Air Pollution Increases Risk of Occurrence of Intracerebral Haemorrhage but Not of Subarachnoid Haemorrhage: Time-Series Cross-Sectional Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Incidence and Environmental Data
2.3. Statistical Methods
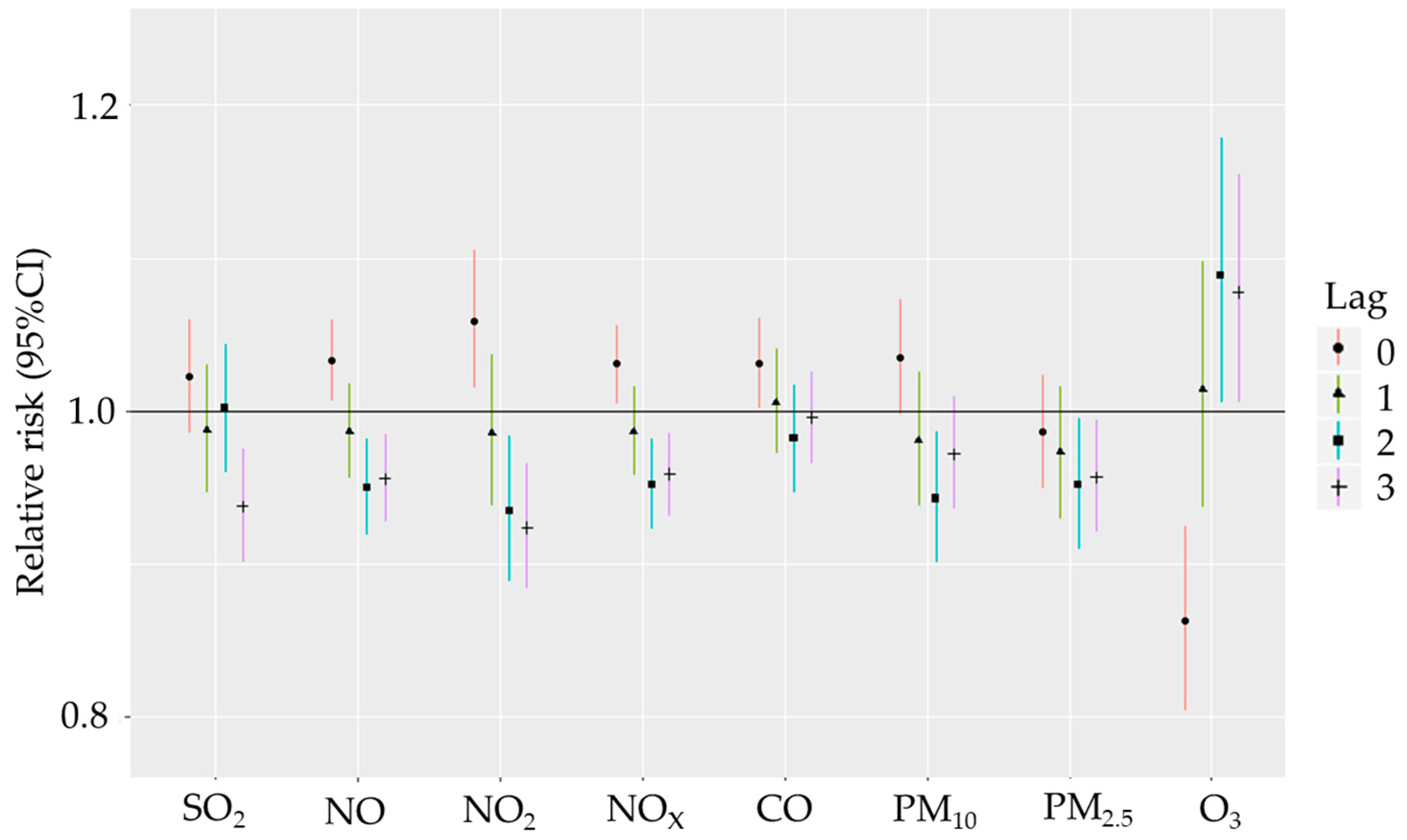
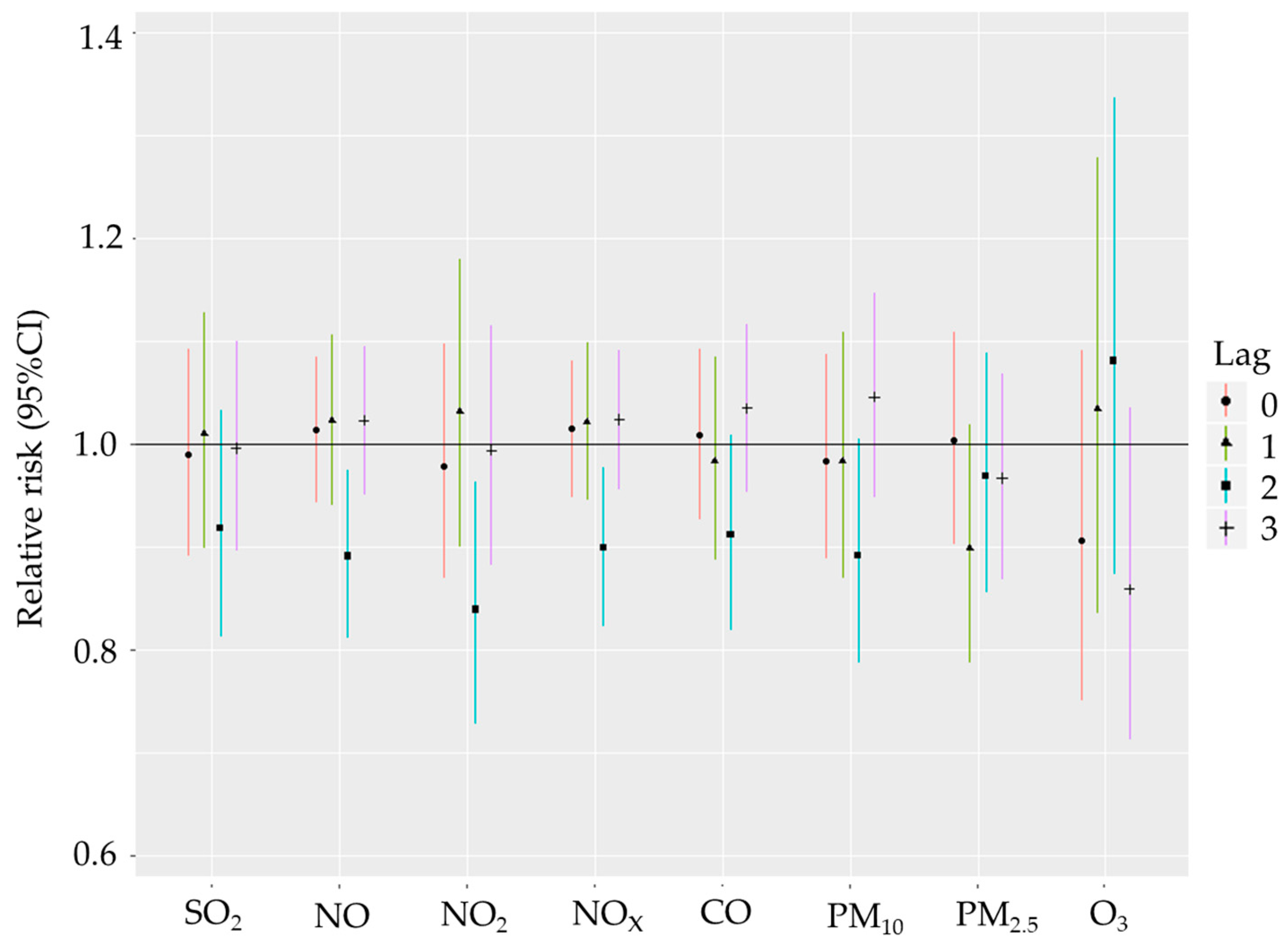
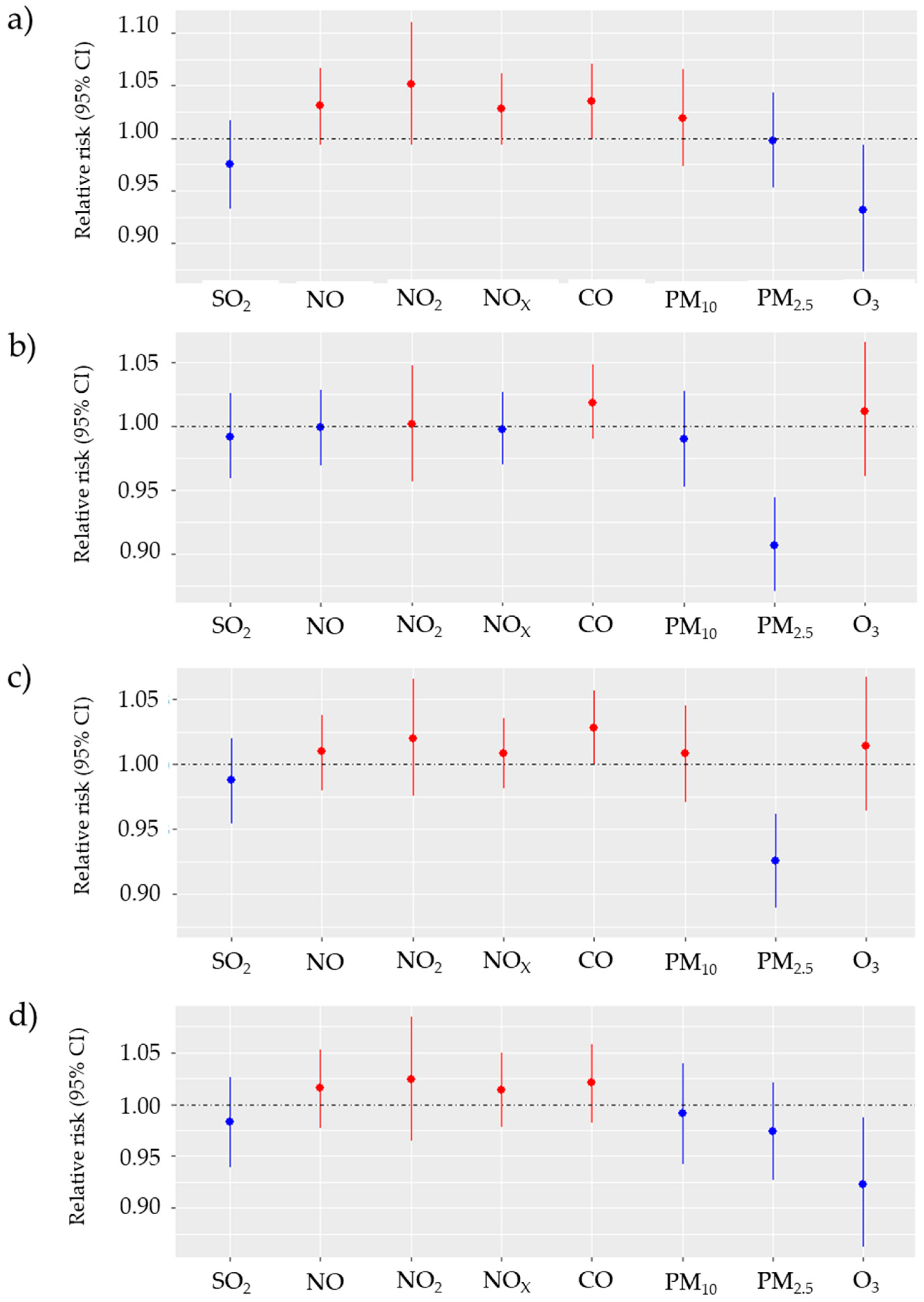
3. Results
Descriptive Analysis and Correlation Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- van Asch, C.J.; Luitse, M.J.; Rinkel, G.J.; van der Tweel, I.; Algra, A.; Klijn, C.J. Incidence, Case Fatality, and Functional Outcome of Intracerebral Haemorrhage over Time, according to Age, Sex, and Ethnic Origin: A Systematic Review and Meta-Analysis. Lancet Neurol. 2010, 9, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Feigin, V.L.; Krishnamurthi, R.V.; Parmar, P.; Norrving, B.; Mensah, G.A.; Bennett, D.A.; Barker-Collo, S.; Moran, A.E.; Sacco, R.L.; Truelsen, T.; et al. Update on the Global Burden of Ischemic and Hemorrhagic Stroke in 1990–2013: The GBD 2013 Study. Neuroepidemiology 2015, 45, 161–176. [Google Scholar] [CrossRef] [PubMed]
- Poon, M.T.C.; Fonville, A.F.; Al-Shahi Salman, R. Long-Term Prognosis after Intracerebral Haemorrhage: Systematic Review and Meta-Analysis. J. Neurol. Neurosurg. Psychiatry 2014, 85, 660–667. [Google Scholar] [CrossRef] [PubMed]
- Butland, B.K.; Atkinson, R.W.; Crichton, S.; Barratt, B.; Beevers, S.; Spiridou, A.; Hoang, U.; Kelly, F.J.; Wolfe, C.D. Air Pollution and the Incidence of Ischaemic and Haemorrhagic Stroke in the South London Stroke Register: A Case–Cross-over Analysis. J. Epidemiol. Community Health 2017, 71, 707–712. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.S.V.; Lee, K.K.; McAllister, D.A.; Hunter, A.; Nair, H.; Whiteley, W.; Langrish, J.P.; Newby, D.E.; Mills, N.L. Short Term Exposure to Air Pollution and Stroke: Systematic Review and Meta-Analysis. BMJ 2015, 350, h1295. [Google Scholar] [CrossRef] [PubMed]
- Chan, C.-C.; Chuang, K.-J.; Chien, L.-C.; Chen, W.-J.; Chang, W.-T. Urban Air Pollution and Emergency Admissions for Cerebrovascular Diseases in Taipei, Taiwan. Eur. Heart J. 2006, 27, 1238–1244. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Ding, R.; Xiao, C.; Xu, Y.; Cheng, H.; Zhu, F.; Lei, R.; Di, D.; Zhao, Q.; Cao, J. Association between Air Pollution and Cardiovascular Mortality in Hefei, China: A Time-Series Analysis. Environ. Pollut. 2017, 229, 790–797. [Google Scholar] [CrossRef] [PubMed]
- Nzwalo, H.; Guilherme, P.; Nogueira, J.; Félix, C.; André, A.; Teles, J.; Mouzinho, M.; Ferreira, F.; Marreiros, A.; Logallo, N.; et al. Fine Particulate Air Pollution and Occurrence of Spontaneous Intracerebral Hemorrhage in an Area of Low Air Pollution. Clin. Neurol. Neurosurg. 2019, 176, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Qian, Y.; Yu, H.; Cai, B.; Fang, B.; Wang, C. Association between Incidence of Fatal Intracerebral Hemorrhagic Stroke and Fine Particulate Air Pollution. Environ. Health Prev. Med. 2019, 24, 38. [Google Scholar] [CrossRef]
- Yorifuji, T.; Kawachi, I.; Sakamoto, T.; Doi, H. Associations of Outdoor Air Pollution with Hemorrhagic Stroke Mortality. J. Occup. Environ. Med. 2011, 53, 124–126. [Google Scholar] [CrossRef]
- Chien, T.-Y.; Ting, H.-W.; Chan, C.-L.; Yang, N.-P.; Pan, R.-H.; Lai, K.R.; Hung, S.-I. Does the Short-Term Effect of Air Pollution Influence the Incidence of Spontaneous Intracerebral Hemorrhage in Different Patient Groups? Big Data Analysis in Taiwan. Int. J. Environ. Res. Public Health 2017, 14, 1547. [Google Scholar] [CrossRef] [PubMed]
- Jun Rui, M.L.; Tan, J.; Tan, B.Y.-Q.; Yeo, T.T.; Sharma, V.K. Air Pollution and Intracranial Hemorrhage. Ann. Indian Acad. Neurol. 2022, 25, S22–S25. [Google Scholar] [CrossRef] [PubMed]
- Tykhonova, S.; Shtanko, V.; Khyzhnyak, O.; Tofan, N. The Effect of Pollution on Hypertension and on the Total Risk Score in Hypertensive Patients. E J. Cardiol. Pract. 2022, 22, 17. [Google Scholar]
- Choi, Y.-J.; Kim, S.-H.; Kang, S.-H.; Kim, S.-Y.; Kim, O.-J.; Yoon, C.-H.; Lee, H.-Y.; Youn, T.-J.; Chae, I.-H.; Kim, C.-H. Short-Term Effects of Air Pollution on Blood Pressure. Sci. Rep. 2019, 9, 20298. [Google Scholar] [CrossRef] [PubMed]
- Brook, R.D.; Rajagopalan, S. Inhaling Hypertension. Hypertension 2020, 76, 32–34. [Google Scholar] [CrossRef] [PubMed]
- Camilli, M.; Russo, M.; Rinaldi, R.; Caffè, A.; La Vecchia, G.; Bonanni, A.; Iannaccone, G.; Basile, M.; Vergallo, R.; Aurigemma, C.; et al. Air Pollution and Coronary Vasomotor Disorders in Patients with Myocardial Ischemia and Unobstructed Coronary Arteries. J. Am. Coll. Cardiol. 2022, 80, 1818–1828. [Google Scholar] [CrossRef] [PubMed]
- Di, Q.; Wang, Y.; Zanobetti, A.; Wang, Y.; Koutrakis, P.; Choirat, C.; Dominici, F.; Schwartz, J.D. Air Pollution and Mortality in the Medicare Population. N. Engl. J. Med. 2017, 376, 2513–2522. [Google Scholar] [CrossRef] [PubMed]
- Crowley, R.A.; Moyer, D.V.; DeLong, D.M. Climate Change and Health. Ann. Intern. Med. 2016, 165, 747–748. [Google Scholar] [CrossRef] [PubMed]
- Cheong, K.H.; Ngiam, N.J.; Morgan, G.G.; Pek, P.P.; Tan, B.Y.-Q.; Lai, J.W.; Koh, J.M.; Ong, M.E.H.; Ho, A.F.W. Acute Health Impacts of the Southeast Asian Transboundary Haze Problem—A Review. Int. J. Environ. Res. Public Health 2019, 16, 3286. [Google Scholar] [CrossRef]
- Ljungman, P.L.; Mittleman, M.A. Ambient Air Pollution and Stroke. Stroke 2014, 45, 3734–3741. [Google Scholar] [CrossRef]
- Verhoeven, J.I.; Allach, Y.; Vaartjes, I.C.H.; Klijn, C.J.M.; de Leeuw, F.-E. Ambient Air Pollution and the Risk of Ischaemic and Haemorrhagic Stroke. Lancet Planet. Health 2021, 5, e542–e552. [Google Scholar] [CrossRef]
- Ho, A.F.W.; Lim, M.J.R.; Zheng, H.; Leow, A.S.-T.; Tan, B.Y.-Q.; Pek, P.P.; Raju, Y.; Seow, W.-J.; Yeo, T.T.; Sharma, V.K.; et al. Association of Ambient Air Pollution with Risk of Hemorrhagic Stroke: A Time-Stratified Case Crossover Analysis of the Singapore Stroke Registry. Int. J. Hyg. Environ. Health 2022, 240, 113908. [Google Scholar] [CrossRef] [PubMed]
- Dąbrowiecki, P.; Chciałowski, A.; Dąbrowiecka, A.; Badyda, A. Ambient Air Pollution and Risk of Admission due to Asthma in the Three Largest Urban Agglomerations in Poland: A Time-Stratified, Case-Crossover Study. Int. J. Environ. Res. Public Health 2022, 19, 5988. [Google Scholar] [CrossRef] [PubMed]
- Dąbrowiecki, P.; Badyda, A.; Chciałowski, A.; Czechowski, P.O.; Wrotek, A. Influence of Selected Air Pollutants on Mortality and Pneumonia Burden in Three Polish Cities over the Years 2011–2018. J. Clin. Med. 2022, 11, 3084. [Google Scholar] [CrossRef]
- Dąbrowiecki, P.; Chciałowski, A.; Dąbrowiecka, A.; Piórkowska, A.; Badyda, A. Air Pollution and Long-Term Risk of Hospital Admission due to Chronic Obstructive Pulmonary Disease Exacerbations in Poland: A Time-Stratified, Case-Crossover Study. Pol. Arch. Med. Wewnętrznej 2023, 133, 16444. [Google Scholar] [CrossRef]
- Dąbrowiecki, P.; Chciałowski, A.; Dąbrowiecka, A.; Piórkowska, A.; Badyda, A. Exposure to Ambient Air Pollutants and Short-Term Risk for Exacerbations of Allergic Rhinitis: A Time-Stratified, Case-Crossover Study in the Three Largest Urban Agglomerations in Poland. Respir. Physiol. Neurobiol. 2023, 315, 104095. [Google Scholar] [CrossRef]
- Gdansk Climate: Weather by Month, Temperature, Precipitation, When to Go. Available online: https://www.climatestotravel.com/climate/poland/gdansk (accessed on 10 August 2022).
- Raporty o Stanie Środowiska. Available online: https://www.gios.gov.pl/images/dokumenty/pms/raporty/stan_srodowiska_2020_pomorskie.pdf (accessed on 18 August 2022).
- WHO. Definition of an Older or Elderly Person|PDF|Ageing|Old Age. Available online: https://www.scribd.com/document/190077600/WHO-Definition-of-an-Older-or-Elderly-Person (accessed on 30 June 2023).
- Rubin, D.B. Multiple Imputation for Nonresponse in Surveys; John Wiley & Sons: Hoboken, NJ, USA, 2004; ISBN 978-0-471-65574-9. [Google Scholar]
- Li, J.; Huang, J.; Wang, Y.; Yin, P.; Wang, L.; Liu, Y.; Pan, X.; Zhou, M.; Li, G. Years of Life Lost from Ischaemic and Haemorrhagic Stroke Related to Ambient Nitrogen Dioxide Exposure: A Multicity Study in China. Ecotoxicol. Environ. Saf. 2020, 203, 111018. [Google Scholar] [CrossRef]
- Wolf, K.; Hoffmann, B.; Andersen, Z.J.; Atkinson, R.W.; Bauwelinck, M.; Bellander, T.; Brandt, J.; Brunekreef, B.; Cesaroni, G.; Chen, J.; et al. Long-Term Exposure to Low-Level Ambient Air Pollution and Incidence of Stroke and Coronary Heart Disease: A Pooled Analysis of Six European Cohorts within the ELAPSE Project. Lancet Planet. Health 2021, 5, e620–e632. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Wang, B.; Hu, Y.; Dai, L.; Liu, Y.; Wang, J.; Cao, X.; Wu, Y.; Zhou, T.; Cui, X.; et al. Short-Term Effects of Low-Level Ambient Air NO2 on the Risk of Incident Stroke in Enshi City, China. Int. J. Environ. Res. Public Health 2022, 19, 6683. [Google Scholar] [CrossRef]
- Yin, P.; Chen, R.; Wang, L.; Meng, X.; Liu, C.; Niu, Y.; Lin, Z.; Liu, Y.; Liu, J.; Qi, J.; et al. Ambient Ozone Pollution and Daily Mortality: A Nationwide Study in 272 Chinese Cities. Environ. Health Perspect. 2017, 125, 117006. [Google Scholar] [CrossRef]
- Wang, F.; Liu, H.; Li, H.; Liu, J.; Guo, X.; Yuan, J.; Hu, Y.; Wang, J.; Lu, L. Ambient Concentrations of Particulate Matter and Hospitalization for Depression in 26 Chinese Cities: A Case-Crossover Study. Environ. Int. 2018, 114, 115–122. [Google Scholar] [CrossRef]
- Wang, L.; Liu, C.; Meng, X.; Niu, Y.; Lin, Z.; Liu, Y.; Liu, J.; Qi, J.; You, J.; Tse, L.A.; et al. Associations between Short-Term Exposure to Ambient Sulfur Dioxide and Increased Cause-Specific Mortality in 272 Chinese Cities. Environ. Int. 2018, 117, 33–39. [Google Scholar] [CrossRef]
- Fisher, J.A.; Puett, R.C.; Laden, F.; Wellenius, G.A.; Sapkota, A.; Liao, D.; Yanosky, J.D.; Carter-Pokras, O.; He, X.; Hart, J.E. Case-Crossover Analysis of Short-Term Particulate Matter Exposures and Stroke in the Health Professionals Follow-up Study. Environ. Int. 2019, 124, 153–160. [Google Scholar] [CrossRef]
- Tian, Y.; Liu, H.; Wu, Y.; Si, Y.; Song, J.; Cao, Y.; Li, M.; Wu, Y.; Wang, X.; Chen, L.; et al. Association between Ambient Fine Particulate Pollution and Hospital Admissions for Cause Specific Cardiovascular Disease: Time Series Study in 184 Major Chinese Cities. BMJ 2019, 367, l6572. [Google Scholar] [CrossRef]
- Byrne, C.P.; Bennett, K.E.; Hickey, A.; Kavanagh, P.; Broderick, B.; O’Mahony, M.; Williams, D.J. Short-Term Air Pollution as a Risk for Stroke Admission: A Time-Series Analysis. Cerebrovasc. Dis. 2020, 49, 404–411. [Google Scholar] [CrossRef]
- Gu, J.; Shi, Y.; Chen, N.; Wang, H.; Chen, T. Ambient Fine Particulate Matter and Hospital Admissions for Ischemic and Hemorrhagic Strokes and Transient Ischemic Attack in 248 Chinese Cities. Sci. Total Environ. 2020, 715, 136896. [Google Scholar] [CrossRef]
- Wilker, E.H.; Mostofsky, E.; Fossa, A.; Koutrakis, P.; Warren, A.; Charidimou, A.; Mittleman, M.A.; Viswanathan, A. Ambient Pollutants and Spontaneous Intracerebral Hemorrhage in Greater Boston. Stroke 2018, 49, 2764–2766. [Google Scholar] [CrossRef]
- Sun, S.; Stewart, J.D.; Eliot, M.N.; Yanosky, J.D.; Liao, D.; Tinker, L.F.; Eaton, C.B.; Whitsel, E.A.; Wellenius, G.A. Short-Term Exposure to Air Pollution and Incidence of Stroke in the Women’s Health Initiative. Environ. Int. 2019, 132, 105065. [Google Scholar] [CrossRef]
- Liu, H.; Tian, Y.; Xu, Y.; Huang, Z.; Huang, C.; Hu, Y.; Zhang, J. Association between Ambient Air Pollution and Hospitalization for Ischemic and Hemorrhagic Stroke in China: A Multicity Case-Crossover Study. Environ. Pollut. 2017, 230, 234–241. [Google Scholar] [CrossRef]
- Chen, R.; Zhang, Y.; Yang, C.; Zhao, Z.; Xu, X.; Kan, H. Acute Effect of Ambient Air Pollution on Stroke Mortality in the China Air Pollution and Health Effects Study. Stroke 2013, 44, 954–960. [Google Scholar] [CrossRef]
- Andersen, Z.J.; Olsen, T.S.; Andersen, K.K.; Loft, S.; Ketzel, M.; Raaschou-Nielsen, O. Association between Short-Term Exposure to Ultrafine Particles and Hospital Admissions for Stroke in Copenhagen, Denmark. Eur. Heart J. 2010, 31, 2034–2040. [Google Scholar] [CrossRef]
- Wang, Y.; Eliot, M.N.; Wellenius, G.A. Short-Term Changes in Ambient Particulate Matter and Risk of Stroke: A Systematic Review and Meta-Analysis. J. Am. Heart Assoc. 2014, 3, e000983. [Google Scholar] [CrossRef]
- Chiu, H.-F.; Chang, C.-C.; Yang, C.-Y. Relationship Between Hemorrhagic Stroke Hospitalization and Exposure to Fine Particulate Air Pollution in Taipei, Taiwan. J. Toxicol. Environ. Health Part A 2014, 77, 1154–1163. [Google Scholar] [CrossRef]
- Han, M.-H.; Yi, H.-J.; Ko, Y.; Kim, Y.-S.; Lee, Y.-J. Association between Hemorrhagic Stroke Occurrence and Meteorological Factors and Pollutants. BMC Neurol. 2016, 16, 59. [Google Scholar] [CrossRef]
- Niu, Z.; Liu, F.; Yu, H.; Wu, S.; Xiang, H. Association between Exposure to Ambient Air Pollution and Hospital Admission, Incidence, and Mortality of Stroke: An Updated Systematic Review and Meta-Analysis of More than 23 Million Participants. Environ. Health Prev. Med. 2021, 26, 15. [Google Scholar] [CrossRef]
- Liu, C.; Yin, P.; Chen, R.; Meng, X.; Wang, L.; Niu, Y.; Lin, Z.; Liu, Y.; Liu, J.; Qi, J.; et al. Ambient Carbon Monoxide and Cardiovascular Mortality: A Nationwide Time-Series Analysis in 272 Cities in China. Lancet Planet. Health 2018, 2, e12–e18. [Google Scholar] [CrossRef]
- Dahmann, D.; Morfeld, P.; Monz, C.; Noll, B.; Gast, F. Exposure Assessment for Nitrogen Oxides and Carbon Monoxide in German Hard Coal Mining. Int. Arch. Occup. Environ. Health 2009, 82, 1267–1279. [Google Scholar] [CrossRef]
- Chen, T.-M.; Kuschner, W.G.; Gokhale, J.; Shofer, S. Outdoor Air Pollution: Nitrogen Dioxide, Sulfur Dioxide, and Carbon Monoxide Health Effects. Am. J. Med. Sci. 2007, 333, 249–256. [Google Scholar] [CrossRef]
- Xu, J.; Geng, W.; Geng, X.; Cui, L.; Ding, T.; Xiao, C.; Zhang, J.; Tang, J.; Zhai, J. Study on the Association between Ambient Air Pollution and Daily Cardiovascular Death in Hefei, China. Environ. Sci. Pollut. Res. Int. 2020, 27, 547–561. [Google Scholar] [CrossRef]
- Carlsen, H.K.; Forsberg, B.; Meister, K.; Gíslason, T.; Oudin, A. Ozone Is Associated with Cardiopulmonary and Stroke Emergency Hospital Visits in Reykjavík, Iceland 2003–2009. Environ. Health 2013, 12, 28. [Google Scholar] [CrossRef]
- Atkinson, R.W.; Butland, B.K.; Dimitroulopoulou, C.; Heal, M.R.; Stedman, J.R.; Carslaw, N.; Jarvis, D.; Heaviside, C.; Vardoulakis, S.; Walton, H.; et al. Long-Term Exposure to Ambient Ozone and Mortality: A Quantitative Systematic Review and Meta-Analysis of Evidence from Cohort Studies. BMJ Open 2016, 6, e009493. [Google Scholar] [CrossRef]
- Montresor-López, J.A.; Yanosky, J.D.; Mittleman, M.A.; Sapkota, A.; He, X.; Hibbert, J.D.; Wirth, M.D.; Puett, R.C. Short-Term Exposure to Ambient Ozone and Stroke Hospital Admission: A Case-Crossover Analysis. J. Expo. Sci. Environ. Epidemiol. 2016, 26, 162–166. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Zhang, Y.; Ma, R.; Liu, X.; Liang, J.; Lin, H.; Shen, P.; Zhang, J.; Lu, P.; Tang, X.; et al. Long-Term Exposure to Ozone and Cardiovascular Mortality in a Large Chinese Cohort. Environ. Int. 2022, 165, 107280. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Liu, H.; Zhao, Z.; Xiang, X.; Li, M.; Juan, J.; Song, J.; Cao, Y.; Wang, X.; Chen, L.; et al. Association between Ambient Air Pollution and Daily Hospital Admissions for Ischemic Stroke: A Nationwide Time-Series Analysis. PLoS Med. 2018, 15, e1002668. [Google Scholar] [CrossRef]
- Hahad, O.; Lelieveld, J.; Birklein, F.; Lieb, K.; Daiber, A.; Münzel, T. Ambient Air Pollution Increases the Risk of Cerebrovascular and Neuropsychiatric Disorders through Induction of Inflammation and Oxidative Stress. Int. J. Mol. Sci. 2020, 21, 4306. [Google Scholar] [CrossRef] [PubMed]
- Perez, C.M.; Hazari, M.S.; Farraj, A.K. Role of Autonomic Reflex Arcs in Cardiovascular Responses to Air Pollution Exposure. Cardiovasc. Toxicol. 2015, 15, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Hackman, J.L.; Nelson, A.M.; Ma, O.J. Spontaneous Subarachnoid and Intracerebral Hemorrhage. In Tintinalli’s Emergency Medicine: A Comprehensive Study Guide; Tintinalli, J.E., Stapczynski, J.S., Ma, O.J., Yealy, D.M., Meckler, G.D., Cline, D.M., Eds.; McGraw-Hill Education: New York, NY, USA, 2016. [Google Scholar]
- Vasquez, H.E.; Prasad, L.; Moscote-Salazar, L.R.; Agrawal, A. Atmospheric Variables and Subarachnoid Hemorrhage: Narrative Review. Egypt. J. Neurosurg. 2021, 36, 17. [Google Scholar] [CrossRef]
- Wang, X.; Cao, Y.; Hong, D.; Zheng, D.; Richtering, S.; Sandset, E.C.; Leong, T.H.; Arima, H.; Islam, S.; Salam, A.; et al. Ambient Temperature and Stroke Occurrence: A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2016, 13, 698. [Google Scholar] [CrossRef] [PubMed]
- Guo, P.; Wang, Y.; Feng, W.; Wu, J.; Fu, C.; Deng, H.; Huang, J.; Wang, L.; Zheng, M.; Liu, H. Ambient Air Pollution and Risk for Ischemic Stroke: A Short-Term Exposure Assessment in South China. Int. J. Environ. Res. Public Health 2017, 14, 1091. [Google Scholar] [CrossRef]
- Hertwig, D.; Grimmond, S.; Kotthaus, S.; Vanderwel, C.; Gough, H.; Haeffelin, M.; Robins, A. Variability of Physical Meteorology in Urban Areas at Different Scales: Implications for Air Quality. Faraday Discuss. 2021, 226, 149–172. [Google Scholar] [CrossRef]
- Czernych, R.; Badyda, A.J.; Kozera, G.; Zagożdżon, P. Assessment of Low-Level Air Pollution and Cardiovascular Incidence in Gdansk, Poland: Time-Series Cross-Sectional Analysis. J. Clin. Med. 2023, 12, 2206. [Google Scholar] [CrossRef] [PubMed]

| Count (% of All) | Mean (SD) | Min | Med | Max | IQR | |
|---|---|---|---|---|---|---|
| All haemorrhagic strokes (I60, I61) | 5181 | 2.84 (2.43) | 0 | 2 | 14 | 2.5 |
| Women | 2178 (42%) | 1.19 (1.29) | 0 | 1 | 7 | 2 |
| Men | 3003 (58%) | 1.65 (1.68) | 0 | 1 | 9 | 2 |
| age ≥ 65 years | 1877 (36%) | 1.03 (1.17) | 0 | 1 | 6 | 1 |
| age < 65 years | 3304 (64%) | 1.81 (1.85) | 0 | 1 | 9 | 2 |
| Subarachnoid haemorrhages (I60) | 639 (12%) | 0.35 (0.64) | 0 | 0 | 3 | 1 |
| Women | 403 (63%) | 0.22 (0.53) | 0 | 0 | 3 | 0 |
| Men | 236 (37%) | 0.13 (0.39) | 0 | 0 | 2 | 0 |
| age ≥ 65 years | 217 (34%) | 0.12 (0.38) | 0 | 0 | 2 | 0 |
| age < 65 years | 422 (66%) | 0.23 (0.54) | 0 | 0 | 3 | 0 |
| Intracerebral haemorrhages (I61) | 4542 (88%) | 2.49 (2.31) | 0 | 2 | 13 | 2 |
| Women | 1775 (39%) | 0.97 (1.15) | 0 | 1 | 5 | 1 |
| Men | 2767 (61%) | 1.52 (1.62) | 0 | 1 | 9 | 2 |
| age ≥ 65 years | 1660 (37%) | 0.91 (1.10) | 0 | 1 | 6 | 1 |
| age < 65 years | 2882 (63%) | 1.58 (1.75) | 0 | 1 | 9 | 2 |
| Chemical Compounds [μg/m3] | Missing Values | Mean (SD) | Min | Max | IQR |
|---|---|---|---|---|---|
| SO2 | 0 | 6.31 (4.08) | 1.98 | 57.73 | 3.65 |
| NO | 0 | 22.83 (17.57) | 4.46 | 170.94 | 14.12 |
| NO2 | 0 | 23.55 (11.96) | 5.09 | 96.49 | 14.62 |
| NOx | 0 | 36.47(29.65) | 7.05 | 294.11 | 22.52 |
| CO | 0 | 496.09 (203.26) | 244.32 | 2280.12 | 164.04 |
| PM10 | 0 | 26.87 (16.68) | 5.66 | 151.17 | 16.76 |
| PM2.5 | 393 | 20.07 (14.27) | 3.58 | 178.83 | 12.84 |
| O3 | 2 | 55.53 (22.8) | 2.36 | 130 | 31.7 |
| SO2 | NO | NO2 | NOX | CO | PM10 | PM2.5 | O3 | |
|---|---|---|---|---|---|---|---|---|
| SO2 | 1 | |||||||
| NO | 0.47 * | 1 | ||||||
| NO2 | 0.56 * | 0.88 * | 1 | |||||
| NOX | 0.46 * | 1 * | 0.86 * | 1 | ||||
| CO | 0.61 * | 0.86 * | 0.79 * | 0.85 * | 1 | |||
| PM10 | 0.62 * | 0.73 * | 0.75 * | 0.73 * | 0.79 * | 1 | ||
| PM2.5 | 0.64 * | 0.75 * | 0.75 * | 0.74 * | 0.85 * | 0.92 * | 1 | |
| O3 | −0.21 * | −0.37 * | −0.26 * | −0.38 * | −0.45 * | −0.2 * | −0.33 * | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Czernych, R.; Kozera, G.; Badyda, A.J.; Bieniaszewski, L.; Zagożdżon, P. Air Pollution Increases Risk of Occurrence of Intracerebral Haemorrhage but Not of Subarachnoid Haemorrhage: Time-Series Cross-Sectional Study. Biomedicines 2024, 12, 1562. https://doi.org/10.3390/biomedicines12071562
Czernych R, Kozera G, Badyda AJ, Bieniaszewski L, Zagożdżon P. Air Pollution Increases Risk of Occurrence of Intracerebral Haemorrhage but Not of Subarachnoid Haemorrhage: Time-Series Cross-Sectional Study. Biomedicines. 2024; 12(7):1562. https://doi.org/10.3390/biomedicines12071562
Chicago/Turabian StyleCzernych, Radosław, Grzegorz Kozera, Artur Jerzy Badyda, Leszek Bieniaszewski, and Paweł Zagożdżon. 2024. "Air Pollution Increases Risk of Occurrence of Intracerebral Haemorrhage but Not of Subarachnoid Haemorrhage: Time-Series Cross-Sectional Study" Biomedicines 12, no. 7: 1562. https://doi.org/10.3390/biomedicines12071562







