Apoptotic Induction by Biosynthesized Gold Nanoparticles Using Phormidesmis communis Strain AB_11_10 against Osteosarcoma Cancer
Abstract
:1. Introduction
2. Experimental Section
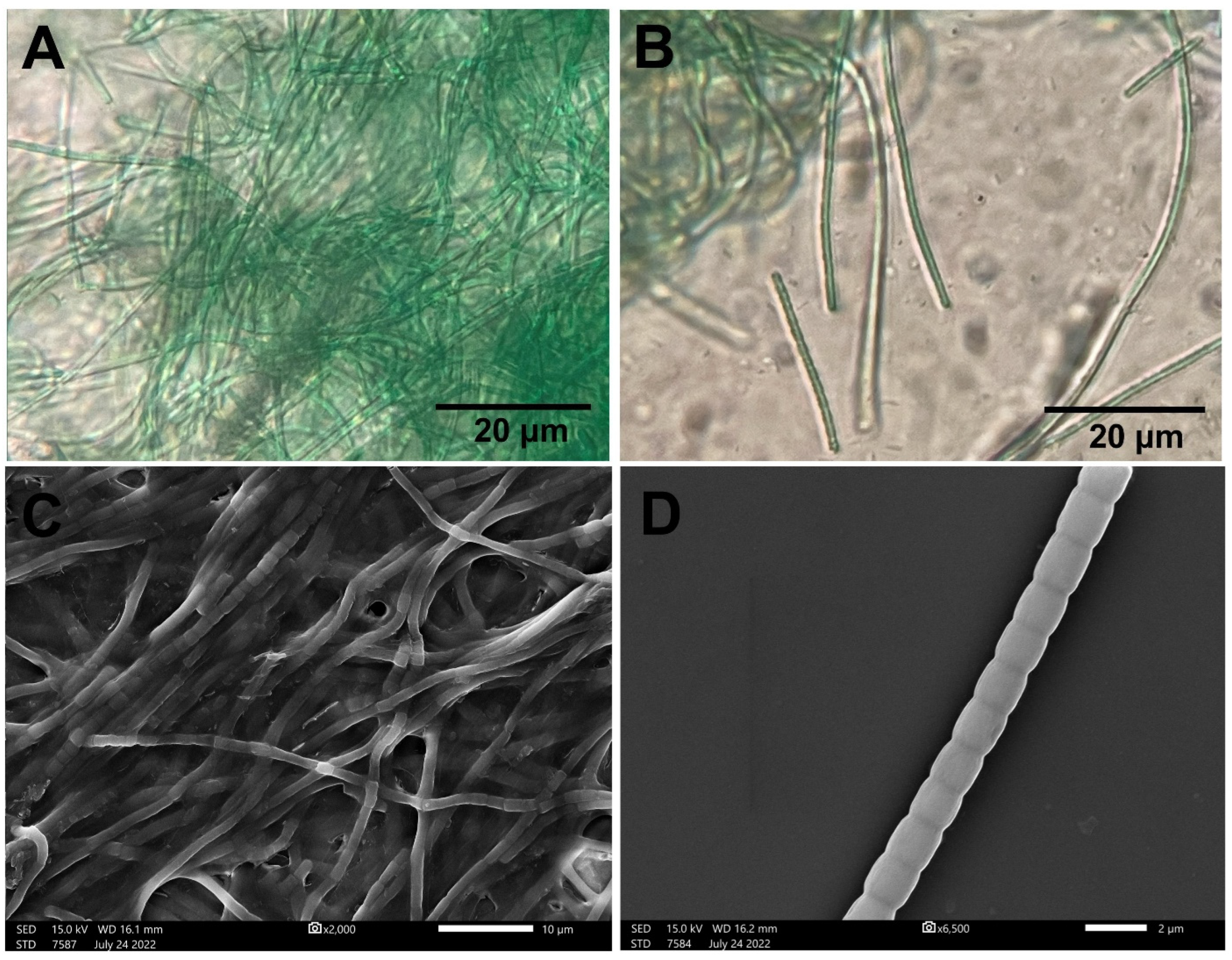
2.1. Cyanobacteria Isolation and Identification
2.2. Algal Extract
2.3. Chemical Composition Using Liquid Chromatography Quadrupole Time-of-Flight Mass Spectrometry (LC-QTOF-MS)
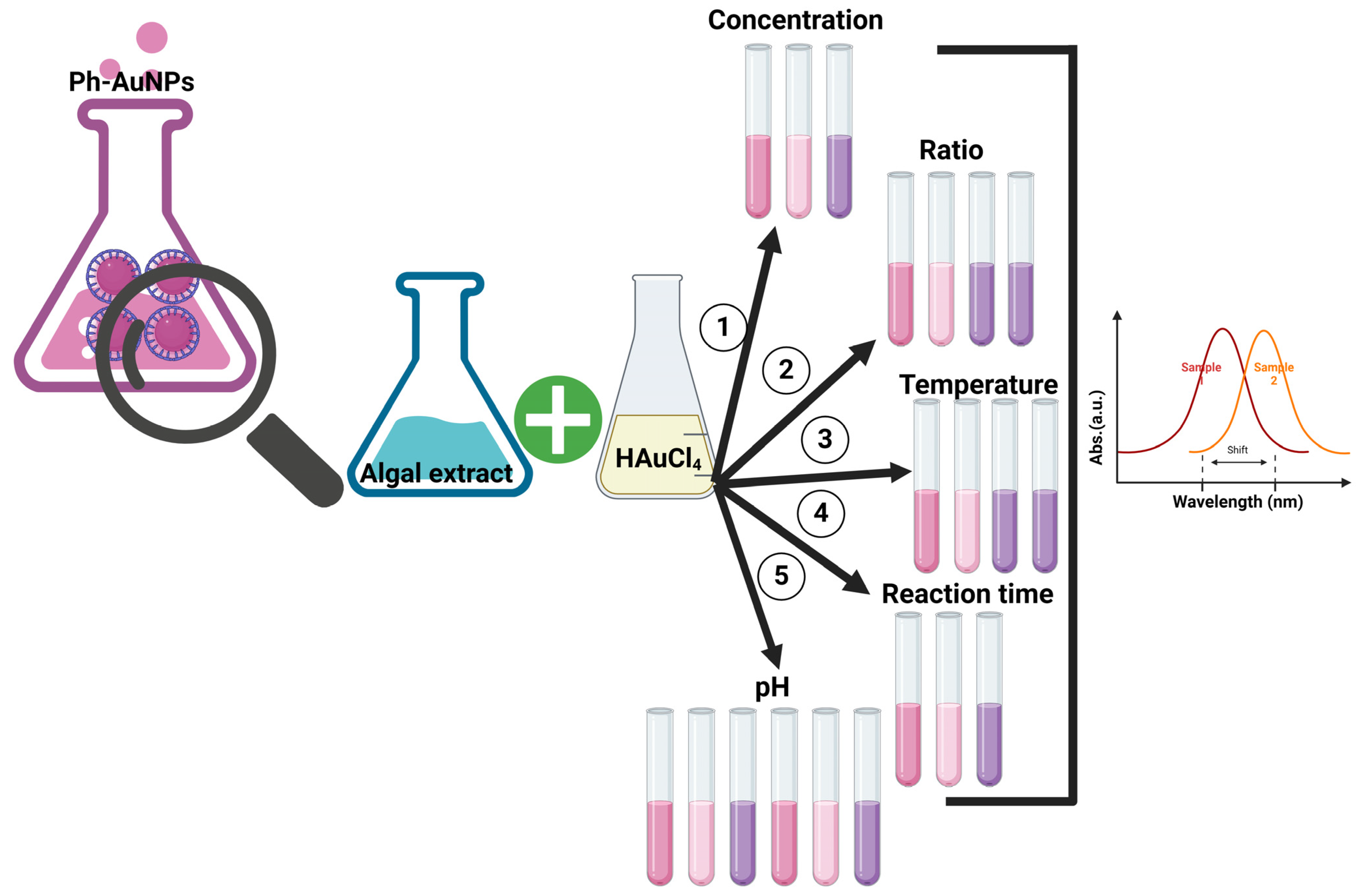
2.4. Optimization of Ph-AuNP Synthesis and Physicochemical Characterizations
3. In Vitro Study
3.1. Cell Culture
3.2. Sulforhodamine B (SRB) Essay
3.3. Apoptotic Analysis Using Annexin VI and Propidium Iodide (PI)
3.4. Cell Cycle Arrest
3.5. Statistical Analysis
4. Results and Discussion
4.1. P. communis Strain AB_11_10 Identification
4.2. Chemical Compositions of P. communis Strain AB_11_10 Algal Extract
4.3. AuNP Synthesis Using P. communis Strain AB_11_10
4.4. Physicochemical Synthesis of AuNPs Synthesized by P. communis Strain AB_11_10
4.5. Anticancer Activity of AuNPs Synthesized by P. communis Strain AB_11_10
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cotterill, S.J.; Wright, C.M.; Pearce, M.S.; Craft, A.W. Stature of young people with malignant bone tumors. Pediatr. Blood Cancer 2004, 42, 59–63. [Google Scholar] [CrossRef] [PubMed]
- Luetke, A.; Meyers, P.A.; Lewis, I.; Juergens, H. Osteosarcoma treatment—Where do we stand? A state of the art review. Cancer Treat. Rev. 2014, 40, 523–532. [Google Scholar] [CrossRef] [PubMed]
- Okinaka, Y.; Takahashi, M. Osteosarcoma of the maxilla: Report of a case and review of the literature concerning metastasis. J. Oral Maxillofac. Surg. 1997, 55, 1177–1181. [Google Scholar] [CrossRef] [PubMed]
- Meazza, C.; Scanagatta, P. Metastatic osteosarcoma: A challenging multidisciplinary treatment. Expert Rev. Anticancer Ther. 2016, 16, 543–556. [Google Scholar] [CrossRef] [PubMed]
- Mirabello, L.; Troisi, R.J.; Savage, S.A. Osteosarcoma incidence and survival rates from 1973 to 2004: Data from the Surveillance, Epidemiology, and End Results Program. Cancer Interdiscip. Int. J. Am. Cancer Soc. 2009, 115, 1531–1543. [Google Scholar] [CrossRef]
- Kansara, M.; Teng, M.W.; Smyth, M.J.; Thomas, D.M. Translational biology of osteosarcoma. Nat. Rev. Cancer 2014, 14, 722–735. [Google Scholar] [CrossRef] [PubMed]
- Gianferante, D.M.; Mirabello, L.; Savage, S.A. Germline and somatic genetics of osteosarcoma—Connecting aetiology, biology and therapy. Nat. Rev. Endocrinol. 2017, 13, 480–491. [Google Scholar] [CrossRef]
- Mirabello, L.; Troisi, R.J.; Savage, S.A. International osteosarcoma incidence patterns in children and adolescents, middle ages and elderly persons. Int. J. Cancer 2009, 125, 229–234. [Google Scholar] [CrossRef]
- Sadykova, L.R.; Ntekim, A.I.; Muyangwa-Semenova, M.; Rutland, C.S.; Jeyapalan, J.N.; Blatt, N.; Rizvanov, A.A. Epidemiology and risk factors of osteosarcoma. Cancer Investig. 2020, 38, 259–269. [Google Scholar] [CrossRef]
- Jafari, F.; Javdansirat, S.; Sanaie, S.; Naseri, A.; Shamekh, A.; Rostamzadeh, D.; Dolati, S. Osteosarcoma: A comprehensive review of management and treatment strategies. Ann. Diagn. Pathol. 2020, 49, 151654. [Google Scholar] [CrossRef]
- Isakoff, M.S.; Bielack, S.S.; Meltzer, P.; Gorlick, R. Osteosarcoma: Current treatment and a collaborative pathway to success. J. Clin. Oncol. 2015, 33, 3029. [Google Scholar] [CrossRef] [PubMed]
- Parveen, S.; Misra, R.; Sahoo, S.K. Nanoparticles: A boon to drug delivery, therapeutics, diagnostics and imaging. In Nanomedicine in Cancer; Jenny Stanford Publishing: Dubai, United Arab Emirates, 2017; pp. 47–98. [Google Scholar]
- Huang, X.; Wu, W.; Yang, W.; Qing, X.; Shao, Z. Surface engineering of nanoparticles with ligands for targeted delivery to osteosarcoma. Colloids Surf. B Biointerfaces 2020, 190, 110891. [Google Scholar] [CrossRef] [PubMed]
- Harish, V.; Tewari, D.; Gaur, M.; Yadav, A.B.; Swaroop, S.; Bechelany, M.; Barhoum, A. Review on nanoparticles and nanostructured materials: Bioimaging, biosensing, drug delivery, tissue engineering, antimicrobial, and agro-food applications. Nanomaterials 2022, 12, 457. [Google Scholar] [CrossRef] [PubMed]
- Pandit, C.; Roy, A.; Ghotekar, S.; Khusro, A.; Islam, M.N.; Emran, T.B.; Lam, S.E.; Khandaker, M.U.; Bradley, D.A. Biological agents for synthesis of nanoparticles and their applications. J. King Saud Univ.-Sci. 2022, 34, 101869. [Google Scholar] [CrossRef]
- Hemlata; Meena, P.R.; Singh, A.P.; Tejavath, K.K. Biosynthesis of silver nanoparticles using Cucumis prophetarum aqueous leaf extract and their antibacterial and antiproliferative activity against cancer cell lines. ACS Omega 2020, 5, 5520–5528. [Google Scholar] [CrossRef] [PubMed]
- Chow, J.C.L. Synthesis and applications of functionalized nanoparticles in biomedicine and radiotherapy. In Additive Manufacturing with Functionalized Nanomaterials; Elsevier: Amsterdam, The Netherlands, 2021; pp. 193–218. [Google Scholar]
- Siddique, S.; Chow, J.C. Recent advances in functionalized nanoparticles in cancer theranostics. Nanomaterials 2022, 12, 2826. [Google Scholar] [CrossRef] [PubMed]
- Burlec, A.F.; Corciova, A.; Boev, M.; Batir-Marin, D.; Mircea, C.; Cioanca, O.; Danila, G.; Danila, M.; Bucur, A.F.; Hancianu, M. Current Overview of Metal Nanoparticles’ Synthesis, Characterization, and Biomedical Applications, with a Focus on Silver and Gold Nanoparticles. Pharmaceuticals 2023, 16, 1410. [Google Scholar] [CrossRef]
- Santhosh, P.B.; Genova, J.; Chamati, H. Green synthesis of gold nanoparticles: An eco-friendly approach. Chemistry 2022, 4, 345–369. [Google Scholar] [CrossRef]
- Amina, S.J.; Guo, B. A review on the synthesis and functionalization of gold nanoparticles as a drug delivery vehicle. Int. J. Nanomed. 2020, 15, 9823–9857. [Google Scholar] [CrossRef]
- Hammami, I.; Alabdallah, N.M. Gold nanoparticles: Synthesis properties and applications. J. King Saud Univ.-Sci. 2021, 33, 101560. [Google Scholar] [CrossRef]
- Tuli, H.S.; Joshi, R.; Kaur, G.; Garg, V.K.; Sak, K.; Varol, M.; Kaur, J.; Alharbi, S.A.; Alahmadi, T.A.; Aggarwal, D. Metal nanoparticles in cancer: From synthesis and metabolism to cellular interactions. J. Nanostruct. Chem. 2023, 13, 321–348. [Google Scholar] [CrossRef]
- Chakraborty, A.; Das, A.; Raha, S.; Barui, A. Size-dependent apoptotic activity of gold nanoparticles on osteosarcoma cells correlated with SERS signal. J. Photochem. Photobiol. B Biol. 2020, 203, 111778. [Google Scholar] [CrossRef]
- Steckiewicz, K.P.; Barcinska, E.; Malankowska, A.; Zauszkiewicz–Pawlak, A.; Nowaczyk, G.; Zaleska-Medynska, A.; Inkielewicz-Stepniak, I. Impact of gold nanoparticles shape on their cytotoxicity against human osteoblast and osteosarcoma in in vitro model. Evaluation of the safety of use and anti-cancer potential. J. Mater. Sci. Mater. Med. 2019, 30, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Rahim, M.; Iram, S.; Khan, M.S.; Khan, M.S.; Shukla, A.R.; Srivastava, A.; Ahmad, S. Glycation-assisted synthesized gold nanoparticles inhibit growth of bone cancer cells. Colloids Surf. B Biointerfaces 2014, 117, 473–479. [Google Scholar] [CrossRef] [PubMed]
- Ghahremani, F.H.; Sazgarnia, A.; Bahreyni-Toosi, M.H.; Rajabi, O.; Aledavood, A. Efficacy of microwave hyperthermia and chemotherapy in the presence of gold nanoparticles: An in vitro study on osteosarcoma. Int. J. Hyperth. 2011, 27, 625–636. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Rashid, R.; Murtaza, G.; Zahra, A. Gold nanoparticles: Synthesis and applications in drug delivery. Trop. J. Pharm. Res. 2014, 13, 1169–1177. [Google Scholar] [CrossRef]
- Patil, M.P.; Kang, M.-J.; Niyonizigiye, I.; Singh, A.; Kim, J.-O.; Seo, Y.B.; Kim, G.-D. Extracellular synthesis of gold nanoparticles using the marine bacterium Paracoccus haeundaensis BC74171T and evaluation of their antioxidant activity and antiproliferative effect on normal and cancer cell lines. Colloids Surf. B Biointerfaces 2019, 183, 110455. [Google Scholar] [CrossRef] [PubMed]
- Patil, M.P.; Kim, G.-D. Eco-friendly approach for nanoparticles synthesis and mechanism behind antibacterial activity of silver and anticancer activity of gold nanoparticles. Appl. Microbiol. Biotechnol. 2017, 101, 79–92. [Google Scholar] [CrossRef]
- Ahmed, I.; Mir, F.A.; Banday, J.A. Synthesis of Metal and Metal Oxide Nanoparticles using Plant Extracts—Characterization and Applications. BioNanoScience 2023, 13, 1541–1557. [Google Scholar] [CrossRef]
- Saravanan, A.; Kumar, P.S.; Karishma, S.; Vo, D.-V.N.; Jeevanantham, S.; Yaashikaa, P.; George, C.S. A review on biosynthesis of metal nanoparticles and its environmental applications. Chemosphere 2021, 264, 128580. [Google Scholar] [CrossRef]
- Ovais, M.; Khalil, A.T.; Ayaz, M.; Ahmad, I.; Nethi, S.K.; Mukherjee, S. Biosynthesis of metal nanoparticles via microbial enzymes: A mechanistic approach. Int. J. Mol. Sci. 2018, 19, 4100. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Kim, Y.-J.; Zhang, D.; Yang, D.-C. Biological synthesis of nanoparticles from plants and microorganisms. Trends Biotechnol. 2016, 34, 588–599. [Google Scholar] [CrossRef] [PubMed]
- Hamida, R.S.; Ali, M.A.; Redhwan, A.; Bin-Meferij, M.M. Cyanobacteria—A promising platform in green nanotechnology: A review on nanoparticles fabrication and their prospective applications. Int. J. Nanomed. 2020, 14, 6033–6066. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Sharma, S.; Sharma, K.; Chetri, S.P.; Vashishtha, A.; Singh, P.; Kumar, R.; Rathi, B.; Agrawal, V. Algae as crucial organisms in advancing nanotechnology: A systematic review. J. Appl. Phycol. 2016, 28, 1759–1774. [Google Scholar] [CrossRef]
- MubarakAli, D.; Arunkumar, J.; Nag, K.H.; SheikSyedIshack, K.; Baldev, E.; Pandiaraj, D.; Thajuddin, N. Gold nanoparticles from Pro and eukaryotic photosynthetic microorganisms—Comparative studies on synthesis and its application on biolabelling. Colloids Surf. B Biointerfaces 2013, 103, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Lengke, M.F.; Ravel, B.; Fleet, M.E.; Wanger, G.; Gordon, R.A.; Southam, G. Mechanisms of gold bioaccumulation by filamentous cyanobacteria from gold (III)−chloride complex. Environ. Sci. Technol. 2006, 40, 6304–6309. [Google Scholar] [CrossRef] [PubMed]
- Ebadi, M.; Zolfaghari, M.R.; Aghaei, S.S.; Zargar, M.; Shafiei, M.; Zahiri, H.S.; Noghabi, K.A. A bio-inspired strategy for the synthesis of zinc oxide nanoparticles (ZnO NPs) using the cell extract of cyanobacterium Nostoc sp. EA03: From biological function to toxicity evaluation. RSC Adv. 2019, 9, 23508–23525. [Google Scholar] [CrossRef] [PubMed]
- Saran, S.; Sharma, G.; Kumar, M.; Ali, M. Biosynthesis of copper oxide nanoparticles using cyanobacteria spirulina platensis and its antibacterial activity. Int. J. Pharm. Sci. Res. 2017, 8, 3887–3892. [Google Scholar]
- Hamida, R.S.; Ali, M.A.; Almohawes, Z.N.; Alahdal, H.; Momenah, M.A.; Bin-Meferij, M.M. Green synthesis of hexagonal silver nanoparticles using a novel microalgae coelastrella aeroterrestrica strain BA_Chlo4 and resulting anticancer, antibacterial, and antioxidant activities. Pharmaceutics 2022, 14, 2002. [Google Scholar] [CrossRef]
- Bolch, C.J.; Orr, P.T.; Jones, G.J.; Blackburn, S.I. Genetic, morphological, and toxicological variation among globally distributed strains of Nodularia (Cyanobacteria). J. Phycol. 1999, 35, 339–355. [Google Scholar] [CrossRef]
- Hamida, R.S.; Ali, M.A.; Mugren, N.; Al-Zaban, M.I.; Bin-Meferij, M.M.; Redhwan, A. Planophila laetevirens-Mediated Synthesis of Silver Nanoparticles: Optimization, Characterization, and Anticancer and Antibacterial Potentials. ACS Omega 2023, 8, 29169–29188. [Google Scholar] [CrossRef] [PubMed]
- Skehan, P.; Storeng, R.; Scudiero, D.; Monks, A.; McMahon, J.; Vistica, D.; Warren, J.T.; Bokesch, H.; Kenney, S.; Boyd, M.R. New colorimetric cytotoxicity assay for anticancer-drug screening. JNCI J. Natl. Cancer Inst. 1990, 82, 1107–1112. [Google Scholar] [CrossRef] [PubMed]
- Fekry, M.I.; Ezzat, S.M.; Salama, M.M.; Alshehri, O.Y.; Al-Abd, A.M. Bioactive glycoalkaloides isolated from Solanum melongena fruit peels with potential anticancer properties against hepatocellular carcinoma cells. Sci. Rep. 2019, 9, 1746. [Google Scholar] [CrossRef] [PubMed]
- Davydov, D.; Vilnet, A. Review of the Cyanobacterial Genus Phormidesmis (Leptolyngbyaceae) with the Description of Apatinema gen. nov. Diversity 2022, 14, 731. [Google Scholar] [CrossRef]
- Asif, N.; Ahmad, R.; Fatima, S.; Shehzadi, S.; Siddiqui, T.; Zaki, A.; Fatma, T. Toxicological assessment of Phormidium sp. derived copper oxide nanoparticles for its biomedical and environmental applications. Sci. Rep. 2023, 13, 6246. [Google Scholar] [CrossRef] [PubMed]
- Birla, S.S.; Gaikwad, S.C.; Gade, A.K.; Rai, M.K. Rapid synthesis of silver nanoparticles from Fusarium oxysporum by optimizing physicocultural conditions. Sci. World J. 2013, 2013, 796018. [Google Scholar] [CrossRef] [PubMed]
- Kumari, M.; Mishra, A.; Pandey, S.; Singh, S.P.; Chaudhry, V.; Mudiam, M.K.R.; Shukla, S.; Kakkar, P.; Nautiyal, C.S. Physico-chemical condition optimization during biosynthesis lead to development of improved and catalytically efficient gold nano particles. Sci. Rep. 2016, 6, 27575. [Google Scholar] [CrossRef]
- Princy, K.; Gopinath, A. Optimization of physicochemical parameters in the biofabrication of gold nanoparticles using marine macroalgae Padina tetrastromatica and its catalytic efficacy in the degradation of organic dyes. J. Nanostruct. Chem. 2018, 8, 333–342. [Google Scholar] [CrossRef]
- Mountrichas, G.; Pispas, S.; Kamitsos, E.I. Effect of temperature on the direct synthesis of gold nanoparticles mediated by poly (dimethylaminoethyl methacrylate) homopolymer. J. Phys. Chem. C 2014, 118, 22754–22759. [Google Scholar] [CrossRef]
- Subbulakshmi, A.; Durgadevi, S.; Anitha, S.; Govarthanan, M.; Biruntha, M.; Rameshthangam, P.; Kumar, P. Biogenic gold nanoparticles from Gelidiella acerosa: Bactericidal and photocatalytic degradation of two commercial dyes. Appl. Nanosci. 2023, 13, 4033–4042. [Google Scholar] [CrossRef]
- Amutha, R.; Arumugam, P.; Berchmans, S. Synthesis of gold nanoparticles: An ecofriendly approach using Hansenula anomala. ACS Appl. Mater. Interfaces 2011, 3, 1418–1425. [Google Scholar]
- González-Ballesteros, N.; Diego-González, L.; Lastra-Valdor, M.; Grimaldi, M.; Cavazza, A.; Bigi, F.; Rodríguez-Argüelles, M.C.; Simón-Vázquez, R. Immunomodulatory and antitumoral activity of gold nanoparticles synthesized by red algae aqueous extracts. Mar. Drugs 2022, 20, 182. [Google Scholar] [CrossRef] [PubMed]
- Parial, D.; Patra, H.K.; Dasgupta, A.K.; Pal, R. Screening of different algae for green synthesis of gold nanoparticles. Eur. J. Phycol. 2012, 47, 22–29. [Google Scholar] [CrossRef]
- Lenartowicz, M.; Marek, P.H.; Madura, I.D.; Lipok, J. Formation of variously shaped gold nanoparticles by Anabaena laxa. J. Clust. Sci. 2017, 28, 3035–3055. [Google Scholar] [CrossRef]
- Parial, D.; Gopal, P.K.; Paul, S.; Pal, R. Gold (III) bioreduction by cyanobacteria with special reference to in vitro biosafety assay of gold nanoparticles. J. Appl. Phycol. 2016, 28, 3395–3406. [Google Scholar] [CrossRef]
- Kowsalya, E.; MosaChristas, K.; Jaquline, C.R.I.; Balashanmugam, P.; Devasena, T. Gold nanoparticles induced apoptosis via oxidative stress and mitochondrial dysfunctions in MCF-7 breast cancer cells. Appl. Organomet. Chem. 2021, 35, e6071. [Google Scholar] [CrossRef]
- Ajdari, Z.; Rahman, H.; Shameli, K.; Abdullah, R.; Abd Ghani, M.; Yeap, S.; Abbasiliasi, S.; Ajdari, D.; Ariff, A. Novel gold nanoparticles reduced by Sargassum glaucescens: Preparation, characterization and anticancer activity. Molecules 2016, 21, 123. [Google Scholar] [CrossRef]









| No | RT | −/+ | Formula | Precursor Mass | Found at Mass | Compound |
|---|---|---|---|---|---|---|
| 1 | 1.92 | + | C8H4O3 | 149.023 | 149.0234 | Phthalic anhydride |
| 2 | 2.11 | − | CH4O8S | 174.957 | 174.9560 | Hydroxy trihydroxymethyl sulfate |
| 3 | 2.26 | + | C8H4O3 | 149.023 | 149.0233 | Phthalic anhydride |
| 4 | 11.43 | + | C8H10 | 107.085 | 107.0852 | O-xylene |
| 5 | 10.5 | − | C9H11NO2 | 164.072 | 164.0713 | Phenylalanine |
| 6 | 11.96 | + | C16H22O4 | 279.172 | 279.1594 | Dibutyl phthalate |
| 7 | 14.06 | + | C11H14O2 | 179.107 | 179.1063 | Methyl eugenol |
| 8 | 14.23 | + | C6H11NO | 114.091 | 114.0909 | Caprolactam |
| 9 | 15.78 | + | C5H5N | 80.049 | 80.0492 | Pyridine |
| 10 | 17.04 | − | C8H10O3S | 185.119 | 185.1177 | Benzenesulfonic acid, dimethyl- |
| 11 | 19.44 | − | C39H70O3 | 585.526 | 585.5253 | 2-(4-Hydroxyphenyl)ethylhentriacontanoate |
| 12 | 19.68 | − | C12H26O4S | 265.18 | 265.1465 | Dodecyl sulfate |
| 13 | 19.84 | + | C8H4O3 | 149.023 | 149.0231 | Phthalic anhydride |
| 14 | 20.29 | + | C8H16O3S2 | 225.061 | 225.0613 | 1,6,9-Trioxa-3,12-dithiacyclotridecane |
| 15 | 21.25 | + | C22H43NS | 354.319 | 354.3191 | (Z,6R)-N-(2,5-dimethylhex-5-enyl)-8-methylsulfanyl-6-propyldec-8-en-2-amine |
| 16 | 21.63 | + | C28H22O4 | 423.160 | 423.1590 | ptychantol A |
| 17 | 22.33 | + | C7H7N | 106.065 | 106.0653 | 4-Vinylpyridine |
| 18 | 23.36 | − | C18H30O2 | 577.422 | 577.4247 | Linolenic acid |
| Element | Line | Mass% | Atom% |
|---|---|---|---|
| C | K | 17.90 ± 3.36 | 71.37 ± 0.37 |
| O | K | 2.37 ± 1.04 | 7.09 ± 0.09 |
| Al | K | 0.21 ± 0.25 | 0.37 ± 0.01 |
| Ca | K | 0.16 ± 0.30 | 0.19 ± 0.01 |
| Ni | K | 0.41 ± 0.47 | 0.34 ± 0.01 |
| Cu | K | 2.85 ± 0.85 | 2.15 ± 0.02 |
| Au | M | 76.10 ± 3.14 | 18.50 ± 0.02 |
| Total | 100.00 | 100.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hamida, R.S.; AlMotwaa, S.M.; Al-Otaibi, W.A.; Alqhtani, H.A.; Ali, M.A.; Bin-Meferij, M.M. Apoptotic Induction by Biosynthesized Gold Nanoparticles Using Phormidesmis communis Strain AB_11_10 against Osteosarcoma Cancer. Biomedicines 2024, 12, 1570. https://doi.org/10.3390/biomedicines12071570
Hamida RS, AlMotwaa SM, Al-Otaibi WA, Alqhtani HA, Ali MA, Bin-Meferij MM. Apoptotic Induction by Biosynthesized Gold Nanoparticles Using Phormidesmis communis Strain AB_11_10 against Osteosarcoma Cancer. Biomedicines. 2024; 12(7):1570. https://doi.org/10.3390/biomedicines12071570
Chicago/Turabian StyleHamida, Reham Samir, Sahar M. AlMotwaa, Waad A. Al-Otaibi, Haifa A. Alqhtani, Mohamed Abdelaal Ali, and Mashael Mohammed Bin-Meferij. 2024. "Apoptotic Induction by Biosynthesized Gold Nanoparticles Using Phormidesmis communis Strain AB_11_10 against Osteosarcoma Cancer" Biomedicines 12, no. 7: 1570. https://doi.org/10.3390/biomedicines12071570





