Design of Alginate/Gelatin Hydrogels for Biomedical Applications: Fine-Tuning Osteogenesis in Dental Pulp Stem Cells While Preserving Other Cell Behaviors
Abstract
:1. Introduction
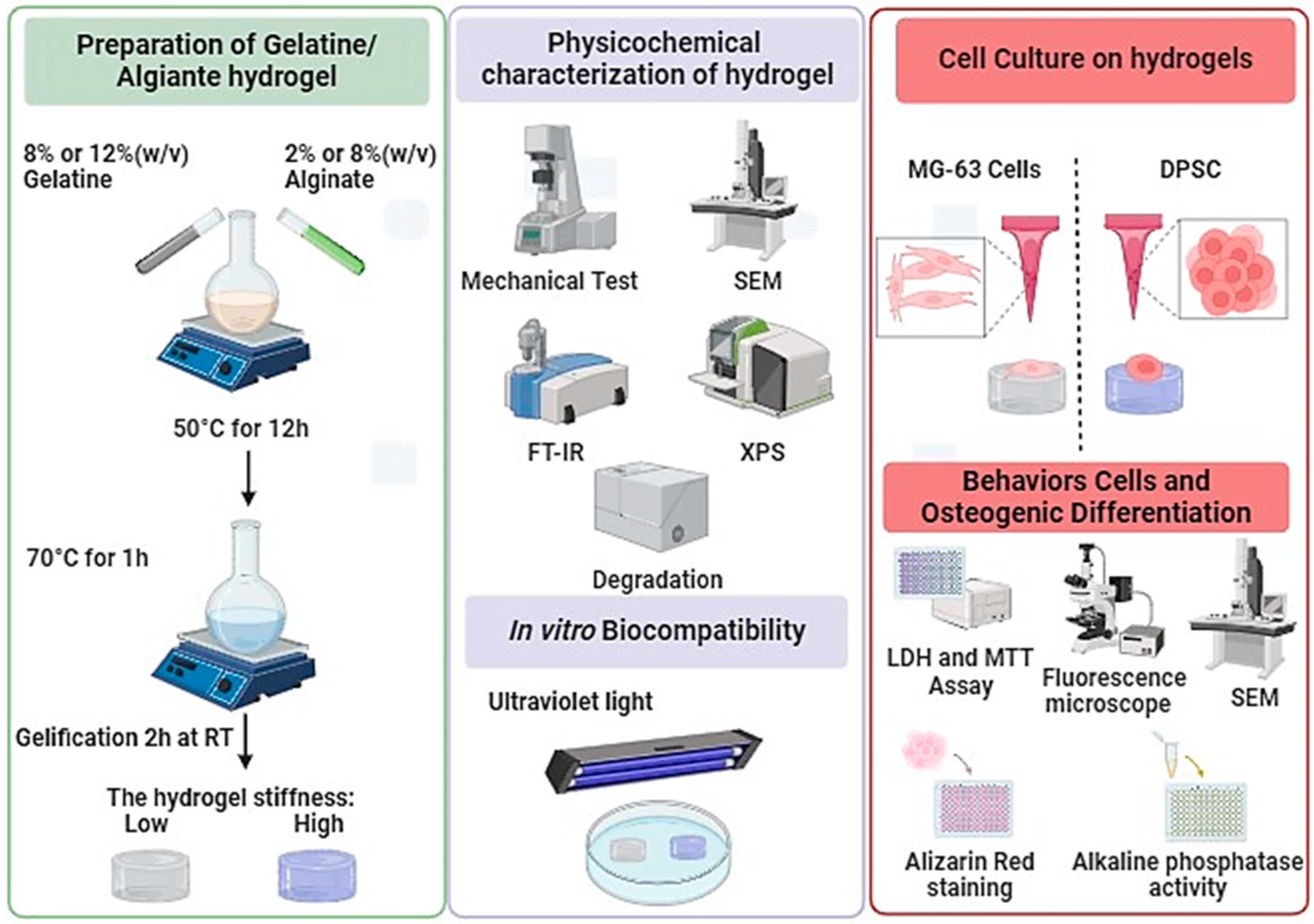
2. Materials and Methods
2.1. Materials
2.2. Preparation of Alginate/Gelatin Hydrogels
2.3. Hydrogels Physicochemical Characterizations
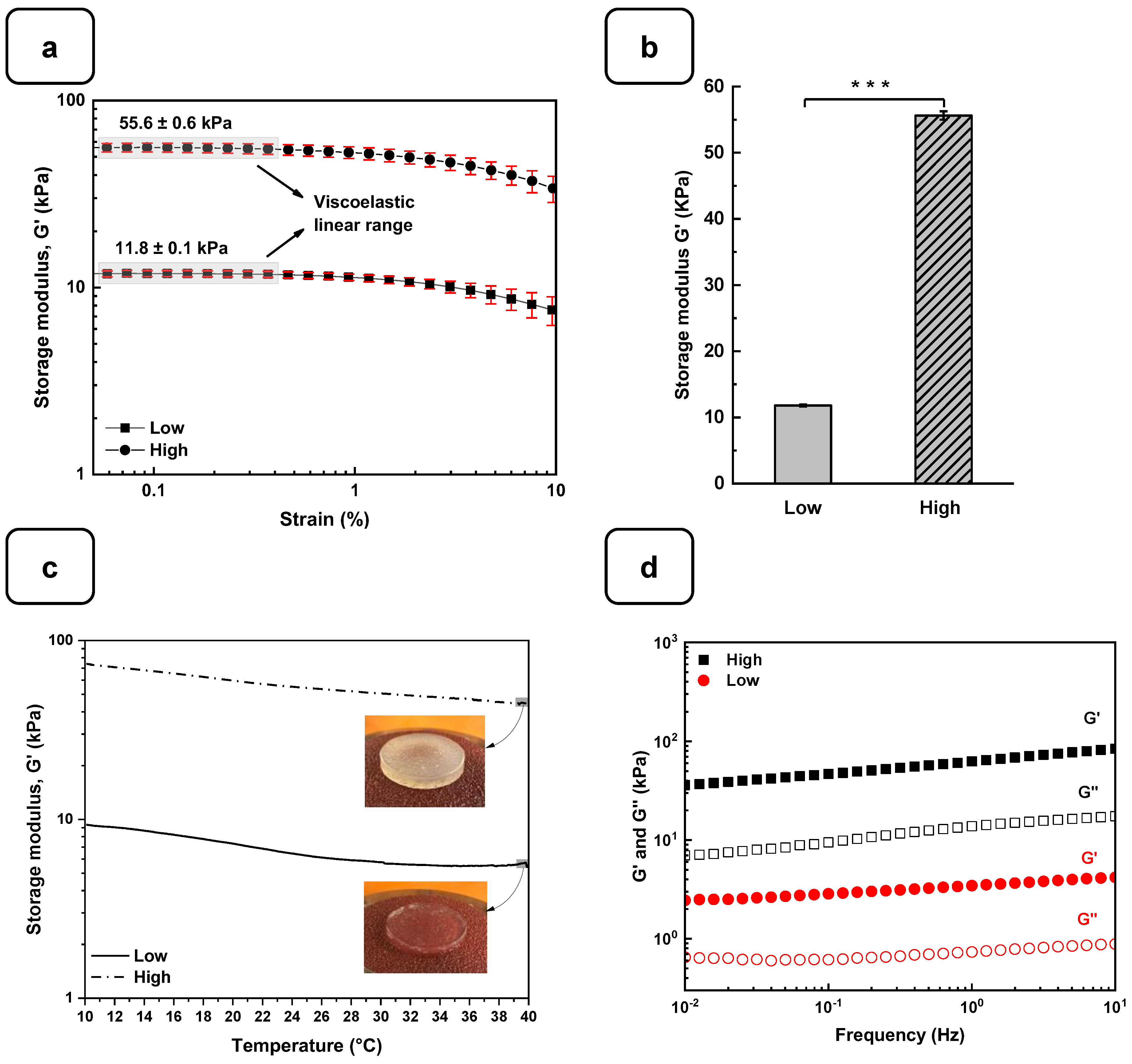
2.4. Rheological Characterizations
2.5. Swelling Properties
2.6. Degradation Behavior
2.7. Cell Culture
2.7.1. Osteoblast Adhesion
2.7.2. Cell Viability
2.7.3. Cytotoxicity Assessment
2.7.4. Cell Morphology
2.8. Osteogenic Activity
2.9. Statistical Analysis
3. Results and Discussion
3.1. Preparation and Characterization of Alg-Gel Hydrogels
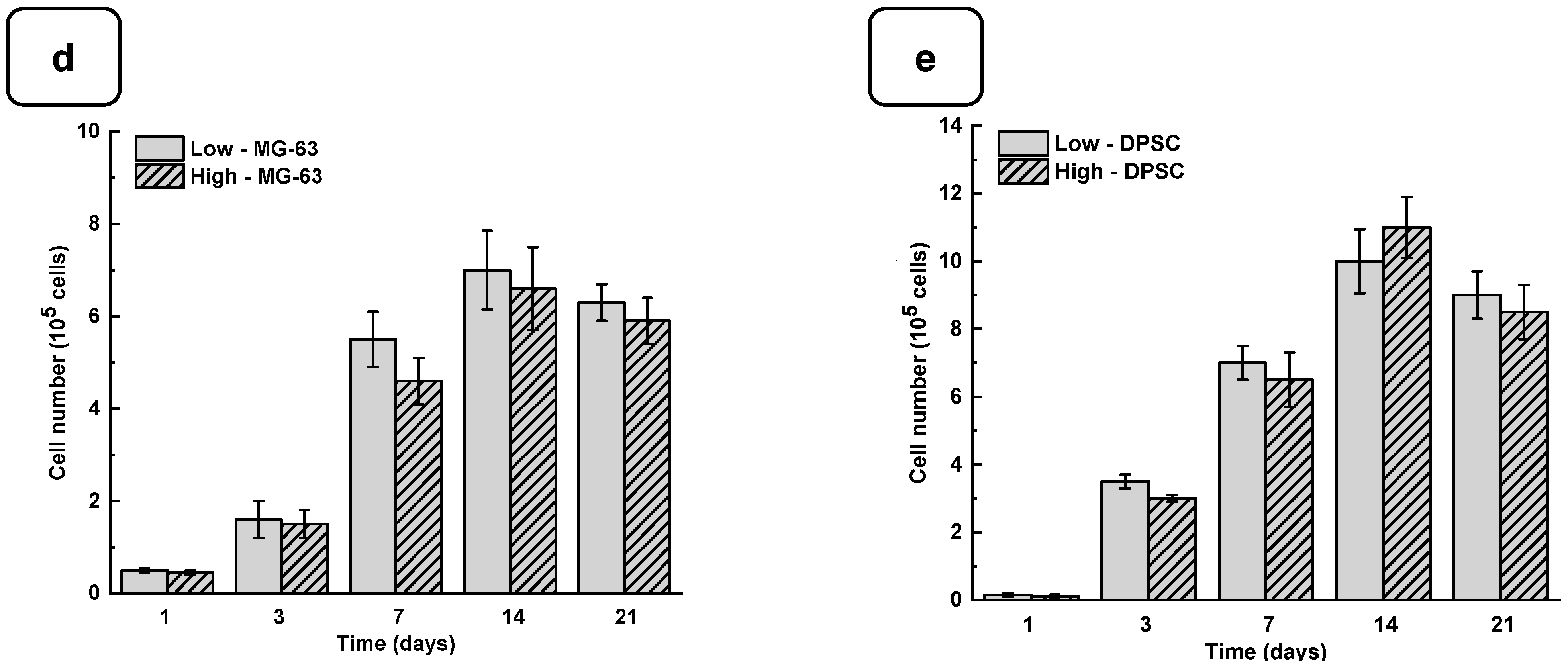
3.2. Biological Performances of Alg-Gel Hydrogels
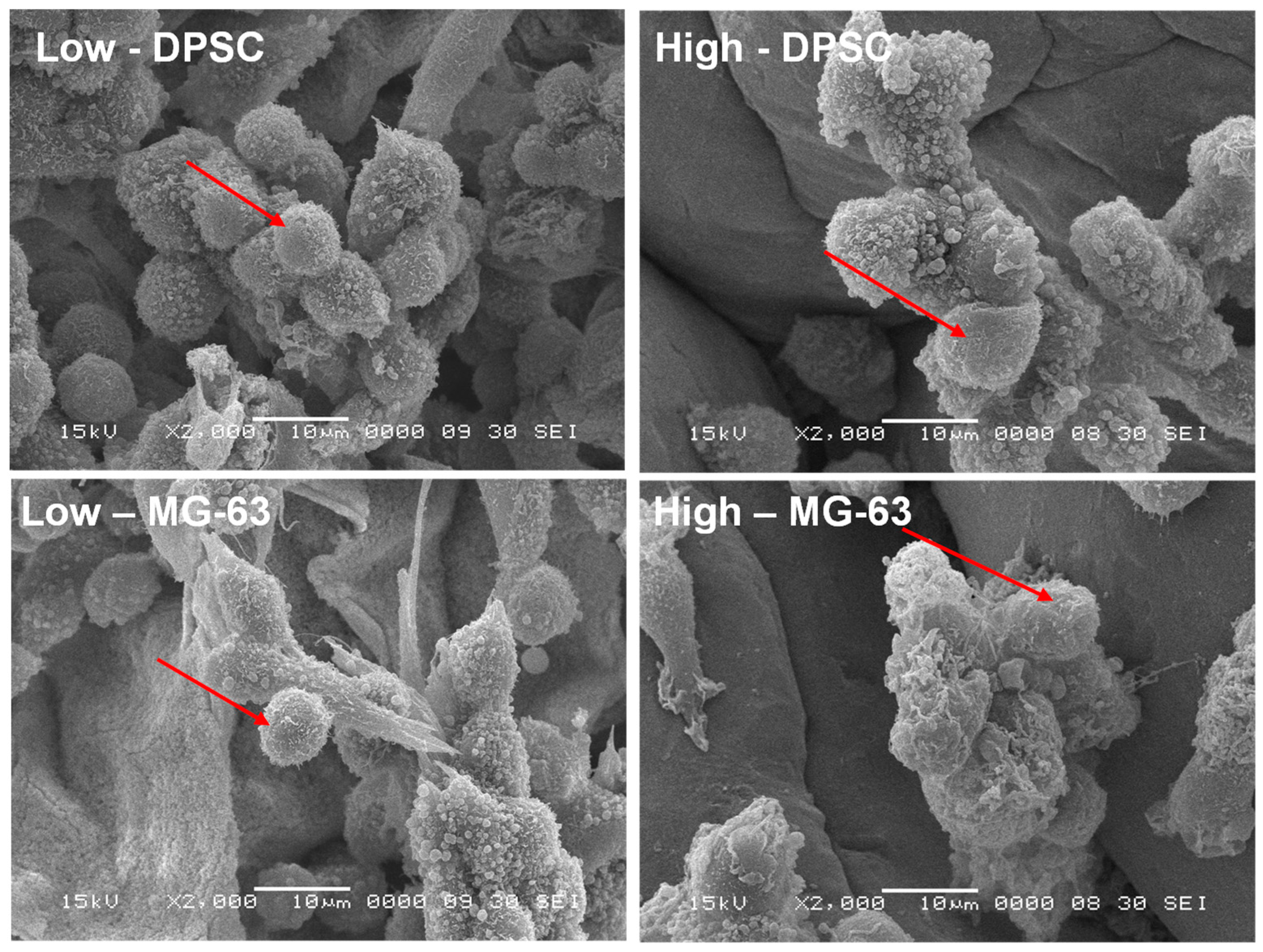
3.3. Cell Morphology
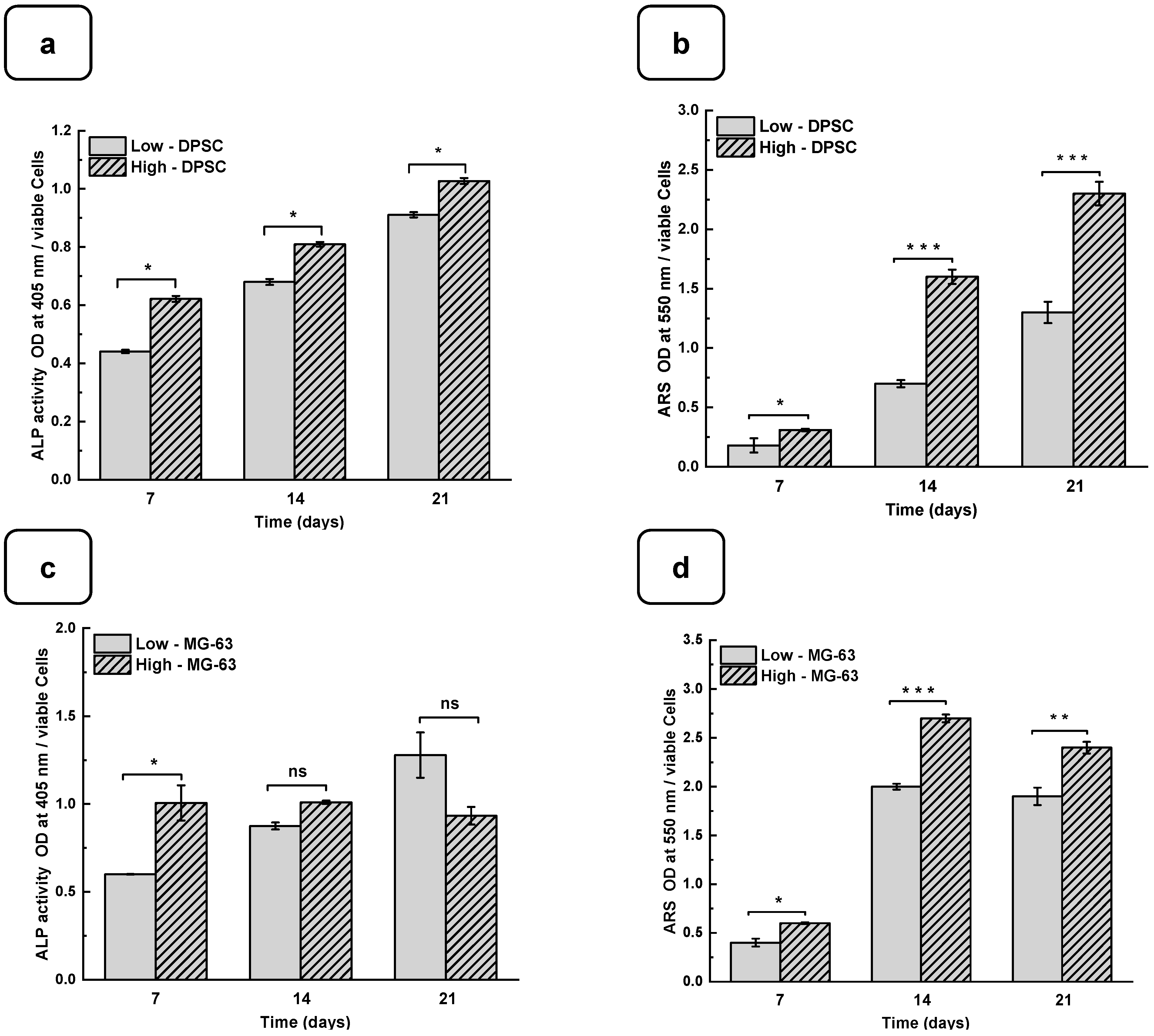
3.4. Mineralization of Cells on Hydrogels
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Arjmand, M.; Ardeshirylajimi, A.; Maghsoudi, H.; Azadian, E. Osteogenic Differentiation Potential of Mesenchymal Stem Cells Cultured on Nanofibrous Scaffold Improved in the Presence of Pulsed Electromagnetic Field. J. Cell. Physiol. 2018, 233, 1061–1070. [Google Scholar] [CrossRef] [PubMed]
- Chopra, H.; Mohanta, Y.K.; Mahanta, S.; Mohanta, T.K.; Singh, I.; Avula, S.K.; Mallick, S.P.; Rabaan, A.A.; AlSaihati, H.; Alsayyah, A.; et al. Recent Updates in Nanotechnological Advances for Wound Healing: A Narrative Review. Nanotechnol. Rev. 2023, 12, 20230129. [Google Scholar] [CrossRef]
- Ye, J.; Yang, G.; Zhang, J.; Xiao, Z.; He, L.; Zhang, H.; Liu, Q. Preparation and Characterization of Gelatin-Polysaccharide Composite Hydrogels for Tissue Engineering. PeerJ 2021, 9, e11022. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Abdeen, A.A.; Zhang, D.; Kilian, K.A. Directing Stem Cell Fate on Hydrogel Substrates by Controlling Cell Geometry, Matrix Mechanics and Adhesion Ligand Composition. Biomaterials 2013, 34, 8140–8148. [Google Scholar] [CrossRef]
- Nabavinia, M.; Khoshfetrat, A.B.; Naderi-Meshkin, H. Nano-Hydroxyapatite-Alginate-Gelatin Microcapsule as a Potential Osteogenic Building Block for Modular Bone Tissue Engineering. Mater. Sci. Eng. C 2019, 97, 67–77. [Google Scholar] [CrossRef]
- Zhao, W.; Li, X.; Liu, X.; Zhang, N.; Wen, X. Effects of Substrate Stiffness on Adipogenic and Osteogenic Differentiation of Human Mesenchymal Stem Cells. Mater. Sci. Eng. C 2014, 40, 316–323. [Google Scholar] [CrossRef]
- Žigon-Branc, S.; Markovic, M.; Van Hoorick, J.; Van Vlierberghe, S.; Dubruel, P.; Zerobin, E.; Baudis, S.; Ovsianikov, A. Impact of Hydrogel Stiffness on Differentiation of Human Adipose-Derived Stem Cell Microspheroids. Tissue Eng. Part A 2019, 25, 1369–1380. [Google Scholar] [CrossRef] [PubMed]
- Kamguyan, K.; Katbab, A.A.; Mahmoudi, M.; Thormann, E.; Zajforoushan Moghaddam, S.; Moradi, L.; Bonakdar, S. An Engineered Cell-Imprinted Substrate Directs Osteogenic Differentiation in Stem Cells. Biomater. Sci. 2018, 6, 189–199. [Google Scholar] [CrossRef]
- Witkowska-Zimny, M.; Walenko, K.; Wrobel, E.; Mrowka, P.; Mikulska, A.; Przybylski, J. Effect of Substrate Stiffness on the Osteogenic Differentiation of Bone Marrow Stem Cells and Bone-Derived Cells: Substrate Stiffness Affects Cell Osteogenesis. Cell Biol. Int. 2013, 37, 608–616. [Google Scholar] [CrossRef]
- Smułek, W.; Grząbka-Zasadzińska, A.; Kilian, A.; Ciesielczyk, F.; Borysiak, S.; Baranowska, H.M.; Walkowiak, K.; Kaczorek, E.; Jarzębski, M. Design of Vitamin-Loaded Emulsions in Agar Hydrogel Matrix Dispersed with Plant Surfactants. Food Biosci. 2023, 53, 102559. [Google Scholar] [CrossRef]
- Fernandez de Grado, G.; Keller, L.; Idoux-Gillet, Y.; Wagner, Q.; Musset, A.-M.; Benkirane-Jessel, N.; Bornert, F.; Offner, D. Bone Substitutes: A Review of Their Characteristics, Clinical Use, and Perspectives for Large Bone Defects Management. J. Tissue Eng. 2018, 9, 2041731418776819. [Google Scholar] [CrossRef]
- Gonzalez-Fernandez, T.; Sikorski, P.; Leach, J.K. Bio-Instructive Materials for Musculoskeletal Regeneration. Acta Biomater. 2019, 96, 20–34. [Google Scholar] [CrossRef]
- Gonzalez-Fernandez, T.; Tenorio, A.J.; Campbell, K.T.; Silva, E.A.; Leach, J.K. Alginate-Based Bioinks for 3D Bioprinting and Fabrication of Anatomically Accurate Bone Grafts. Tissue Eng. Part A 2021, 27, 1168–1181. [Google Scholar] [CrossRef] [PubMed]
- Maisani, M.; Pezzoli, D.; Chassande, O.; Mantovani, D. Cellularizing Hydrogel-Based Scaffolds to Repair Bone Tissue: How to Create a Physiologically Relevant Micro-Environment? J. Tissue Eng. 2017, 8, 204173141771207. [Google Scholar] [CrossRef] [PubMed]
- Vining, K.H.; Mooney, D.J. Mechanical Forces Direct Stem Cell Behaviour in Development and Regeneration. Nat. Rev. Mol. Cell Biol. 2017, 18, 728–742. [Google Scholar] [CrossRef] [PubMed]
- Łabowska, M.B.; Cierluk, K.; Jankowska, A.M.; Kulbacka, J.; Detyna, J.; Michalak, I. A Review on the Adaption of Alginate-Gelatin Hydrogels for 3D Cultures and Bioprinting. Materials 2021, 14, 858. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and Biomedical Applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef] [PubMed]
- Sahiner, N.; Sagbas, S.; Sahiner, M.; Silan, C. P(TA) Macro-, Micro-, Nanoparticle-Embedded Super Porous p(HEMA) Cryogels as Wound Dressing Material. Mater. Sci. Eng. C 2017, 70, 317–326. [Google Scholar] [CrossRef]
- Rongen, J.J.; van Bochove, B.; Hannink, G.; Grijpma, D.W.; Buma, P. Degradation Behavior of, and Tissue Response to Photo-Crosslinked Poly(Trimethylene Carbonate) Networks: TISSUE RESPONSE TO PHOTO-CROSSLINKED POLY(TRIMETHYLENE CARBONATE) NETWORKS. J. Biomed. Mater. Res. A 2016, 104, 2823–2832. [Google Scholar] [CrossRef]
- Wei, B.; Wang, W.; Liu, X.; Xu, C.; Wang, Y.; Wang, Z.; Xu, J.; Guan, J.; Zhou, P.; Mao, Y. Gelatin Methacrylate Hydrogel Scaffold Carrying Resveratrol-Loaded Solid Lipid Nanoparticles for Enhancement of Osteogenic Differentiation of BMSCs and Effective Bone Regeneration. Regen. Biomater. 2021, 8, rbab044. [Google Scholar] [CrossRef]
- Yu, H.; Zhang, X.; Song, W.; Pan, T.; Wang, H.; Ning, T.; Wei, Q.; Xu, H.H.K.; Wu, B.; Ma, D. Effects of 3-Dimensional Bioprinting Alginate/Gelatin Hydrogel Scaffold Extract on Proliferation and Differentiation of Human Dental Pulp Stem Cells. J. Endod. 2019, 45, 706–715. [Google Scholar] [CrossRef] [PubMed]
- Akbari, S.; Taghavi, S.M. From Breakup to Coiling and Buckling Regimes in Buoyant Viscoplastic Injections. J. Fluid Mech. 2022, 940, A42. [Google Scholar] [CrossRef]
- Zahedi Tehrani, T.; Irani, S.; Ardeshirylajimi, A.; Seyedjafari, E. Natural Based Hydrogels Promote Chondrogenic Differentiation of Human Mesenchymal Stem Cells. Front. Bioeng. Biotechnol. 2024, 12, 1363241. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Li, J.; Song, X.; Sun, T.; Mi, L.; Liu, J.; Xia, X.; Bai, N.; Li, X. Alginate/Gelatin Hydrogel Scaffold Containing nCeO2 as a Potential Osteogenic Nanomaterial for Bone Tissue Engineering. Int. J. Nanomed. 2022, 17, 6561–6578. [Google Scholar] [CrossRef]
- Filipović, V.V.; Babić Radić, M.M.; Vuković, J.S.; Vukomanović, M.; Rubert, M.; Hofmann, S.; Müller, R.; Tomić, S.L. Biodegradable Hydrogel Scaffolds Based on 2-Hydroxyethyl Methacrylate, Gelatin, Poly(β-Amino Esters), and Hydroxyapatite. Polymers 2021, 14, 18. [Google Scholar] [CrossRef]
- Sosnik, A. Alginate Particles as Platform for Drug Delivery by the Oral Route: State-of-the-Art. ISRN Pharm. 2014, 2014, 1–17. [Google Scholar] [CrossRef]
- Chen, Z.; Lv, Y. Gelatin/Sodium Alginate Composite Hydrogel with Dynamic Matrix Stiffening Ability for Bone Regeneration. Compos. Part B Eng. 2022, 243, 110162. [Google Scholar] [CrossRef]
- Naghieh, S.; Karamooz-Ravari, M.R.; Sarker, M.; Karki, E.; Chen, X. Influence of Cross-linking on the Mechanical Behavior of 3D Printed Alginate Scaffolds: Experimental and Numerical Approaches. J. Mech. Behav. Biomed. Mater. 2018, 80, 111–118. [Google Scholar] [CrossRef]
- Stojkov, G.; Niyazov, Z.; Picchioni, F.; Bose, R.K. Relationship between Structure and Rheology of Hydrogels for Various Applications. Gels 2021, 7, 255. [Google Scholar] [CrossRef]
- Gregory, T.; Benhal, P.; Scutte, A.; Quashie, D.; Harrison, K.; Cargill, C.; Grandison, S.; Savitsky, M.J.; Ramakrishnan, S.; Ali, J. Rheological Characterization of Cell-Laden Alginate-Gelatin Hydrogels for 3D Biofabrication. J. Mech. Behav. Biomed. Mater. 2022, 136, 105474. [Google Scholar] [CrossRef]
- Serafin, A.; Culebras, M.; Collins, M.N. Synthesis and Evaluation of Alginate, Gelatin, and Hyaluronic Acid Hybrid Hydrogels for Tissue Engineering Applications. Int. J. Biol. Macromol. 2023, 233, 123438. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Aledia, A.S.; Popson, S.A.; Him, L.; Hughes, C.C.W.; George, S.C. Rapid Anastomosis of Endothelial Progenitor Cell–Derived Vessels with Host Vasculature Is Promoted by a High Density of Cotransplanted Fibroblasts. Tissue Eng. Part A 2010, 16, 585–594. [Google Scholar] [CrossRef] [PubMed]
- Chai, Q.; Jiao, Y.; Yu, X. Hydrogels for Biomedical Applications: Their Characteristics and the Mechanisms behind Them. Gels 2017, 3, 6. [Google Scholar] [CrossRef] [PubMed]
- Luo, T.; Tan, B.; Zhu, L.; Wang, Y.; Liao, J. A Review on the Design of Hydrogels with Different Stiffness and Their Effects on Tissue Repair. Front. Bioeng. Biotechnol. 2022, 10, 817391. [Google Scholar] [CrossRef] [PubMed]
- Loessner, D.; Stok, K.S.; Lutolf, M.P.; Hutmacher, D.W.; Clements, J.A.; Rizzi, S.C. Bioengineered 3D Platform to Explore Cell–ECM Interactions and Drug Resistance of Epithelial Ovarian Cancer Cells. Biomaterials 2010, 31, 8494–8506. [Google Scholar] [CrossRef] [PubMed]
- Discher, D.E.; Janmey, P.; Wang, Y. Tissue Cells Feel and Respond to the Stiffness of Their Substrate. Science 2005, 310, 1139–1143. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Chi, G.; Li, P.; Lv, S.; Xu, J.; Xu, Z.; Xia, Y.; Tan, Y.; Xu, J.; Li, L.; et al. Effects of Matrix Stiffness on the Morphology, Adhesion, Proliferation and Osteogenic Differentiation of Mesenchymal Stem Cells. Int. J. Med. Sci. 2018, 15, 257–268. [Google Scholar] [CrossRef] [PubMed]
- Pelham, R.J.; Wang, Y. Cell Locomotion and Focal Adhesions Are Regulated by Substrate Flexibility. Proc. Natl. Acad. Sci. USA 1997, 94, 13661–13665. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, U.S.; Safran, S.A. Physics of Adherent Cells. Rev. Mod. Phys. 2013, 85, 1327–1381. [Google Scholar] [CrossRef]
- Trappmann, B.; Gautrot, J.E.; Connelly, J.T.; Strange, D.G.T.; Li, Y.; Oyen, M.L.; Cohen Stuart, M.A.; Boehm, H.; Li, B.; Vogel, V.; et al. Extracellular-Matrix Tethering Regulates Stem-Cell Fate. Nat. Mater. 2012, 11, 642–649. [Google Scholar] [CrossRef]
- Chaudhuri, O.; Koshy, S.T.; Branco da Cunha, C.; Shin, J.-W.; Verbeke, C.S.; Allison, K.H.; Mooney, D.J. Extracellular Matrix Stiffness and Composition Jointly Regulate the Induction of Malignant Phenotypes in Mammary Epithelium. Nat. Mater. 2014, 13, 970–978. [Google Scholar] [CrossRef] [PubMed]
- Beck, G.R.; Zerler, B.; Moran, E. Phosphate Is a Specific Signal for Induction of Osteopontin Gene Expression. Proc. Natl. Acad. Sci. USA 2000, 97, 8352–8357. [Google Scholar] [CrossRef] [PubMed]
- Ginebra, M.P.; Traykova, T.; Planell, J.A. Calcium Phosphate Cements as Bone Drug Delivery Systems: A Review. J. Control. Release 2006, 113, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Akkouch, A.; Zhang, Z.; Rouabhia, M. Engineering Bone Tissue Using Human Dental Pulp Stem Cells and an Osteogenic Collagen-Hydroxyapatite-Poly(L-Lactide-Co-ε-Caprolactone) Scaffold. J. Biomater. Appl. 2014, 28, 922–936. [Google Scholar] [CrossRef] [PubMed]
- Alipour, M.; Firouzi, N.; Aghazadeh, Z.; Samiei, M.; Montazersaheb, S.; Khoshfetrat, A.B.; Aghazadeh, M. The Osteogenic Differentiation of Human Dental Pulp Stem Cells in Alginate-Gelatin/Nano-Hydroxyapatite Microcapsules. BMC Biotechnol. 2021, 21, 6. [Google Scholar] [CrossRef]
- Jones, J.R.; Tsigkou, O.; Coates, E.E.; Stevens, M.M.; Polak, J.M.; Hench, L.L. Extracellular Matrix Formation and Mineralization on a Phosphate-Free Porous Bioactive Glass Scaffold Using Primary Human Osteoblast (HOB) Cells. Biomaterials 2007, 28, 1653–1663. [Google Scholar] [CrossRef] [PubMed]
- Ahearne, M. Introduction to Cell–Hydrogel Mechanosensing. Interface Focus 2014, 4, 20130038. [Google Scholar] [CrossRef] [PubMed]
- Labouesse, C.; Tan, B.X.; Agley, C.C.; Hofer, M.; Winkel, A.K.; Stirparo, G.G.; Stuart, H.T.; Verstreken, C.M.; Mulas, C.; Mansfield, W.; et al. StemBond Hydrogels Control the Mechanical Microenvironment for Pluripotent Stem Cells. Nat. Commun. 2021, 12, 6132. [Google Scholar] [CrossRef]
- Yi, B.; Xu, Q.; Liu, W. An Overview of Substrate Stiffness Guided Cellular Response and Its Applications in Tissue Regeneration. Bioact. Mater. 2022, 15, 82–102. [Google Scholar] [CrossRef]
- Revete, A.; Aparicio, A.; Cisterna, B.A.; Revete, J.; Luis, L.; Ibarra, E.; Segura González, E.A.; Molino, J.; Reginensi, D. Advancements in the Use of Hydrogels for Regenerative Medicine: Properties and Biomedical Applications. Int. J. Biomater. 2022, 2022, 1–16. [Google Scholar] [CrossRef]
- Engler, A.J.; Sen, S.; Sweeney, H.L.; Discher, D.E. Matrix Elasticity Directs Stem Cell Lineage Specification. Cell 2006, 126, 677–689. [Google Scholar] [CrossRef] [PubMed]
- Solbu, A.A.; Caballero, D.; Damigos, S.; Kundu, S.C.; Reis, R.L.; Halaas, Ø.; Chahal, A.S.; Strand, B.L. Assessing Cell Migration in Hydrogels: An Overview of Relevant Materials and Methods. Mater. Today Bio 2023, 18, 100537. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferjaoui, Z.; López-Muñoz, R.; Akbari, S.; Chandad, F.; Mantovani, D.; Rouabhia, M.; D. Fanganiello, R. Design of Alginate/Gelatin Hydrogels for Biomedical Applications: Fine-Tuning Osteogenesis in Dental Pulp Stem Cells While Preserving Other Cell Behaviors. Biomedicines 2024, 12, 1510. https://doi.org/10.3390/biomedicines12071510
Ferjaoui Z, López-Muñoz R, Akbari S, Chandad F, Mantovani D, Rouabhia M, D. Fanganiello R. Design of Alginate/Gelatin Hydrogels for Biomedical Applications: Fine-Tuning Osteogenesis in Dental Pulp Stem Cells While Preserving Other Cell Behaviors. Biomedicines. 2024; 12(7):1510. https://doi.org/10.3390/biomedicines12071510
Chicago/Turabian StyleFerjaoui, Zied, Roberto López-Muñoz, Soheil Akbari, Fatiha Chandad, Diego Mantovani, Mahmoud Rouabhia, and Roberto D. Fanganiello. 2024. "Design of Alginate/Gelatin Hydrogels for Biomedical Applications: Fine-Tuning Osteogenesis in Dental Pulp Stem Cells While Preserving Other Cell Behaviors" Biomedicines 12, no. 7: 1510. https://doi.org/10.3390/biomedicines12071510
APA StyleFerjaoui, Z., López-Muñoz, R., Akbari, S., Chandad, F., Mantovani, D., Rouabhia, M., & D. Fanganiello, R. (2024). Design of Alginate/Gelatin Hydrogels for Biomedical Applications: Fine-Tuning Osteogenesis in Dental Pulp Stem Cells While Preserving Other Cell Behaviors. Biomedicines, 12(7), 1510. https://doi.org/10.3390/biomedicines12071510





