High Glucose Increases Lactate and Induces the Transforming Growth Factor Beta-Smad 1/5 Atherogenic Pathway in Primary Human Macrophages
Abstract
1. Introduction
2. Materials and Methods
2.1. Primary Human Macrophage Culture
2.2. Lactate Assay
2.3. Phosphofructokinase (PFK) Assay
2.4. Quantitative Real-Time Reverse Transcriptase PCR (qRT-PCR)
2.5. Analysis of the Phosphorylation of Smads Proteins
2.6. Flow Cytometry
2.7. Statistical Analysis
3. Results
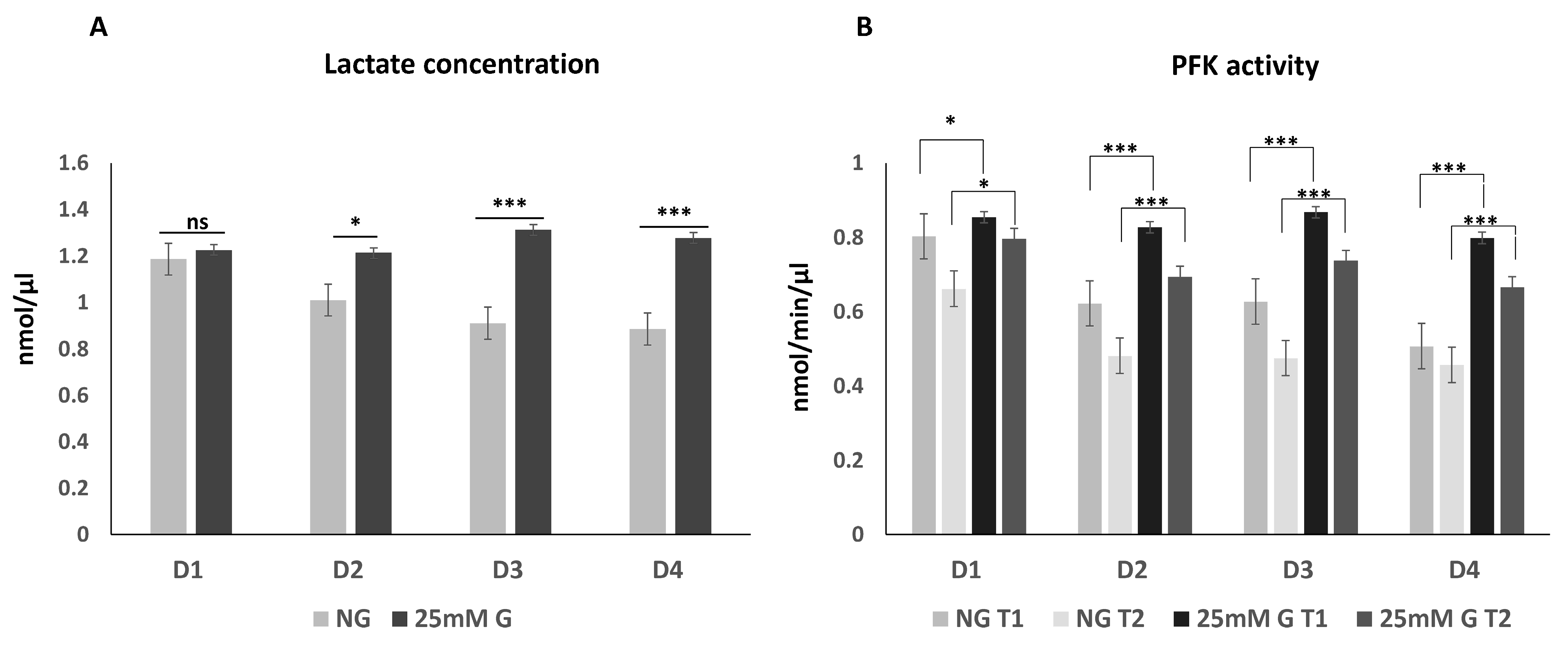
3.1. Lactate Concentration and Phosphofructokinase Enzyme Activity in Macrophages Cultured under Normal or High Glucose Levels
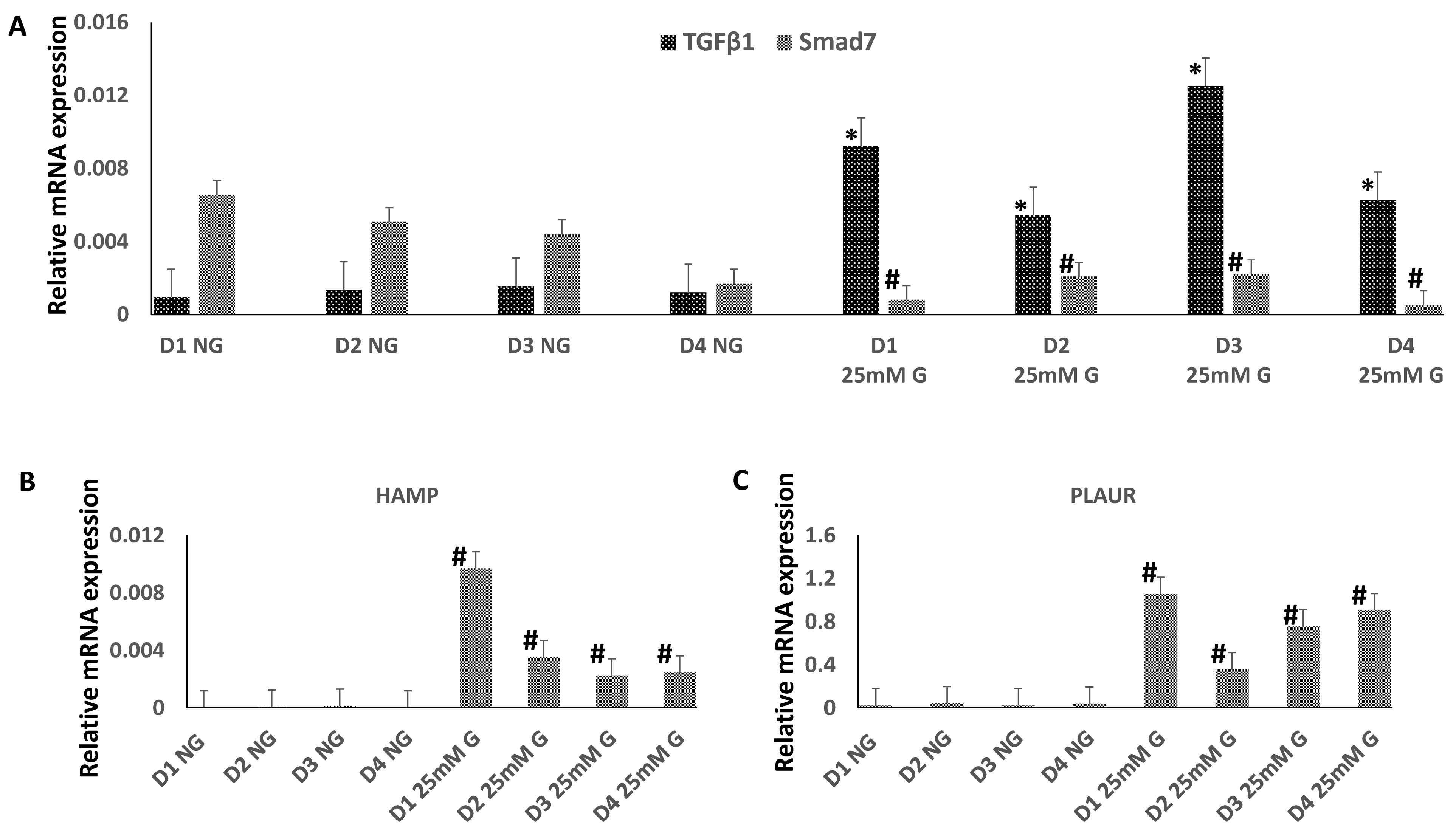
3.2. The Expression of TGFβ1, Smad7, HAMP, and PLAUR mRNA in Macrophges Cultured under Normal or High-Glucose Conditions
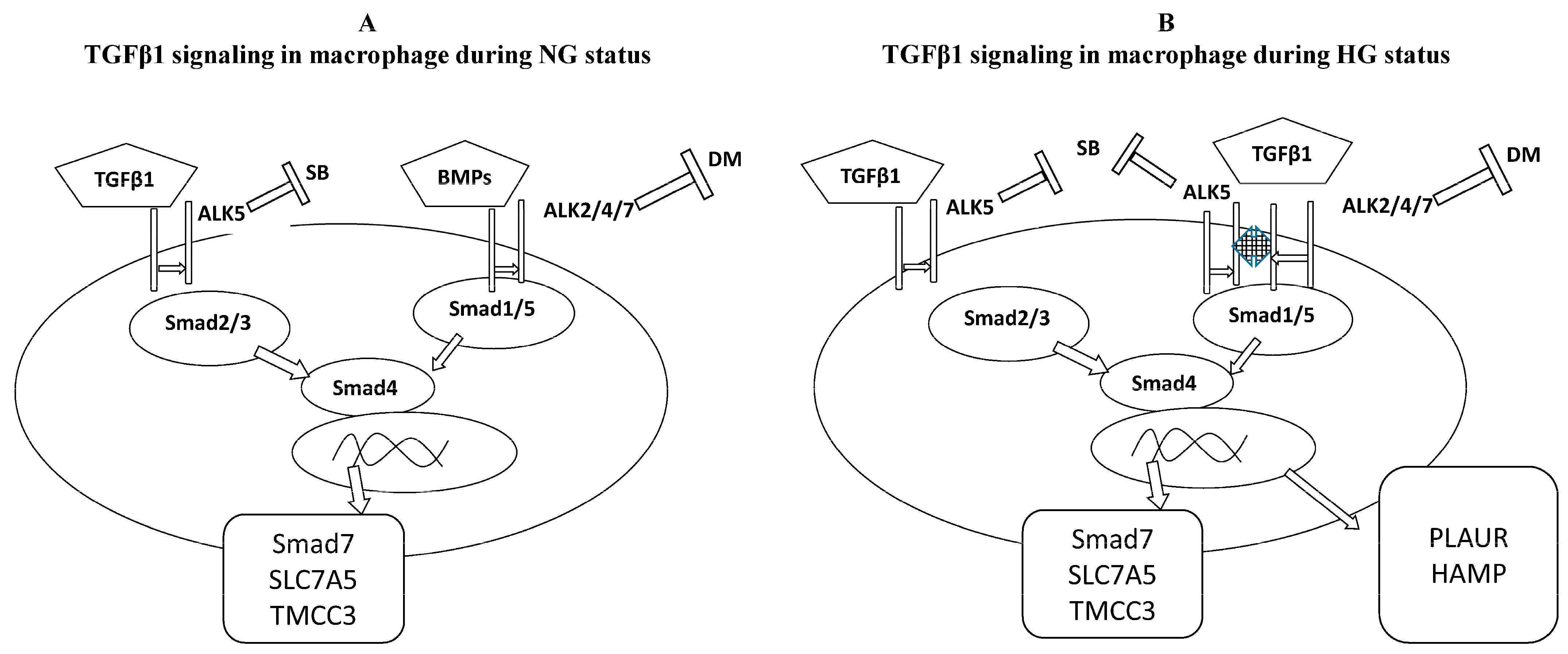
3.3. TGFβ1/Smad Signaling in Macrophages Cultured under Normal or High Glucose
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| TGFβ1 | transforming growth factor beta 1 |
| NG | normal glucose |
| HG | high glucose |
| PFK | phosphofructokinase |
| uPAR | urokinase plasminogen activator surface receptor |
| GM-CSF | granulocyte-MQ colony-stimulating factor |
| PBS | phosphate-buffered saline |
References
- Ahmad, E.; Lim, S.; Lamptey, R.; Webb, D.R.; Davies, M.J. Type 2 diabetes. Lancet 2022, 400, 1803–1820. [Google Scholar] [CrossRef]
- Motawi, T.K.; Shahin, N.N.; Maghraby, A.S.; Kirschfink, M.; Abd-Elshafy, D.N.; Awad, K.; Bahgat, M.M. H1N1 Infection Reduces Glucose Level in Human U937 Monocytes Culture. Viral Immunol. 2020, 33, 384–390. [Google Scholar] [CrossRef]
- Nurgazieva, D.; Mickley, A.; Moganti, K.; Ming, W.; Ovsyi, I.; Popova, A.; Sachindra; Awad, K.; Wang, N.; Bieback, K.; et al. TGF-β1, but not bone morphogenetic proteins, activates Smad1/5 pathway in primary human macrophages and induces expression of proatherogenic genes. J. Immunol. 2015, 194, 709–718. [Google Scholar] [CrossRef]
- Awad, K.; Maghraby, A.S.; Abd-Elshafy, D.N.; Bahgat, M.M. Carbohydrates metabolic signatures in immune cells: Response to infection. Front. Immunol. 2022, 13, 912899. [Google Scholar] [CrossRef]
- Raggi, F.; Pelassa, S.; Pierobon, D.; Penco, F.; Gattorno, M.; Novelli, F.; Eva, A.; Varesio, L.; Giovarelli, M.; Bosco, M.C. Regulation of Human Macrophage M1-M2 Polarization Balance by Hypoxia and the Triggering Receptor Expressed on Myeloid Cells-1. Front. Immunol. 2017, 8, 1097. [Google Scholar] [CrossRef]
- Wang, L.; Yi, X.; Xiao, X.; Zheng, Q.; Ma, L.; Li, B. Exosomal miR-628-5p from M1 polarized macrophages hinders m6A modification of circFUT8 to suppress hepatocellular carcinoma progression. Cell. Mol. Biol. Lett. 2022, 27, 106. [Google Scholar] [CrossRef]
- Massague, J. TGFbeta signalling in context. Nat. Rev. Mol. Cell Biol. 2012, 13, 616–630. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Huang, J.; Jian, X.; Wang, H.; Lan, H.; Liao, Z.; Gu, R.; Hu, J.; Liao, H. IRE1α arm of unfolded protein response in muscle-specific TGF-β signaling-mediated regulation of muscle cell immunological properties. Cell. Mol. Biol. Lett. 2023, 28, 15. [Google Scholar] [CrossRef] [PubMed]
- David, C.J.; Massagué, J. Contextual determinants of TGFβ action in development, immunity and cancer. Nat. Rev. Mol. Cell Biol. 2018, 19, 419–435. [Google Scholar] [CrossRef] [PubMed]
- Aigner, L.; Bogdahn, U. TGF-beta in neural stem cells and in tumors of the central nervous system. Cell Tissue Res. 2008, 331, 225–241. [Google Scholar] [CrossRef] [PubMed]
- Brissot, P.; Pietrangelo, A.; Adams, P.C.; de Graaff, B.; McLaren, C.E.; Loréal, O. Haemochromatosis. Nat. Rev. Dis. Primers 2018, 4, 18016. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Real, J.M.; Manco, M. Effects of iron overload on chronic metabolic diseases. Lancet Diabetes Endocrinol. 2014, 2, 513–526. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Luo, G.; Guo, X.; Jiang, C.; Zeng, H.; Zhou, F.; Li, Y.; Yu, J.; Yao, P. Macrophage iron retention aggravates atherosclerosis: Evidence for the role of autocrine formation of hepcidin in plaque macrophages. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2020, 1865, 158531. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Zhang, M.; Liu, Y.; Li, H.; Shang, L.; Xu, T.; Chen, Z.; Wang, F.; Qiao, T.; Li, K. Iron accumulation in macrophages promotes the formation of foam cells and development of atherosclerosis. Cell Biosci. 2020, 10, 137. [Google Scholar] [CrossRef] [PubMed]
- Hindy, G.; Hindy, G.; Tyrrell, D.J.; Tyrrell, D.J.; Vasbinder, A.; Vasbinder, A.; Wei, C.; Wei, C.; Presswalla, F.; Presswalla, F.; et al. Increased soluble urokinase plasminogen activator levels modulate monocyte function to promote atherosclerosis. J. Clin. Investig. 2022, 132, e158788. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Wang, B.; Su, F.; Gu, B.; Xiang, L.; Gao, L.; Zheng, P.; Li, X.-M.; Chen, H. TCF7L2 promotes anoikis resistance and metastasis of gastric cancer by transcriptionally activating PLAUR. Int. J. Biol. Sci. 2022, 18, 4560–4577. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Fan, H.; Zhou, X.; Xiang, Y.; Liu, Y. Prognostic significance and gene co-expression network of plau and plaur in gliomas. Front. Oncol. 2022, 11, 602321. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, K.; Gao, X.; Liu, Z.; Xing, Z. Comprehensive analysis of the importance of PLAUR in the progression and immune microenvironment of renal clear cell carcinoma. PLoS ONE 2022, 17, e0269595. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Sun, Z.; Lu, S.; Zhang, X.; Xiao, C.; Li, T.; Wu, J. Identification of PLAUR-related ceRNA and immune prognostic signature for kidney renal clear cell carcinoma. Front. Oncol. 2022, 12, 834524. [Google Scholar] [CrossRef]
- Goodchild, T.T.; Li, Z.; Lefer, D.J. Soluble urokinase plasminogen activator receptor: From biomarker to active participant in atherosclerosis and cardiovascular disease. J. Clin. Investig. 2022, 132, e165868. [Google Scholar] [CrossRef]
- Liu, H.; Chen, Y.G. The interplay between TGF-β signaling and cell metabolism. Front. Cell Dev. Biol. 2022, 10, 846723. [Google Scholar] [CrossRef] [PubMed]
- Österlund, P.; Jiang, M.; Westenius, V.; Kuivanen, S.; Järvi, R.; Kakkola, L.; Lundberg, R.; Melén, K.; Korva, M.; Županc, T.A.; et al. Asian and African lineage Zika viruses show differential replication and innate immune responses in human dendritic cells and macrophages. Sci. Rep. 2019, 9, 15710. [Google Scholar] [CrossRef] [PubMed]
- Gratchev, A.; Kzhyshkowska, J.; Kannookadan, S.; Ochsenreiter, M.; Popova, A.; Yu, X.; Mamidi, S.; Stonehouse-Usselmann, E.; Muller-Molinet, I.; Gooi, L.; et al. Activation of a TGF-beta-specific multistep gene expression program in mature macrophages requires glucocorticoid-mediated surface expression of TGF-beta receptor II. J. Immunol. 2008, 180, 6553–6565. [Google Scholar] [CrossRef] [PubMed]
- Waldhäusl, W.; Gries, A.; Scherbaum, W. Diabetes in der Praxis, 2nd ed.; Springer: Berlin, Germany, 1992; pp. 3–15. ISBN 3-540-58982-1. [Google Scholar]
- Nawroth, P. Innovative models for investigation of pathomechanisms leading to late diabetic complications. Exp. Clin. Endocrinol. Diabetes 2012, 120, 177–178. [Google Scholar] [CrossRef]
- Ward, R.; Ergul, A. Relationship of endothelin-1 and NLRP3 inflammasome activation in HT22 hippocampal cells in diabetes. Life Sci. 2016, 159, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Zhou, F.; Liu, Y.; Yi, Z.; Wang, X.; Wu, Y.; Gong, P. 1α,25 Dihydroxyvitamin D3 promotes angiogenesis by alleviating AGEs-induced autophagy. Arch. Biochem. Biophys. 2021, 712, 109041. [Google Scholar] [CrossRef] [PubMed]
- Batlle, E.; Massagué, J. Transforming Growth Factor-β signaling in immunity and cancer. Immunity 2019, 50, 924–940. [Google Scholar] [CrossRef]
- Alrasheed, N.M.; Alammari, R.B.; Alshammari, T.K.; Alamin, M.A.; Alharbi, A.O.; Alonazi, A.S.; Bin Dayel, A.F.; Alrasheed, N.M. α1A Adrenoreceptor blockade attenuates myocardial infarction by modulating the integrin-linked kinase/TGF-β/Smad signaling pathways. BMC Cardiovasc. Disord. 2023, 23, 153. [Google Scholar] [CrossRef] [PubMed]
- Misiakos, E.; Kouraklis, G.; Agapitos, E.; Perrea, D.; Karatzas, G.; Boudoulas, H.; Karayannakos, P. Expression of PDGF-A, TGFb and VCAM-1 during the developmental stages of experimental atherosclerosis. Eur. Surg. Res. 2001, 33, 264–269. [Google Scholar] [CrossRef]
- Saratzis, A.; Bown, M.J. The genetic basis for aortic aneurysmal disease. Heart 2014, 100, 916–922. [Google Scholar] [CrossRef]
- Kukhtina, N.B.; Aref’eva, T.I.; Aref’eva, A.M.; Akchurin, R.S.; Krasnikova, T.L. Expression of chemokines and cytokines in atherosclerotic plaques and internal membrane of the arteries in patients with coronary artery disease. Ter. Arkh. 2008, 80, 63–69. [Google Scholar]
- Heinzmann, D.; Fuß, S.; Ungern-Sternberg, S.V.; Schreieck, J.; Gawaz, M.; Gramlich, M.; Seizer, P. TGFβ is specifically upregulated on circulating CD14++ CD16+ and CD14+ CD16++ monocytes in patients with atrial fibrillation and severe atrial fibrosis. Cell. Physiol. Biochem. 2018, 49, 226–234. [Google Scholar] [CrossRef] [PubMed]
- El-Remessy, A.B.; Behzadian, M.A.; Abou-Mohamed, G.; Franklin, T.; Caldwell, R.W.; Caldwell, R.B. Experimental diabetes causes breakdown of the blood-retina barrier by a mechanism involving tyrosine nitration and increases in expression of vascular endothelial growth factor and urokinase plasminogen activator receptor. Am. J. Pathol. 2003, 162, 1995–2004. [Google Scholar] [CrossRef] [PubMed]
- Hayek, S.S.; Divers, J.; Raad, M.; Xu, J.; Bowden, D.W.; Tracy, M.; Reiser, J.; Freedman, B.I. Predicting mortality in african americans with type 2 diabetes mellitus: Soluble urokinase plasminogen activator receptor, coronary artery calcium, and high-sensitivity c-reactive protein. J. Am. Heart Assoc. 2018, 7, e008194. [Google Scholar] [CrossRef]
- Curovic, V.R.; Theilade, S.; Winther, S.A.; Tofte, N.; Eugen-Olsen, J.; Persson, F.; Hansen, T.W.; Jeppesen, J.; Rossing, P. Soluble urokinase plasminogen activator receptor predicts cardiovascular events, kidney function decline, and mortality in patients with type 1 diabetes. Diabetes Care 2019, 42, 1112–1119. [Google Scholar] [CrossRef] [PubMed]
- Sherif, E.M.; El Maksood, A.A.A.; Youssef, O.I.; Salah El-Din, N.Y.; Khater, O.K.M. Soluble urokinase plasminogen activator receptor in type 1 diabetic children, relation to vascular complications. J. Diabetes Complicat. 2019, 33, 628–633. [Google Scholar] [CrossRef] [PubMed]
- Müller, J.; Radej, J.; Horak, J.; Karvunidis, T.; Valesova, L.; Kriz, M.; Matejovic, M. Lactate: The Fallacy of Oversimplification. Biomedicines 2023, 11, 3192. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, L.; Slimani, R.; Zizmare, L.; Ehlers, J.; Borgmann, F.K.; Fitzgerald, J.C.; Fallier-Becker, P.; Beckmann, A.; Grißmer, A.; Meier, C.; et al. TGF-Beta Modulates the Integrity of the Blood Brain Barrier In Vitro, and Is Associated with Metabolic Alterations in Pericytes. Biomedicines 2023, 11, 214. [Google Scholar] [CrossRef] [PubMed]
- Oliver, M.A.; Davis, X.D.; Bohannon, J.K. TGFbeta macrophage reprogramming: A new dimension of macrophage plasticity. J. Leukoc. Biol. 2024, 115, 411–414. [Google Scholar] [CrossRef]
- Wang, H.; Liu, F.; Zhao, W.; Guo, Y.; Mai, P.; Zhao, S.; Wen, Z.; Su, J.; Li, X.; Wang, Y.; et al. High glucose promotes atherosclerosis by regulating miRNA let7d-5p level. J. Diabetes Investig. 2024, 15, 711–724. [Google Scholar] [CrossRef]
- Sun, H.; Ma, X.; Ma, H.; Li, S.; Xia, Y.; Yao, L.; Wang, Y.; Pang, X.; Zhong, J.; Yao, G.; et al. High glucose levels accelerate atherosclerosis via NLRP3-IL/ MAPK/NF-kappaB-related inflammation pathways. Biochem. Biophys. Res. Commun. 2024, 704, 149702. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Cao, J.; Gao, Y.; Xie, X.; Zhao, Z.; Yuan, Q.; He, Y.; Zu, X.; Liu, J. High glucose promotes macrophage M1 polarization through miR-32/Mef2d/cAMP signaling pathway. Genes Dis. 2023, 11, 539–541. [Google Scholar] [CrossRef] [PubMed]

| Gene | Primers Forward/Reverse | Accession Number |
|---|---|---|
| TGFβ1 | TTATTGAGCACCTTGGGCAC | NM_000660.7 |
| TCTCTGGGCTTGTTTCCTCAC | ||
| Smad7 | TTCCTCGGAAGTCAAGAGGCT | NM_005904.4 |
| CCATCGGGTATCTGGAGTAA | ||
| HAMP | ACAACTTGCAGAGCTGCAAC | NM_021175.4 |
| GCAGCAGAAAATGCAGATGG | ||
| PLAUR | TGAAGATCACCAGCCTTACC | NM_002659.4 |
| TGATGAGCCACAGGAAATGC | ||
| GAPDH | GGTGGTCTCCTCTGACTTCAAC | NM_002046.7 |
| GTTGCTGTAGCCAAATTCGTTG |
| Donor | pSmad2 | pSmad1/5 |
|---|---|---|
| 1 | LG: ++ HG: +++ | LG: −− HG: ++ |
| 2 | LG: ++ HG: ++ | LG: −− HG: ++ |
| 3 | LG: +++ HG: +++ | LG: −− HG: +++ |
| 4 | LG: ++ HG: ++ | LG: −− HG: + |
| 5 | LG: +++ HG: +++ | LG: −− HG: ++ |
| 6 | LG: +++ HG: +++ | LG: −− HG: + |
| 7 | LG: +++ HG: − | LG: −− HG: +++ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Awad, K.; Kakkola, L.; Julkunen, I. High Glucose Increases Lactate and Induces the Transforming Growth Factor Beta-Smad 1/5 Atherogenic Pathway in Primary Human Macrophages. Biomedicines 2024, 12, 1575. https://doi.org/10.3390/biomedicines12071575
Awad K, Kakkola L, Julkunen I. High Glucose Increases Lactate and Induces the Transforming Growth Factor Beta-Smad 1/5 Atherogenic Pathway in Primary Human Macrophages. Biomedicines. 2024; 12(7):1575. https://doi.org/10.3390/biomedicines12071575
Chicago/Turabian StyleAwad, Kareem, Laura Kakkola, and Ilkka Julkunen. 2024. "High Glucose Increases Lactate and Induces the Transforming Growth Factor Beta-Smad 1/5 Atherogenic Pathway in Primary Human Macrophages" Biomedicines 12, no. 7: 1575. https://doi.org/10.3390/biomedicines12071575
APA StyleAwad, K., Kakkola, L., & Julkunen, I. (2024). High Glucose Increases Lactate and Induces the Transforming Growth Factor Beta-Smad 1/5 Atherogenic Pathway in Primary Human Macrophages. Biomedicines, 12(7), 1575. https://doi.org/10.3390/biomedicines12071575






