Time-Dependent Shifts in Intestinal Bacteriome, Klebsiella Colonization and Incidence of Antibiotic-Resistance Genes after Allogeneic Hematopoietic Stem Cell Transplantation
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Statement
2.2. Patients and Study Design
2.3. Laboratory Studies
2.3.1. Bacteriology
2.3.2. Multiplex PCR
2.3.3. NGS-Based Assessment of Gut Bacteriome
2.3.4. Bioinformatics
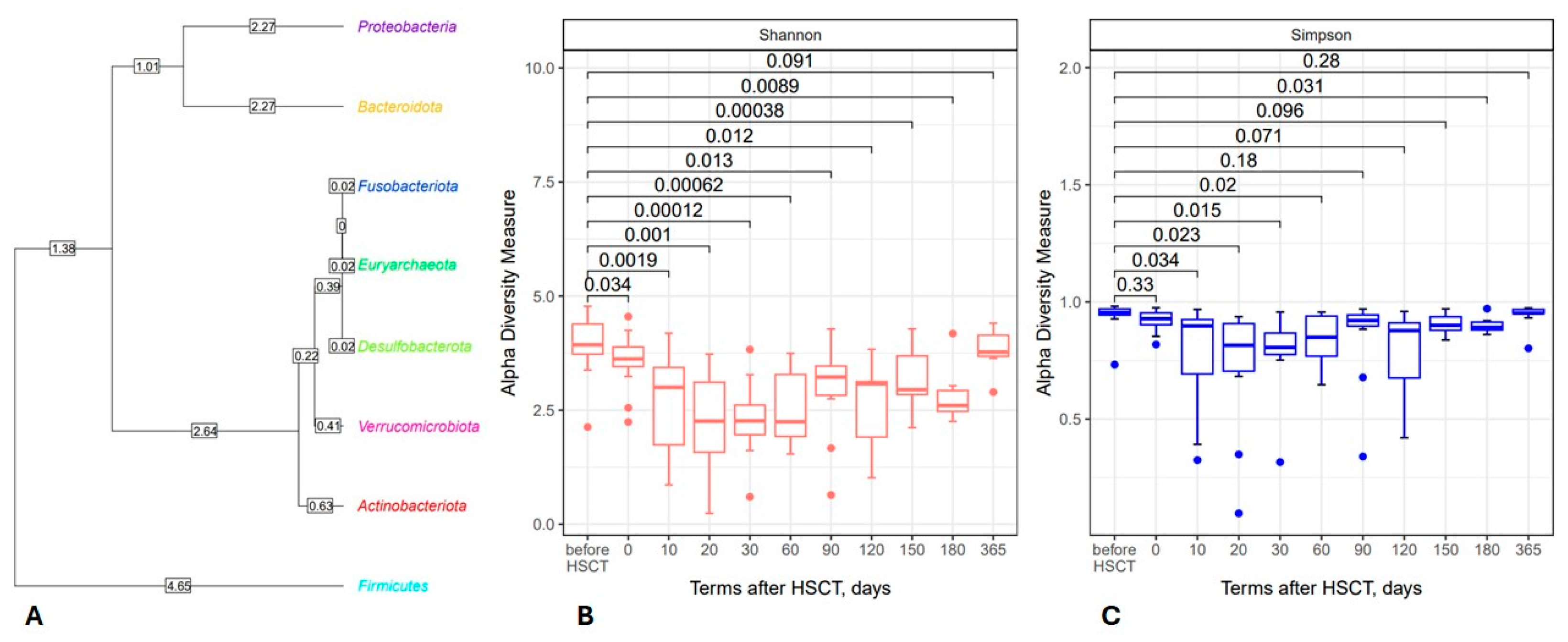
3. Results
3.1. Posttransplant Changes in Intestinal Microbiota
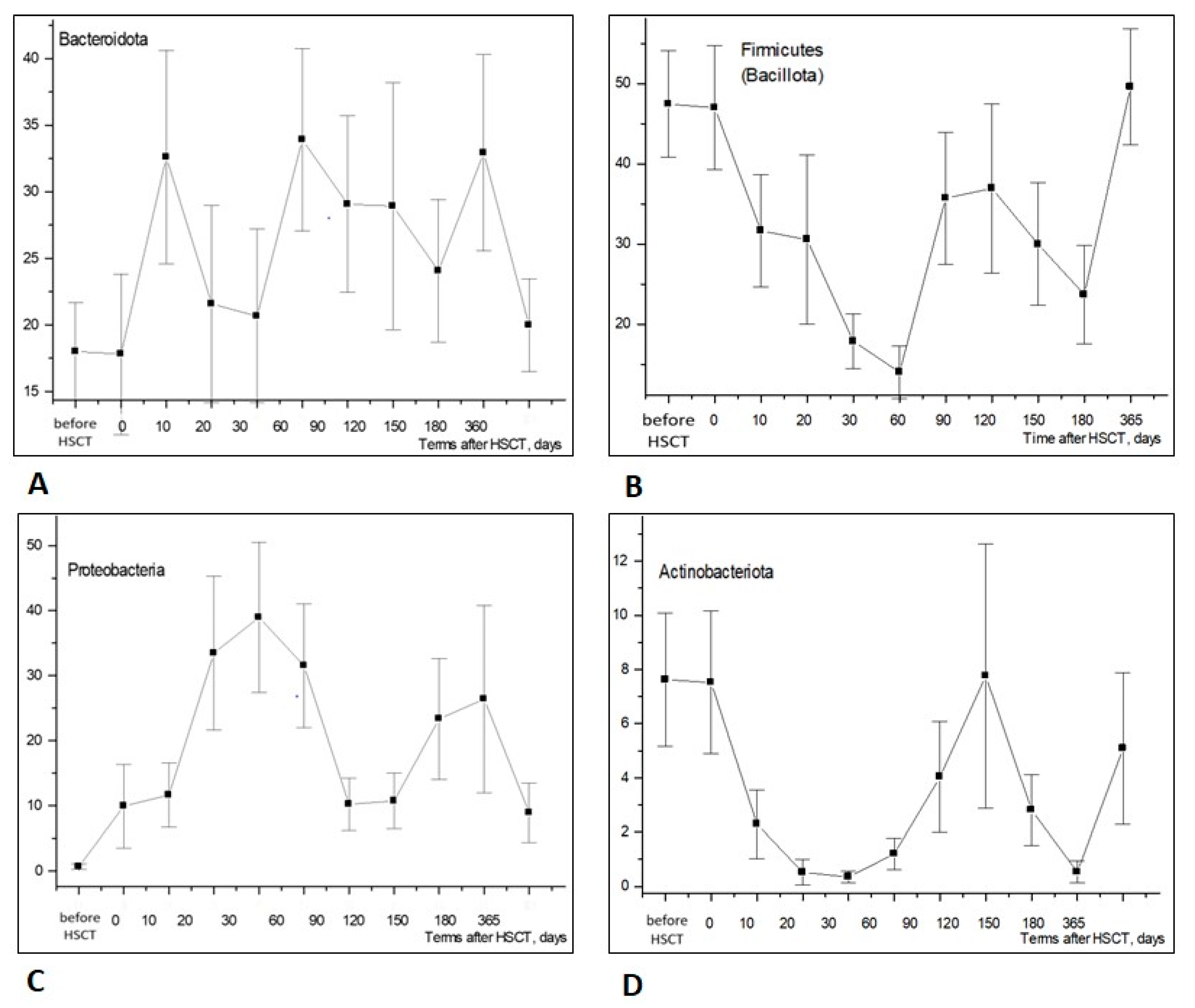
3.1.1. Phylum Level
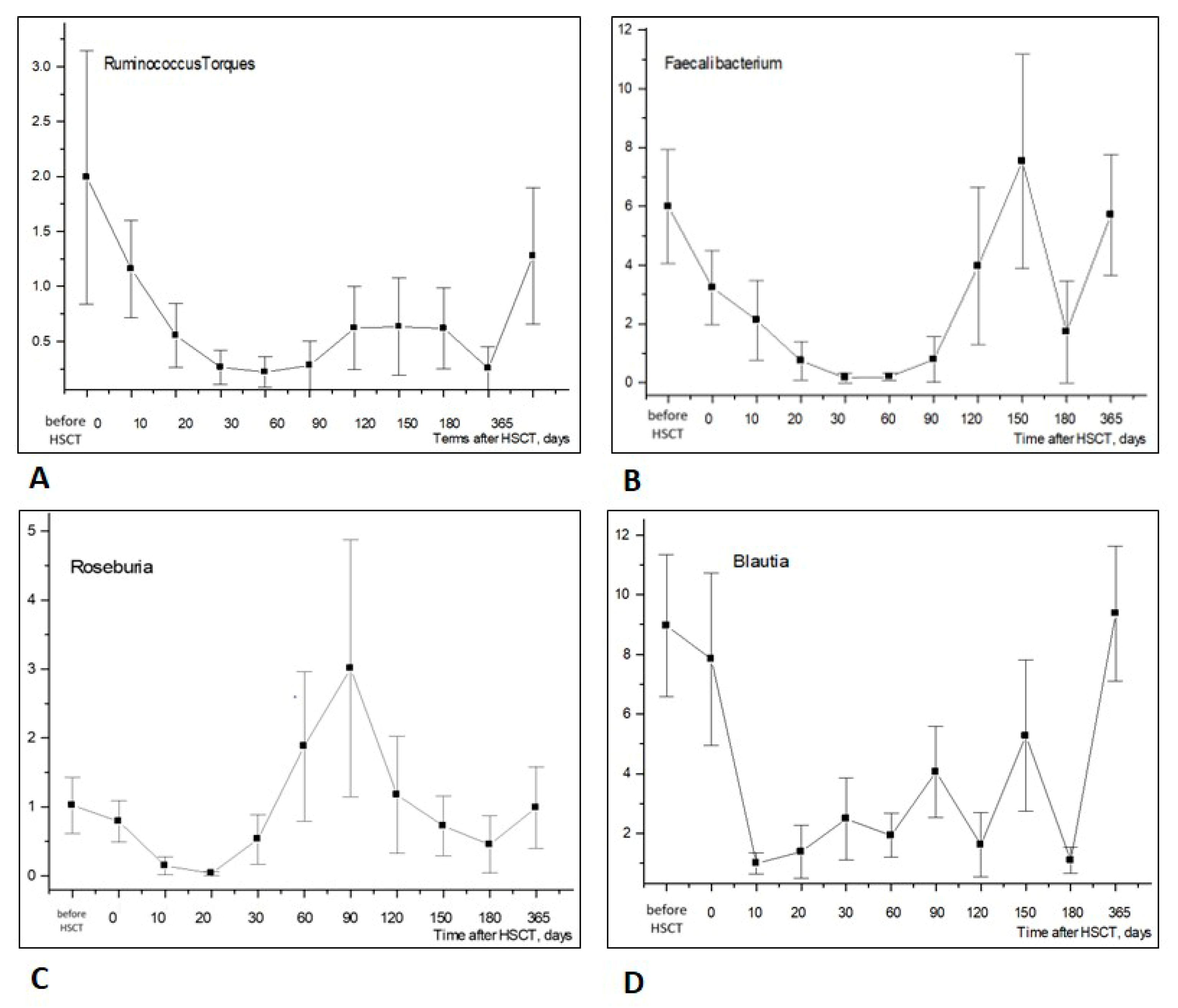
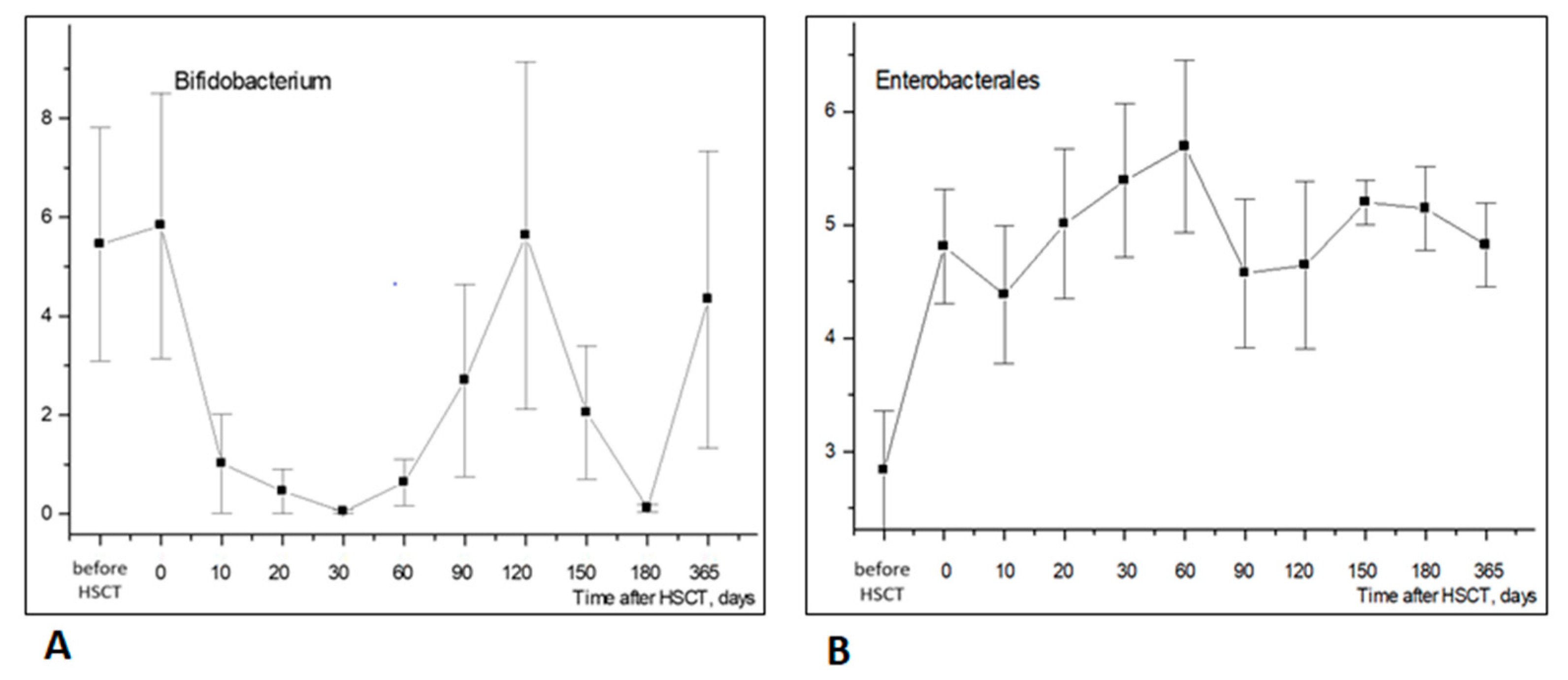
3.1.2. Changes in Bacterial Genera
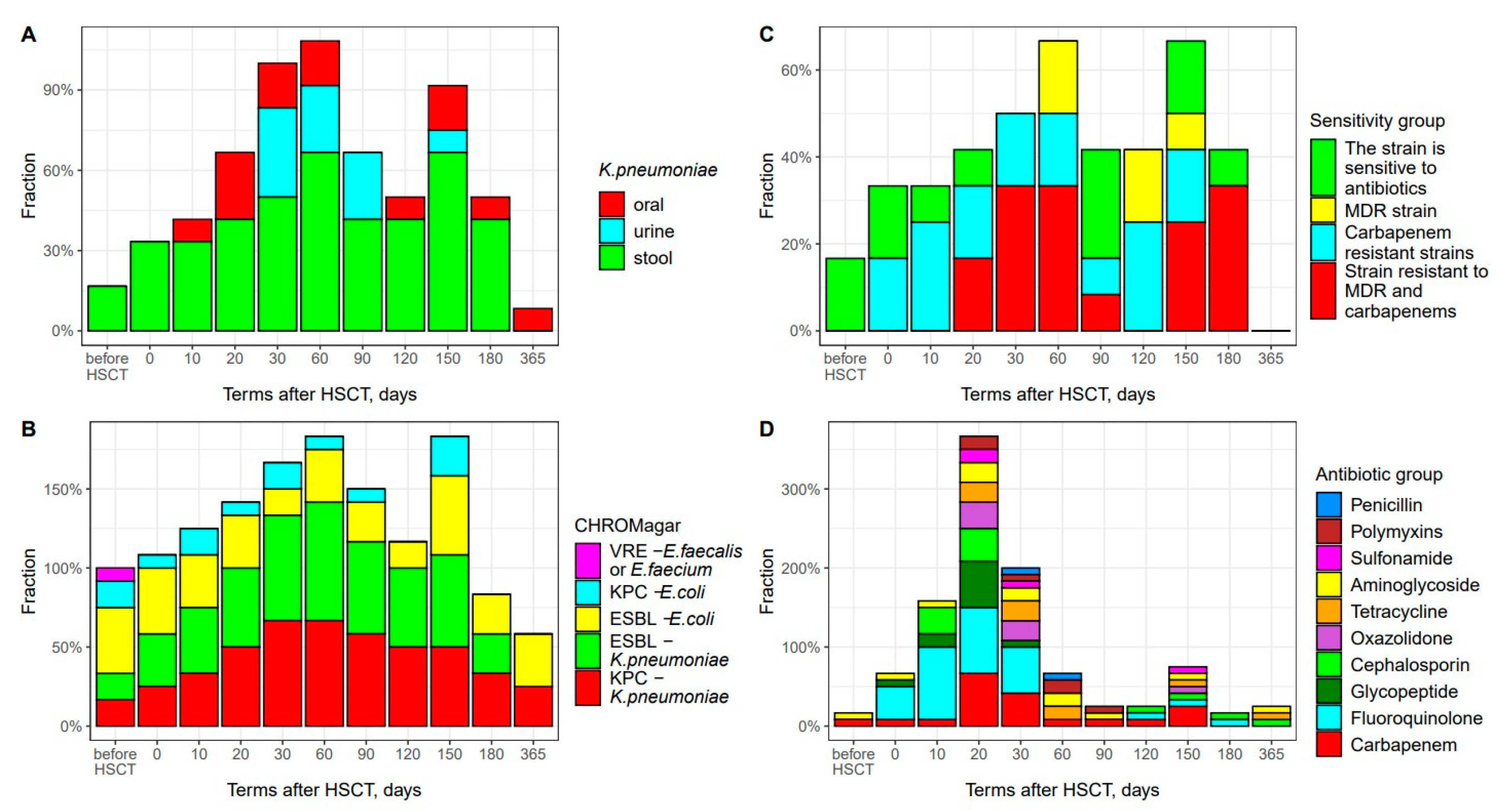
3.2. K. pneumoniae Colonization and Antibiotic Resistance
3.3. Antibacterial Therapy and Infectious Complications
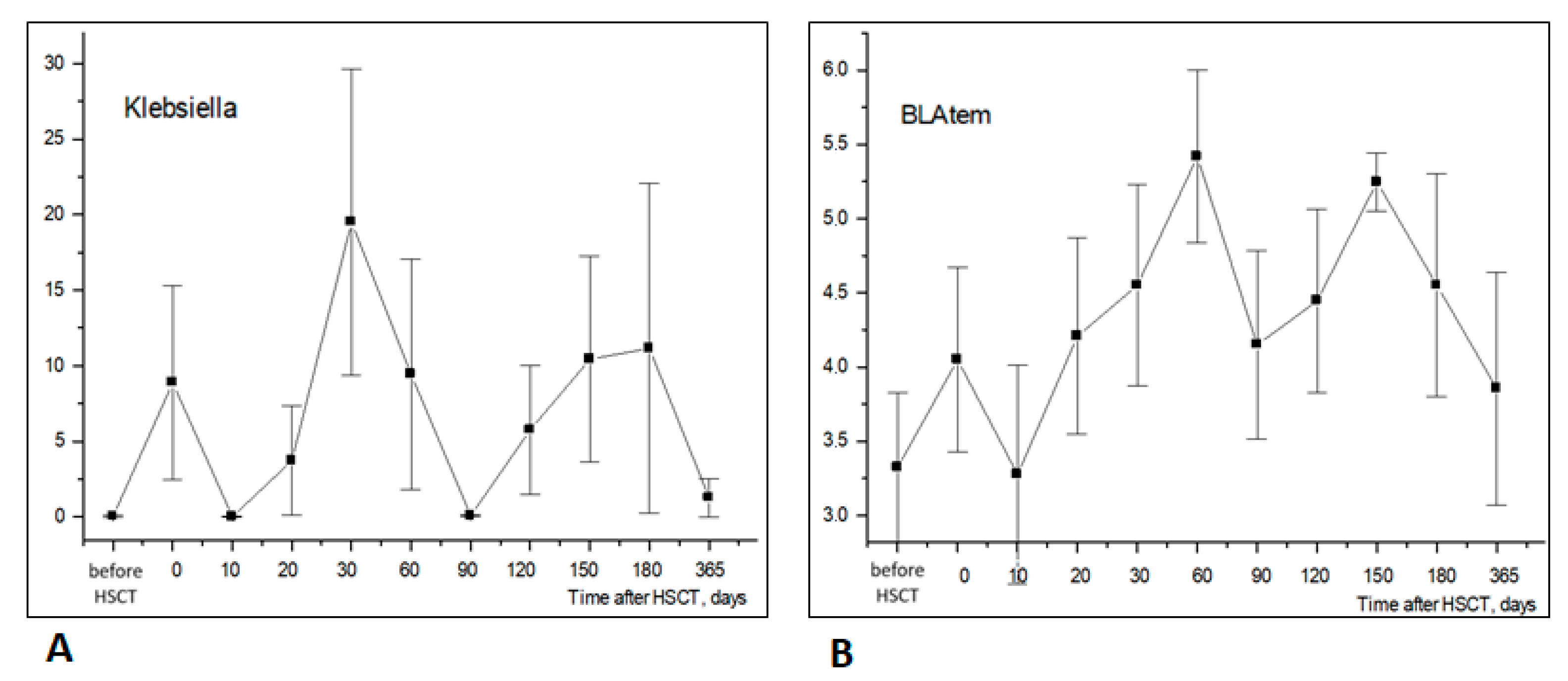
3.4. Temporal Shifts in Enterobacterales and Antimicrobial Resistance Genes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kikuchi, M.; Akahoshi, Y.; Nakano, H.; Ugai, T.; Wada, H.; Yamasaki, R.; Sakamoto, K.; Kawamura, K.; Ishihara, Y.; Sato, M.; et al. Risk factors for pre- and post-engraftment bloodstream infections after allogeneic hematopoietic stem cell transplantation. Transpl. Infect. Dis. 2015, 17, 56–65. [Google Scholar] [CrossRef]
- Chukhlovin, A.B.; Spiridonova, A.A.; Baranova, I.B.; Grigoriants, A.P.; Vladovskaya, M.D.; Zubarovskaya, L.S.; Afanasyev, B.V. Common bacterial cultures from oral mucosa after hematopoietic stem cell transplantation: Dependence on the patient characteristics and therapeutic factors. Cell. Ther. Transplant. 2019, 7, 49–56. [Google Scholar] [CrossRef]
- Malard, F.; Jenq, R.R. The microbiome and its impact on allogeneic hematopoietic cell transplantation. Cancer J. 2023, 29, 75–83. [Google Scholar] [CrossRef]
- Peled, J.U.; Devlin, S.M.; Staffas, A.; Lumish, M.; Khanin, R.; Littmann, E.R.; Ling, L.; Kosuri, S.; Maloy, M.; Slingerland, J.B.; et al. Intestinal microbiota and relapse after hematopoietic-cell transplantation. J. Clin. Oncol. 2017, 35, 1650–1659. [Google Scholar] [CrossRef] [PubMed]
- Spiridonova, A.A.; Volkova, A.G.; Chukhlovin, A.B.; Moiseev, I.S.; Zubarovakaya, L.S.; Kulagin, A.D. Bronchoalveolar microbiota in oncohematological patients: Age dependence and microbiota shifts following hematopoietic stem cell transplantation. Cell. Ther. Transplant. 2022, 11, 45–53. [Google Scholar] [CrossRef]
- World Health Organization. WHO Handbook for Reporting Results of Cancer Treatment; World Health Organization Offset Publication No. 48; World Health Organization: Geneva, Switzerland, 1979; pp. 15–22. Available online: https://iris.who.int/handle/10665/37200 (accessed on 5 July 2024).
- Schoemans, H.M.; Lee, S.J.; Ferrara, J.; Wolff, D.; Levine, J.; Schultz, K.; Shaw, B.; Flowers, M.E.; Ruutu, T.; Greinix, H.; et al. EBMT (European Society for Blood and Marrow Transplantation) Transplant Complications Working Party and the “EBMT−NIH (National Institutes of Health)−CIBMTR (Center for International Blood and Marrow Transplant Research) GvHD Task Force”. EBMT-NIH-CIBMTR Task Force position statement on standardized terminology & guidance for graft-versus-host disease assessment. Bone Marrow Transplant. 2018, 53, 1401–1415. [Google Scholar] [CrossRef]
- The European Committee on Antimicrobial Susceptibility Testing. Breakpoint Tables for Interpretation of MICs and Zone Diameters, Version 10.0. 2020. Available online: http://www.eucast.org/clinical_breakpoints/ (accessed on 5 July 2024).
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef] [PubMed]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA ribosomal RNA gene database project: Improved data processing and Web-based tools. Nucleic Acids Res. 2013, 41, D590–D596. [Google Scholar] [CrossRef] [PubMed]
- McMurdie, P.J.; Holmes, S. phyloseq: An R package for reproducible interactive analysis and graphics of microbiome census data. PLoS ONE 2013, 8, e61217. [Google Scholar] [CrossRef]
- Davis, N.M.; Proctor, D.M.; Holmes, S.P.; Relman, D.A.; Callahan, B.J. Simple statistical identification and removal of contaminant sequences in marker-gene and metagenomics data. Microbiome 2018, 6, 226. [Google Scholar] [CrossRef]
- Kleine Bardenhorst, S.K.; Vital, M.; Karch, A.; Rübsamen, N. Richness estimation in microbiome data obtained from denoising pipelines. Comput. Struct. Biotechnol. J. 2022, 10, 508–520. [Google Scholar] [CrossRef]
- Shono, Y.; van den Brink, M.R.M. Gut microbiota injury in allogeneic haematopoietic stem cell transplantation. Nat. Rev. Cancer 2018, 18, 283–295. [Google Scholar] [CrossRef] [PubMed]
- Chukhlovin, A.B.; Pankratova, O.S. Opportunistic microflora at unusual sites: Marker pathogens in severe posttransplant immune deficiency. Cell. Ther. Transplant. 2017, 6, 28–41. [Google Scholar] [CrossRef]
- Lehouritis, P.; Cummins, J.; Stanton, M.; Murphy, C.T.; McCarthy, F.O.; Reid, G.; Urbaniak, C.; Byrne, W.L.; Tangney, M. Local bacteria affect the efficacy of chemotherapeutic drugs. Sci. Rep. 2015, 5, 14554. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Chen, Y.; Huang, W.; Zhou, H.; Zhang, W. Drug-microbiota interactions: An emerging priority for precision medicine. Signal Transduct. Target Ther. 2023, 9, 386. [Google Scholar] [CrossRef]
- Jørgensen, M.; Nørgaard, J.C.; Ilett, E.E.; Marandi, R.Z.; Noguera-Julian, M.; Paredes, R.; Murray, D.D.; Lundgren, J.; MacPherson, C.R.; Sengeløv, H. Metabolic potential of the gut microbiome is significantly impacted by conditioning regimen in allogeneic hematopoietic stem cell transplantation recipients. Int. J. Mol. Sci. 2022, 23, 11115. [Google Scholar] [CrossRef] [PubMed]
- Shouval, R.; Waters, N.R.; Gomes, A.L.C.; Zuanelli Brambilla, C.; Fei, T.; Devlin, S.M.; Nguyen, C.L.; Markey, K.A.; Dai, A.; Slingerland, J.B.; et al. Conditioning regimens are associated with distinct patterns of microbiota injury in allogeneic hematopoietic cell transplantation. Clin. Cancer Res. 2023, 29, 165–173. [Google Scholar] [CrossRef] [PubMed]
- van Lier, Y.F.; van den Brink, M.R.; Hazenberg, M.D.; Markey, K.A. The post-hematopoietic cell transplantation microbiome: Relationships with transplant outcome and potential therapeutic targets. Haematologica 2021, 106, 2042–2053. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Sun, P.; Vamathevan, J.; Li, Y.; Ingraham, K.; Palmer, L.; Huang, J.; Brown, J.R. Comparative genomics of Klebsiella pneumoniae strains with different antibiotic resistance profiles. Antimicrob. Agents Chemother. 2011, 55, 4267. [Google Scholar] [CrossRef]
- Snitkin, E.S.; Zelazny, A.M.; Thomas, P.J.; Stock, F.; NISC Comparative Sequencing Program Group; Henderson, D.K.; Palmore, T.N.; Segre, J.A. Tracking a hospital outbreak of carbapenem-resistant Klebsiella pneumoniae with whole-genome sequencing. Sci. Transl. Med. 2012, 4, 148ra116. [Google Scholar] [CrossRef]
- Rashidi, A.; Ebadi, M.; Rehman, T.U.; Elhusseini, H.; Kazadi, D.; Halaweish, H.; Khan, M.H.; Hoeschen, A.; Cao, Q.; Luo, X.; et al. Long- and short-term effects of fecal microbiota transplantation on antibiotic resistance genes: Results from a randomized placebo-controlled trial. Gut Microbes 2024, 16, 2327442. [Google Scholar] [CrossRef] [PubMed]
- Blanco-Picazo, P.; Gómez-Gómez, C.; Tormo, M.; Ramos-Barbero, M.D.; Rodríguez-Rubio, L.; Muniesa, M. Prevalence of bacterial genes in the phage fraction of food viromes. Food Res. Int. 2022, 156, 111342. [Google Scholar] [CrossRef] [PubMed]
- Mahdavi, S.; Sadeghi, M.; Shokri, R.; Sadegh, B. The role of bacteriophages as important reservoirs of extended-spectrum beta-lactamase genes in Azerbaijan hospitals. Microb. Drug Resist. 2022, 28, 436–443. [Google Scholar] [CrossRef] [PubMed]
- Pluta, A.; Krawiec, K.; Wieczorkiewicz-Kabut, A.; Helbig, G.; Strzałka, P.; Dutka, M.; Zaucha, J.; Sobczyk-Kruszelnicka, M.; Giebel, S.; Czerw, T.; et al. P1535: Assessment of colonization and infection epidemiology in patients undergoing allogeneic stem cell transplantation—A prospective multi-center study of Polish Adult Leukemia Group (PALG). Hemasphere 2023, 7, e875498b. [Google Scholar] [CrossRef]
- Rogacheva, Y.A.; Popova, M.O.; Siniaev, A.A.; Spiridonova, A.A.; Markelov, V.V.; Vlasova, Y.Y.; Bondarenko, S.N.; Zubarovskaya, L.S.; Kulagin, A.D. Epidemiology and impact of colonization by multidrug-resistant Gram-negative bacteria on bloodstream infections in early phase of allogeneic hematopoietic stem cell transplantation. Clin. Microbiol. Antimicrob. Chemother. 2022, 24, 375–382. [Google Scholar] [CrossRef]


| Characteristic | Overall, N = 12 1 | Female, N = 4 1 | Male, N = 8 1 | p-Value 2 |
|---|---|---|---|---|
| age | 33 (30.25, 40) | 30 (26.75, 34) | 36 (31.75, 45) | 0.2 |
| HSCT donor type | 0.2 | |||
| Allogeneic related | 3 (25.00%) | 0 (0.00%) | 3 (37.50%) | |
| Allogeneic unrelated | 8 (66.67%) | 3 (75.00%) | 5 (62.50%) | |
| Haploidentical | 1 (8.33%) | 1 (25.00%) | 0 (0.00%) | |
| Conditioning regimen | 0.7 | |||
| FluBe | 1 (8.33%) | 0 (0.00%) | 1 (12.50%) | |
| FluBu10 | 2 (16.67%) | 0 (0.00%) | 2 (25.00%) | |
| FluBu12 | 9 (75.00%) | 4 (100.00%) | 5 (62.50%) | |
| Mucositis cases | 0.7 | |||
| 0 | 2 (16.67%) | 0 (0.00%) | 2 (25.00%) | |
| 1 | 2 (16.67%) | 0 (0.00%) | 2 (25.00%) | |
| 2 | 2 (16.67%) | 1 (25.00%) | 1 (12.50%) | |
| 3 | 5 (41.67%) | 2 (50.00%) | 3 (37.50%) | |
| 4 | 1 (8.33%) | 1 (25.00%) | 0 (0.00%) | |
| Skin HVHD | 0.7 | |||
| 0 | 7 (58.33%) | 2 (50.00%) | 5 (62.50%) | |
| 2 | 1 (8.33%) | 0 (0.00%) | 1 (12.50%) | |
| 3 | 4 (33.33%) | 2 (50.00%) | 2 (25.00%) | |
| Hepatic HVHD | 0.2 | |||
| 0 | 9 (75.00%) | 2 (50.00%) | 7 (87.50%) | |
| 1 | 1 (8.33%) | 1 (25.00%) | 0 (0.00%) | |
| 2 | 2 (16.67%) | 1 (25.00%) | 1 (12.50%) | |
| Intestinal GVHD | >0.9 | |||
| 0 | 10 (83.33%) | 3 (75.00%) | 7 (87.50%) | |
| 2 | 2 (16.67%) | 1 (25.00%) | 1 (12.50%) | |
| Chronic Skin GVHD | 0.7 | |||
| 0 | 9 (75.00%) | 3 (75.00%) | 6 (75.00%) | |
| 1 | 1 (8.33%) | 1 (25.00%) | 0 (0.00%) | |
| 2 | 1 (8.33%) | 0 (0.00%) | 1 (12.50%) | |
| 3 | 1 (8.33%) | 0 (0.00%) | 1 (12.50%) | |
| Chronic Hepatic GVHD | 0.5 | |||
| 0 | 10 (83.33%) | 4 (100.00%) | 6 (75.00%) | |
| 1 | 2 (16.67%) | 0 (0.00%) | 2 (25.00%) | |
| Chronic Oral GVHD | 0.6 | |||
| 0 | 10 (83.33%) | 3 (75.00%) | 7 (87.50%) | |
| 1 | 1 (8.33%) | 1 (25.00%) | 0 (0.00%) | |
| 2 | 1 (8.33%) | 0 (0.00%) | 1 (12.50%) | |
| Sepsis | 0.067 | |||
| 0 | 8 (66.67%) | 1 (25.00%) | 7 (87.50%) | |
| 1 | 4 (33.33%) | 3 (75.00%) | 1 (12.50%) | |
| Septic shock | >0.9 | |||
| 0 | 10 (83.33%) | 3 (75.00%) | 7 (87.50%) | |
| 1 | 2 (16.67%) | 1 (25.00%) | 1 (12.50%) | |
| Bacteremia | 0.2 | |||
| 0 | 9 (75.00%) | 2 (50.00%) | 7 (87.50%) | |
| 1 | 3 (25.00%) | 2 (50.00%) | 1 (12.50%) |
| Bacterial Genera | Antibiotic Resistance Genes (Multiplex qPCR ) | |||||
|---|---|---|---|---|---|---|
| (16S rRNA Gene, NGS Assay) | ||||||
| OXA-48-like | KPC | NDM | CTX-M-1 | TEM | SHV | |
| E. coli/Shigella | r = 0.154 | r = −0.305 | r = 0.146 | r = 0.325 | r = 0.286 | r = 0.024 |
| p = 0.05 | p = 3.5 × 10−4 | p = 0.05 | p = 1.5 × 10−4 | p = 8 × 10−4 | p = 0.40 | |
| Klebsiella | r = 0.444 | r = 0.249 | r = 0.176 | r = 0.468 | r = 0.515 | r = 0.680 |
| p = 2 × 10−7 | p = 0.003 | p = 0.03 | p = 3.5 × 10−8 | p = 9 × 10−10 | p = 6 × 10−18 | |
| Pseudomonas | r = −0.061 | r = −0.043 | r = 0.268 | r = 0.023 | r = −0.064 | r = 0.024 |
| p = 0.25 | p = 0.32 | p = 0.002 | p = 0.40 | p = 0.24 | p = 0.40 | |
| Ruminococcus | r = −0.378 | r = −0.149 | r = −0.210 | r = −0.356 | r = −0.238 | r = −0.410 |
| p = 1 × 10−5 | p = 0.05 | p = 0.01 | p = 5 × 10−5 | p = 0.004 | p = 2 × 10−6 | |
| Faecalibacter | r = −0.284 | r = −0.140 | r = −0.183 | r = −0.348 | r = −0.257 | r = −0.367 |
| p = 0.001 | p = 0.06 | p = 0.02 | p = 5 × 10−5 | p = 0.002 | p = 2 × 10−5 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goloshchapov, O.V.; Chukhlovin, A.B.; Polev, D.E.; Eismont, Y.A.; Bug, D.S.; Kusakin, A.V.; Kosarev, O.V.; Klementeva, R.V.; Gostev, V.V.; Ageevets, V.A.; et al. Time-Dependent Shifts in Intestinal Bacteriome, Klebsiella Colonization and Incidence of Antibiotic-Resistance Genes after Allogeneic Hematopoietic Stem Cell Transplantation. Biomedicines 2024, 12, 1566. https://doi.org/10.3390/biomedicines12071566
Goloshchapov OV, Chukhlovin AB, Polev DE, Eismont YA, Bug DS, Kusakin AV, Kosarev OV, Klementeva RV, Gostev VV, Ageevets VA, et al. Time-Dependent Shifts in Intestinal Bacteriome, Klebsiella Colonization and Incidence of Antibiotic-Resistance Genes after Allogeneic Hematopoietic Stem Cell Transplantation. Biomedicines. 2024; 12(7):1566. https://doi.org/10.3390/biomedicines12071566
Chicago/Turabian StyleGoloshchapov, Oleg V., Alexey B. Chukhlovin, Dmitrii E. Polev, Yury A. Eismont, Dmitry S. Bug, Alexey V. Kusakin, Oleg V. Kosarev, Ruslana V. Klementeva, Vladimir V. Gostev, Vladimir A. Ageevets, and et al. 2024. "Time-Dependent Shifts in Intestinal Bacteriome, Klebsiella Colonization and Incidence of Antibiotic-Resistance Genes after Allogeneic Hematopoietic Stem Cell Transplantation" Biomedicines 12, no. 7: 1566. https://doi.org/10.3390/biomedicines12071566
APA StyleGoloshchapov, O. V., Chukhlovin, A. B., Polev, D. E., Eismont, Y. A., Bug, D. S., Kusakin, A. V., Kosarev, O. V., Klementeva, R. V., Gostev, V. V., Ageevets, V. A., Volkov, N. P., Ipatova, A. S., Moiseev, I. S., Spiridonova, A. A., Sidorenko, S. V., & Kulagin, A. D. (2024). Time-Dependent Shifts in Intestinal Bacteriome, Klebsiella Colonization and Incidence of Antibiotic-Resistance Genes after Allogeneic Hematopoietic Stem Cell Transplantation. Biomedicines, 12(7), 1566. https://doi.org/10.3390/biomedicines12071566






