRKO, PR knockout; RIP, receptor interacting protein; s.c., subcutaneous; SCI, spinal cord injury; TGF-β1, transforming growth factor beta 1; TNFα, tumor necrosis factor alpha; T, thoracic vertebrae; TrkB, tropomyosin receptor kinase B; VF, ventral funiculus; YFP, yellow fluorescent protein.
3.3. Neuroprotective Effects of Androgens on Spinal Cord Injury in Animal Models
Inflammation promotes the exacerbation of damage in the pathophysiology of SCI. Therefore, the regulation of the immune system could control inflammation in SCI. Rouleau et al. provided evidence of the correlation between testosterone levels and immune deficiencies in acute SCI. In CD1 mice studied for 4 weeks after an SCI by transection between the 9th and 10th thoracic vertebrae, a decrease in serum testosterone levels was identified during the first 2 weeks, and an increase in growth hormone (GH) levels occurred one week after the injury. These changes were correlated to a decrease in total leukocyte, lymphocyte, and eosinophil counts in the blood and lymphocytes in the bone marrow. Significant differences were also observed in the hormonal and immune systems, providing evidence for the role of hormones (i.e., GH, insulin, parathyroid hormone, and dehydroepiandrosterone) in immune dysfunction following SCI. Thus, this study highlights a direct correlation between reduced serum testosterone levels and immune dysfunction after SCI [106]. Moreover, inflammation is an essential host defense mechanism. However, although its effects are contradictory—inflammation is central in modulating the pathological progression of acute and chronic SCI but also regulates neuronal damage and regeneration—testosterone could influence both processes [107].
Furthermore, the immune response to trauma also shows sexual dimorphism [108]. In this regard, Hauben et al. demonstrated that male mice and rats show worse locomotor recovery after incomplete SCI than their female littermates. Evaluation with the BBB locomotion scale after a severe spinal cord contusion showed that hindlimb motor performance was significantly better in females [46]. Additionally, these authors reported that castrated males recover better than intact males and females treated post-injury with dihydrotestosterone. Notably, post-traumatic administration of flutamide (a testosterone antagonist) produced a better recovery in male rats [46]. These results could be explained by the inhibitory effect of testosterone on T cell-mediated immunity, suggesting that testosterone may contribute to poor recovery in males with SCI.
To learn whether testosterone could regulate any of the pathophysiological events in SCI, Gürer et al. (2015) showed that a single intraperitoneal dose of testosterone (15 mg/kg) decreased the activity of caspase-3, myeloperoxidase, and xanthine oxidase enzymes. Testosterone also decreased malondialdehyde levels, whereas catalase levels increased following transient global SCI by ischemia in rabbits. Testosterone was adminsistered immediately after the occlusion clamp of the aorta. A mixture of four testosterone esters (testosterone propionate, testosterone phenylpropionate, testosterone isocaproate, and testosterone decanoate) with different half-lives was preferred to provide more stable serum testosterone levels [109]. After testosterone administration, caspase-3, myeloperoxidase, and xanthine oxidase enzyme activity decreased, as did malondialdehyde levels, while catalase levels increased. The authors concluded that testosterone exhibits significant neuroprotective activity after a spinal cord ischemia-reperfusion injury.
In addition to its effects on the spinal cord, androgen treatment attenuates sublesional muscle loss and other phenotypic changes associated with impaired muscle function after SCI [7,110,111,112,113,114] and may promote a slight improvement in locomotor recovery in rodent models [115]. Byers et al. (2012) implanted testosterone-filled Silastic capsules in female rats after SCI at the T9 level. Four weeks later, testosterone treatment prevented the decrease in the dendritic length of quadriceps motoneurons observed in untreated SCI animals. Similarly, the vastus lateralis muscle weights and fiber cross-sectional areas of untreated SCI animals were smaller, and testosterone treatment also prevented these reductions. However, testosterone showed no effect on reducing lesion volume or increasing tissue sparing [113]. These findings support the role of testosterone as a neurotherapeutic agent (Table 3).

Table 3.
Neuroprotective effects of androgens (testosterone) on spinal cord injury.
Table 3.
Neuroprotective effects of androgens (testosterone) on spinal cord injury.
| SCI Animal Model | Treatment | Evaluated Parameters | Outcome vs. Controls | Conclusions | Authors (Year) [Ref] |
|---|---|---|---|---|---|
| Male and female rats with severe spinal cord contusions at T8 | No treatment | Functional recovery (locomotor hindlimb performance) |
| The better spontaneous recovery of female rats and mice after SCI than that of males is related to the suppressive effect of androgens on the ability to sustain a T-cell-mediated protective response to a CNS insult | Hauben et al. (2002) [46] |
| Male and female rats with severe or mild spinal cord contusions at T8 | No treatment | Functional recovery (locomotor hindlimb performance) Neurological function Morphological analysis of the lesion site |
| The better functional recovery observed in females may be attributable to improved tissue preservation, possibly due to endogenous neuroprotective processes that do not occur in males | |
| Male and female WT and nude Balb/c mice with SCI at T12 | No treatment | Functional recovery |
| T-cell immune response helps the body overcome the effects of destructive self-compounds that emerge from injured tissues | |
| Male nude Balb/c mice with SCI at T12 | Castrated | Functional recovery |
| Sexual dimorphism observed in functional recovery from ISCI may, at least partially, be androgen-dependent | |
| Male rats with mild SCI at T8 | Castrated | Functional recovery |
| ||
| Female rats with severe SCI at T8 | DHT (100 mg) s.c pellet (21-day-release) 10 days post-injury | Functional recovery |
| DHT has an adverse impact on SCI recovery | |
| Male rats with severe SCI at T8 | Flutamide (testosterone-antagonist) i.p. injection (25 mg/kg weight) immediately after SCI and 5 mg/kg every other day for ten days | Functional recovery |
| Testosterone has an adverse effect on SCI recovery | |
| Male mice with spinal cord transection at T9/10 | No treatment | Serum levels of testosterone, GH, PTH, DHEA, insulin, and a complete immune cell count from blood and bone marrow samples | Two weeks after SCI:
| Significant changes occur rapidly (<1–2 weeks) in both the hormonal and immune systems after SCI | Rouleau et al. (2007) [106] |
| Young adult female rats with SCI at T9 | Testosterone-filled Silastic capsules | Soma volume, motoneuron number, lesion volume, and tissue-sparing |
| Regressive changes in motoneuron and muscle morphology were observed with testosterone treatment | Byers et al. (2012) [113] |
| Male rats with complete spinal cord transection | Low (2.8 mg/kg) or high (7.5 mg/kg) 24 h doses of testosterone provided with an Alzet pump | Expression of MAFbx, MuRF1, REDD1, and FOXO1 Weight of muscles with different fiber-type compositions | High-dose
| High-dose of testosterone partially protected against muscle atrophy and gene expression changes caused by MP | Wu et al. (2012) [112] |
| Male rabbits with ischemia/reperfusion SCI | Testosterone i.p. injection (15 mg/kg) | Malondialdehyde and catalase levels Activities of caspase-3, myeloperoxidase, and xanthine oxidase Histopathological, ultrastructural, and neurological studies |
| Biochemical, histopathological, ultrastructural, and neurological examination findings showed that testosterone has neuroprotective effects on ischemia/reperfusion SCI | Gürer et al. (2015) [109] |
| Young adult female rats with SCI at T9 | DHT-filled Silastic capsules | Functional recovery Lesion volume and tissue sparing, quadriceps muscle fiber cross-sectional area, and motoneuron dendritic morphology |
| DHT treatment ameliorated deficits in micturition and regressive changes in motoneuron and muscle morphology seen after SCI | Sengelaub et al. (2018) [49] |
| Clinical studies | |||||
| Men with SCI | No treatment | Serum levels of FSH, LH, testosterone, estradiol, and PRL The LHRH stimulation test Semen analysis and testicular volumes |
| SCI patients showed hypogonadotropism due to secondary neural or hormonal pathway alteration, leading to semen quality impairment | Naderi and Safarinejad (2003) [116] |
| Men with SCI | No treatment | Testosterone and LH serum levels Free testosterone levels Level of disability (FIM instrument and ASIA exams) |
| A negative androgen status is notable, especially in the first year after a spinal cord injury Testosterone substitution therapy should be considered during the first year after injury to induce neural regeneration and preserve muscle strength | Celik et al. (2007) [117] |
| Men with SCI | No treatment | Serum testosterone levels Time since SCI Selected laboratory values |
| Men with SCI are at risk of low serum testosterone | Clark et al. (2008) [118] |
| Men with SCI | Testosterone-cypionate (200 mg) i.m. injection, monthly | Motor function (ASIA motor index discharge and FIM total discharge scores) |
| Muscle size and strength increased with testosterone | Clark et al. (2008) [119] |
| Men with chronic SCI | No treatment | Serum total testosterone, albumin, LH, FSH, and prolactin levels |
| Testosterone levels were significantly associated with the severity of SCI | Dunga et al. (2011) [120] |
| Men with SCI | No treatment | Serum testosterone, insulin, triglyceride levels HOMA-IR, BMI Hypogonadism-related symptoms (AMS questionnaire) LTPA |
| Poor LTPA, high BMI, and low sexual desire are independent predictors of low testosterone levels in men with chronic SCI | Barbonetti et al. (2014) [121] |
| Men with SCI | No treatment | Testosterone levels by decade of life |
| Low serum total testosterone concentration occurs earlier in life in men with SCI, with a higher prevalence by a decade of life | Bauman et al. (2014) [122] |
ASIA, American Spinal Injury Association; BBB, Basso, Beattie, and Bresnahan scale; BMI, body mass index; DHEA, dehydroepiandrosterone; DHT, dihydrotestosterone; F, female; FIM, Functional Independence Measure; FSH: follicle-stimulant hormone; GH, growth hormone; HOMA-IR, homeostatic model assessment of insulin resistance; IM, intramuscular; i.p., intraperitoneal; LH, luteinizing hormone; LHRH, LH-releasing hormone; LTPA, leisure time physical activity; M, male; MP, methylprednisolone; PRL, prolactin; PTH, parathyroid hormone; SCI, spinal cord injury; T, thoracic vertebrae; WT, wild type.
4. Neuroprotective Effects of Sexual Hormones on Spinal Cord Injury in Humans
Although research on hormone replacement as a treatment for SCI has been developed, this type of therapy has not been performed in the clinical phase. Only a few studies on the effects of sexual hormones have been conducted in humans with any SCI condition. Among these studies are those using androgen treatment to correlate testosterone levels with SCI.
Some reports indicate an association between SCI clinical characteristics and low testosterone levels. In two clinical studies, a considerable percentage of men with SCI (60% and 43.3%, respectively) showed low serum testosterone levels [118,120]. Durga et al. (2011) reported an inverse association between low serum testosterone and the severity of SCI. In this study, the participants with complete motor injuries presented a more significant testosterone deficiency than those with incomplete motor injuries [120].
Low testosterone levels in men with SCI have been associated with lower hemoglobin and higher prolactin levels in healthy subjects. From a sample of 102 men with SCI, 60% had low testosterone levels. The mean testosterone concentration was 220 ng/dL (normal reference range: 241 to 827 ng/dL). Testosterone levels < 241 ng/dL were considered abnormally low. Less time since injury was also correlated with low testosterone levels; those men with low testosterone levels were more likely to be acutely injured than men with higher testosterone levels [118].
Androgen levels decrease more notably during the first year after SCI. Celik et al. (2007) conducted a prospective case study that included 44 male patients with SCI. Values from the control group of healthy individuals were considered as references. Testosterone and free testosterone concentrations were evaluated in both groups. Total testosterone levels were significantly higher in SCI patients with a time since injury > 12 months than in the control group. Free testosterone levels were lower in SCI patients with time since injury < 12 months than in patients with time since injury >12 months. Consequently, these authors concluded that during the first year after SCI, patients could show a negative androgenic status [117].
Another study found a similar correlation between low testosterone levels and advancing age in men with SCI. Bauman et al. (2014) found lower serum testosterone levels earlier in SCI patients and a higher prevalence per decade compared to the general population [122]. In the same year (2014), Barbonetti et al. found that one-third of patients showed biochemical androgen deficiency defined by a total testosterone concentration < 300 ng/dL. In the search for the determinants of androgen deficiency, these authors found that low leisure-time physical activity, high body mass index (BMI), and low sexual desire are independent predictors of low testosterone in men with chronic SCI [121]. Overall, these results indicate that the severity of injury, time since injury, and leisure-time physical activity are correlated with androgen deficiency.
Some studies associate the increase of systemic and metabolic complications, such as obesity, cardiovascular diseases, diabetes, osteoporosis, and severe infections, with chronic immobilization due to SCI and changes in hormone levels that can regulate metabolic functions, such as testosterone [118,120,123].
In humans, testosterone increases energy, improves muscle mass and strength, enhances cognition, modifies mood/libido, and sexual function, increases bone mineral density, and has anti-inflammatory effects [123,124]. Consequently, low testosterone levels in patients with SCI lead to other complications, such as alterations in mood/libido, sexual function, and fertility problems. Naderi and Safarinejad (2003) reported low serum testosterone in 16% of men with SCI. In addition, they found hypogonadotropism secondary to the alteration in the hypothalamus/pituitary neural/hormonal pathway in these patients. This mechanism could contribute to the altered semen quality reported in men with SCI [116].
Decreased musculoskeletal integrity and neuromuscular impairment are also significant complications of SCI. Men with low testosterone exhibit low muscle mass, impaired muscle function [125], and worsened walking biomechanics [126]. In contrast, testosterone replacement therapy improves muscle mass, neuromuscular function [127], and walking speed in older ambulatory hypogonadal men [128].
During the first year after injury, when the most significant neurological recovery occurs, testosterone replacement therapy might be considered an option to induce neural regeneration and preserve muscle strength in patients with SCI [117]. Notably, men with incomplete SCI treated monthly with testosterone cypionate (200 mg) showed higher American Spinal Injury Association (ASIA) discharge motor scores. However, these results should be interpreted cautiously, as the groups were not randomized and differed by ethnicity and length of stay [119]. Prospective studies are needed to validate these findings.
5. Conclusions
Although the pathophysiology of SCI is highly complex, evidence supports the neuroprotective role of sex steroid hormones. Several mechanisms have been proposed by which particular pathways related to neuroprotection may lead to therapeutic targets. E2- and P4-mediated neuroprotection is related to the interactions with their receptors and signaling systems, effects on astrocytes and microglia, modulation of the inflammatory response, and their antioxidant effects. Conversely, testosterone-mediated neuroprotection is more controversial since contrasting results can be found depending on the animal model or patients analyzed. Therefore, it is imperative to develop further studies in experimental models to elucidate the neuroprotective effect of testosterone on SCI.
The optimal doses and duration of hormone therapy in humans are currently unknown due to limited clinical studies. Although E2, P4, and testosterone treatments are generally considered safe, multicenter, randomized, prospective, and larger human clinical studies s are needed to further evaluate their clinical role as neuroprotective agents after acute traumatic spinal cord injury.
Author Contributions
Conceptualization, A.C.-S. and C.G.-A.; methodology, C.O.-B. and G.M.d.l.B.; investigation, A.C.-S., C.G.-A., J.S.-U., C.O.-B., R.P.-A., T.C.-M., H.S.-C., S.S.-T., X.F.-T., S.O.-S., I.F.-R. and G.M.d.l.B.; data curation, A.C.-S., C.G.-A., G.M.d.l.B., H.S.-C. and J.S.-U.; writing—original draft preparation, A.C.-S., C.G.-A., J.S.-U., C.O.-B., R.P.-A., T.C.-M., H.S.-C., S.S.-T., X.F.-T., S.O.-S., I.F.-R. and G.M.d.l.B.; writing—review and editing, A.C.-S., C.G.-A., G.M.d.l.B., H.S.-C., J.S.-U., A.C.-S. and C.G.-A.; supervision, A.C.-S. and C.G.-A. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by FORDECYT-PRONACES (grant number 845110).
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors are grateful for the founding administration of Fundación IMSS. Stephanie Sánchez-Torres and Tzayaka Castillo-Mendieta thank the Post-Doctoral Fellowship Program of CONAHCYT.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Donovan, W.H. Spinal cord injury—Past, present, and future. J. Spinal Cord Med. 2007, 30, 85–100. [Google Scholar] [CrossRef] [PubMed]
- Pickelsimer, E.; Shiroma, E.J.; Wilson, D.A. Statewide investigation of medically attended adverse health conditions of persons with spinal cord injury. J. Spinal Cord. Med. 2010, 33, 221–231. [Google Scholar] [CrossRef]
- Cao, H.Q.; Dong, E.D. An update on spinal cord injury research. Neurosci. Bull. 2013, 29, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Samantaray, S.; Das, A.; Matzelle, D.C.; Yu, S.P.; Wei, L.; Varma, A.; Ray, S.K.; Banik, N.L. Administration of low dose estrogen attenuates gliosis and protects neurons in acute spinal cord injury in rats. J. Neurochem. 2016, 136, 1064–1073. [Google Scholar] [CrossRef]
- Bracken, M.B.; Holford, T.R. Neurological and functional status 1 year after acute spinal cord injury: Estimates of functional recovery in National Acute Spinal Cord Injury Study II from results modeled in National Acute Spinal Cord Injury Study III. J. Neurosurg. 2002, 96, 259–266. [Google Scholar] [CrossRef]
- Silva, N.A.; Sousa, N.; Reis, R.L.; Salgado, A.J. From basics to clinical: A comprehensive review on spinal cord injury. Prog. Neurobiol. 2014, 114, 25–57. [Google Scholar] [CrossRef] [PubMed]
- Sengelaub, D.R.; Xu, X.M. Protective effects of gonadal hormones on spinal motoneurons following spinal cord injury. Neural Regen. Res. 2018, 13, 971–976. [Google Scholar] [CrossRef]
- Sribnick, E.A.; Wingrave, J.M.; Matzelle, D.D.; Wilford, G.G.; Ray, S.K.; Banik, N.L. Estrogen attenuated markers of inflammation and decreased lesion volume in acute spinal cord injury in rats. J. Neurosci. Res. 2005, 82, 283–293. [Google Scholar] [CrossRef]
- Kachadroka, S.; Hall, A.M.; Niedzielko, T.L.; Chongthammakun, S.; Floyd, C.L. Effect of endogenous androgens on 17β-estradiol-mediated protection after spinal cord injury in male rats. J. Neurotrauma 2010, 27, 611–626. [Google Scholar] [CrossRef]
- Samantaray, S.; Smith, J.A.; Das, A.; Matzelle, D.D.; Varma, A.K.; Ray, S.K.; Banik, N.L. Low-dose estrogen prevents neuronal degeneration and microglial reactivity in an acute model of spinal cord injury: Effect of dosing, route of administration, and therapy delay. Neurochem. Res. 2011, 36, 1809–1816. [Google Scholar] [CrossRef]
- Letaif, O.; Cristante, A.; Barros Filho, T.; Ferreira, R.; Santos, G.; Rocha, I.; Marcon, R.M. Effects of estrogen on functional and neurological recovery after spinal cord injury: An experimental study with rats. Clinics 2015, 70, 700–705. [Google Scholar] [CrossRef] [PubMed]
- De Nicola, A.F.; Gonzalez, S.L.; Labombarda, F.; González Deniselle, M.C.; Garay, L.; Guennoun, R.; Schumacher, M. Progesterone treatment of spinal cord injury: Effects on receptors, neurotrophins, and myelination. J. Mol. Neurosci. 2006, 28, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Labombarda, F.; Gonzalez, S.; Gonzalez Deniselle, M.C.; Garay, L.; Guennoun, R.; Schumacher, M.; De Nicola, A.F. Progesterone increases the expression of myelin basic protein and the number of cells showing NG2 immunostaining in the lesioned spinal cord. J. Neurotrauma 2006, 23, 181–192. [Google Scholar] [CrossRef] [PubMed]
- Sribnick, E.A.; Samantaray, S.; Das, A.; Smith, J.; Matzelle, D.D.; Ray, S.K.; Banik, N.L. Post-injury estrogen treatment of chronic spinal cord injury improves locomotor function in rats. J. Neurosci. Res. 2010, 88, 1738–1750. [Google Scholar] [CrossRef] [PubMed]
- Hu, R.; Sun, H.; Zhang, Q.; Chen, J.; Wu, N.; Meng, H.; Cui, G.; Hu, S.; Li, F.; Lin, J.; et al. G-protein coupled estrogen receptor 1 mediated estrogenic neuroprotection against spinal cord injury. Crit. Care Med. 2012, 40, 3230–3237. [Google Scholar] [CrossRef] [PubMed]
- Mosquera, L.; Colón, J.M.; Santiago, J.M.; Torrado, A.I.; Meléndez, M.; Segarra, A.C.; Rodríguez-Orengo, J.F.; Miranda, J.D. Tamoxifen and estradiol improved locomotor function and increased spared tissue in rats after spinal cord injury: Their antioxidant effect and role of estrogen receptor alpha. Brain Res. 2014, 1561, 11–22. [Google Scholar] [CrossRef]
- González, S.L.; Labombarda, F.; González Deniselle, M.C.; Guennoun, R.; Schumacher, M.; De Nicola, A.F. Progesterone up-regulates neuronal brain-derived neurotrophic factor expression in the injured spinal cord. Neuroscience 2004, 125, 605–614. [Google Scholar] [CrossRef] [PubMed]
- Labombarda, F.; Jure, I.; Gonzalez, S.; Lima, A.; Roig, P.; Guennoun, R.; Schumacher, M.; De Nicola, A.F. A functional progesterone receptor is required for immunomodulation, reduction of reactive gliosis and survival of oligodendrocyte precursors in the injured spinal cord. J. Steroid Biochem. Mol. Biol. 2015, 154, 274–284. [Google Scholar] [CrossRef] [PubMed]
- Tator, C.H. Update on the pathophysiology and pathology of acute spinal cord injury. Brain Pathol. 1995, 5, 407–413. [Google Scholar] [CrossRef]
- McDonald, J.W.; Sadowsky, C. Spinal-cord injury. Lancet 2002, 359, 417–425. [Google Scholar] [CrossRef]
- Ackery, A.; Tator, C.; Krassioukov, A. A global perspective on spinal cord injury epidemiology. J. Neurotrauma 2004, 21, 1355–1370. [Google Scholar] [CrossRef]
- Norenberg, M.D.; Smith, J.; Marcillo, A. The pathology of human spinal cord injury: Defining the problems. J. Neurotrauma 2004, 1, 429–440. [Google Scholar] [CrossRef] [PubMed]
- Yip, P.K.; Malaspina, A. Spinal cord trauma and the molecular point of no return. Mol. Neurodegener. 2012, 7, 6. [Google Scholar] [CrossRef]
- Cramer, S.C.; Lastra, L.; Lacourse, M.G.; Cohen, M.J. Brain motor system function after chronic, complete spinal cord injury. Brain 2005, 128, 2941–2950. [Google Scholar] [CrossRef] [PubMed]
- Yiu, G.; He, Z. Glial inhibition of CNS axon regeneration. Nat. Rev. Neurosci. 2006, 7, 617–627. [Google Scholar] [CrossRef] [PubMed]
- Ulndreaj, A.; Chio, J.C.; Ahuja, C.S.; Fehlings, M.G. Modulating the immune response in spinal cord injury. Expert. Rev. Neurother. 2016, 16, 1127–1129. [Google Scholar] [CrossRef]
- Tator, C.H.; Fehlings, M.G. Review of the secondary injury theory of acute spinal cord trauma with emphasis on vascular mechanisms. J. Neurosurg. 1991, 75, 15–26. [Google Scholar] [CrossRef]
- Tator, C.H. Review of experimental spinal cord injury with emphasis on the local and systemic circulatory effects. Neurochirurgie 1991, 37, 291–302. [Google Scholar]
- Bareyre, F.M.; Schwab, M.E. Inflammation, degeneration and regeneration in the injured spinal cord: Insights from DNA microarrays. Trends Neurosci. 2003, 26, 555–563. [Google Scholar] [CrossRef]
- Jamme, I.; Petit, E.; Divoux, D.; Gerbi, A.; Maixent, J.M.; Nouvelot, A. Modulation of mouse cerebral Na+, K (+)-ATPase activity by oxygen free radicals. Neuroreport 1995, 7, 333–337. [Google Scholar]
- Hall, E.D.; Braughler, J.M. Thyrotropin-releasing hormone for spinal trauma. N. Engl. J. Med. 1982, 306, 429–430. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, H.; Tao, Y.; Zhang, S.; Wang, J.; Feng, X. Necroptosis inhibitor necrostatin-1 promotes cell protection and physiological function in traumatic spinal cord injury. Neuroscience 2014, 266, 91–101. [Google Scholar] [CrossRef]
- Liu, M.; Wu, W.; Li, H.; Li, S.; Huang, L.T.; Yang, Y.Q.; Sun, Q.; Wang, C.X.; Yu, Z.; Hang, C.H. Necroptosis, a novel type of programmed cell death, contributes to early neural cells damage after spinal cord injury in adult mice. J. Spinal Cord Med. 2015, 38, 745–753. [Google Scholar] [CrossRef]
- Li, S.; Stys, P.K. Mechanisms of ionotropic glutamate receptor-mediated excitotoxicity in isolated spinal cord white matter. J. Neurosci. 2000, 20, 1190–1198. [Google Scholar] [CrossRef]
- Crowe, M.J.; Bresnahan, J.C.; Shuman, S.L.; Masters, J.N.; Beattie, M.S. Apoptosis and delayed degeneration after spinal cord injury in rats and monkeys. Nat. Med. 1997, 3, 73–76. [Google Scholar] [CrossRef]
- Dong, H.; Fazzaro, A.; Xiang, C.; Korsmeyer, S.J.; Jacquin, M.F.; McDonald, J.W. Enhanced oligodendrocyte survival after spinal cord injury in Bax-deficient mice and mice with delayed Wallerian degeneration. J. Neurosci. 2003, 23, 8682–8691. [Google Scholar] [CrossRef] [PubMed]
- Shuman, S.L.; Bresnahan, J.C.; Beattie, M.S. Apoptosis of microglia and oligodendrocytes after spinal cord contusion in rats. J. Neurosci. Res. 1997, 50, 798–808. [Google Scholar] [CrossRef]
- Gallo, V.; Bertolotto, A.; Levi, G. The proteoglycan chondroitin sulfate is present in a subpopulation of cultured astrocytes and in their precursors. Dev. Biol. 1987, 123, 282–285. [Google Scholar] [CrossRef]
- Katoh-Semba, R.; Matsuda, M.; Kato, K.; Oohira, A. Chondroitin sulfate proteoglycans in the rat brain: Candidates for axon barriers of sensory neurons and the possible modification by laminin of their actions. Eur. J. Neurosci. 1995, 7, 613–621. [Google Scholar] [CrossRef]
- Jones, D.G.; Anderson, E.R.; Galvin, K.A. Spinal cord regeneration: Moving tentatively towards new perspectives. Neurorehabilitation 2003, 18, 339–351. [Google Scholar] [CrossRef]
- Schumacher, M.; Weill-Engerer, S.; Liere, P.; Robert, F.; Franklin, R.J.M.; Garcia-Segura, L.M.; Lambert, J.J.; Mayo, W.; Melcangi, R.C.; Parducz, A.; et al. Steroid hormones and neurosteroids in normal and pathological aging of the nervous system. Prog. Neurobiol. 2003, 71, 3–29. [Google Scholar] [CrossRef]
- Meffre, D.; Labombarda, F.; Delespierre, B.; Chastre, A.; De Nicola, A.F.; Stein, D.G.; Schumacher, M.; Guennoun, R. Distribution of membrane progesterone receptor alpha in the male mouse and rat brain and its regulation after traumatic brain injury. Neuroscience 2013, 231, 111–124. [Google Scholar] [CrossRef]
- Persky, R.W.; Liu, F.; Xu, Y.; Weston, G.; Levy, S.; Roselli, C.E.; McCullough, L.D. Neonatal testosterone exposure protects adult male rats from stroke. Neuroendocrinology 2013, 97, 271–282. [Google Scholar] [CrossRef]
- Schumacher, M.; Mattern, C.; Ghoumari, A.; Oudinet, J.P.; Liere, P.; Labombarda, F.; Sitruk-Ware, R.; De Nicola, A.F.; Guennoun, R. Revisiting the roles of progesterone and allopregnanolone in the nervous system: Resurgence of the progesterone receptors. Prog. Neurobiol. 2014, 113, 6–39. [Google Scholar] [CrossRef]
- Dominguez, R.; Zitting, M.; Liu, Q.; Patel, A.; Babadjouni, R.; Hodis, D.M.; Chow, R.H.; Mack, W.J. Estradiol protects white matter of male C57BL6J mice against experimental chronic cerebral hypoperfusion. J. Stroke Cerebrovasc. Dis. 2018, 27, 1743–1775. [Google Scholar] [CrossRef]
- Hauben, E.; Mizrahi, T.; Agranov, E.; Schwartz, M. Sexual dimorphism in the spontaneous recovery from spinal cord injury: A gender gap in beneficial autoimmunity? Eur. J. Neurosci. 2002, 16, 1731–1740. [Google Scholar] [CrossRef]
- Farooque, M.; Suo, Z.; Arnold, P.M.; Wulser, M.J.; Chou, C.T.; Vancura, R.W.; Fowler, S.; Festoff, B.W. Gender-related differences in recovery of locomotor function after spinal cord injury in mice. Spinal Cord 2006, 44, 182–187. [Google Scholar] [CrossRef]
- Brotfain, E.; Gruenbaum, S.E.; Boyko, M.; Kutz, R.; Zlotnik, A.; Klein, M. Neuroprotection by estrogen and progesterone in traumatic brain injury and spinal cord injury. Curr. Neuropharmacol. 2016, 14, 641–653. [Google Scholar] [CrossRef]
- Sengelaub, D.R.; Han, Q.; Liu, N.K.; Maczuga, M.A.; Szalavari, V.; Valencia, S.A.; Xu, X.M. Protective effects of estradiol and dihydrotestosterone following spinal cord injury. J. Neurotrauma 2018, 35, 825–841. [Google Scholar] [CrossRef]
- Ludwig, P.E.; Patil, A.A.; Chamczuk, A.J.; Agrawal, D.K. Hormonal therapy in traumatic spinal cord injury. Am. J. Transl. Res. 2017, 9, 3881–3895. [Google Scholar] [PubMed] [PubMed Central]
- Hammes, S.R.; Levin, E.R. Extranuclear steroid receptors: Nature and actions. Endocr. Rev. 2007, 28, 726–741. [Google Scholar] [CrossRef] [PubMed]
- Labombarda, F.; Meffre, D.; Delespierre, B.; Krivokapic-Blondiaux, S.; Chastre, A.; Thomas, P.; Pang, Y.; Lydon, J.P.; Gonzalez, S.L.; De Nicola, A.F.; et al. Membrane progesterone receptors localization in the mouse spinal cord. Neuroscience 2010, 166, 94–106. [Google Scholar] [CrossRef]
- Barth, C.; Villringer, A.; Sacher, J. Sex hormones affect neurotransmitters and shape the adult female brain during hormonal transition periods. Front. Neurosci. 2015, 9, 37. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Despaigne, D.A. Selective estrogen receptor modulators: Their benefit in postmenopausal women. Rev. Cuba. Endocrinol. 2001, 2, 124–127. (In Spanish) [Google Scholar]
- Diez-Perez, A. Selective estrogen receptor modulators (SERMS). Arq. Bras. Endocrinol. Metab. 2006, 50, 720–734. [Google Scholar] [CrossRef] [PubMed]
- McEwen, B.S. Non-genomic and genomic effects of steroids on neural activity. Trends Pharmacol. Sci. 1991, 12, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Lösel, R.; Wehling, M. Nongenomic actions of steroid hormones. Nat. Rev. Mol. Cell Biol. 2003, 4, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Cornil, C.A.; Ball, G.F.; Balthazart, J. Functional significance of the rapid regulation of brain estrogen action: Where do the estrogens come from? Brain Res. 2006, 1126, 2–26. [Google Scholar] [CrossRef] [PubMed]
- Gundlah, C.; Lu, N.Z.; Mirkes, S.J.; Bethea, C.L. Estrogen receptor beta (ERbeta) mRNA and protein in serotonin neurons of macaques. Brain Res. Mol. Brain Res. 2001, 91, 14–22. [Google Scholar] [CrossRef]
- Mitra, S.W.; Hoskin, E.; Yudkovitz, J.; Pear, L.; Wilkinson, H.A.; Hayashi, S.; Pfaff, D.W.; Ogawa, S.; Rohrer, S.P.; Schaeffer, J.M.; et al. Immunolocalization of estrogen receptor beta in the mouse brain: Comparison with estrogen receptor alpha. Endocrinology 2003, 144, 2055–2067. [Google Scholar] [CrossRef]
- Brinton, R.D.; Thompson, R.F.; Foy, M.R.; Baudry, M.; Wang, J.; Finch, C.E.; Morgan, T.E.; Pike, C.J.; Mack, W.J.; Stanczyk, F.Z.; et al. Progesterone receptors: Form and function in the brain. Front. Neuroendocrinol. 2008, 29, 313–339. [Google Scholar] [CrossRef]
- Pang, Y.; Dong, J.; Thomas, P. Characterization, neurosteroid binding and brain distribution of human membrane progesterone receptors δ and ε (mPRδ and mPRε) and mPRδ involvement in neurosteroid inhibition of apoptosis. Endocrinology 2013, 154, 283–295. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.F.; McKenna, S.E.; Cologer-Clifford, A.; Nau, E.A.; Simon, N.G. Androgen receptor in mouse brain: Sex differences and similarities in autoregulation. Endocrinology 1998, 139, 1594–1601. [Google Scholar] [CrossRef] [PubMed]
- Burke, K.A.; Schroeder, D.M.; Abel, R.A.; Richardson, S.C.; Bigsby, R.M.; Nephew, K.P. Immunohistochemical detection of estrogen receptor alpha in male rat spinal cord during development. J. Neurosci. Res. 2000, 61, 329–337. [Google Scholar] [CrossRef] [PubMed]
- Papka, R.E.; Storey-Workley, M.; Shughrue, P.J.; Merchenthaler, I.; Collins, J.J.; Usip, S.; Saunders, P.T.; Shupnik, M. Estrogen receptor-alpha and beta-immunoreactivity and mRNA in neurons of sensory and autonomic ganglia and spinal cord. Cell Tissue Res. 2001, 304, 193–214. [Google Scholar] [CrossRef] [PubMed]
- Labombarda, F.; Guennoun, R.; Gonzalez, S.; Roig, P.; Lima, A.; Schumacher, M.; De Nicola, A.F. Immunocytochemical evidence for a progesterone receptor in neurons and glial cells of the rat spinal cord. Neurosci. Lett. 2000, 288, 29–32. [Google Scholar] [CrossRef]
- Guennoun, R.; Meffre, D.; Labombarda, F.; Gonzalez, S.L.; Deniselle, M.C.; Stein, D.G.; De Nicola, A.F.; Schumacher, M. The membrane-associated progesterone-binding protein 25-Dx: Expression, cellular localization and up-regulation after brain and spinal cord injuries. Brain Res. Rev. 2008, 57, 493–505. [Google Scholar] [CrossRef] [PubMed]
- Labombarda, F.; Gonzalez, S.L.; Deniselle, M.C.; Vinson, G.P.; Schumacher, M.; De Nicola, A.F.; Guennoun, R. Effects of injury and progesterone treatment on progesterone receptor and progesterone binding protein 25-Dx expression in the rat spinal cord. J. Neurochem. 2003, 87, 902–913. [Google Scholar] [CrossRef]
- Freeman, L.M.; Padgett, B.A.; Prins, G.S.; Breedlove, S.M. Distribution of androgen receptor immunoreactivity in the spinal cord of wild-type, androgen-insensitive and gonadectomized male rats. J. Neurobiol. 1995, 27, 51–59. [Google Scholar] [CrossRef]
- Ray, S.K.; Samantaray, S.; Smith, J.A.; Matzelle, D.D.; Das, A.; Banik, N.L. Inhibition of cysteine proteases in acute and chronic spinal cord injury. Neurotherapeutics 2011, 8, 180–186. [Google Scholar] [CrossRef]
- Hurlbert, R.J. Methylprednisolone for acute spinal cord injury: An inappropriate standard of care. J. Neurosurg. 2000, 93, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Pointillart, V.; Petitjean, M.E.; Wiart, L.; Vital, J.M.; Lassié, P.; Thicoipé, M.; Dabadie, P. Pharmacological therapy of spinal cord injury during the acute phase. Spinal Cord 2000, 38, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Choi, H.Y.; Na, W.H.; Ju, B.G.; Yune, T.Y. 17β-Estradiol inhibits MMP-9 and SUR1/TrpM4 expression and activation and thereby attenuates BSCB disruption/hemorrhage after spinal cord injury in male rats. Endocrinology 2015, 156, 1838–1850. [Google Scholar] [CrossRef] [PubMed]
- Benyi, E.; Kieler, H.; Linder, M.; Ritzén, M.; Carlstedt-Duke, J.; Tuvemo, T.; Westphal, O.; Sävendahl, L. Risks of malignant and non-malignant tumours in tall women treated with high-dose oestrogen during adolescence. Horm. Res. Paediatr. 2014, 82, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Wibowo, E.; Schellhammer, P.; Wassersug, R.J. Role of estrogen in normal male function: Clinical implications for patients with prostate cancer on androgen deprivation therapy. J. Urol. 2011, 185, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Cox, A.; Varma, A.; Barry, J.; Vertegel, A.; Banik, N. Nanoparticle estrogen in rat spinal cord injury elicits rapid anti-inflammatory effects in plasma, cerebrospinal fluid, and tissue. J. Neurotrauma 2015, 32, 1413–1421. [Google Scholar] [CrossRef] [PubMed]
- Samantaray, S.; Das, A.; Matzelle, D.C.; Yu, S.P.; Wei, L.; Varma, A.; Ray, S.K.; Banik, N.L. Administration of low dose estrogen attenuates persistent inflammation, promotes angiogenesis, and improves locomotor function following chronic spinal cord injury in rats. J. Neurochem. 2016, 137, 604–617. [Google Scholar] [CrossRef]
- Tian, D.S.; Liu, J.L.; Xie, M.J.; Zhan, Y.; Qu, W.S.; Yu, Z.Y.; Tang, Z.P.; Pan, D.J.; Wang, W. Tamoxifen attenuates inflammatory-mediated damage and improves functional outcome after spinal cord injury in rats. J. Neurochem. 2009, 109, 1658–1667. [Google Scholar] [CrossRef]
- Sánchez-Torres, S.; Orozco-Barrios, C.; Salgado-Ceballos, H.; Segura-Uribe, J.J.; Guerra-Araiza, C.; León-Cholula, Á.; Morán, J.; Coyoy-Salgado, A. Tibolone improves locomotor function in a rat model of spinal cord injury by modulating apoptosis and autophagy. Int. J. Mol. Sci. 2023, 24, 15285. [Google Scholar] [CrossRef]
- Meffre, D.; Delespierre, B.; Gouézou, M.; Leclerc, P.; Vinson, G.P.; Schumacher, M.; Stein, D.G.; Guennoun, R. The membrane-associated progesterone-binding protein 25-Dx is expressed in brain regions involved in water homeostasis and is up-regulated after traumatic brain injury. J. Neurochem. 2005, 93, 1314–1326. [Google Scholar] [CrossRef]
- De Nicola, A.F.; Labombarda, F.; Gonzalez Deniselle, M.C.; Gonzalez, S.L.; Garay, L.; Meyer, M.; Gargiulo, G.; Guennoun, R.; Schumacher, M. Progesterone neuroprotection in traumatic CNS injury and motoneuron degeneration. Front. Neuroendocrinol. 2009, 30, 173–187. [Google Scholar] [CrossRef] [PubMed]
- Labombarda, F.; Gonzalez, S.L.; Lima, A.; Roig, P.; Guennoun, R.; Schumacher, M.; De Nicola, A.F. Effects of progesterone on oligodendrocyte progenitors, oligodendrocyte transcription factors, and myelin proteins following spinal cord injury. Glia 2009, 57, 884–897. [Google Scholar] [CrossRef] [PubMed]
- Labombarda, F.; Gonzalez, S.; Lima, A.; Roig, P.; Guennoun, R.; Schumacher, M.; De Nicola, A.F. Progesterone attenuates astro- and microgliosis and enhances oligodendrocyte differentiation following spinal cord injury. Exp. Neurol. 2011, 231, 135–146. [Google Scholar] [CrossRef]
- Guennoun, R.; Benmessahel, Y.; Delespierre, B.; Gouézou, M.; Rajkowski, K.M.; Baulieu, E.E.; Schumacher, M. Progesterone stimulates Krox-20 gene expression in Schwann cells. Mol. Brain Res. 2001, 90, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Jure, I.; De Nicola, A.F.; Labombarda, F. Progesterone effects on oligodendrocyte differentiation in injured spinal cord. Brain Res. 2019, 1708, 36–46. [Google Scholar] [CrossRef] [PubMed]
- Mitew, S.; Hay, C.M.; Peckham, H.; Xiao, J.; Koenning, M.; Emery, B. Mechanisms regulating the development of oligodendrocytes and central nervous system myelin. Neuroscience 2014, 276, 29–47. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Zhu, Q.; Zheng, K.; Li, H.; Qi, Y.; Cao, Q.; Qiu, M. Co-localization of Nkx6.2 and Nkx2.2 homeodomain proteins in differentiated myelinating oligodendrocytes. Glia 2010, 58, 458–468. [Google Scholar] [CrossRef]
- Sugimori, M.; Nagao, M.; Parras, C.M.; Nakatani, H.; Lebel, M.; Guillemot, F.; Nakafuku, M. Ascl1 is required for oligodendrocyte development in the spinal cord. Development 2008, 135, 1271–1281. [Google Scholar] [CrossRef]
- Emery, B.; Agalliu, D.; Cahoy, J.D.; Watkins, T.A.; Dugas, J.C.; Mulinyawe, S.B.; Ibrahim, A.; Ligon, K.L.; Rowitch, D.H.; Barres, B.A. Myelin gene regulatory factor is a critical transcriptional regulator required for CNS myelination. Cell 2009, 138, 172–185. [Google Scholar] [CrossRef]
- Hornig, J.; Frob, F.; Vogl, M.R.; Hermans-Borgmeyer, I.; Tamm, E.R.; Wegner, M. The transcription factors Sox10 and Myrf define an essential regulatory network module in differentiating oligodendrocytes. PLoS Genet. 2013, 9, e1003907. [Google Scholar] [CrossRef]
- García-Ovejero, D.; González, S.; Paniagua-Torija, B.; Lima, A.; Molina-Holgado, E.; De Nicola, A.F.; Labombarda, F. Progesterone reduces secondary damage, preserves white matter, and improves locomotor outcome after spinal cord contusion. J. Neurotrauma 2014, 31, e857–e871. [Google Scholar] [CrossRef] [PubMed]
- Garay, L.; Tüngler, V.; Deniselle, M.C.; Lima, A.; Roig, P.; De Nicola, A.F. Progesterone attenuates demyelination and microglial reaction in the lysolecithin-injured spinal cord. Neuroscience 2011, 192, 588–597. [Google Scholar] [CrossRef] [PubMed]
- Ogata, T.; Nakamura, Y.; Tsuji, K.; Shibata, T.; Kataoka, K. Steroid hormones protect spinal cord neurons from glutamate toxicity. Neuroscience 1993, 55, 445–449. [Google Scholar] [CrossRef]
- Thomas, A.J.; Nockels, R.P.; Pan, H.Q.; Shaffrey, C.I.; Chopp, M. Progesterone is neuroprotective after acute experimental spinal cord trauma in rats. Spine 1999, 24, 2134–2138. [Google Scholar] [CrossRef] [PubMed]
- Labombarda, F.; Gonzalez, S.L.; Gonzalez, D.M.; Guennoun, R.; Schumacher, M.; De Nicola, A.F. Cellular basis for progesterone neuroprotection in the injured spinal cord. J. Neurotrauma 2002, 19, 343–355. [Google Scholar] [CrossRef] [PubMed]
- González, S.L.; Labombarda, F.; Gonzalez Deniselle, M.C.; Mougel, A.; Guennoun, R.; Schumacher, M.; De Nicola, A.F. Progesterone neuroprotection in spinal cord trauma involves up-regulation of brain-derived neurotrophic factor in motoneurons. J. Steroid Biochem. Mol. Biol. 2005, 94, 143–149. [Google Scholar] [CrossRef]
- González, S.L.; López-Costa, J.J.; Labombarda, F.; Gonzalez Deniselle, M.C.; Guennoun, R.; Schumacher, M.; De Nicola, A.F. Progesterone effects on neuronal ultrastructure and expression of microtubule-associated protein 2 (MAP2) in rats with acute spinal cord injury. Cell Mol. Neurobiol. 2009, 29, 27–39. [Google Scholar] [CrossRef]
- Labombarda, F.; Ghoumari, A.M.; Liere, P.; De Nicola, A.F.; Schumacher, M.; Guennoun, R. Neuroprotection by steroids after neurotrauma in organotypic spinal cord cultures: A key role for progesterone receptors and steroidal modulators of GABA(A) receptors. Neuropharmacology 2013, 71, 46–55. [Google Scholar] [CrossRef]
- Yang, Z.; Xie, W.; Ju, F.; Khan, A.; Zhang, S. In vivo two-photon imaging reveals a role of progesterone in reducing axonal dieback after spinal cord injury in mice. Neuropharmacology 2017, 116, 30–37. [Google Scholar] [CrossRef]
- Coronel, M.F.; Labombarda, F.; Villar, M.J.; De Nicola, A.F.; González, S.L. Progesterone prevents allodynia after experimental spinal cord injury. J. Pain 2011, 12, 71–83. [Google Scholar] [CrossRef]
- Coronel, M.F.; Labombarda, F.; De Nicola, A.F.; González, S.L. Progesterone reduces the expression of spinal cyclooxygenase-2 and inducible nitric oxide synthase and prevents allodynia in a rat model of central neuropathic pain. Eur. J. Pain 2014, 18, 348–359. [Google Scholar] [CrossRef]
- Coronel, M.F.; Raggio, M.C.; Adler, N.S.; De Nicola, A.F.; Labombarda, F.; González, S.L. Progesterone modulates pro-inflammatory cytokine expression profile after spinal cord injury: Implications for neuropathic pain. J. Neuroimmunol. 2016, 292, 85–92. [Google Scholar] [CrossRef]
- Coronel, M.F.; Villar, M.J.; Brumovsky, P.R.; González, S.L. Spinal neuropeptide expression and neuropathic behavior in the acute and chronic phases after spinal cord injury: Effects of progesterone administration. Peptides 2017, 88, 189–195. [Google Scholar] [CrossRef]
- Cavalcante, L.P.; Ferreira, S.G.; Pereira, D.R.; Moraes, S.R.; Simas, R.; Sannomiya, P.; Breithaupt-Faloppa, A.C.; Moreira, L.F.P. Acute administration of oestradiol or progesterone in a spinal cord ischaemia-reperfusion model in rats. Interact. Cardiovasc. Thorac. Surg. 2018, 26, 196–201. [Google Scholar] [CrossRef]
- Fee, D.B.; Swartz, K.R.; Joy, K.M.; Roberts, K.N.; Scheff, N.N.; Scheff, S.W. Effects of progesterone on experimental spinal cord injury. Brain Res. 2007, 1137, 146–152. [Google Scholar] [CrossRef]
- Rouleau, P.; Ung, R.-V.; Lapointe, N.P.; Guertin, P.A. Hormonal and immunological changes in mice after spinal cord injury. J. Neurotrauma 2007, 24, 367–378. [Google Scholar] [CrossRef]
- Freyermuth-Trujillo, X.; Segura-Uribe, J.J.; Salgado-Ceballos, H.; Orozco-Barrios, C.E.; Coyoy-Salgado, A. Inflammation: A target for treatment in spinal cord injury. Cells 2022, 11, 2692. [Google Scholar] [CrossRef]
- Angele, M.K.; Schwacha, M.G.; Ayala, A.; Chaudry, I.H. Effect of gender and sex hormones on immune responses following shock. Shock 2000, 14, 81–90. [Google Scholar] [CrossRef]
- Gürer, B.; Kertmen, H.; Kasim, E.; Yilmaz, E.R.; Kanat, B.H.; Sargon, M.F.; Arikok, A.T.; Ergüder, B.I.; Sekerci, Z. Neuroprotective effects of testosterone on ischemia/reperfusion injury of the rabbit spinal cord. Injury 2015, 46, 240–248. [Google Scholar] [CrossRef]
- Yarrow, J.F.; Conover, C.F.; Beggs, L.A.; Beck, D.T.; Otzel, D.M.; Balaez, A.; Combs, S.M.; Miller, J.R.; Ye, F.; Aguirre, J.I.; et al. Testosterone dose dependently prevents bone and muscle loss in rodents after spinal cord injury. J. Neurotrauma 2014, 31, 834–845. [Google Scholar] [CrossRef]
- Yarrow, J.F.; Phillips, E.G.; Conover, C.F.; Bassett, T.E.; Chen, C.; Teurlings, T.; Vasconez, A.; Alerte, J.; Prock, H.; Jiron, J.M.; et al. Testosterone plus finasteride prevents bone loss without prostate growth in a rodent spinal cord injury model. J. Neurotrauma 2017, 34, 2972–2981. [Google Scholar] [CrossRef]
- Wu, Y.; Zhao, J.; Zhao, W.; Pan, J.; Bauman, W.A.; Cardozo, C.P. Nandrolone normalizes determinants of muscle mass and fiber type after spinal cord injury. J. Neurotrauma 2012, 29, 1663–1675. [Google Scholar] [CrossRef]
- Byers, J.S.; Huguenard, A.L.; Kuruppu, D.; Liu, N.K.; Xu, X.M.; Sengelaub, D.R. Neuroprotective effects of testosterone on muscle and motoneurons morphology following spinal cord injury. J. Comp. Neurol. 2012, 520, 2683–2696. [Google Scholar] [CrossRef] [PubMed]
- Gregory, C.M.; Vandenborne, K.; Huang, H.F.; Ottenweller, J.E.; Dudley, G.A. Effects of testosterone replacement therapy on skeletal muscle after spinal cord injury. Spinal Cord 2003, 41, 23–28. [Google Scholar] [CrossRef]
- Yarrow, J.F.; Kok, H.J.; Phillips, E.G.; Conover, C.F.; Lee, J.; Bassett, T.E.; Buckley, K.H.; Reynolds, M.C.; Wnek, R.D.; Otzel, D.M.; et al. Locomotor training with adjuvant testosterone preserves cancellous bone and promotes muscle plasticity in male rats after severe spinal cord injury. J. Neurosci. Res. 2020, 98, 843–868. [Google Scholar] [CrossRef]
- Naderi, A.R.; Safarinejad, M.R. Endocrine profiles and semen quality in spinal cord injured men. Clin. Endocrinol. 2003, 58, 177–184. [Google Scholar] [CrossRef]
- Celik, B.; Sahin, A.; Caglar, N.; Erhan, B.; Gunduz, B.; Gultekin, O.; Karabulut, M. Sex hormone levels and functional outcomes: A controlled study of patients with spinal cord injury compared with healthy subjects. Am. J. Phys. Med. Rehabil. 2007, 86, 784–790. [Google Scholar] [CrossRef]
- Clark, M.J.; Schopp, L.H.; Mazurek, M.O.; Zaniletti, I.; Lammy, A.B.; Martin, T.A.; Thomas, F.P.; Acuff, M.E. Testosterone levels among men with spinal cord injury: Relationship between time since injury and laboratory values. Am. J. Phys. Med. Rehabil. 2008, 87, 758–767. [Google Scholar] [CrossRef]
- Clark, M.J.; Petroski, G.F.; Mazurek, M.O.; Hagglund, K.J.; Sherman, A.K.; Lammy, A.B.; Childers, M.K.; Acuff, M.E. Testosterone replacement therapy and motor function in men with spinal cord injury. Am. J. Phys. Med. Rehabil. 2008, 87, 281–284. [Google Scholar] [CrossRef]
- Durga, A.; Sepahpanah, F.; Regozzi, M.; Hastings, J.; Crane, D.A. Prevalence of testosterone deficiency after spinal cord injury. PM&R 2011, 3, 929–932. [Google Scholar] [CrossRef]
- Barbonetti, A.; Vassallo, M.R.C.; Pacca, F.; Cavallo, F.; Costanzo, M.; Felzani, G.; Francavilla, S.; Francavilla, F. Correlates of low testosterone in men with chronic spinal cord injury. Andrology 2014, 2, 721–728. [Google Scholar] [CrossRef]
- Bauman, W.A.; La Fountaine, M.F.; Spungen, A.M. Age-related prevalence of low testosterone in men with spinal cord injury. J. Spinal Cord Med. 2014, 37, 32–39. [Google Scholar] [CrossRef]
- Bianchi, V.E. The anti-inflammatory effects of testosterone. J. Endocr. Soc. 2018, 3, 91–107. [Google Scholar] [CrossRef]
- Podlasek, C.A.; Mulhall, J.; Davies, K.; Wingard, C.J.; Hannan, J.L.; Bivalacqua, T.J.; Musicki, B.; Khera, M.; González-Cadavid, N.F.; Burnett, A.L. Translational perspective on the role of testosterone in sexual function and dysfunction. J. Sex. Med. 2016, 13, 1183–1198. [Google Scholar] [CrossRef]
- Borst, S.E.; Yarrow, J.F. Injection of testosterone may be safer and more effective than transdermal administration for combating loss of muscle and bone in older men. Am. J. Physiol. Endocrinol. Metab. 2015, 308, E1035–E1042. [Google Scholar] [CrossRef] [PubMed]
- Cheung, A.S.; Gray, H.; Schache, A.G.; Hoermann, R.; Lim, J.D.; Zajac, J.D.; Pandy, M.G.; Grossmann, M. Androgen deprivation causes selective deficits in the biomechanical leg muscle function of men during walking: A prospective case-control study. J. Cachexia Sarcopenia Muscle 2017, 8, 102–112. [Google Scholar] [CrossRef]
- Skinner, J.W.; Otzel, D.M.; Bowser, A.; Nargi, D.; Agarwal, S.; Peterson, M.D.; Zou, B.; Borst, S.E.; Yarrow, J.F. Muscular responses to testosterone replacement vary by administration route: A systematic review and meta-analysis. J. Cachexia Sarcopenia Muscle 2018, 9, 465–481. [Google Scholar] [CrossRef]
- Snyder, P.J.; Bhasin, S.; Cunningham, G.R.; Matsumoto, A.M.; Stephens-Shields, A.J.; Cauley, J.A.; Gill, T.M.; Barrett-Connor, E.; Swerdloff, R.S.; Wang, C.; et al. Effects of testosterone treatment in older men. N. Engl. J. Med. 2016, 374, 611–624. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).