Quality of Sleep and Mental Symptoms Contribute to Health-Related Quality of Life after COVID-19 Pneumonia, a Follow-Up Study of More than 2 Years
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Evaluation Time Points
2.2.1. Daily Agendas
2.2.2. Questionnaires
2.3. Procedures
2.3.1. Daily Agendas

| Variable | Men (n = 30) | Women (n = 42) | All (n = 72) |
|---|---|---|---|
| Mean ± S.D. (95% C.I.) | Mean ± S.D. (95% C.I.)) | Mean ± S.D. (95% C.I.) | |
| Years of age | 52.4 ± 12.2 (47.8–57.0) | 52.5 ± 14.8 (47.9–57.2) | 52.5 ± 13.7 (49.2–55.7) |
| Companion/no companion | 20/10 | 32/10 | 52/20 |
| Body mass index at hospital admission | 32.1 ± 7.19 (29.4–34.0) | 29.3 ± 5.1 (27.7–30.9) | 30.5 ± 6.2 (29.1–31.8) |
| Body mass index at hospital discharge | 31.5 ± 6.9 (29.0–34.1) | 28.8 ± 5.0 (27.3–30.4) | 29.9 ± 5.8 (28.6–32.2) |
| Days from symptom onset to hospitalization | 8.1 ± 3.6 (6.7–9.4) | 9.9 ± 4.3 (8.6–11.2) | 9.1 ± 4.1 (8.2–10.1) |
| Days in hospital | 11.6 ± 12.8 (6.7–16.4) | 10.0 ± 7.4 (7.9–12.5) | 10.8 ± 10.0 (8.4–13.1) |
| Oxygen saturation at hospital admission (%) | 79.8 ± 12.6 (49.0–96.0) | 83.1 ± 11.0 (79.6–86.5) | 81.7 ± 11.7 (79.0–84.5) |
| Oxygen saturation at hospital discharge (%) | 93.4 ± 2.6 (88.0–98.0) | 93.9 ± 2.2 (93.2–94.6) | 93.6 ± 2.4 (93.1–94.2) |
| Heart rate per minute at hospital admission | 92.8 ± 19.7 (85.4–100.1) | 98.0 ± 19.1 (92.0–104.0() | 95.8 ± 19.4 (91.3–100.4) |
| Heart rate per minute at hospital discharge | 80.8 ± 10.8 (76.7–84.8) | 72.5 ± 10.6 (69.2–75.8) | 75.9 ± 11.3 (73.2–78.6) |
| Leucocytes count at hospital admission | 9.1 ± 4.8 (7.2–109) | 10.1 ± 4.0 (8.8–11.3) | 9.6 ± 4.3 (8.6–10.7) |
| Neutrophils at hospital admission | 82.8 ± 10.5 (76.3–84.3) | 85.3 ± 7.3 (83.0–87.6) | 83.4 ± 9.0 (81.2–85.5) |
| Lymphocytes at hospital admission | 13.7 ± 9.0 (10.3–17.2) | 8.8 ± 4.7 (7.3–10.2) | 10.7 ± 7.1 (9.0 12.4) |
| Leucocytes count at hospital discharge | 8.0 ± 2.3 (7.1–8.9) | 8.0 ± 2.4 (7.2–8.7) | 8.0 ± 2.3 (7.4–8.5) |
| Neutrophils at hospital discharge | 70.8 ± 14.5 (65.3–76.3) | 72.7 ± 13.3 (68.5–76.9) | 72.0 ± 13.6 (68.8–75.2) |
| Lymphocytes at hospital discharge | 21.9 ± 11.8 (17.3–26.4) | 18.6 ± 10.3 (15.4–21.9) | 19.9 ± 10.9 (17.3–22.5) |
| n (%, 95% C.I.) | n (%, 95% C.I.) | n (%, 95% C.I.) | |
| Intensive care during hospitalization | 6 (20%, 5.6–34.3%) | 7 (16%, 4.9–27%) | 13 (18%, 9.1–26.8%) |
| Systemic high blood pressure | 14 (46%, 28.1–63.8%) | 12 (28%, 14.4–41.5%) | 26 (36%, 24.9–47.0%) |
| Type 2 diabetes | 17 (56%, 38.2–73.7%) | 13 (31%, 17.0–44.9%) | 30 (41%, 29.6–52.3%) |
| Ever smokers | 5 (16%, 2.8–29.1%) | 11 (26%, 12.7–39.2%) | 16 (22%, 12.4–31.5%) |
| Alcohol use | 7 (23%, 7.9–38.0%) | 24 (57%, 42.0–71.9%) | 31 (43%, 31.5–54.4%) |
| Schooling | |||
| <6 years | 3 (10%, 0–20.7%) | 1 (2%, -2.2–6.2%) | 4 (5%, 0–10.0%) |
| 6 years | 4 (13%, 0–25.0%) | 6 (14%, 3.5–24.4%) | 10 (14%, 5.9–22.0%) |
| 9 years | 8 (27%, 11–42%) | 13 (31%, 17.0–44.9%) | 21 (29%, 18.5–39.4%) |
| 12 years | 8 (27%, 11–42%) | 6 (14%, 3.5–24.4%) | 14 (19%, 9.9–28.0%) |
| >12 years | 7 (23%, 7.9–38.0%) | 16 (38%, 23.3–52.6%) | 23 (32%, 21.2–42.7%) |
2.3.2. Questionnaires
- The Pittsburgh Sleep Quality Index [22], which comprises 19 items on 7 components: subjective sleep quality, sleep latency, sleep duration, habitual sleep efficiency, sleep disturbances, use of sleeping medication, and daytime dysfunction. A total score is calculated according to instructions by the developers [22]; a cut-off score > 5 is used to distinguish poor sleep, with Cronbach’s alpha coefficient ≥ 0.70 [23,24].
- The Hospital Anxiety and Depression Scale (HADS) [25], which comprises 14 items (7 for anxiety and 7 for depression) rated on a scale from 0 to 4. A total score is calculated by the sum of the ratings for all the items, and the two subscores are calculated by summing the ratings of the corresponding items. Cut-off scores of ≥8 for the subscores and ≥11 for the total score have shown sensitivity and specificity ≥ 0.70 [22,23], with a Cronbach’s alpha coefficient ≥ 0.67 [26], and test–retest reliability of the Spanish version > 0.85 [27].
- The Dissociative Experiences Scale [29], which comprises 28 items on disturbances in memory, identity, and cognition, and feelings of depersonalization, derealization, absorption, and imaginative involvement. Scores on each item may range from 0% to 100% (using multiples of ten: 10%, 20%, 30%, etc.). A total score is calculated by dividing the sum of the individual scores by 28 (range 0% to 100%); a cut-off score of 8 is considered for the low normal range. The Spanish version has a Cronbach alpha coefficient of 0.96 [30].
- The Post-traumatic Stress Disorder Symptom Severity Scale-Revised by Echeburúa (2016) [31], which is a 21-item structured interview based on DSM-5 criteria, with a Cronbach alpha coefficient of 0.91 [31]. Individual diagnosis was performed according to both the scale and face-to-face psychiatric interview.
- The Montreal Cognitive Assessment (MoCA) [32], which comprises six domains: Visuo-spatial, Naming, Attention, Language, Abstraction, Memory, and Orientation. The items in each domain yield individual index scores, with a maximum total score of 30 points, where a 24/25 cut-off has shown higher specificity than a 25/26 cut-off [33]. It has shown a Cronbach’s alpha coefficient > 0.70 [34], with the reliability of the Spanish version of 0.89 [35].
- The Short-Form Health Survey (SF-36) [36], which is composed of 36 items on eight domains of health-related quality of life: physical function, social functioning, role limitations due to physical problems, role limitations due to emotional problems, mental health, vitality, pain, and general health perception. Scores on each domain range from 0 to 100 [37]. The Spanish version has a Cronbach’s alpha coefficient > 0.7 for all dimensions, except for social functioning (alpha coefficient = 0.45) [38].
2.4. Statistical Analysis
3. Results
3.1. Descriptive Statistics and Exploratory Bivariate Analyses
3.1.1. Agendas
3.1.2. Questionnaires
3.2. Multivariate Analysis
3.2.1. Cognitive Performance
3.2.2. Health-Related Quality of Life
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ceban, F.; Ling, S.; Lui, L.M.W.; Lee, Y.; Gill, H.; Teopiz, K.M.; Rodrigues, N.B.; Subramaniapillai, M.; Di Vincenzo, J.D.; Cao, B.; et al. Fatigue and cognitive impairment in post-COVID-19 Syndrome: A systematic review and meta-analysis. Brain. Behav. Immun. 2022, 101, 93–135. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization Coronavirus (COVID-19) Dashboard. Available online: https://covid19.who.int/ (accessed on 31 May 2024).
- Sk Abd Razak, R.; Ismail, A.; Abdul Aziz, A.F.; Suddin, L.S.; Azzeri, A.; Insyirah Sha’ari, N. Post-COVID syndrome prevalence: A systematic review and meta-analysis. BMC Public Health 2024, 24, 1785. [Google Scholar] [CrossRef] [PubMed]
- National Institute for Health and Care Excellence (NICE). COVID-19 Rapid Guideline: Managing the Long-Term Effects of COVID-19; NICE Guideline 188; National Institute for Health and Care Excellence (NICE): London, UK, 2020. [Google Scholar]
- CDC. Long COVID-Household Pulse Survey-COVID-19. CDC. Available online: https://www.cdc.gov/nchs/covid19/pulse/long-covid.htm (accessed on 27 April 2023).
- Davis, H.E.; McCorkell, L.; Vogel, J.M.; Topol, J. Long COVID: Major findings, mechanisms, and recommendations. Nat. Rev. Microbiol. 2023, 21, 133–146. [Google Scholar] [CrossRef] [PubMed]
- Morioka, S.; Nikaido, M.; Tsuzuki, S.; Kutsuna, S.; Saito, S.; Hayakawa, K.; Sugiyama, M.; Ohmagari, N. Epidemiology of post-COVID conditions beyond 3 years and factors associated with their persistence longer than 2 years: A cross-sectional study. J. Infect. Chemother. 2024, 30, 734–740. [Google Scholar] [CrossRef] [PubMed]
- Baskett, W.I.; Qureshi, A.I.; Shyu, D.; Armer, J.M.; Shyu, C.R. COVID-Specific Long-term Sequelae in Comparison to Common Viral Respiratory Infections: An Analysis of 17 487 Infected Adult Patients. Open Forum Infect. Dis. 2023, 10, ofac683. [Google Scholar] [CrossRef] [PubMed]
- Taquet, M.; Geddes, J.R.; Husain, M.; Luciano, S.; Harrison, P.J. 6-month neurological and psychiatric outcomes in 236 379 survivors of COVID-19: A retrospective cohort study using electronic health records. Lancet Psychiatry 2021, 8, 416–427. [Google Scholar] [CrossRef] [PubMed]
- Stavem, K.; Heir, T.; Dammen, T.; Brønstad, E.; Lerum, T.V.; Durheim, M.T.; Lund, K.M.A. The course and determinants of post-traumatic stress over 12 months after hospitalization for COVID-19. Front. Psychiatry 2022, 13, 931349. [Google Scholar] [CrossRef] [PubMed]
- Al-Aly, Z.; Xie, Y.; Bowe, B. High-dimensional characterization of post-acute sequelae of COVID-19. Nature 2021, 594, 259–264. [Google Scholar] [CrossRef]
- Woo, M.S.; Malsy, J.; Pöttgen, J.; Zai, S.; Ufer, F.; Hadjilaou, A.; Schmiedel, S.; Addo, M.M.; Gerloff, C.; Heesen, C.; et al. Frequent neurocognitive deficits after recovery from mild COVID-19. Brain Commun. 2020, 2, fcaa205. [Google Scholar] [CrossRef]
- Greene, C.; Connolly, R.; Brennan, D.; Laffan, A.; O’Keeffe, E.; Zaporojan, L.; O’Callaghan, J.; Thomson, B.; Connolly, E.; Argue, R.; et al. Blood-brain barrier disruption and sustained systemic inflammation in individuals with long COVID-associated cognitive impairment. Nat. Neurosci. 2024, 27, 421–432. [Google Scholar] [CrossRef]
- Oka, N.; Shimada, K.; Ishii, A.; Kobayashi, N.; Kondo, K. SARS-CoV-2 S1 protein causes brain inflammation by reducing intracerebral acetylcholine production. Science 2023, 26, 106954. [Google Scholar] [CrossRef] [PubMed]
- Jackson, C.; Stewart, I.D.; Plekhanova, T.; Cunningham, P.S.; Hazel, A.L.; Al-Sheklly, B.; Aul, R.; Bolton, C.E.; Chalder, T.; Chalmers, J.D.; et al. Effects of sleep disturbance on dyspnoea and impaired lung function following hospital admission due to COVID-19 in the UK: A prospective multicentre cohort study. Lancet Respir. Med. 2023, 11, 673–684. [Google Scholar] [CrossRef] [PubMed]
- Pellitteri, G.; Surcinelli, A.; De Martino, M.; Fabris, M.; Janes, F.; Bax, F.; Marini, A.; Marini, A.; Milanic, R.; Piani, A.; et al. Sleep alterations following COVID-19 are associated with both neuroinflammation and psychological disorders, although at different times. Front. Neurol. 2022, 13, 929480. [Google Scholar] [CrossRef] [PubMed]
- Pataka, A.; Kotoulas, S.; Sakka, E.; Katsaounou, P.; Pappa, S. Sleep Dysfunction in COVID-19 Patients: Prevalence, Risk Factors, Mechanisms, and Management. J. Pers. Med. 2021, 11, 1203. [Google Scholar] [CrossRef] [PubMed]
- Semyachkina-Glushkovskaya, O.; Mamedova, A.; Vinnik, V.; Klimova, M.; Saranceva, E.; Ageev, V.; Penzel, T.; Kurths, J. Brain mechanisms of COVID-19 sleep disorders. Int. J. Mol. Sci. 2021, 22, 6917. [Google Scholar] [CrossRef] [PubMed]
- Sheehan, D.V.; Lecrubier, Y.; Sheehan, K.H.; Amorim, P.; Janavs, J.; Weiller, E.; Hergueta, T.; Baker, R.; Dunbar, G.C. The Mini-International Neuropsychiatric Interview (M.I.N.I.): The development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J. Clin. Psychiatry 1998, 59 (Suppl. S20), 22–33. [Google Scholar] [PubMed]
- Cummings, J.L.; Mega, M.; Gray, K.; Rosenberg-Thompson, S.; Carusi, D.A.; Gornbein, J. The Neuropsychiatric Inventory: Comprehensive assessment of psychopathology in dementia. Neurology 1994, 44, 2308–2314. [Google Scholar] [CrossRef] [PubMed]
- Borg, G.; Hassmén, P.; Lagerström, M. Perceived exertion related to heart rate and blood lactate during arm and leg exercise. Eur. J. Appl. Physiol. Occup. Physiol. 1987, 56, 679–685. [Google Scholar] [CrossRef] [PubMed]
- Buysse, D.J.; Reynolds, C.F., III; Monk, T.H.; Berman, S.R.; Kupfer, D.J. The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989, 28, 193–213. [Google Scholar] [CrossRef]
- Mollayeva, T.; Thurairajah, P.; Burton, K.; Mollayeva, S.; Shapiro, C.M.; Colantonio, A. The Pittsburgh sleep quality index as a screening tool for sleep dysfunction in clinical and non-clinical samples: A systematic review and meta-analysis. Sleep Med. Rev. 2016, 25, 52–73. [Google Scholar] [CrossRef]
- Hita-Contreras, F.; Martínez-López, E.; Latorre-Román, P.A.; Garrido, F.; Santos, M.A.; Martínez-Amat, A. Reliability and validity of the Spanish version of the Pittsburgh Sleep Quality Index (PSQI) in patients with fibromyalgia. Rheumatol. Int. 2014, 34, 929–936. [Google Scholar] [CrossRef] [PubMed]
- Zigmond, A.S.; Snaith, R.P. The Hospital Anxiety and Depression Scale. Acta Psychiatr. Scand. 1983, 67, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Bjelland, I.; Dahl, A.A.; Haug, T.T.; Neckelmann, D. The validity of the Hospital Anxiety and Depression Scale: An updated literature review. J. Psychosom. Res. 2002, 52, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Quintana, J.M.; Padierna, A.; Esteban, C.; Arostegui, I.; Bilbao, A.; Ruiz, I. Evaluation of the psychometric characteristics of the Spanish version of the Hospital Anxiety and Depression Scale. Acta Psychiatr. Scand. 2003, 107, 216–221. [Google Scholar] [CrossRef] [PubMed]
- Cox, B.J.; Swinson, R.P. Instrument to assess depersonalization-derealization in panic disorder. Depress. Anxiety 2002, 15, 172–175. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, E.M.; Putnam, F.W. Development, Reliability, and Validity of a Dissociation Scale. J. Nerv. Ment. Dis. 1986, 174, 727–735. [Google Scholar] [CrossRef] [PubMed]
- Robles Garcia, R.; Garibay Rico, S.E.; Paez Agraz, F. Evaluación de trastornos disociativos en población psiquiátrica mexicana: Prevalencia, comorbilidad y características psicométricas de la Escala de Experiencias Disociativas. Salud Ment. 2006, 29, 38–43. [Google Scholar]
- Echeburúa, E.; Amor, P.J.; Sarasua, B.; Zubizarreta, I.; Holgado-Tello, F.P.; Muñoz, J.M. Posttraumatic Stress Disorder Symptom Severity Scale-Revised (EGS-R) according to DSM-5 criteria: Psychometric Properties. Ter. Psicol. 2016, 34, 111–128. [Google Scholar] [CrossRef]
- Nasreddine, Z.S.; Phillips, N.A.; Bédirian, V.; Charbonneau, S.; Whitehead, V.; Collin, I.; Cummings, J.L.; Chertkow, H. The Montreal Cognitive Assessment, MoCA: A brief screening tool for mild cognitive impairment. J. Am. Geriatr. Soc. 2005, 53, 695–699. [Google Scholar] [CrossRef]
- Sun, R.; Ge, B.; Wu, S.; Li, H.; Lin, L. Optimal cut-off MoCA score for screening for mild cognitive impairment in elderly individuals in China: A systematic review and meta-analysis. Asian J. Psychiatry 2023, 87, 103691. [Google Scholar] [CrossRef]
- Koski, L. Validity and Applications of the Montreal Cognitive Assessment for the Assessment of Vascular Cognitive Impairment. Cerebrovasc. Dis. 2013, 36, 6–18. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-Navarro, S.G.; Mimenza-Alvarado, A.J.; Palacios-García, A.A.; Samudio-Cruz, A.; Gutiérrez-Gutiérrez, L.A.; Ávila-Funes, J.A. Validity and Reliability of the Spanish Version of the Montreal Cognitive Assessment (MoCA) for the Detection of Cognitive Impairment in Mexico. Rev. Colomb. Psiquiatr. 2018, 47, 237–243. [Google Scholar] [CrossRef] [PubMed]
- McHorney, C.A.; Ware, J.E.; Raczek, A.E. The MOS 36-Item Short-Form Health Survey (SF-36): II. Psychometric and clinical tests of validity in measuring physical and mental health constructs. Med. Care 1993, 31, 247–263. [Google Scholar] [CrossRef]
- Ware, J.E.; Kosinski, M.; Keller, S.D. SF-36 Physical and Mental Health Summary Scales: A User’s Manual; The Health Institute: Boston, MA, USA, 1994. [Google Scholar]
- Alonso, J.; Prieto, L.; Anto, J. La versión española del SF-36 Health Survey (Cuestionario de Salud SF-36): Un instrumento para la medida de los resultados clínicos. Med. Clin. 1995, 104, 771–776. [Google Scholar]
- Cohen, J. Statistical Power Analysis. Curr. Dir. Psychol. Sci. 1992, 1, 98–101. [Google Scholar] [CrossRef]
- Shah, A.S.; Ryu, M.H.; Hague, C.J.; Murphy, D.T.; Johnston, J.C.; Ryerson, C.J.; Carlsten, C.; Wong, A.W. Changes in pulmonary function and patient-reported outcomes during COVID-19 recovery: A longitudinal, prospective cohort study. ERJ Open Res. 2021, 7, 00243–02021. [Google Scholar] [CrossRef]
- Seeßle, J.; Waterboer, T.; Hippchen, T.; Simon, J.; Kirchner, M.; Lim, A.; Müller, B.; Merle, U. Persistent Symptoms in Adult Patients 1 Year after Coronavirus Disease 2019 (COVID-19): A Prospective Cohort Study. Clin. Infect. Dis. 2022, 74, 1191–1198. [Google Scholar] [CrossRef]
- Zheng, B.; Daines, L.; Han, Q.; Hurst, J.R.; Pfeffer, P.; Shankar-Hari, M.; Elneima, O.; Walker, S.; Brown, J.S.; Siddiqui, S.; et al. Prevalence, risk factors, and treatments for post-COVID breathlessness: A systematic review and meta-analysis. Eur. Respir. Rev. 2022, 31, 220071. [Google Scholar] [CrossRef]
- Lim, R.K.; Rosentreter, R.; Chen, Y.; Mehta, R.; McLeod, G.; Wan, M.; Krett, J.D.; Mahjoub, Y.; Lee, A.; Schwartz, I. Quality of life, respiratory symptoms, and health care utilization 1 year following outpatient management of COVID-19: A prospective cohort study. Sci. Rep. 2022, 12, 12988. [Google Scholar] [CrossRef]
- Zheng, B.; Vivaldi, G.; Daines, L.; Leavy, O.C.; Richardson, M.; Elneima, O.; McAuley, H.J.C.; Shikotra, A.; Singapuri, A.; Sereno, M. Determinants of recovery from post-COVID-19 dyspnoea: Analysis of UK prospective cohorts of hospitalized COVID-19 patients and community-based controls. Lancet Reg. Health Eur. 2023, 29, 100635. [Google Scholar] [CrossRef]
- Romanet, C.; Wormser, J.; Fels, A.; Lucas, P.; Prudat, C.; Sacco, E.; Bruel, C.; Plantefève, G.; Pene, F.; Chatellier, G.; et al. Effectiveness of exercise training on the dyspnoea of individuals with long COVID: A randomised controlled multicentre trial. Ann. Physic. Rehab. Med. 2023, 66, 101765. [Google Scholar] [CrossRef] [PubMed]
- Kalamara, E.; Pataka, A.; Boutou, A.; Panagiotidou, E.; Georgopoulou, A.; Ballas, E.; Chloros, D.; Metallidis, S.; Kioumis, I.; Pitsiou, G. Persistent Sleep Quality Deterioration among Post-COVID-19 Patients: Results from a 6-Month Follow-Up Study. J. Pers. Med. 2022, 12, 1909. [Google Scholar] [CrossRef] [PubMed]
- Linh, T.T.D.; Ho, D.K.N.; Nguyen, N.N.; Hu, C.-J.; Yang, C.-H.; Wu, D. Global prevalence of post-COVID-19 sleep disturbances in adults at different follow-up time points: A systematic review and meta-analysis. Sleep Med. Rev. 2023, 71, 101833. [Google Scholar] [CrossRef] [PubMed]
- Seighali, N.; Abdollahi, A.; Shafiee, A.; Amini, J.; Athar, M.M.T.; Safari, O.; Faghfouri, P.; Eskandari, A.; Rostaii, O.; Salehi, A.H.; et al. The global prevalence of depression, anxiety, and sleep disorder among patients coping with Post COVID-19 syndrome (long COVID): A systematic review and meta-analysis. BMC Psychiatry 2024, 24, 105. [Google Scholar] [CrossRef] [PubMed]
- Chinvararak, C.; Chalder, T. Prevalence of sleep disturbances in patients with long COVID assessed by standardized questionnaires and diagnostic criteria: A systematic review and meta-analysis. J. Psychosom. Res. 2023, 223, 111535. [Google Scholar] [CrossRef] [PubMed]
- Han, Q.; Zheng, B.; Daines, L.; Sheikh, A. Long-Term Sequelae of COVID-19: A Systematic Review and Meta-Analysis of One-Year Follow-Up Studies on Post-COVID Symptoms. Pathogens 2022, 11, 269. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.S.; Pedersini, C.A.; Misra, R.; Gandhi, T.K.; Rokers, B.; Biswal, B.B. Tracts in the limbic system show microstructural alterations post COVID-19 recovery. Brain Commun. 2024, 6, fcae139. [Google Scholar] [CrossRef] [PubMed]
- Mazza, M.G.; De Lorenzo, R.; Conte, C.; Poletti, S.; Vai, B.; Bollettini, I.; Melloni, E.M.T.; Furlan, R.; Ciceri, F.; Rovere-Querini, P.; et al. Anxiety and depression in COVID-19 survivors: Role of inflammatory and clinical predictors. Brain Behav. Immun. 2020, 89, 594–600. [Google Scholar] [CrossRef]
- Zielinski, M.R.; Kim, Y.; Karpova, S.A.; McCarley, R.W.; Strecker, R.E.; Gerashchenko, D. Chronic sleep restriction elevates brain interleukin-1 beta and tumor necrosis factor-alpha and attenuates brain-derived neurotrophic factor expression. Neurosci. Lett. 2014, 580, 27–31. [Google Scholar] [CrossRef]
- Medina-Flores, F.; Hurtado-Alvarado, G.; Contis-Montes de Oca, A.; López-Cervantes, S.P.; Konigsberg, M.; Deli, M.A.; Gómez-González, B. Sleep loss disrupts pericyte-brain endothelial cell interactions impairing blood-brain barrier function. Brain Behav. Immun. 2020, 89, 118–132. [Google Scholar] [CrossRef]
- Rustenhoven, J.; Jansson, D.; Smyth, L.C.; Dragunow, M. Brain pericytes as mediators of neuroinflammation. Trends Pharmacol. Sci. 2017, 38, 291–304. [Google Scholar] [CrossRef] [PubMed]
- Stein, D.J.; Simeon, D. Cognitive-Affective Neuroscience of Depersonalization. CNS Spectr. 2009, 14, 467–471. [Google Scholar] [CrossRef] [PubMed]
- Michal, M.; Wiltink, J.; Subic-Wrana, C.; Zwerenz, R.; Tuin, I.; Lichy, M.; Brähler, E.; Beutel, M.E. Prevalence, correlates, and predictors of depersonalization experiences in the German general population. J. Nerv. Ment. Dis. 2009, 197, 499–506. [Google Scholar] [CrossRef] [PubMed]
- van der Kloet, D.; Merckelbach, H.; Giesbrecht, T.; Lynn, S.J. Fragmented Sleep, Fragmented Mind: The Role of Sleep in Dissociative Symptoms. Perspect. Psychol. Sci. 2012, 7, 159–175. [Google Scholar] [CrossRef]
- Fernández-de-las-Peñas, C.; Cancela-Cilleruelo, I.; Rodríguez-Jiménez, J.; Arias-Navalón, J.A.; Martín-Guerrero, J.D.; Pellicer-Valero, O.J.; Arendt-Nielsen, L.; Cigarán-Méndez, M. Trajectory of post-COVID brain fog, memory loss, and concentration loss in previously hospitalized COVID-19 survivors: The LONG-COVID-EXP multicenter study. Front. Hum. Neurosci. 2023, 17, 1259660. [Google Scholar] [CrossRef] [PubMed]
- Chee, M.W.L.; Goh, C.S.F.; Namburi, P.; Parimal, S.; Seidl, K.N.; Kastner, S. Effects of sleep deprivation on cortical activation during directed attention in the absence and presence of visual stimuli. NeuroImage 2011, 58, 595–604. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Zhang, L.; Zhang, Y.; Chen, Y.; Peng, J.; Shao, Y.; Zhang, X. Decreased Functional Connectivity between the Right Precuneus and Middle Frontal Gyrus Is Related to Attentional Decline Following Acute Sleep Deprivation. Front. Neurosci. 2020, 14, 530257. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Liu, G.; Zhang, X.; Zhang, M.; Lu, J.; Li, H. Altered intrinsic brain activity and functional connectivity in COVID-19 hospitalized patients at 6-month follow-up. BMC Infect. Dis. 2023, 23, 521. [Google Scholar]
- Boboshko, M.Y.; Garbaruk, E.S.; Vikhnina, S.M.; Golovanova, L.E.; Ogorodnikova, E.A.; Rabchevskaya, A.V.; Zhilinskaia, E.V. The New Coronavirus Infection (COVID-19) and Hearing Function in Adults. J. Otorhinolaryngol. Hear Balance Med. 2022, 3, 5. [Google Scholar] [CrossRef]
- Saraiva, R.; Matos Silva, C. COVID-19 and central auditory processing. J. Hear. Sci. 2022, 12, 8. [Google Scholar]
- Hewala, S.H.; Kolkaila, E.A.; Emara, A.A.; Nada, N. Central auditory functions in post-COVID-19 adults; a case control study. Egypt J. Otolaryngol. 2024, 40, 47. [Google Scholar] [CrossRef]
- Kotz, S.A.; Schwartze, M. Cortical speech processing unplugged: A timely subcortico-cortical framework. Trends Cognit. Sci. 2010, 14, 392–399. [Google Scholar] [CrossRef] [PubMed]
- Cervós-Navarro, J.; Diemer, N.H. Selective vulnerability in brain hypoxia. Crit. Rev. Neurobiol. 1991, 6, 149–182. [Google Scholar] [PubMed]
- Manca, R.; De Marco, M.; Ince, P.G.; Venneri, A. Heterogeneity in regional damage detected by neuroimaging and neuropathologic al studies in older adults with COVID-19: A cognitive neuroscience systematic review to inform the long-term impact of the virus on neurocognitive trajectories. Front. Aging Neurosci. 2021, 13, 646908. [Google Scholar] [CrossRef]
- Alhazmi, F.H.; Alsharif, W.M.; Alshoabi, S.A.; Gameraddin, M.; Aloufi, K.M.; Abdulaal, O.M.; Qurashi, A.A. Identifying cerebral microstructural changes in patients with COVID-19 using MRI: A systematic review. Brain Circ. 2023, 9, 6–15. [Google Scholar] [PubMed]
- Hampshire, A.; Trendera, W.; Chamberlainb, S.R.; Jollya, A.E.; Grantd, J.E.; Patricke, F.; Mazibuko, N.; Williams, S.C.; Barnby, J.M.; Hellyer, P.; et al. Cognitive deficits in people who have recovered from COVID-19. ClinicalMedicine 2021, 39, 101044. [Google Scholar] [CrossRef] [PubMed]
- Guedj, E.; Morbelli, S.; Kaphan, E.; Campion, J.Y.; Dudouet, P.; Ceccaldi, M.; Cammilleri, S.; Nobili, F.; Eldin, C. From early limbic inflammation to long COVID sequelae. Brain 2021, 144, 1–3. [Google Scholar]
- Huang, S.; Zhou, X.; Zhao, W.; Du, Y.; Yang, D.; Huang, Y.; Chen, Y.; Zhang, H.; Yang, G.; Liu, J.; et al. Dynamic white matter changes in recovered COVID-19 patients: A two-year follow-up study. Theranostics 2023, 13, 724–735. [Google Scholar] [CrossRef]
- Fernández-Castañeda, A.; Lu, P.; Geraghty, A.C.; Song, E.; Lee, M.H.; Wood, J.; O’Dea, M.R.; Dutton, S.; Shamardani, K.; Nwangwu, K.; et al. Mild respiratory COVID can cause multi-lineage neural cell and myelin dysregulation. Cell 2022, 185, 2452–2468.e16. [Google Scholar] [CrossRef]
- Oliver, S.F.; Lazoff, S.A.; Popovich, J.; Enfield, K.B.; Quigg, M.; Davis, E.M.; Kadl, A. Chronic Neurocognitive, Neuropsychological, and Pulmonary Symptoms in Outpatient and Inpatient Cohorts after COVID-19 Infection. Neurosci. Insights 2023, 18, 1–7. [Google Scholar] [CrossRef]
- Yamamoto, H.; Tanaka, S.; Kasugai, D.; Shimizu, M.; Tsuchikawa, Y.; Fugane, Y.; Inoue, T.; Nagaya, M.; Omote, N.; Higashi, M.; et al. Physical function and mental health trajectories in COVID-19 patients following invasive mechanical ventilation: A prospective observational study. Sci. Rep. 2023, 13, 14529. [Google Scholar] [CrossRef] [PubMed]
- Alemanno, F.; Houdayer, E.; Parma, A.; Spina, A.; Del Forno, A.; Scatolini, A.; Angelone, S.; Brugliera, L.; Tettamanti, A.; Beretta, L.; et al. COVID-19 cognitive deficits after respiratory assistance in the subacute phase: A COVID-rehabilitation unit experience. PLoS ONE 2021, 16, e0246590. [Google Scholar] [CrossRef] [PubMed]
- Schild, A.K.; Goereci, Y.; Scharfenberg, D.; Klein, K.; Lülling, J.; Meiberth, D.; Schweitzer, F.; Stürmer, S.; Zeyen, P.; Sahin, D.; et al. Multidomain cognitive impairment in non-hospitalized patients with the post-COVID-19 syndrome: Results from a prospective monocentric cohort. J. Neurol. 2023, 270, 1215–1223. [Google Scholar] [CrossRef] [PubMed]
- Diotaiuti, P.; Valente, G.; Mancone, S.; Corrado, S.; Bellizzi, F.; Falese, L.; Langiano, E.; Vilarino, G.T.; Andrade, A. Effects of Cognitive Appraisals on Perceived Self-Efficacy and Distress during the COVID-19 Lockdown: An Empirical Analysis Based on Structural Equation Modeling. Int. J. Environ. Res. Public Health 2023, 20, 529. [Google Scholar] [CrossRef] [PubMed]
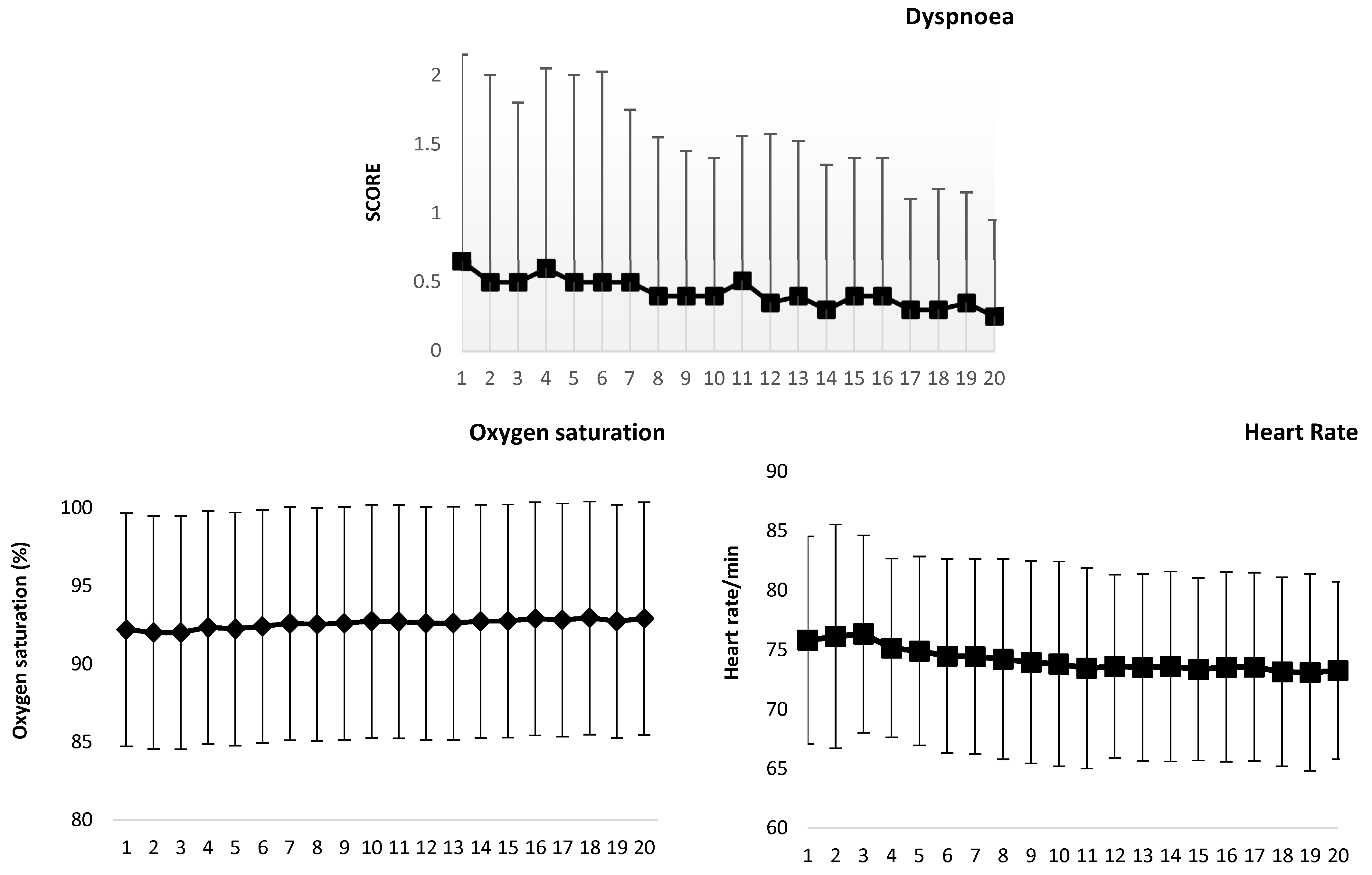
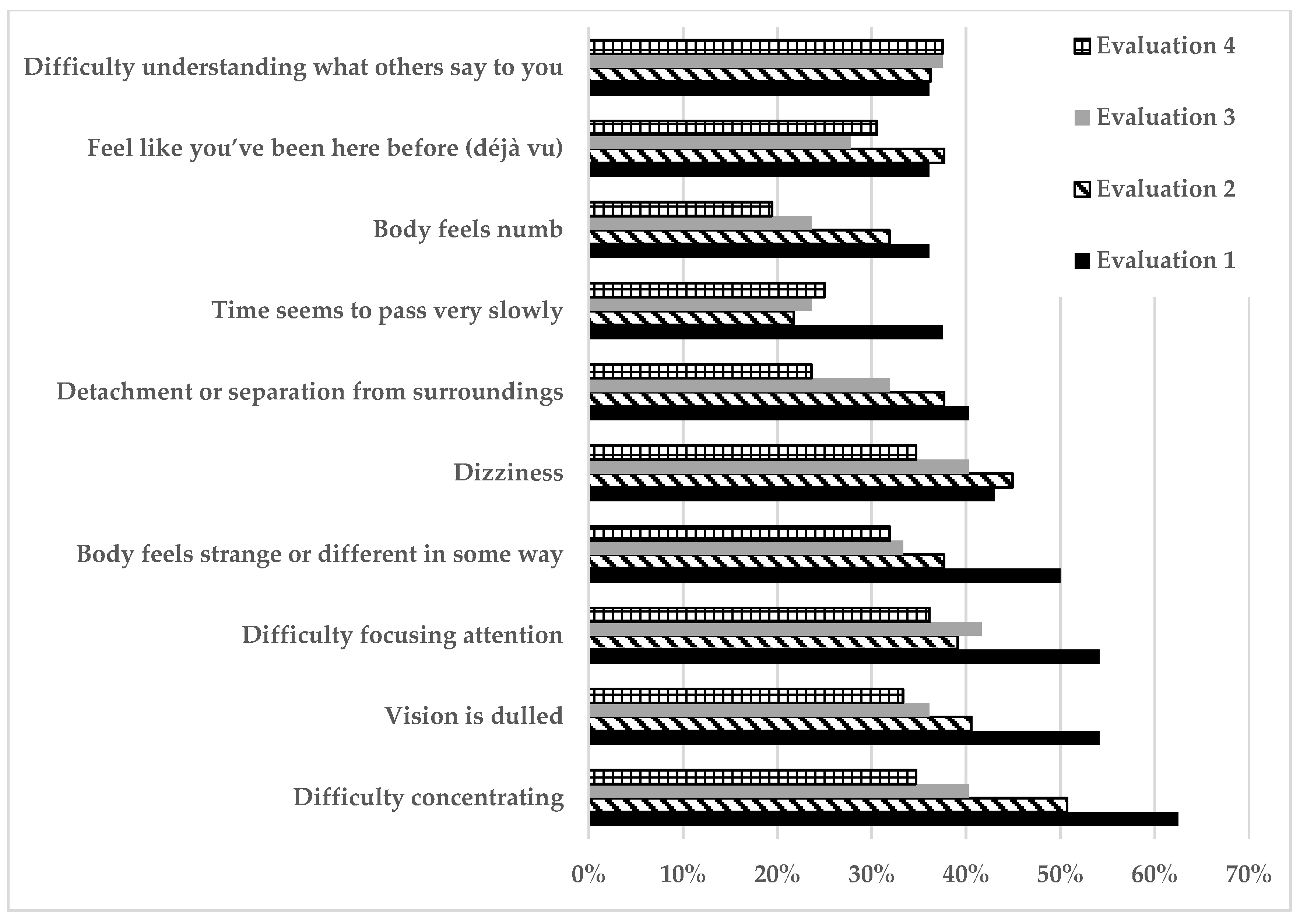

| Assessment Questionnaires | Evaluation 1 (n = 72) | Evaluation 2 (n = 69) | Evaluation 3 (n = 72) | Evaluation 4 (n = 72) | |
|---|---|---|---|---|---|
| Days Since Start of Disease | 143 (69) | 241(66) | 339 (75) | 889 (178) | |
| Mean (Range) | Mean (Range) | Mean (Range) | Mean (Range) | p (X2 Value) | |
| Sleep index | 8.1 (1–19) | 7.9 (0–19) | 7.5 (1–19) | 7.0 (1–15) | 0.002 (14.37) |
| HADS total score | 10.2 (0–29) | 9.3 (0–28) | 7.8/0–28) | 6.0 (0–20) | <0.0001 (16.291) |
| Anxiety subscale | 6.0 (0–18) | 5.6 (0–16) | 4.3 (0–19) | 3.2 (0–15) | <0.0001 (46.54) |
| Depression subscale | 4.1 (0–17) | 3.7 (0–14) | 3.4 (0–15) | 2.7 (0–9) | 0.01 (10.15) |
| Depersonalization/derealization | 10 (0–77) | 7.6 (0–59) | 7 (0–49) | 4 (2–7) | 0.0006 (17.06) |
| Dissociative experience scale | 5.7 (0–50) | 4.1 (0–19.6) | 3.5 (0–32.5) | 3.3 (0–28) | <0.00001(31.79) |
| Post-traumatic stress | 5.4 (0–45) | 3.4 (0–41) | 3.4 (0–52) | 0.66 (0–19) | 0.00002 (8.42) |
| Health-related quality of life (SF-36) | |||||
| Physical function | 66 (20–100) | 73 (20–100) | 75 (15–100) | 80 (45–100) | <0.00001(56.98) |
| Limitations physical problems | 50 (0–100) | 64 (0–100) | 65 (0–100) | 75 (0–100) | <0.00001 (43.23) |
| Pain | 58 (10–100) | 69 (22–100) | 70 (10–100) | 82 (31–100) | <0.00001 (61.24) |
| General health perception | 59 (5–100) | 59 (5–100) | 63 (10–100) | 63 (10–97) | 0.005(12.60) |
| Vitality | 52 (5–95) | 59 (5–100) | 58 (0–100) | 61 (10–100) | 0.0003 (18.72) |
| Social functioning | 67 (0–100) | 75 (0–100) | 74 (0–100) | 82 (50–100) | <0.00001(37.63) |
| Limitations emotional problems | 63 (0–100) | 68 (0–100) | 72 (0–100) | 82 (33–100) | <0.00001 (40.92) |
| Mental health | 73 (16–100) | 77 (36–100) | 75 (4–100) | 76 (36–100) | 0.298 (3.67) |
| SF-36 total score | 62 (17–95) | 68 (24–97) | 69 (12–100) | 75 (38–98) | <0.00001 (69.42) |
| MoCA | |||||
| Visuo-spatial | 3.9 (0–5) | 4.1 (0–5) | 5 (0–5) | 4 (0–5) | 0.092 (6.41) |
| Naming | 2.8 (0–3) | 2.9 (2–3) | 2.9 (1–3) | 2.9 (2–3) | 0.691 (1.45) |
| Attention | 4.6 (1–6) | 4.4 (1–6) | 4.4 (1–6) | 4.6 (1–6) | 0.130 (5.64) |
| Language | 2.3 (0–3) | 2.2 (0–3) | 2.3 (1–3) | 2.6 (1–3) | <0.00001 (29.69) |
| Abstraction | 1.3 (0–2) | 1.4 (0–2) | 1.4 (0–2) | 1.3 (0–2) | 0.582 (1.95) |
| Memory | 3.4 (0–5) | 3.7 (0–5) | 3.7 (0–5) | 3.7 (1–5) | 0.140 (5.46) |
| Orientation | 5.9 (4–6) | 5.9 (5–6) | 5.9 (4–6) | 5.9 (5–6) | 0.491 (2.40) |
| MoCA total score | 24.5 (13–30) | 24.9 (14–30) | 25.1 (16.7–30) | 25.5 (14–30) | 0.002 (14.64) |
| Assessment Questionnaires | Evaluation 1 (n = 72) | Evaluation 2 (n = 69) | Evaluation 3 (n = 72) | Evaluation 4 (n = 72) |
|---|---|---|---|---|
| Spearman’s R (t-Value, p) | Spearman’s R (t-Value, p) | Spearman’s R (t-Value, p) | Spearman’s R (t-Value, p) | |
| HADS total score | 0.58 (6.100, <0.00001) | 0.57 (5.701, <0.00001) | 0.53 (5.229, <0.00001) | 0.58 (6.013, 0.00001) |
| Anxiety subscale | 0.67 (7.572, <0.00001) | 0.57 (5.736, <0.00001) | 0.52 (5.216, <0.00001) | 0.55 (5.511, <0.00001) |
| Depression subscale | 0.35 (3.146, 0.002) | 0.47 (4.380, 0.00004) | 0.49 (4.759, 0.00001) | 0.47 (4.494, 0.00002) |
| Depersonalization/ Derealization | 0.55 (5.543, <0.00001) | 0.55 (5.450, <0.00001) | 0.50 (4.916, <0.00001) | 0.40 (3.673, 004) |
| Dissociative experience scale | 0.43 (4.035, 0.0001) | 0.28 (2.415, 0.018) | 0.36 (3.238, 0.001) | 0.25 (2.227, 0.029) |
| Post traumatic stress scale | 0.42 (3.954, 0.0001) | 0.32 (2.780, 0.007) | 0.41 (3.854, 0.0002) | − |
| SF36 total score | −0.53 (−5.336, <0.00001) | −0.68 (−7.597, <0.00001) | −0.67 (−7.731, <0.00001) | −0.67 (−7.676, <0.00001) |
| Physical function | −0.41 (−3.807, 0.0002) | −0.47 (−4.419, 0.00003) | −0.45 (−4.310, 0.00005) | −0.48 (−4.587, 0.00001) |
| Limitations physical problems | −0.41 (−3.807,0.0002) | −0.60 (−6.153, <0.00001) | −0.56 (−5.689, <0.00001) | −0.53 (−5.346, <0.00001) |
| Pain | −0.48 (−4.647, 0.00001) | −0.55 (−5.421, <0.00001) | −0.57 (−5.830, <0.00001) | −0.56 (−5.698, <0.00001) |
| General health perception | −0.27 (−2.431, 0.017) | −0.47 (−4.402, 0.00003) | −0.48 (−4.636, 0.00001) | −0.37 (−3.403, 0.001) |
| Vitality | −0.46 (−4.366, 0.00004) | −0.55 (−5.438, <0.00001) | −0.59 (−6.272, <0.00001) | −0.66 (−7.417, <0.00001) |
| Social functioning | −0.32 (−2.893, 0.005) | −0.56 (−5.664, <0.00001) | −0.60 (−6.420, <0.00001) | −0.50 (−4.949, <0.00001) |
| Limitations emotional problems | −0.36 (−3.310, 0.001) | −0.47 (−4.386, 0.00004) | −0.58 (−6.029, <0.00001) | −0.50 (−4.914, <0.00001) |
| Mental health | −0.51 (−5.025, <0.00001) | −0.56 (−5.641, <0.00001) | −0.57 (−5.846, <0.00001) | −0.57 (−5.885, <0.00001) |
| Variable | p (F) |
|---|---|
| Intercept | <0.00001 (86.699) |
| Age | 0.030(4.952) |
| Schooling | 0.004(8.785) |
| Oxygen saturation at hospital admission | 0.215 (1.567) |
| HADS total score | 0.001 (10.927) |
| Leukocyte count at hospital discharge | 0.006 (8.079) |
| Tobacco use | 0.011 (6.846) |
| Obesity | 0.441 (0.601) |
| Intubation | 0.266 (1.259) |
| Tobacco use × Obesity | 0.404 (0.704) |
| Tobacco use × Intubation | 0.662 (0.192) |
| Obesity × Intubation | 0.121 (2.471) |
| Tobacco use × Obesity × Intubation | 0.608 (0.265) |
| Repeated Measures (R) | 0.001 (5.569) |
| R × Age | 0.149 (1.796) |
| R × Schooling | 0.720 (0.445) |
| R × Oxygen saturation at hospital admission | 0.0005 (6.186) |
| R × HADS total score | 0.60 (2.515) |
| R × Leukocyte count at hospital discharge | 0.124 (1.945) |
| R × Tobacco use | 0.096 (2.141) |
| R × Obesity | 0.001 (5.165) |
| R × Intubation | 0.026 (3.162) |
| R × Tobacco use × Obesity | 0.286 (1.268) |
| R × Tobacco use × Intubation | 0.455 (0.875) |
| R × Obesity × Intubation | 0.004 (4.572) |
| R × Tobacco use × Obesity × Intubation | 0.284 (1.275) |
| Variable | TOTAL Score | Physical Function | Physical Problems | Pain | General Health | Vitality | Social Functioning | Emotional Problems | Mental Health |
|---|---|---|---|---|---|---|---|---|---|
| p (F) | p (F) | p (F) | p (F) | p (F) | p (F) | p (F) | p (F) | p (F) | |
| Intercept | <0.00001 (413.98) | <0.00001 (303.40) | <0.00001 (102.45) | <0.00001 (276.22) | <0.00001 (194.70) | <0.00001 (242.08) | <0.00001 (423.52) | <0.00001 (166.12) | <0.00001 (682.67) |
| First Pittsburgh Sleep Quality Index score | 0.009 (7.236) | 0.072 (3.3460) | 0.035 (4.610) | 0.013 (6.438) | 0.453 (0.569) | 0.020 (5.632) | 0.020 (5.676) | 0.200 (1.674) | 0.00025 (15.055) |
| First Depersonalization/ Derealization score | 0.0002 (15.519) | 0.152 (2.097) | 0.063 (3.566) | 0.032 (4.763) | 0.0005 (13.286) | 0.004 (8.803) | 0.002 (9.939) | <0.00001 (28.300) | 0.001 (10.780) |
| First 10 days median dyspnea | 0.756 (0.096) | 0.463 (0.544) | 0.478 (0.508) | 0.388 (0.754) | 0.823 (0.050) | 0.219 (1.537) | 0.137 (2.263) | 0.812 (0.056) | 0.017 (6.003) |
| Gender | 0.225 (1.499) | 0.0006 (7.804) | 0.287 (1.153) | 0.173 (1.898) | 0.125 (2.411) | 0.166 (1.963) | 0.288 (1.147) | 0.933 (0.007) | 0.492 (0.477) |
| Repeated measures (R) | 0.004 (4.511) | 0.208 (1.526) | 0.034 (2.928) | 0.886 (0.214) | 0.228 (1.455) | 0.0008 (5.774) | 0.305 (1.214) | 0.040 (2.819) | 0.428 (0.928) |
| R × Pittsburgh Sleep Quality Index score | 0.053 (2.599) | 0.408 (0.968) | 0.150 (1.789) | 0.367 (1.060) | 0.62 (2.482) | 0.001 (5.295) | 0.573 (0.667) | 0.286 (1.268) | 0.142 (1.836) |
| R × Depersonalization/ Derealization score | 0.0001 (7.116) | 0.001 (5.119) | 0.187 (1.613) | 0.108 (2.047) | 0.016 (3.513) | 0.006 (4.258) | 0.279 (1.289) | 0.002 (4.851) | 0.005 (4.363) |
| R × First 10 days dyspnoea score | 0.00009 (7.455) | 0.0001 (7.094) | 0.113 (2.010) | 0.00009 (7.450) | 0.647 (0.552) | 0.247 (1.389) | 0.075 (2.335) | 0.0006 (5.994) | 0.620 (0.593) |
| R × Gender | 0.002 (5.091) | 0.289 (1.261) | 0.045 (2.717) | 0.885 (0.215) | 0.004 (4.453) | 0.004 (4.493) | 0.786 (0.353) | 0.247 (1.401) | 0.001 (5.615) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jáuregui-Renaud, K.; Cooper-Bribiesca, D.; Miguel-Puga, J.A.; Alcantara-Calderón, Y.; Roaro-Figueroa, M.F.; Herrera-Ocampo, M.; Guzmán-Chacón, M.J. Quality of Sleep and Mental Symptoms Contribute to Health-Related Quality of Life after COVID-19 Pneumonia, a Follow-Up Study of More than 2 Years. Biomedicines 2024, 12, 1574. https://doi.org/10.3390/biomedicines12071574
Jáuregui-Renaud K, Cooper-Bribiesca D, Miguel-Puga JA, Alcantara-Calderón Y, Roaro-Figueroa MF, Herrera-Ocampo M, Guzmán-Chacón MJ. Quality of Sleep and Mental Symptoms Contribute to Health-Related Quality of Life after COVID-19 Pneumonia, a Follow-Up Study of More than 2 Years. Biomedicines. 2024; 12(7):1574. https://doi.org/10.3390/biomedicines12071574
Chicago/Turabian StyleJáuregui-Renaud, Kathrine, Davis Cooper-Bribiesca, José Adán Miguel-Puga, Yadira Alcantara-Calderón, María Fernanda Roaro-Figueroa, Mariana Herrera-Ocampo, and Melodie Jedid Guzmán-Chacón. 2024. "Quality of Sleep and Mental Symptoms Contribute to Health-Related Quality of Life after COVID-19 Pneumonia, a Follow-Up Study of More than 2 Years" Biomedicines 12, no. 7: 1574. https://doi.org/10.3390/biomedicines12071574






