Plasma Neurofilament Light Chain: A Potential Biomarker for Neurological Dysfunction in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants and Demographic Data
2.2. Laboratory Procedures
2.3. Neuropsychologic and Neuropsychiatric Assessment
2.4. Peripheral Nervous System
2.5. Statistical Analysis
3. Results
3.1. Participants
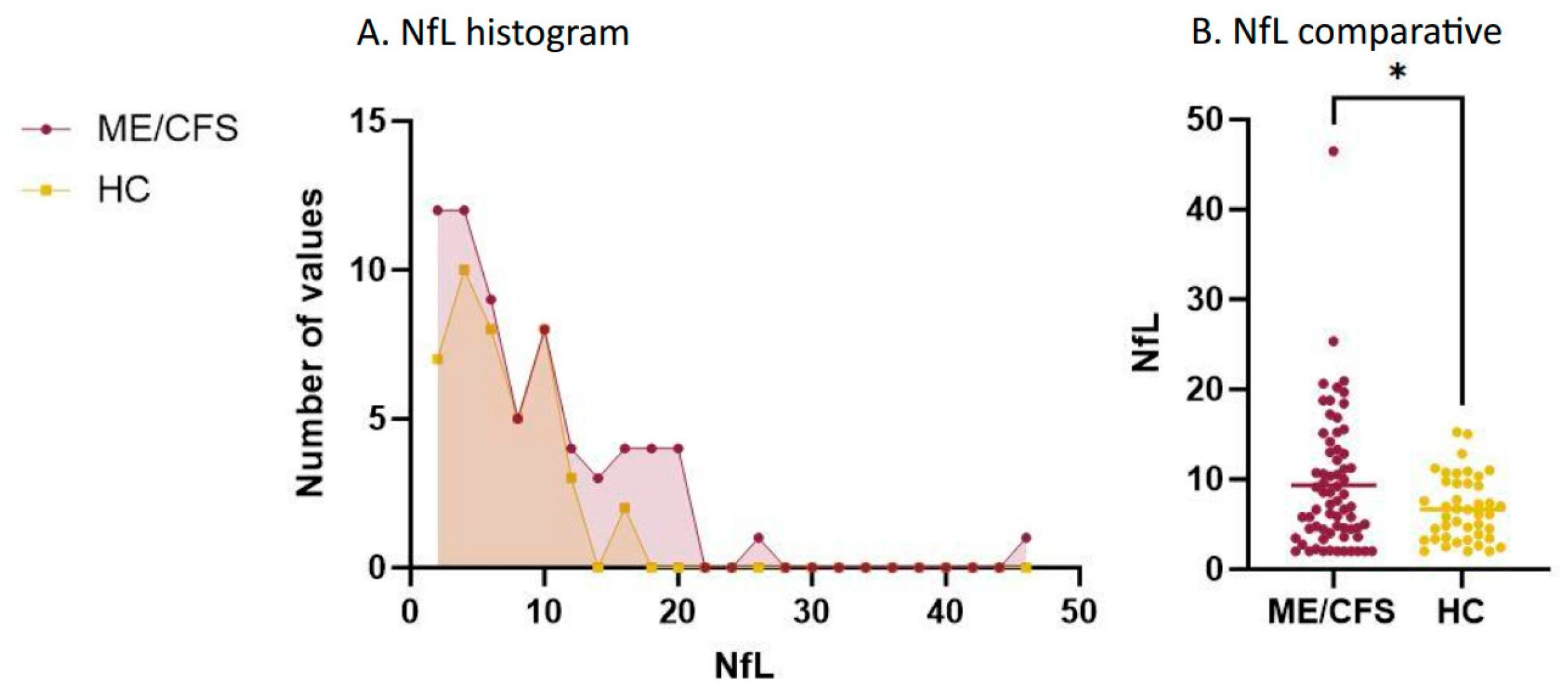
3.2. Plasma NfL
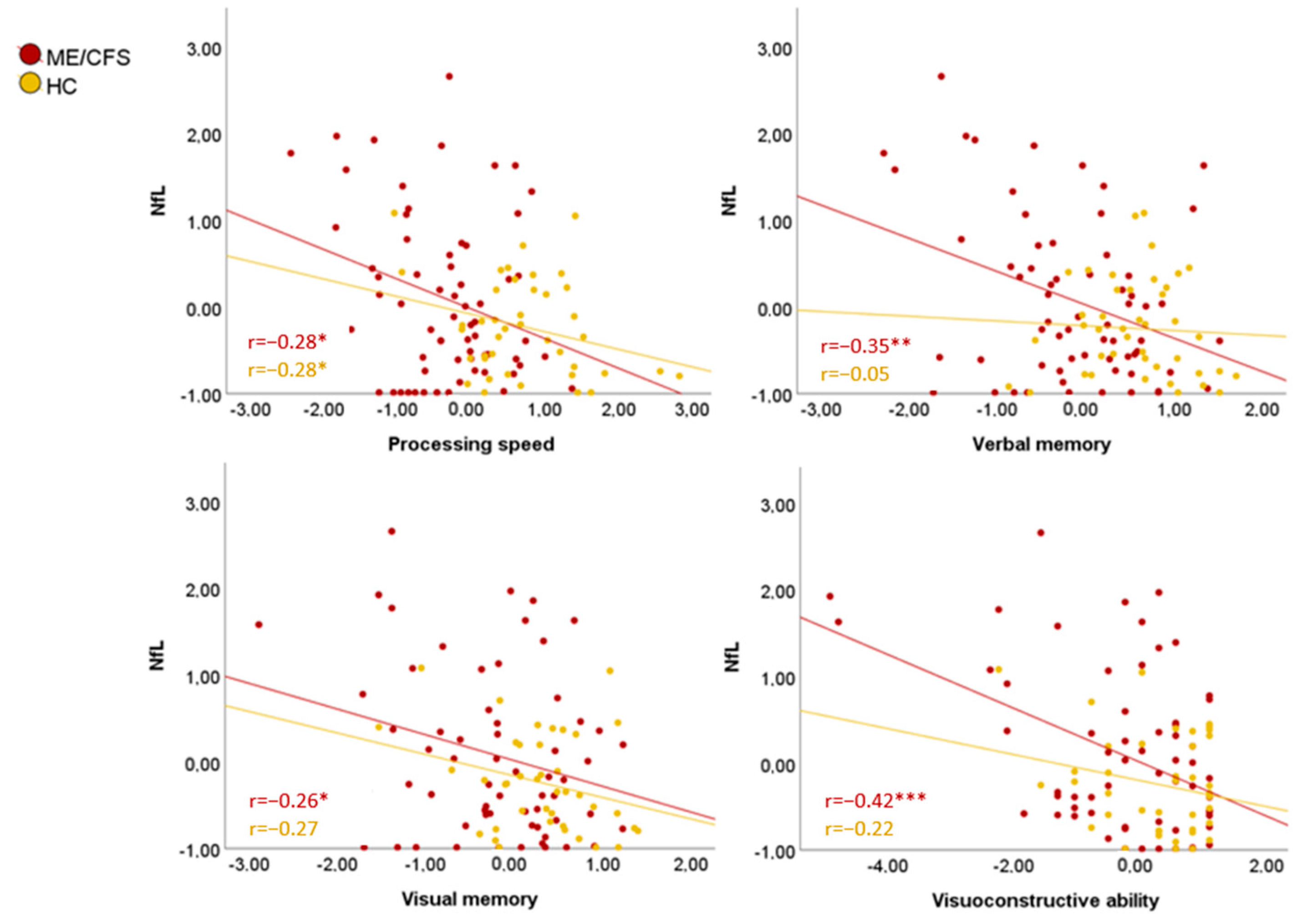
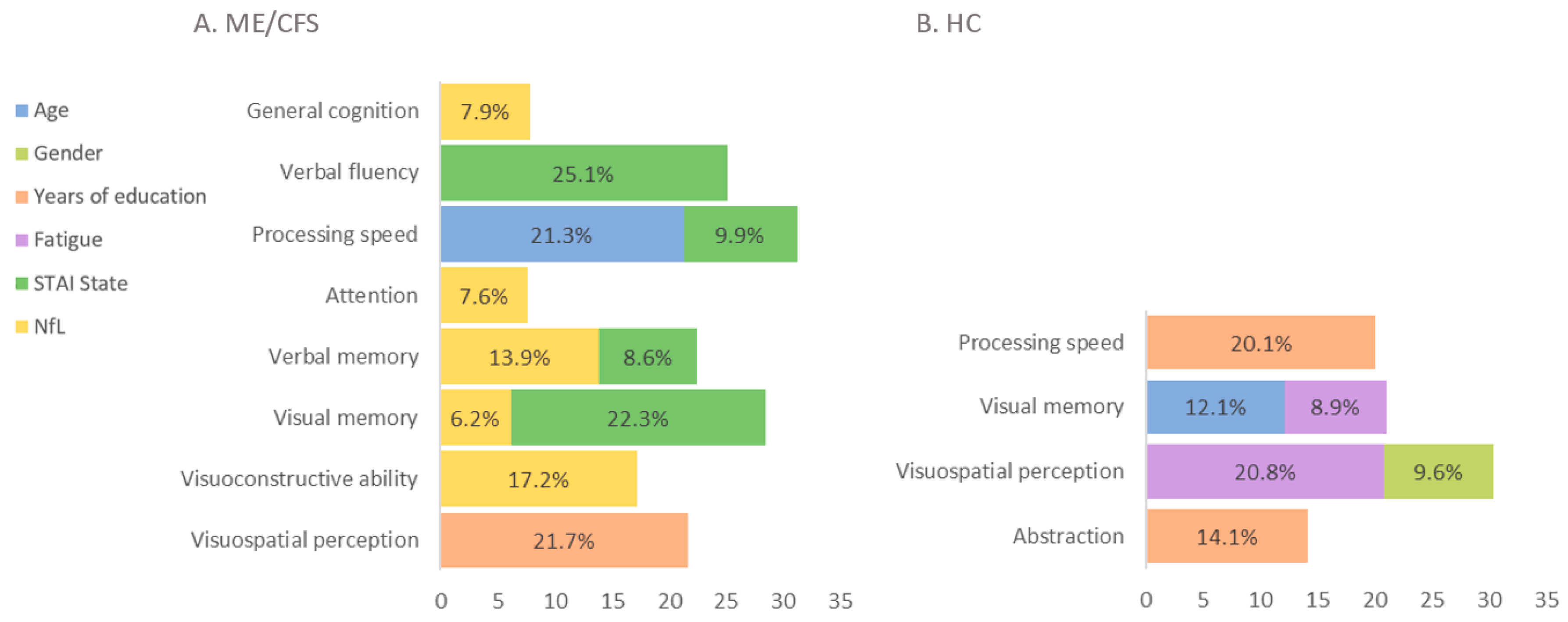
3.3. Neuropsychologic and Neuropsychiatric Results
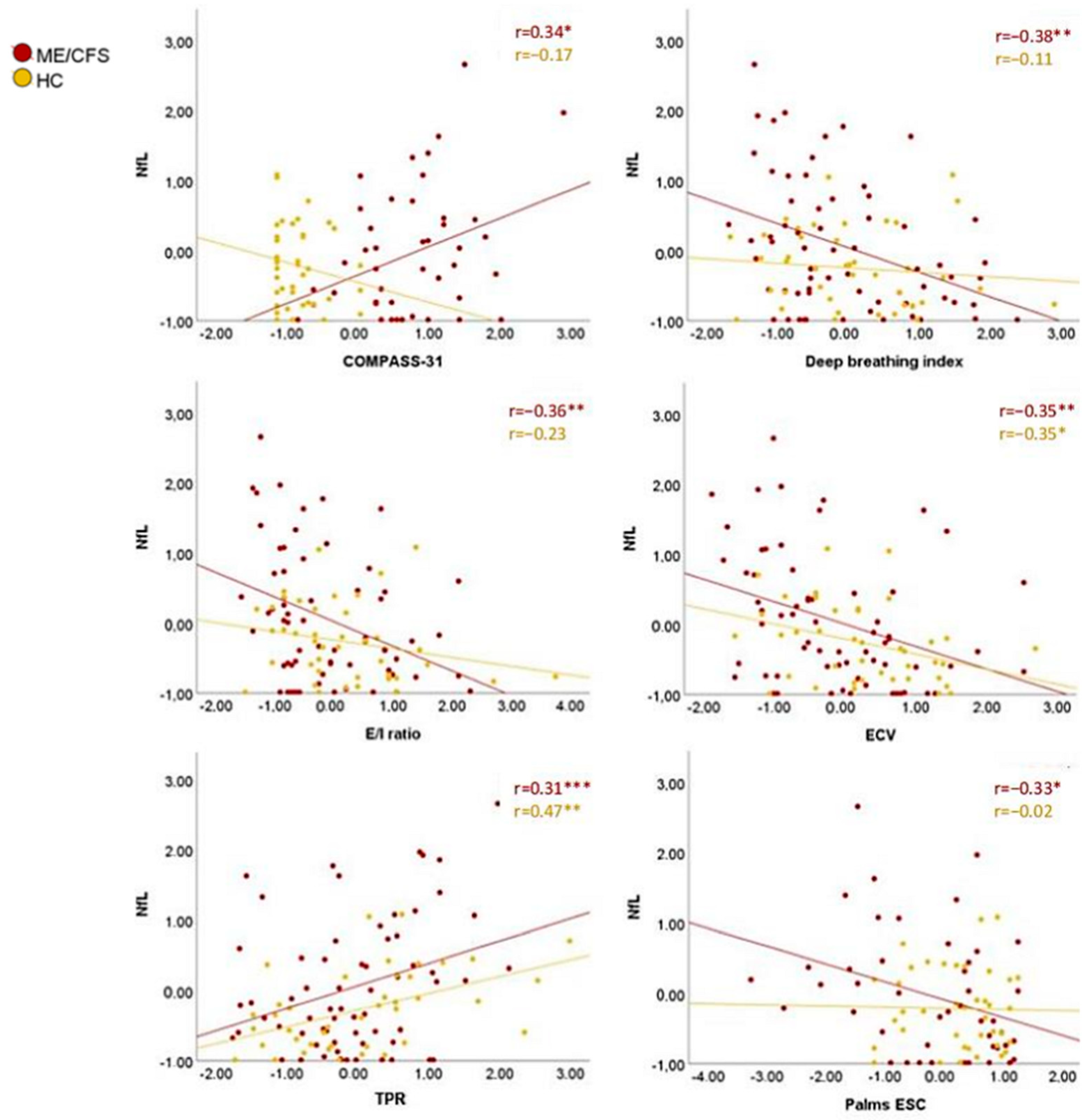
3.4. Peripheral Nervous System
3.5. Plasma NfL Implications
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Azcue, N.; Gómez-Esteban, J.C.; Acera, M.; Tijero, B.; Fernandez, T.; Ayo-Mentxakatorre, N.; Pérez-Concha, T.; Murueta-Goyena, A.; Lafuente, J.V.; Prada, Á.; et al. Brain fog of post-COVID-19 condition and Chronic Fatigue Syndrome, same medical disorder? J. Transl. Med. 2022, 20, 569. [Google Scholar] [CrossRef] [PubMed]
- Azcue, N.; Del Pino, R.; Acera, M.; Fernández-Valle, T.; Ayo-Mentxakatorre, N.; Pérez-Concha, T.; Murueta-Goyena, A.; Lafuente, J.V.; Prada, A.; de Munain, A.L.; et al. Dysautonomia and small fiber neuropathy in post-COVID condition and Chronic Fatigue Syndrome. J. Transl. Med. 2023, 21, 814. [Google Scholar] [CrossRef] [PubMed]
- Deumer, U.S.; Varesi, A.; Floris, V.; Savioli, G.; Mantovani, E.; López-Carrasco, P.; Rosati, G.M.; Prasad, S.; Ricevuti, G. Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS): An Overview. J. Clin. Med. 2021, 10, 4786. [Google Scholar] [CrossRef]
- Lim, E.-J.; Son, C.-G. Review of case definitions for myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS). J. Transl. Med. 2020, 18, 289. [Google Scholar] [CrossRef] [PubMed]
- Maksoud, R.; Magawa, C.; Eaton-Fitch, N.; Thapaliya, K.; Marshall-Gradisnik, S. Biomarkers for myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS): A systematic review. BMC Med. 2023, 21, 189. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, K.; Straus, S.E.; Hickie, I.; Sharpe, M.C.; Dobbins, J.G.; Komaroff, A.; International Chronic Fatigue Syndrome Study Group. The chronic fatigue syndrome: A comprehensive approach to its definition and study. Ann. Intern. Med. 1994, 121, 953–959. [Google Scholar] [CrossRef]
- Carruthers, B.M.; Van de Sande, M.I. Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: A Clinical Case Definition and Guidelines for Medical Practitioners; An Overview of the Canadian Consensus Document; The National Library of Canada: Ottawa, ON, Canada, 2003. [Google Scholar]
- Carruthers, B.M.; Van De Sande, M.I.; De Meirleir, K.L.; Klimas, N.G.; Broderick, G.; Mitchell, T.; Staines, D.; Powles, A.C.; Speight, N.; Vallings, R.; et al. Myalgic encephalomyelitis: International Consensus Criteria. J. Intern. Med. 2011, 270, 327–338. [Google Scholar] [CrossRef] [PubMed]
- Blomberg, J.; Gottfries, C.-G.; Elfaitouri, A.; Rizwan, M.; Rosén, A. Infection elicited autoimmunity and Myalgic encephalomyelitis/chronic fatigue syndrome: An explanatory model. Front. Immunol. 2018, 9, 229. [Google Scholar] [CrossRef]
- Brenu, E.W.; van Driel, M.L.; Staines, D.R.; Ashton, K.J.; Ramos, S.B.; Keane, J.; Klimas, N.G.; Marshall-Gradisnik, S.M. Immunological abnormalities as potential biomarkers in Chronic Fatigue Syndrome/Myalgic Encephalomyelitis. J. Transl. Med. 2011, 9, 81. [Google Scholar] [CrossRef] [PubMed]
- Gravelsina, S.; Vilmane, A.; Svirskis, S.; Rasa-Dzelzkaleja, S.; Nora-Krukle, Z.; Vecvagare, K.; Krumina, A.; Leineman, I.; Shoenfeld, Y.; Murovska, M. Biomarkers in the diagnostic algorithm of myalgic encephalomyelitis/chronic fatigue syndrome. Front. Immunol. 2022, 13, 928945. [Google Scholar] [CrossRef]
- Vernon, S.D.; Unger, E.R.; Dimulescu, I.M.; Rajeevan, M.; Reeves, W.C. Utility of the Blood for Gene Expression Profiling and Biomarker Discovery in Chronic Fatigue Syndrome. Dis. Markers 2002, 18, 193–199. [Google Scholar] [CrossRef]
- Fletcher, M.A.; Rosenthal, M.; Antoni, M.; Ironson, G.; Zeng, X.R.; Barnes, Z.; Harvey, J.M.; Hurwitz, B.; Levis, S.; Broderick, G.; et al. Plasma neuropeptide Y: A biomarker for symptom severity in chronic fatigue syndrome. Behav. Brain Funct. 2010, 6, 76. [Google Scholar] [CrossRef]
- Poenaru, S.; Abdallah, S.J.; Corrales-Medina, V.; Cowan, J. COVID-19 and post-infectious myalgic encephalomyelitis/chronic fatigue syndrome: A narrative review. Ther. Adv. Infect. Dis. 2021, 8, 20499361211009385. [Google Scholar] [CrossRef] [PubMed]
- Kedor, C.; Freitag, H.; Meyer-Arndt, L.; Wittke, K.; Hanitsch, L.G.; Zoller, T.; Steinbeis, F.; Haffke, M.; Rudolf, G.; Heidecker, B.; et al. A prospective observational study of post-COVID-19 chronic fatigue syndrome following the first pandemic wave in Germany and biomarkers associated with symptom severity. Nat. Commun. 2022, 13, 5104. [Google Scholar] [CrossRef]
- Komaroff, A.L.; Lipkin, W.I. Insights from myalgic encephalomyelitis/chronic fatigue syndrome may help unravel the pathogenesis of postacute COVID-19 syndrome. Trends Mol. Med. 2021, 27, 895–906. [Google Scholar] [CrossRef]
- Gutman, E.G.; Salvio, A.L.; Fernandes, R.A.; Duarte, L.A.; Raposo-Vedovi, J.V.; Alcaraz, H.F.; Teixeira, M.A.; Passos, G.F.; de Medeiros, K.Q.M.; Hammerle, M.B.; et al. Long COVID: Plasma levels of neurofilament light chain in mild COVID-19 patients with neurocognitive symptoms. Mol. Psychiatry 2024, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Ashton, N.J.; Janelidze, S.; Al Khleifat, A.; Leuzy, A.; van der Ende, E.L.; Karikari, T.K.; Benedet, A.L.; Pascoal, T.A.; Lleó, A.; Parnetti, L.; et al. A multicentre validation study of the diagnostic value of plasma neurofilament light. Nat. Commun. 2021, 12, 3400. [Google Scholar] [CrossRef]
- Budelier, M.M.; He, Y.; Barthelemy, N.R.; Jiang, H.; Li, Y.; Park, E.; Henson, R.L.; Schindler, S.E.; Holtzman, D.M.; Bateman, R.J. A map of neurofilament light chain species in brain and cerebrospinal fluid and alterations in Alzheimer’s disease. Brain Commun. 2022, 4, fcac045. [Google Scholar] [CrossRef]
- Verde, F.; Otto, M.; Silani, V. Neurofilament Light Chain as Biomarker for Amyotrophic Lateral Sclerosis and Frontotemporal Dementia. Front. Neurosci. 2021, 15, 679199. [Google Scholar] [CrossRef] [PubMed]
- Bhan, A.; Jacobsen, C.; Myhr, K.M.; Dalen, I.; Lode, K.; Farbu, E. Neurofilaments and 10-year follow-up in multiple sclerosis. Mult. Scler. J. 2018, 24, 1301–1307. [Google Scholar] [CrossRef]
- Osborn, K.E.; Khan, O.A.; Kresge, H.A.; Bown, C.W.; Liu, D.; Moore, E.E.; Gifford, K.A.; Acosta, L.M.Y.; Bell, S.P.; Hohman, T.J.; et al. Cerebrospinal fluid and plasma neurofilament light relate to abnormal cognition. Alzheimer’s Dement. Diagn. Assess. Dis. Monit. 2019, 11, 700–709. [Google Scholar] [CrossRef] [PubMed]
- Barro, C.; Chitnis, T.; Weiner, H.L. Blood neurofilament light: A critical review of its application to neurologic disease. Ann. Clin. Transl. Neurol. 2020, 7, 2508–2523. [Google Scholar] [CrossRef] [PubMed]
- Vermunt, L.; Otte, M.; Verberk, I.M.W.; Killestein, J.; Lemstra, A.W.; van der Flier, W.M.; Pijnenburg, Y.A.L.; Vijverberg, E.G.B.; Bouwman, F.H.; Gravesteijn, G.; et al. Age- and disease-specific reference values for neurofilament light presented in an online interactive support interface. Ann. Clin. Transl. Neurol. 2022, 9, 1832–1837. [Google Scholar] [CrossRef] [PubMed]
- Beltran, T.A. Normative Values for Serum Neurofilament Light Chain in US Adults. J. Clin. Neurol. 2024, 20, 46–49. [Google Scholar] [CrossRef]
- Casellini, C.M.; Parson, H.K.; Richardson, M.S.; Nevoret, M.L.; Vinik, A.I. Sudoscan, a noninvasive tool for detecting diabetic small fiber neuropathy and autonomic dysfunction. Diabetes Technol. Ther. 2013, 15, 948–953. [Google Scholar] [CrossRef]
- Nielsen, H.H.; Soares, C.B.; Høgedal, S.S.; Madsen, J.S.; Hansen, R.B.; Christensen, A.A.; Madsen, C.; Clausen, B.H.; Frich, L.H.; Degn, M.; et al. Acute Neurofilament Light Chain Plasma Levels Correlate with Stroke Severity and Clinical Outcome in Ischemic Stroke Patients. Front. Neurol. 2020, 11, 448. [Google Scholar] [CrossRef] [PubMed]
- Danilenko, O.V.; Gavrilova, N.Y.; Churilov, L.P. Chronic Fatigue Exhibits Heterogeneous Autoimmunity Characteristics Which Reflect Etiology. Pathophysiology 2022, 29, 187–199. [Google Scholar] [CrossRef]
- Berkis, U.; Svirskis, S.; Krumina, A.; Gravelsina, S.; Vilmane, A.; Araja, D.; Nora-Krukle, Z.; Murovska, M. Exploring the joint potential of inflammation, immunity, and receptor-based biomarkers for evaluating ME/CFS progression. Front. Immunol. 2023, 14, 1294758. [Google Scholar] [CrossRef] [PubMed]
- Morris, G.; Berk, M.; Galecki, P.; Maes, M. The emerging role of autoimmunity in myalgic encephalomyelitis/chronic fatigue syndrome (ME/cfs). Mol. Neurobiol. 2014, 49, 741–756. [Google Scholar] [CrossRef]
- Russell, L.; Broderick, G.; Taylor, R.; Fernandes, H.; Harvey, J.; Barnes, Z.; Smylie, A.; Collado, F.; Balbin, E.G.; Katz, B.Z.; et al. Illness progression in chronic fatigue syndrome: A shifting immune baseline. BMC Immunol. 2016, 17, 3. [Google Scholar] [CrossRef]
- Nakatomi, Y.; Mizuno, K.; Ishii, A.; Wada, Y.; Tanaka, M.; Tazawa, S.; Onoe, K.; Fukuda, S.; Kawabe, J.; Takahashi, K.; et al. Neuroinflammation in patients with chronic fatigue syndrome/myalgic encephalomyelitis: An11C-(R)-PK11195 PET study. J. Nucl. Med. 2014, 55, 945–950. [Google Scholar] [CrossRef] [PubMed]
- Glassford, J.A.G. The neuroinflammatory etiopathology of myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS). Front. Physiol. 2017, 8, 88. [Google Scholar] [CrossRef] [PubMed]
- Arnett, S.; Alleva, L.; Korossy-Horwood, R.; Clark, I. Chronic fatigue syndrome—A neuroimmunological model. Med. Hypotheses 2011, 77, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Ferrero, K.; Silver, M.; Cocchetto, A.; Masliah, E.; Langford, D. Cns Findings in Chronic Fatigue Syndrome and a Neuropathological Case Report. J. Investig. Med. 2017, 65, 974–983. [Google Scholar] [CrossRef] [PubMed]
- Nacul, L.; O’Boyle, S.; Palla, L.; Nacul, F.E.; Mudie, K.; Kingdon, C.C.; Cliff, J.M.; Clark, T.G.; Dockrell, H.M.; Lacerda, E.M. How Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS) Progresses: The Natural History of ME/CFS. Front. Neurol. 2020, 11, 826. [Google Scholar] [CrossRef] [PubMed]
- Duindam, H.; Mengel, D.; Kox, M.; Göpfert, J.; Kessels, R.; Synofzik, M.; Pickkers, P.; Abdo, W. Systemic inflammation relates to neuroaxonal damage associated with long-term cognitive dysfunction in COVID-19 patients. Brain Behav. Immun. 2024, 117, 510–520. [Google Scholar] [CrossRef] [PubMed]
- Maia, L.F.; Maceski, A.; Conceição, I.; Obici, L.; Magalhães, R.; Cortese, A.; Leppert, D.; Merlini, G.; Kuhle, J.; Saraiva, M.J. Plasma neurofilament light chain: An early biomarker for hereditary ATTR amyloid polyneuropathy. Amyloid 2020, 27, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Cavaletti, G.; Pizzamiglio, C.; Man, A.; Engber, T.M.; Comi, C.; Wilbraham, D. Studies to Assess the Utility of Serum Neurofilament Light Chain as a Biomarker in Chemotherapy-Induced Peripheral Neuropathy. Cancers 2023, 15, 4216. [Google Scholar] [CrossRef] [PubMed]
- Baka, P.; Steenken, L.; Escolano-Lozano, F.; Steffen, F.; Papagianni, A.; Sommer, C.; Pogatzki-Zahn, E.; Hirsch, S.; Protopapa, M.; Bittner, S.; et al. Studying serum neurofilament light chain levels as a potential new biomarker for small fiber neuropathy. Eur. J. Neurol. 2024, 31, e16192. [Google Scholar] [CrossRef]
- Vicente, M.C.; Humphrey, C.M.; Gargaglioni, L.H.; Ostrowski, T.D. Decreased excitability of locus coeruleus neurons during hypercapnia is exaggerated in the streptozotocin-model of Alzheimer’s disease. Exp. Neurol. 2020, 328, 113250. [Google Scholar] [CrossRef]
- Shan, Z.Y.; Barnden, L.R.; Kwiatek, R.A.; Bhuta, S.; Hermens, D.F.; Lagopoulos, J. Neuroimaging characteristics of myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS): A systematic review. J. Transl. Med. 2020, 18, 335. [Google Scholar] [CrossRef] [PubMed]
- Daniela, M.; Catalina, L.; Ilie, O.; Paula, M.; Daniel-Andrei, I.; Ioana, B. Effects of Exercise Training on the Autonomic Nervous System with a Focus on Anti-Inflammatory and Antioxidants Effects. Antioxidants 2022, 11, 350. [Google Scholar] [CrossRef] [PubMed]

| ME/CFS (n = 67) M (SD) | HC (n = 43) M (SD) | Statistics | |
|---|---|---|---|
| Age, years | 44.63 (10.42) | 43.19 (9.18) | U = 1260.00 |
| Education, years | 15.19 (5.52) | 16.45 (2.72) | U = 1764.50 * |
| Female, n (%) | 61 (91.00%) | 34 (79.10%) | χ2 = 3.19 |
| Disease duration, months | 60.59 (59.07) | - | - |
| COMPASS-31 | 25.24 (10.79) | 4.13 (4.73) | U = 73.00 *** |
| SFNSL | 34.51 (15.86) | 2.46 (3.68) | U = 34.50 *** |
| SF-36 | 30.48 (16.19) | 85.81 (9.04) | U = 2653.00 *** |
| MFIS | 66.04 (14.95) | 10.19 (11.73) | U = 21.50 *** |
| PSQI | 12.84 (4.78) | 5.24 (3.13) | U = 283.00 *** |
| STAI-State | 30.37 (15.49) | 11.47 (4.37) | U = 424.50 *** |
| STAI-Trait | 20.98 (15.64) | 13.21 (8.79) | U = 1010.00 * |
| GDS | 8.92 (3.72) | 1.02 (1.45) | U = 53.00 *** |
| C-SSRS | 1.00 (1.74) | 0.00 (0.00) | U = 840.00 *** |
| Cognitive performance (z-scores) | |||
| General cognition | −0.12 (0.25) | 0.19 (0.07) | F = 22.83 *** |
| Verbal fluency | −0.27 (0.81) | 2.48 (0.88) | F = 55.66 *** |
| Processing speed | −0.34 (0.76) | 2.83 (0.73) | F = 48.43 *** |
| Attention | −0.34 (0.58) | 0.73 (0.07) | F = 16.89 *** |
| Verbal memory | −0.14 (0.85) | 0.57 (0.58) | F = 23.25 *** |
| Visual memory | −0.22 (0.84) | 0.31 (0.63) | F = 12.15 *** |
| Visuoconstructive ability | −0.23 (1.26) | 0.28 (0.84) | F = 5.07 * |
| Visuospatial perception | −0.25 (0.96) | 0.36 (0.74) | F = 11.92 *** |
| Abstraction | −0.40 (0.87) | 0.47 (0.78) | F = 27.76 *** |
| Executive functions | −0.41 (0.26) | −0.37 (0.38) | F = 0.28 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Azcue, N.; Tijero-Merino, B.; Acera, M.; Pérez-Garay, R.; Fernández-Valle, T.; Ayo-Mentxakatorre, N.; Ruiz-López, M.; Lafuente, J.V.; Gómez Esteban, J.C.; Del Pino, R. Plasma Neurofilament Light Chain: A Potential Biomarker for Neurological Dysfunction in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome. Biomedicines 2024, 12, 1539. https://doi.org/10.3390/biomedicines12071539
Azcue N, Tijero-Merino B, Acera M, Pérez-Garay R, Fernández-Valle T, Ayo-Mentxakatorre N, Ruiz-López M, Lafuente JV, Gómez Esteban JC, Del Pino R. Plasma Neurofilament Light Chain: A Potential Biomarker for Neurological Dysfunction in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome. Biomedicines. 2024; 12(7):1539. https://doi.org/10.3390/biomedicines12071539
Chicago/Turabian StyleAzcue, Naiara, Beatriz Tijero-Merino, Marian Acera, Raquel Pérez-Garay, Tamara Fernández-Valle, Naia Ayo-Mentxakatorre, Marta Ruiz-López, Jose Vicente Lafuente, Juan Carlos Gómez Esteban, and Rocio Del Pino. 2024. "Plasma Neurofilament Light Chain: A Potential Biomarker for Neurological Dysfunction in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome" Biomedicines 12, no. 7: 1539. https://doi.org/10.3390/biomedicines12071539
APA StyleAzcue, N., Tijero-Merino, B., Acera, M., Pérez-Garay, R., Fernández-Valle, T., Ayo-Mentxakatorre, N., Ruiz-López, M., Lafuente, J. V., Gómez Esteban, J. C., & Del Pino, R. (2024). Plasma Neurofilament Light Chain: A Potential Biomarker for Neurological Dysfunction in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome. Biomedicines, 12(7), 1539. https://doi.org/10.3390/biomedicines12071539





