Ketosis Suppression and Ageing (KetoSAge) Part 2: The Effect of Suppressing Ketosis on Biomarkers Associated with Ageing, HOMA-IR, Leptin, Osteocalcin, and GLP-1, in Healthy Females
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics
2.2. Participants
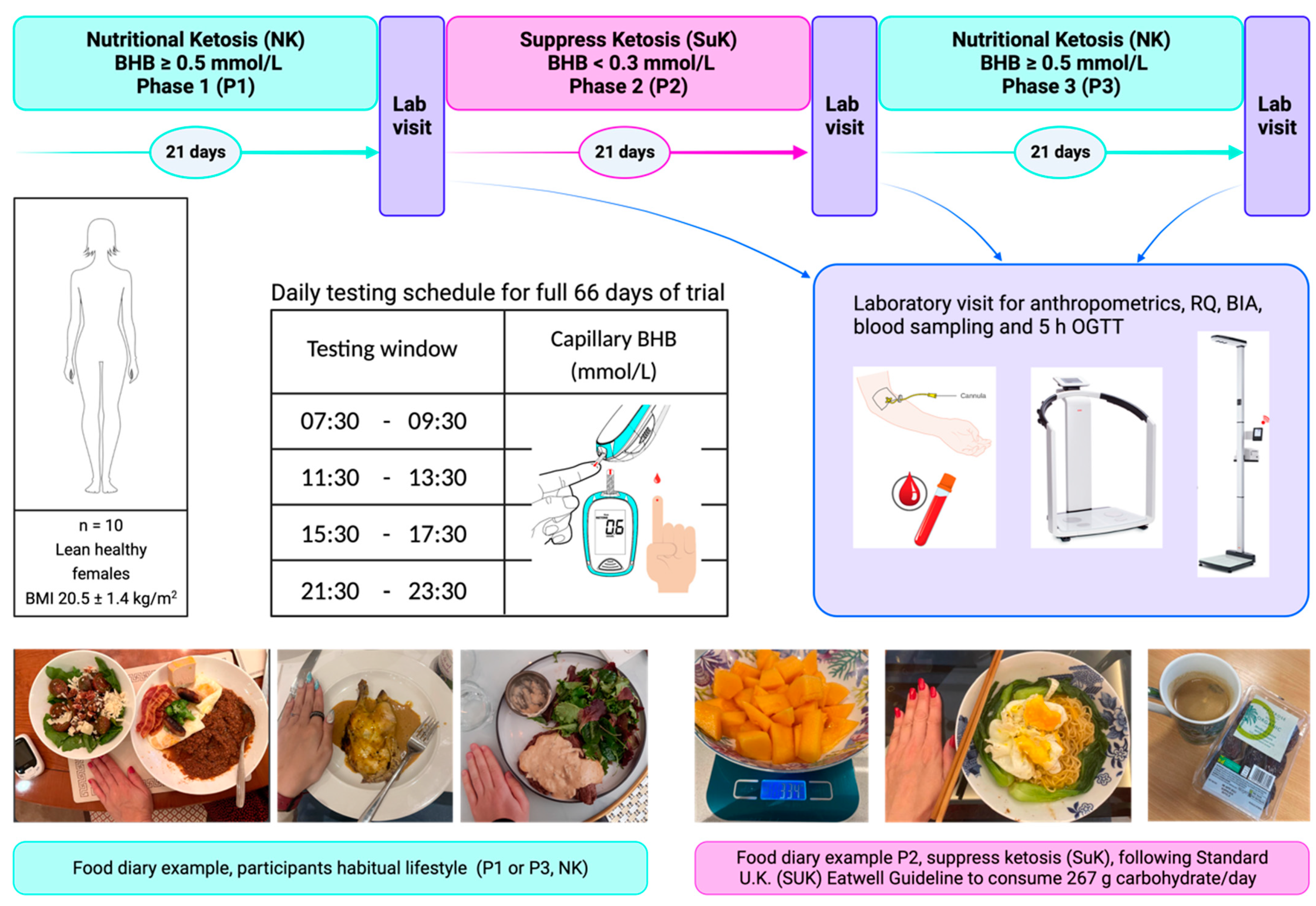
2.3. Study Design
2.4. Anthropometric Measurements
2.5. Blood Collection and Measurement
2.6. Blood Marker Analysis
2.7. Statistical Analysis
3. Results
3.1. Frequency of Rank-Ordered Capillary BHB Level
| Phase 1 NK | |||||||||||
| Participant | 1011 | 1021 | 1031 | 1041 | 1051 | 1061 | 1071 | 1081 | 1091 | 1101 | Rank 4 frequency |
| Test 1 (Wake up) | 2 | 1 | 2 | 1 | 2 | 1 | 1 | 1 | 1 | 1 | 0 |
| Test 2 (Pre-lunch) | 3 | 3 | 4 | 3 | 3 | 4 | 4 | 2 | 4 | 2 | 4 |
| Test 3 (Pre-dinner) | 4 | 4 | 3 | 4 | 4 | 2 | 3 | 4 | 3 | 4 | 6 |
| Test 4 (Bedtime) | 1 | 2 | 1 | 2 | 1 | 3 | 2 | 3 | 2 | 3 | 0 |
| Phase 3 NK | |||||||||||
| Participant | 1011 | 1021 | 1031 | 1041 | 1051 | 1061 | 1071 | 1081 | 1091 | 1101 | Rank 4 frequency |
| Test 1 (Wake up) | 2 | 1 | 2 | 1 | 2 | 1 | 1 | 1 | 1 | 2 | 0 |
| Test 2 (Pre-lunch) | 4 | 3 | 4 | 3 | 2 | 3 | 4 | 3 | 3 | 4 | 4 |
| Test 3 (Pre-dinner) | 3 | 4 | 3 | 4 | 4 | 4 | 3 | 4 | 4 | 3 | 6 |
| Test 4 (Bedtime) | 1 | 2 | 1 | 2 | 3 | 2 | 2 | 2 | 2 | 1 | 0 |
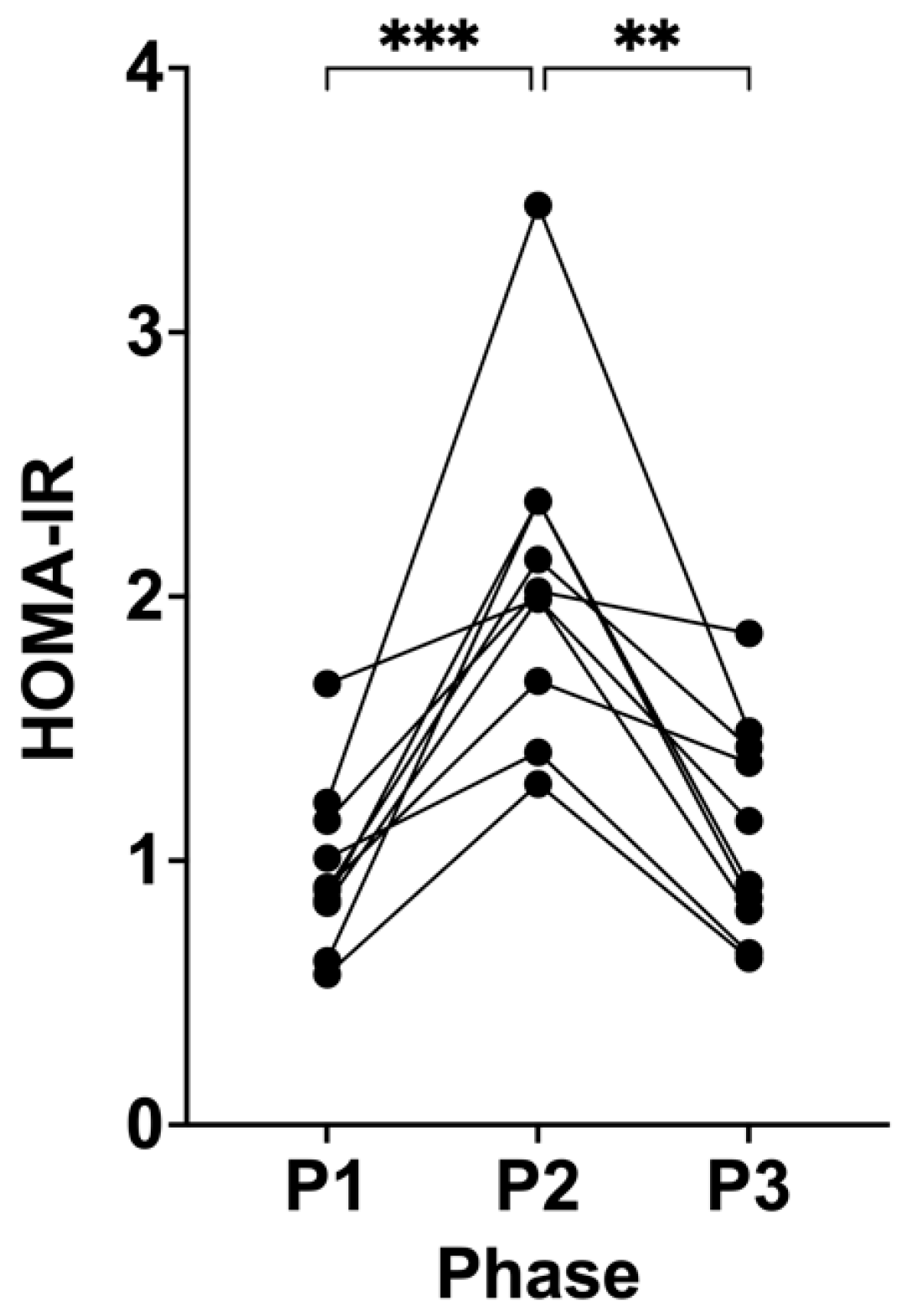
3.2. Suppression of Ketosis is Associated with Increases in HOMA-IR
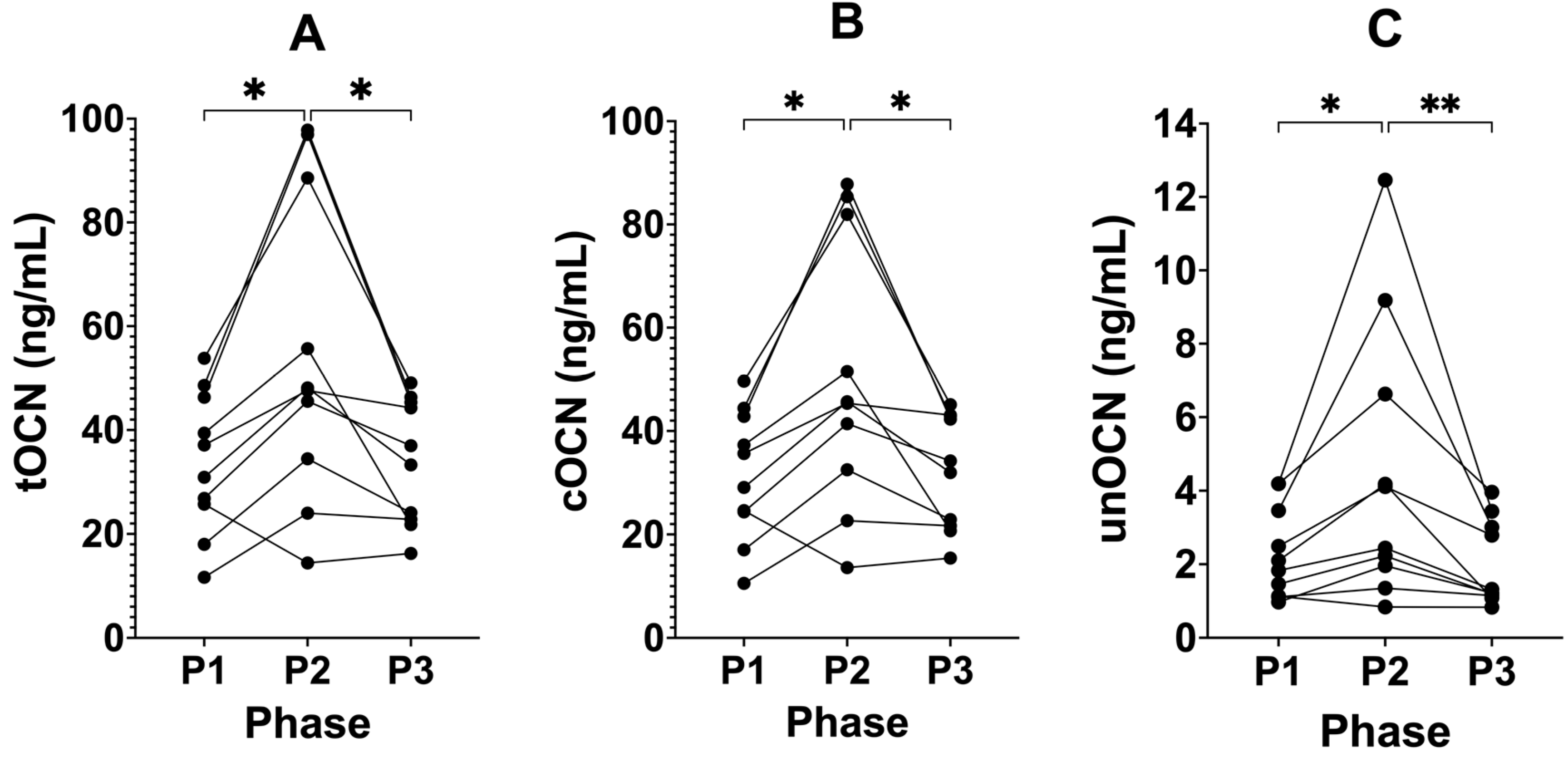
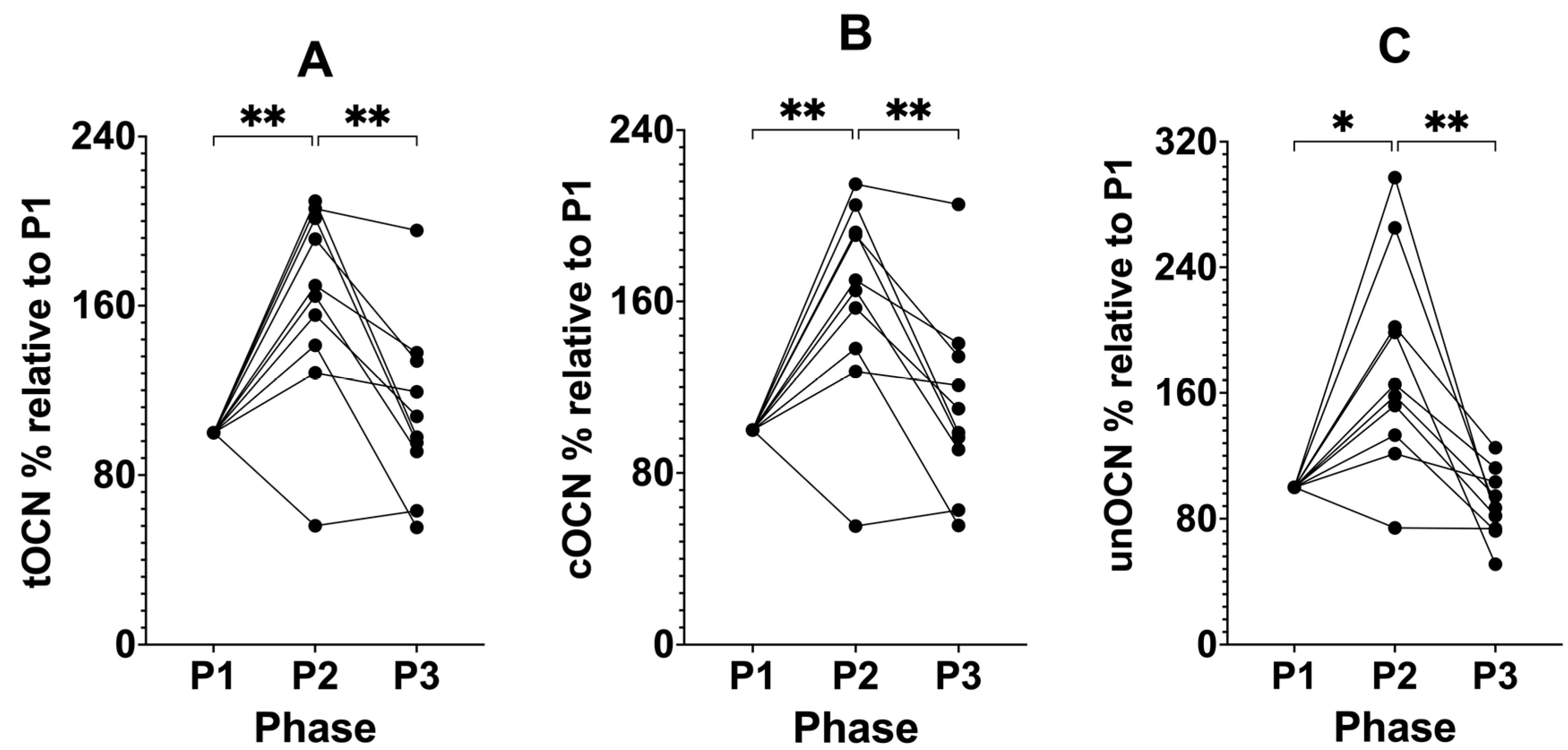
3.3. Suppressing Ketosis Increases all Forms of Osteocalcin
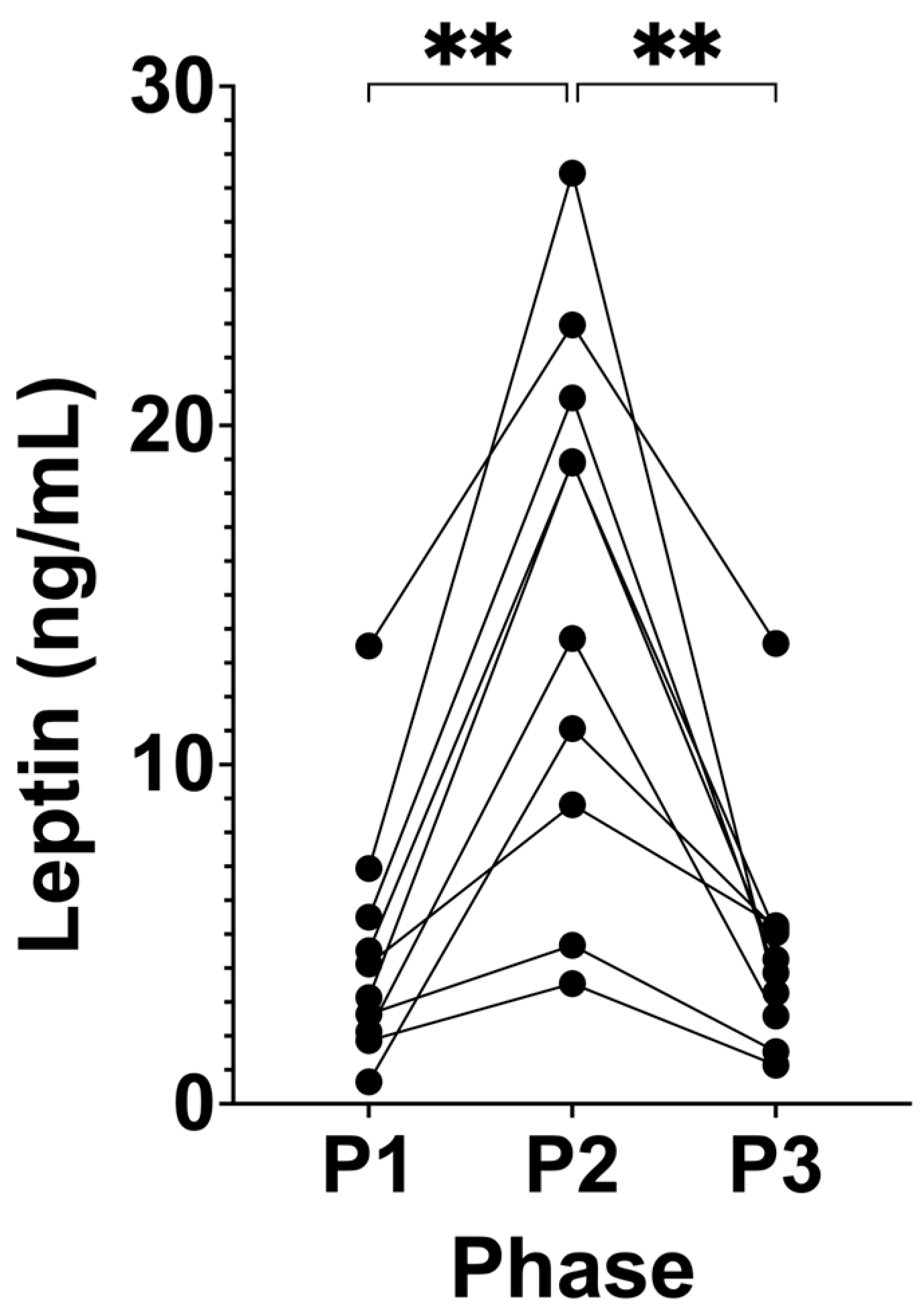
3.4. Suppression of Ketosis is Associated with Increased Leptin
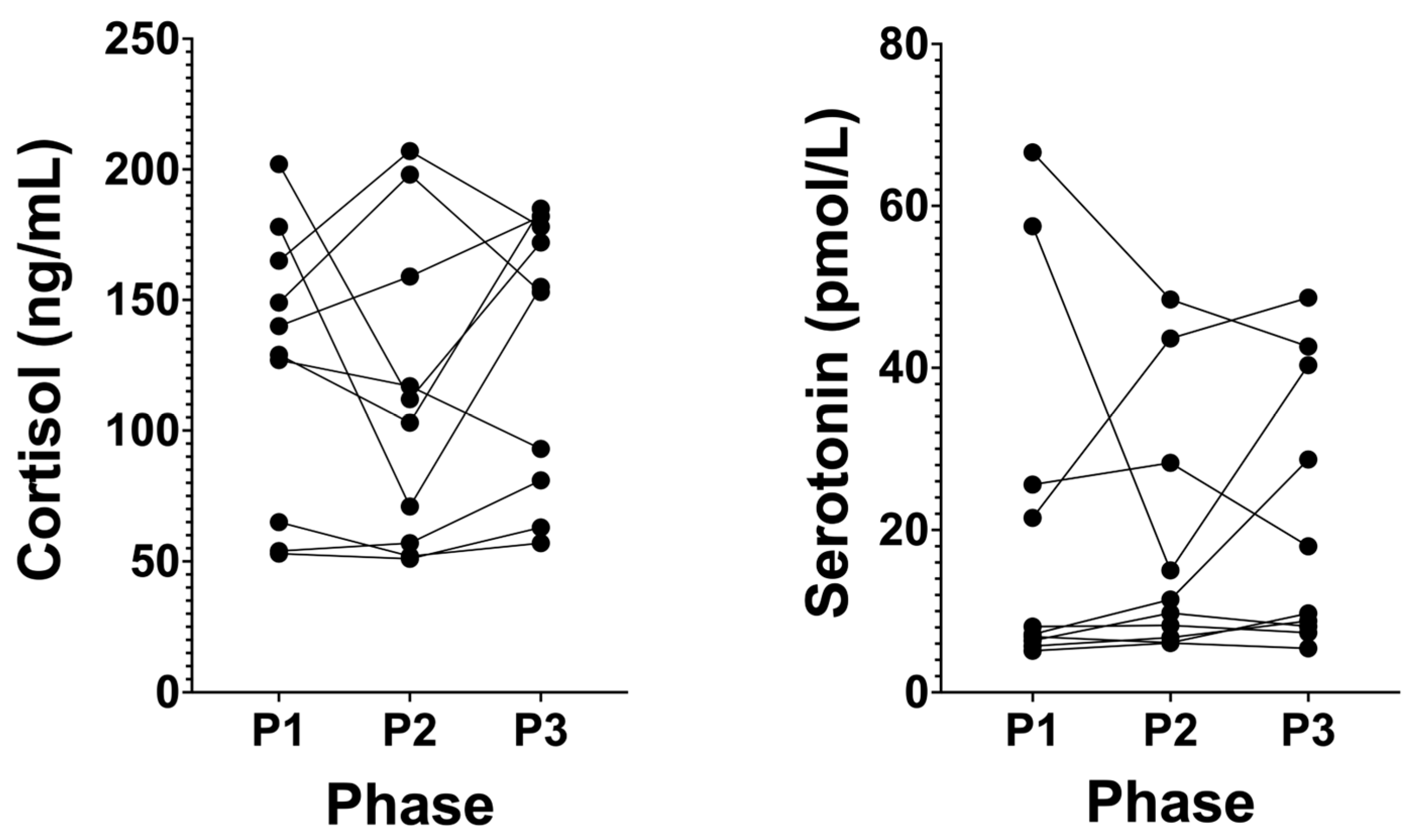
3.5. Cortisol Levels Remain at Healthy Low Levels in Long-Term NK, and Serotonin Does Not Significantly Change
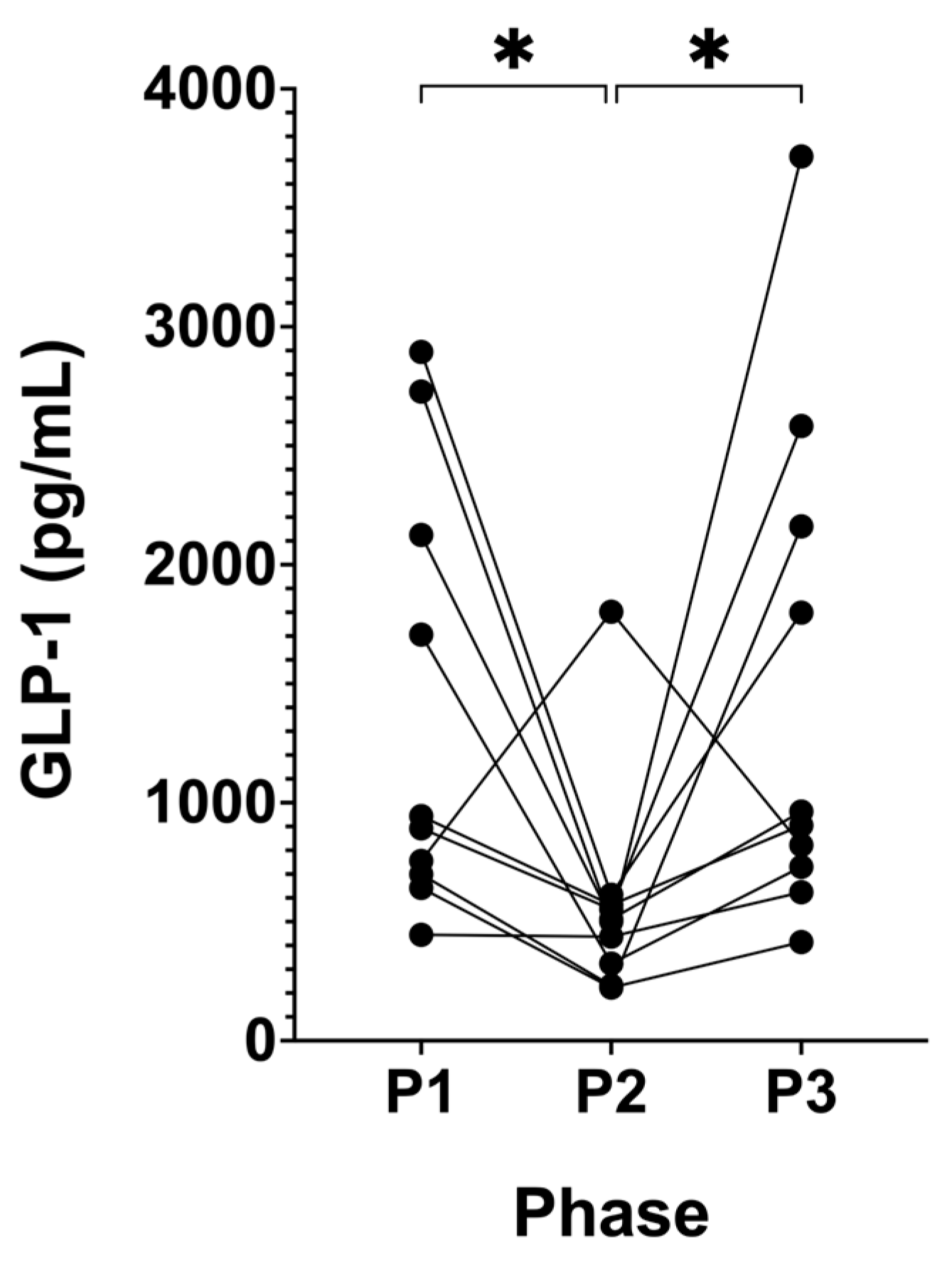
3.6. Suppression of Ketosis is Associated with Decreased GLP-1
4. Discussion
4.1. When to Measure Capillary BHB
4.2. Hypoketonaemia, HOMA-IR, and Fasting Insulin
4.3. Osteocalcin
4.4. Leptin Increases with Chronic Suppression of Ketosis a Risk of Hyperleptinaemia
4.5. Cortisol and Serotonin
4.6. GLP-1
5. Strengths and Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 9-PAHSA | palmitic-acid-9-hydroxy-stearic-acid |
| ACD | all-cause dementia |
| AD | Alzheimer’s disease |
| BA | biological ageing |
| BHB | beta-hydroxybutyrate |
| BMI | body mass index |
| CAC | coronary artery calcification |
| CI | confidence interval |
| cOCN | carboxylated osteocalcin |
| CVD | cardiovascular disease |
| EPC | endothelial progenitor cells |
| FAHFA | fatty acid ester of hydroxy fatty acids |
| GGT | gamma-glutamyl transferase |
| GLP-1 | glucagon like peptide-1 |
| GLP-1R | glucagon like peptide-1 receptor |
| HA | hydroxyapatite |
| HOMA-IR | homeostatic model assessment for insulin resistance |
| IGF-1 | insulin like growth factor-1 |
| L | ileo-colonic |
| MASLD | metabolic-dysfunction-associated steatosis liver disease |
| MCP-1 | monocyte chemotactic protein-1 |
| MetS | metabolic syndrome |
| Mt | mitochondrial |
| MP | metabolic phenotype |
| NK | nutritional ketosis |
| OGTT | oral glucose tolerance test |
| OR | odds ratio |
| OXPHOS | oxidative phosphorylation |
| P1 | Phase 1 |
| P2 | Phase 2 |
| P3 | Phase 3 |
| RCT | randomised control trial |
| SA | sub-clinical atherosclerosis |
| SFA | saturated fatty acid |
| SUK | standard U.K. diet |
| SuK | suppression of ketosis |
| TOFI | thin on the outside fat on the inside |
| T2DM | type 2 diabetes mellitus |
| T3 | triiodothyronine |
| tOCN | total osteocalcin |
| unOCN | uncarboxylated osteocalcin |
References
- Yang, H.; Gong, R.; Liu, M.; Deng, Y.; Zheng, X.; Hu, T. HOMA-IR is positively correlated with biological age and advanced aging in the US adult population. Eur. J. Med. Res. 2023, 28, 470. [Google Scholar] [CrossRef] [PubMed]
- Cooper, I.D.; Kyriakidou, Y.; Petagine, L.; Edwards, K.; Elliott, B.T. Bio-hacking better health—Leveraging metabolic biochemistry to maximise healthspan. Antioxidants 2023, 12, 1749. [Google Scholar] [CrossRef] [PubMed]
- UN World Population Ageing 2019: Highlights. Available online: https://www.un.org/en/development/desa/population/publications/pdf/ageing/WorldPopulationAgeing2019-Report.pdf (accessed on 13 September 2023).
- WHO. Global Health Estimates: Leading Causes of Death. 2019. Available online: https://www.who.int/data/gho/data/themes/mortality-and-global-health-estimates/ghe-leading-causes-of-death (accessed on 13 September 2023).
- ONS. Death Registration Summary Statistics, England and Wales—Office for National Statistics. ONS Website. 2023. Available online: https://www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/deaths/articles/deathregistrationsummarystatisticsenglandandwales/2022 (accessed on 14 September 2023).
- Araújo, J.; Cai, J.; Stevens, J. Prevalence of optimal metabolic ealth in american adults: National health and nutrition examination survey 2009–2016. Metab. Syndr. Relat. Disord. 2018, 17, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Cooper, I.D.; Kyriakidou, Y.; Edwards, K.; Petagine, L.; Seyfried, T.N.; Duraj, T.; Soto-Mota, A.; Scarborough, A.; Jacome, S.L.; Brookler, K.; et al. Ketosis Suppression and Ageing (KetoSAge): The effects of suppressing ketosis in long term keto-adapted non-athletic females. Int. J. Mol. Sci. 2023, 24, 15621. [Google Scholar] [CrossRef] [PubMed]
- Cooper, I.D.; Brookler, K.H.; Kyriakidou, Y.; Elliott, B.T.; Crofts, C.A.P. Metabolic phenotypes and step by step evolution of type 2 diabetes: A new paradigm. Biomedicines 2021, 9, 800. [Google Scholar] [CrossRef] [PubMed]
- Crofts, C.A.P.; Zinn, C.; Wheldon, M.; Schofield, M. Hyperinsulinemia: A unifying theory of chronic disease? Diabesity 2015, 1, 34. [Google Scholar] [CrossRef]
- Petagine, L.; Zariwala, M.G.; Patel, V.B. Non-alcoholic fatty liver disease: Immunological mechanisms and current treatments. World J. Gastroenterol. 2023, 29, 4831–4850. [Google Scholar] [CrossRef] [PubMed]
- Younossi, Z.M.; Rinella, M.E.; Sanyal, A.J.; Harrison, S.A.; Brunt, E.M.; Goodman, Z.; Cohen, D.E.; Loomba, R. From NAFLD to MAFLD: Implications of a Premature Change in Terminology. Hepatology 2021, 73, 1194–1198. [Google Scholar] [CrossRef] [PubMed]
- Salvatore, T.; Galiero, R.; Caturano, A.; Rinaldi, L.; Criscuolo, L.; Di Martino, A.; Albanese, G.; Vetrano, E.; Catalini, C.; Sardu, C.; et al. Current knowledge on the pathophysiology of lean/normal-weight type 2 diabetes. Int. J. Mol. Sci. 2022, 24, 658. [Google Scholar] [CrossRef]
- Nabulsi, A.A.; Folsom, A.R.; Heiss, G.; Weir, S.S.; Chambless, L.E.; Watson, R.L.; Eckfeldt, J.H. Atherosclerosis Risk in Communities Study Investigators Fasting hyperinsulinemia and cardiovascular disease risk factors in nondiabetic adults: Stronger associations in lean versus obese subjects. Metabolism 1995, 44, 914–922. [Google Scholar] [CrossRef]
- Kempf, A.M.; Strother, M.L.; Li, C.; Kaur, H.; Huang, T.T.K. Leptin as a marker of body fat and hyperinsulinemia in college students. J. Am. Coll. Health 2006, 55, 175–180. [Google Scholar] [CrossRef]
- Wiebe, N.; Muntner, P.; Tonelli, M. Associations of body mass index, fasting insulin, and inflammation with mortality: A prospective cohort study. Int. J. Obes. 2022, 46, 2107–2113. [Google Scholar] [CrossRef]
- Moore, A.R.; Holland-Winkler, A.M.; Ansley, J.K.; Boone, E.D.H.; Schulte, M.K.O. Reliability and diagnostic performance of a new blood ketone and glucose meter in humans. J. Int. Soc. Sports Nutr. 2021, 18, 1–9. [Google Scholar] [CrossRef]
- The Emerging Risk Factors Collaboration Diabetes mellitus, fasting glucose, and risk of cause-specific death. N. Engl. J. Med. 2011, 364, 829–841. [CrossRef]
- Sherwani, S.I.; Khan, H.A.; Ekhzaimy, A.; Masood, A.; Sakharkar, M.K. Significance of HbA1c test in diagnosis and prognosis of diabetic patients. Biomark. Insights 2016, 11, 95. [Google Scholar] [CrossRef]
- Ramirez, A.; Lange, C.; Kaduszkiewicz, H.; Weyerer, S.; Werle, J.; Pentzek, M.; Fuchs, A.; Riedel-Heller, S.G.; Luck, T.; Mösch, E.; et al. Elevated HbA1c is associated with increased risk of incident dementia in primary care patients. J. Alzheimers Dis. 2015, 44, 1203–1212. [Google Scholar] [CrossRef]
- Crofts, C.; Schofield, G.; Zinn, C.; Wheldon, M.; Kraft, J. Identifying hyperinsulinaemia in the absence of impaired glucose tolerance: An examination of the Kraft database. Diabetes Res. Clin. Pract. 2016, 118, 50–57. [Google Scholar] [CrossRef]
- Goalstone, M.L.; Leitner, J.W.; Wall, K.; Dolgonos, L.; Rother, K.I.; Accili, D.; Draznin, B. Effect of insulin on farnesyltransferase. J. Biol. Chem. 1998, 273, 23892–23896. [Google Scholar] [CrossRef]
- Draznin, B. Mitogenic action of insulin: Friend, foe or “frenemy”? Diabetologia 2010, 53, 229–233. [Google Scholar] [CrossRef]
- Goalstone, M.L.; Draznin, B. What does insulin do to Ras? Cell. Signal. 1998, 10, 297–301. [Google Scholar] [CrossRef] [PubMed]
- Draznin, B. Mechanism of the mitogenic influence of hyperinsulinemia. Diabetol. Metab. Syndr. 2011, 3, 10. [Google Scholar] [CrossRef] [PubMed]
- Hodson, A.E.; Tippetts, T.S.; Bikman, B.T. Insulin treatment increases myocardial ceramide accumulation and disrupts cardiometabolic function. Cardiovasc. Diabetol. 2015, 14, 153. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.E.; Tippetts, T.S.; Brassfield, E.S.; Tucker, B.J.; Ockey, A.; Swensen, A.C.; Anthonymuthu, T.S.; Washburn, T.D.; Kane, D.A.; Prince, J.T.; et al. Mitochondrial fission mediates ceramide-induced metabolic disruption in skeletal muscle. Biochem. J. 2013, 456, 427–439. [Google Scholar] [CrossRef] [PubMed]
- Hansen, M.E.; Tippetts, T.S.; Anderson, M.C.; Holub, Z.E.; Moulton, E.R.; Swensen, A.C.; Prince, J.T.; Bikman, B.T. Insulin increases ceramide synthesis in skeletal muscle. J. Diabetes Res. 2014, 2014, 765784. [Google Scholar] [CrossRef] [PubMed]
- Action to Control Cardiovascular Risk in Diabetes Study Group; Gerstein, H.C.; Miller, M.E.; Byington, R.P.; Goff, D.C., Jr.; Bigger, J.T.; Buse, J.B.; Cushman, W.C.; Genuth, S.; Ismail-Beigi, F.; et al. Effects of intensive glucose lowering in type 2 diabetes. N. Engl. J. Med. 2008, 358, 2545–2559. [Google Scholar] [CrossRef]
- Zhou, X.; Kang, C.; Hu, Y.H.; Wang, X.C. Study on insulin resistance and ischemic cerebrovascular disease: A bibliometric analysis via CiteSpace. Front. Public Health 2023, 11, 1021378. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.B.M.; Radwan, N.; Amer, S.; Saheb Sharif-Askari, N.; Mahdami, A.; Samara, K.A.; Halwani, R.; Jelinek, H.F. Assessing the link between diabetic metabolic dysregulation and breast cancer progression. Int. J. Mol. Sci. 2023, 24, 11816. [Google Scholar] [CrossRef]
- Cooper, I.D.; Crofts, C.A.P.; DiNicolantonio, J.J.; Malhotra, A.; Elliott, B.; Kyriakidou, Y.; Brookler, K.H. Relationships between hyperinsulinaemia, magnesium, vitamin D, thrombosis and COVID-19: Rationale for clinical management. Open Hear. 2020, 7, e001356. [Google Scholar] [CrossRef]
- Cooper, I.D.; Sanchez-Pizarro, C.; Norwitz, N.G.; Feldman, D.; Kyriakidou, Y.; Edwards, K.; Petagine, L.; Elliot, B.T.; Soto-Mota, A. Thyroid markers and body composition predict LDL-cholesterol change in lean healthy women on a ketogenic diet: Experimental support for the lipid energy model. Front. Endocrinol. 2023, 14, 1326768. [Google Scholar] [CrossRef]
- Paoli, A.; Rubini, A.; Volek, J.S.; Grimaldi, K.A. Beyond weight loss: A review of the therapeutic uses of very-low-carbohydrate (ketogenic) diets. Eur. J. Clin. Nutr. 2013, 67, 789–796. [Google Scholar] [CrossRef]
- Gershuni, V.M.; Yan, S.L.; Medici, V. Nutritional ketosis for weight management and reversal of metabolic syndrome. Curr. Nutr. Rep. 2018, 7, 97–106. [Google Scholar] [CrossRef]
- Janssen, J.A.M.J.L.; Street, E. Hyperinsulinemia and its pivotal role in aging, obesity, type 2 diabetes, cardiovascular disease and cancer. Int. J. Mol. Sci. 2021, 22, 7797. [Google Scholar] [CrossRef] [PubMed]
- Iglesies-Grau, J.; Garcia-Alvarez, A.; Oliva, B.; Mendieta, G.; García-Lunar, I.; Fuster, J.J.; Devesa, A.; Pérez-Herreras, C.; Fernández-Ortiz, A.; Brugada, R.; et al. Early insulin resistance in normoglycemic low-risk individuals is associated with subclinical atherosclerosis. Cardiovasc. Diabetol. 2023, 22, 350. [Google Scholar] [CrossRef] [PubMed]
- Hauschka, P.V.; Lian, J.B.; Cole, D.E.; Gundberg, C.M. Osteocalcin and matrix Gla protein: Vitamin K-dependent proteins in bone. Phys. Rev. 1989, 69, 990–1047. [Google Scholar] [CrossRef] [PubMed]
- Mizokami, A.; Wang, D.G.; Tanaka, M.; Gao, J.; Takeuchi, H.; Matsui, T.; Hirata, M. An extract from pork bones containing osteocalcin improves glucose metabolism in mice by oral administration. Biosci. Biotechnol. Biochem. 2016, 80, 2176–2183. [Google Scholar] [CrossRef] [PubMed]
- Cooper, I.D.; Brookler, K.H.; Crofts, C.A.P. Rethinking fragility fractures in type 2 diabetes: The link between hyperinsulinaemia and osteofragilitas. Biomedicines 2021, 9, 1165. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, M.; Imaoka, M.; Takeda, M. Interaction of bone and brain: Osteocalcin and cognition. Int. J. Neurosci. 2020, 131, 1115–1123. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.S.; Mazur, C.M.; Wein, M.N. Sclerostin and osteocalcin: Candidate bone-produced hormones. Front. Endocrinol. 2021, 12, 584147. [Google Scholar] [CrossRef] [PubMed]
- Puig, J.; Blasco, G.; Daunis-I-Estadella, J.; Moreno, M.; Molina, X.; Alberich-Bayarri, A.; Xifra, G.; Pedraza, S.; Ricart, W.; Fernández-Aranda, F.; et al. Lower serum osteocalcin concentrations are associated with brain microstructural changes and worse cognitive performance. Clin. Endocrinol. 2016, 84, 756–763. [Google Scholar] [CrossRef]
- Ferron, M.; Lacombe, J. Regulation of energy metabolism by the skeleton: Osteocalcin and beyond. Arch. Biochem. Biophys. 2014, 561, 137–146. [Google Scholar] [CrossRef]
- Dede, A.D.; Tournis, S.; Dontas, I.; Trovas, G. Type 2 diabetes mellitus and fracture risk. Metabolism 2014, 63, 1480–1490. [Google Scholar] [CrossRef]
- Foresta, C.; Strapazzon, G.; De Toni, L.; Gianesello, L.; Calcagno, A.; Pilon, C.; Plebani, M.; Vettor, R. Evidence for osteocalcin production by adipose tissue and its role in human metabolism. J. Clin. Endocrinol. Metab. 2010, 95, 3502–3506. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gössl, M.; Mödder, U.I.; Atkinson, E.J.; Lerman, A.; Khosla, S. Osteocalcin expression by circulating endothelial progenitor cells in patients with coronary atherosclerosis. J. Am. Coll. Cardiol. 2008, 52, 1314. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.; Mackenzie, N.C.W.; Millán, J.L.; Farquharson, C.; MacRae, V.E. The appearance and modulation of osteocyte marker expression during calcification of vascular smooth muscle cells. PLoS ONE 2011, 6, e19595. [Google Scholar] [CrossRef] [PubMed]
- Brandenburg, V.M.; Kramann, R.; Koos, R.; Krüger, T.; Schurgers, L.; Mühlenbruch, G.; Hübner, S.; Gladziwa, U.; Drechsler, C.; Ketteler, M. Relationship between sclerostin and cardiovascular calcification in hemodialysis patients: A cross-sectional study. BMC Nephrol. 2013, 14, 219. [Google Scholar] [CrossRef] [PubMed]
- De Maré, A.; D’haese, P.C.; Verhulst, A. The role of sclerostin in bone and ectopic calcification. Int. J. Mol. Sci. 2020, 21, 3199. [Google Scholar] [CrossRef] [PubMed]
- Tyson, J.; Bundy, K.; Roach, C.; Douglas, H.; Ventura, V.; Segars, M.F.; Schwartz, O.; Simpson, C.L. Mechanisms of the osteogenic switch of smooth muscle cells in vascular calcification: WNT signaling, BMPs, mechanotransduction, and EndMT. Bioengineering 2020, 7, 88. [Google Scholar] [CrossRef] [PubMed]
- Saleem, U.; Mosley, T.H.; Kullo, I.J. Serum osteocalcin is associated with measures of insulin resistance, adipokine levels, and the presence of metabolic syndrome. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 1474–1478. [Google Scholar] [CrossRef] [PubMed]
- Yaylali, Y.T.; Fidan-Yaylali, G.; Dedeoglu, O.; Senol, H. Osteocalcin and epicardial adipose tissue in obesity: New hints for epicardial adipose tissue–bone crosstalk. Scand. Cardiovasc. J. 2019, 53, 296–298. [Google Scholar] [CrossRef] [PubMed]
- Bao, Y.; Xiaojing, M.; Yang, R.; Wang, F.; Hao, Y.; Dou, J.; He, H.; Jia, W. Inverse relationship between serum osteocalcin levels and visceral fat area in chinese men. J. Clin. Endocrinol. Metab. 2013, 98, 345–351. [Google Scholar] [CrossRef]
- Lei, H.; Liu, J.; Wang, W.; Yang, X.; Feng, Z.; Zang, P.; Lu, B.; Shao, J. Association between osteocalcin, a pivotal marker of bone metabolism, and secretory function of islet beta cells and alpha cells in Chinese patients with type 2 diabetes mellitus: An observational study. Diabetol. Metab. Syndr. 2022, 14, 160. [Google Scholar] [CrossRef]
- Ferron, M. Endocrine Functions of Bone. In Principles of Endocrinology and Hormone Action; Belfiore, A., LeRoith, D., Eds.; Springer: Cham, Switzerland, 2018; ISBN 978-3-319-44675-2. [Google Scholar]
- Mizokami, A.; Yasutake, Y.; Gao, J.; Matsuda, M.; Takahashi, I.; Takeuchi, H.; Hirata, M. Osteocalcin induces release of glucagon-like peptide-1 and thereby stimulates insulin secretion in mice. PLoS ONE 2013, 8, 57375. [Google Scholar] [CrossRef]
- Mizokami, A.; Mukai, S.; Gao, J.; Kawakubo-Yasukochi, T.; Otani, T.; Takeuchi, H.; Jimi, E.; Hirata, M. GLP-1 signaling is required for improvement of glucose tolerance by osteocalcin. J. Endocrinol. 2020, 244, 285–296. [Google Scholar] [CrossRef] [PubMed]
- Ben-Dor, M.; Gopher, A.; Hershkovitz, I.; Barkai, R. Man the fat hunter: The demise of Homo erectus and the emergence of a new Hominin lineage in the middle pleistocene (ca. 400 kyr) Levant. PLoS ONE 2011, 6, e28689. [Google Scholar] [CrossRef] [PubMed]
- Boden, G.; Chen, X.; Kolaczynski, J.W.; Polansky, M. Effects of prolonged hyperinsulinemia on serum leptin in normal human subjects. J. Clin. Investig. 1997, 100, 1107. [Google Scholar] [CrossRef] [PubMed]
- Pluta, W.; Dudzińska, W.; Lubkowska, A. Metabolic obesity in people with normal body weight (MONW)—review of diagnostic criteria. Int. J. Environ. Res. Public Health 2022, 19, 624. [Google Scholar] [CrossRef]
- Ruderman, N.B.; Schneider, S.H.; Berchtold, P. The “metabolically-obese,” normal-weight individual. Am. J. Clin. Nutr. 1981, 34, 1617–1621. [Google Scholar] [CrossRef] [PubMed]
- Veech, R.L. The therapeutic implications of ketone bodies: The effects of ketone bodies in pathological conditions: Ketosis, ketogenic diet, redox states, insulin resistance, and mitochondrial metabolism. Prostaglandins Leukot. Essent. Fat. Acids 2004, 70, 309–319. [Google Scholar] [CrossRef] [PubMed]
- Reilly, M.P.; Iqbal, N.; Schutta, M.; Wolfe, M.L.; Scally, M.; Localio, A.R.; Rader, D.J.; Kimmel, S.E. Plasma leptin levels are associated with coronary atherosclerosis in type 2 diabetes. J. Clin. Endocrinol. Metab. 2004, 89, 3872–3878. [Google Scholar] [CrossRef]
- Zdrojewicz, Z.; Popowicz, E.; Szyca, M.; Michalik, T.; Śmieszniak, B. TOFI phenotype-its effect on the occurrence of diabetes. Pediatr. Endocrinol. Diabetes. Metab. 2017, 23, 96–100. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, A.; Abdo, S.; Zhao, S.; Wu, C.-H.; Shi, Y.; Lo, C.-S.; Chenier, I.; Alquier, T.; Filep, J.G.; Ingelfinger, J.R.; et al. Insulin Inhibits Nrf2 Gene Expression via Heterogeneous Nuclear Ribonucleoprotein F/K in Diabetic Mice. Endocrinology 2019, 158, 903–919. [Google Scholar] [CrossRef] [PubMed]
- Ruderman, N.; Chisholm, D.; Pi-Sunyer, X.; Schneider, S. The metabolically obese, normal-weight individual revisited. Diabetes 1998, 47, 699–713. [Google Scholar] [CrossRef] [PubMed]
- Lana, A.; Valdés-Bécares, A.; Buño, A.; Rodríguez-Artalejo, F.; Lopez-Garcia, E. Serum leptin concentration is associated with incident frailty in older adults. Aging Dis. 2017, 8, 240–249. [Google Scholar] [CrossRef] [PubMed]
- Shih, Y.L.; Huang, T.C.; Shih, C.C.; Chen, J.Y. Relationship between leptin and insulin resistance among community—dwelling middle-aged and elderly populations in Taiwan. J. Clin. Med. 2022, 11, 5357. [Google Scholar] [CrossRef] [PubMed]
- Stalder, T.; Kirschbaum, C. Cortisol. In Encyclopedia of Behavioral Medicine; Gellman, M.D., Ed.; Springer: Cham, Switzerland, 2020; pp. 561–567. ISBN 978-3-030-39903-0. [Google Scholar]
- Yan, Y.X.; Xiao, H.B.; Wang, S.S.; Zhao, J.; He, Y.; Wang, W.; Dong, J. Investigation of the relationship between chronic stress and Insulin resistance in a chinese population. J. Epidemiol. 2016, 26, 355–360. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, R.M.; Labad, J.; Strachan, M.W.J.; Braun, A.; Fowkes, F.G.R.; Lee, A.J.; Frier, B.M.; Seckl, J.R.; Walker, B.R.; Price, J.F. Elevated fasting plasma cortisol is associated with ischaemic heart disease and its risk factors in people with type 2 diabetes: The Edinburgh Type 2 Diabetes Study. J. Clin. Endocrinol. Metab. 2010, 95, 1602. [Google Scholar] [CrossRef] [PubMed]
- Kushnir, M.M.; Neilson, R.; Roberts, W.L.; Rockwood, A.L. Cortisol and cortisone analysis in serum and plasma by atmospheric pressure photoionization tandem mass spectrometry. Clin. Biochem. 2004, 37, 357–362. [Google Scholar] [CrossRef] [PubMed]
- Seal, S.V.; Turner, J.D. The ‘Jekyll and Hyde’ of gluconeogenesis: Early life adversity, later life stress, and metabolic disturbances. Int. J. Mol. Sci. 2021, 22, 3344. [Google Scholar] [CrossRef] [PubMed]
- Popoviciu, M.S.; Păduraru, L.; Yahya, G.; Metwally, K.; Cavalu, S. Emerging role of GLP-1 agonists in obesity: A comprehensive review of randomised controlled trials. Int. J. Mol. Sci. 2023, 24, 10449. [Google Scholar] [CrossRef] [PubMed]
- Jujić, A.; Godina, C.; Belting, M.; Melander, O.; Juul Holst, J.; Ahlqvist, E.; Gomez, M.F.; Nilsson, P.M.; Jernström, H.; Magnusson, M. Endogenous incretin levels and risk of first incident cancer: A prospective cohort study. Sci. Rep. 2023, 13, 382. [Google Scholar] [CrossRef]
- Drucker, D.J. The biology of incretin hormones. Cell Metab. 2006, 3, 153–165. [Google Scholar] [CrossRef]
- Drucker, D.J.; Philippe, J.; Mojsov, S.; Chick, W.L.; Habener, J.F. Glucagon-like peptide I stimulates insulin gene expression and increases cyclic AMP levels in a rat islet cell line. Proc. Natl. Acad. Sci. USA 1987, 84, 3434. [Google Scholar] [CrossRef] [PubMed]
- Ramracheya, R.; Chapman, C.; Chibalina, M.; Dou, H.; Miranda, C.; González, A.; Moritoh, Y.; Shigeto, M.; Zhang, Q.; Braun, M.; et al. GLP-1 suppresses glucagon secretion in human pancreatic alpha-cells by inhibition of P/Q-type Ca2+ channels. Physiol. Rep. 2018, 6, e13852. [Google Scholar] [CrossRef] [PubMed]
- Kreymann, B.; Ghatei, M.A.; Williams, G.; Bloom, S.R. Glucagon-like peptide-1 7-36: A physiological incretin in man. Lancet 1987, 330, 1300–1304. [Google Scholar] [CrossRef] [PubMed]
- Albrechtsen, N.J.W.; Bak, M.J.; Hartmann, B.; Christensen, L.W.; Kuhre, R.E.; Deacon, C.F.; Holst, J.J. Stability of glucagon-like peptide 1 and glucagon in human plasma. Endocr. Connect. 2015, 4, 50. [Google Scholar] [CrossRef] [PubMed]
- Ebou, M.H.; Singh-Estivalet, A.; Launay, J.M.; Callebert, J.; Tronche, F.; Ferré, P.; Gautier, J.F.; Guillemain, G.; Bréant, B.; Blondeau, B.; et al. Glucocorticoids inhibit basal and hormone-induced serotonin synthesis in pancreatic beta cells. PLoS ONE 2016, 11, 149343. [Google Scholar] [CrossRef] [PubMed]
- Pi, M.; Nishimoto, S.K.; Quarles, L.D. GPRC6A: Jack of all metabolism (or master of none). Mol. Metab. 2017, 6, 185–193. [Google Scholar] [CrossRef]
- Müller, T.D.; Finan, B.; Bloom, S.R.; D’Alessio, D.; Drucker, D.J.; Flatt, P.R.; Fritsche, A.; Gribble, F.; Grill, H.J.; Habener, J.F.; et al. Glucagon-like peptide 1 (GLP-1). Mol. Metab. 2019, 30, 72–130. [Google Scholar] [CrossRef] [PubMed]
- Yore, M.M.; Syed, I.; Moraes-Vieira, P.M.; Zhang, T.; Herman, M.A.; Homan, E.A.; Patel, R.T.; Lee, J.; Chen, S.; Peroni, O.D.; et al. Discovery of a class of endogenous mammalian lipids with anti-diabetic and anti-inflammatory effects. Cell 2014, 159, 318–332. [Google Scholar] [CrossRef] [PubMed]
- Manful, C.F.; Pham, T.H.; Wheeler, E.; Nadeem, M.; Adigun, O.A.; Walsh, N.; Thomas, R.H. Assessing the fate of fatty acid esters of hydroxy fatty acids, diglycerides and monoacetyldiacylglycerides in grilled ruminant meats marinated with unfiltered beer-based marinades. J. Food Drug Anal. 2022, 30, 234. [Google Scholar] [CrossRef]
- An, N.; Wang, Y.; He, D.X.; Mei, P.C.; Zhu, Q.F.; Feng, Y.Q. A dataset of branched fatty acid esters of hydroxy fatty acids diversity in foods. Sci. Data 2023, 10, 790. [Google Scholar] [CrossRef]
- Brezinova, M.; Kuda, O.; Hansikova, J.; Rombaldova, M.; Balas, L.; Bardova, K.; Durand, T.; Rossmeisl, M.; Cerna, M.; Stranak, Z.; et al. Levels of palmitic acid ester of hydroxystearic acid (PAHSA) are reduced in the breast milk of obese mothers. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2018, 1863, 126–131. [Google Scholar] [CrossRef] [PubMed]
- Ott, B.; Skurk, T.; Lagkouvardos, L.; Fischer, S.; Büttner, J.; Lichtenegger, M.; Clavel, T.; Lechner, A.; Rychlik, M.; Haller, D.; et al. Short-term overfeeding with dairy cream does not modify gut permeability, the fecal microbiota, or glucose metabolism in young healthy men. J. Nutr. 2018, 148, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Kellerer, T.; Kleigrewe, K.; Brandl, B.; Hofmann, T.; Hauner, H.; Skurk, T. Fatty acid esters of hydroxy fatty acids (FAHFAs) are associated with diet, BMI, and age. Front. Nutr. 2021, 8, 691401. [Google Scholar] [CrossRef] [PubMed]
- Rollo, F.; Ubaldi, M.; Ermini, L.; Marota, I. Ötzi’s last meals: DNA analysis of the intestinal content of the neolithic glacier mummy from the Alps. Proc. Natl. Acad. Sci. USA 2002, 99, 12594–12599. [Google Scholar] [CrossRef] [PubMed]
- Ben-Dor, M.; Sirtoli, R.; Barkai, R. The evolution of the human trophic level during the Pleistocene. Am. J. Phys. Anthropol. 2021, 175, 27–56. [Google Scholar] [CrossRef] [PubMed]
- Meier, J.J.; Nauck, M.A.; Kranz, D.; Holst, J.J.; Deacon, C.F.; Gaeckler, D.; Schmidt, W.E.; Gallwitz, B. Secretion, Degradation, and Elimination of Glucagon-Like Peptide 1 and Gastric Inhibitory Polypeptide in Patients with Chronic Renal Insufficiency and Healthy Control Subjects. Diabetes 2004, 53, 654–662. [Google Scholar] [CrossRef] [PubMed]
- Fareed, A.; Hussain, A. The expanding role of GLP-1: From diabetes management to cancer treatment. Clin. Med. Insights Endocrinol. Diabetes 2023, 16. [Google Scholar] [CrossRef] [PubMed]
- Hallberg, S.J.; McKenzie, A.L.; Williams, P.T.; Bhanpuri, N.H.; Peters, A.L.; Campbell, W.W.; Hazbun, T.L.; Volk, B.M.; McCarter, J.P.; Phinney, S.D.; et al. Effectiveness and safety of a novel care model for the management of type 2 diabetes at 1 Year: An open-label, non-randomized, controlled study. Diabetes Ther. 2018, 9, 583–612. [Google Scholar] [CrossRef] [PubMed]
- Danan, A.; Westman, E.C.; Saslow, L.R.; Ede, G. The ketogenic diet for refractory mental illness: A retrospective analysis of 31 inpatients. Front. Psychiatry 2022, 13, 951376. [Google Scholar] [CrossRef]
- Norwitz, N.G.; Hu, M.T.; Clarke, K. The mechanisms by which the ketone body D-β-hydroxybutyrate may improve the multiple cellular pathologies of Parkinson’s disease. Front. Nutr. 2019, 6, 63. [Google Scholar] [CrossRef]
- Kelly, T.; Unwin, D.; Finucane, F. Low-carbohydrate diets in the management of obesity and type 2 diabetes: A review from clinicians using the approach in practice. Int. J. Environ. Res. Public Health 2020, 17, 2557. [Google Scholar] [CrossRef] [PubMed]
- Athinarayanan, S.J.; Adams, R.N.; Hallberg, S.J.; McKenzie, A.L.; Bhanpuri, N.H.; Campbell, W.W.; Volek, J.S.; Phinney, S.D.; McCarter, J.P. Long-term effects of a novel continuous remote care intervention including nutritional ketosis for the management of type 2 diabetes: A 2-year nonrandomized clinical trial. Front. Endocrinol. 2019, 10, 348. [Google Scholar] [CrossRef] [PubMed]
- Norwitz, N.G.; Hurn, M.; Forcen, F.E.; Norwitz, N. Animal-based ketogenic diet puts severe anorexia nervosa into multi-year remission: A case series. J. Metab. Health 2023, 6, 8. [Google Scholar] [CrossRef]
| P1 | P2 | P3 | ANOVA p Value | P1 vs. P2 | P2 vs. P3 | P1 vs. P3 | |
|---|---|---|---|---|---|---|---|
| BMI (kg/m2) | 20.52 (±1.39) | 21.54 (±1.30) | 20.82 (±1.46) | <0.0001 | <0.0001 | 0.0025 | 0.0197 |
| Fat mass (kg) | 14.21 (±2.55) | 15.88 (±2.23) | 14.78 (±2.20) | <0.0001 | 0.0008 | 0.0057 | 0.1016 |
| Insulin (µIU/mL) | 4.95 (±1.24) | 9.06 (±2.14) | 5.62 (±1.83) | <0.0001 | 0.0006 | 0.0027 | 0.3995 |
| Glucose (mmol/L) | 4.36 (±0.53) | 5.12 (±0.59) | 4.41 (±0.30) | 0.0015 | 0.0088 | 0.0177 | 0.9469 |
| BHB (mmol/L) | 2.43 (±1.28) | 0.18 (±0.13) | 2.31 (±0.71) | 0.0001 | 0.0012 | <0.0001 | 0.9854 |
| HOMA-IR | 0.97 (±0.32) | 2.07 (±0.61) | 1.11 (±0.41) | <0.0001 | 0.0008 | 0.0013 | 0.4950 |
| tOCN (ng/mL) | 33.84 (±13.66) | 55.31 (±29.71) | 34.02 (±12.05) | 0.0049 | 0.0138 | 0.0253 | 0.9978 |
| cOCN (ng/mL) | 31.54 (±12.59) | 50.78 (±26.22) | 32.02 (±11.13) | 0.0040 | 0.0120 | 0.0246 | 0.9840 |
| unOCN (ng/mL) | 2.29 (±1.25) | 4.54 (±3.79) | 2.00 (±1.16) | 0.0004 | 0.0417 | 0.0010 | 0.7907 |
| tOCN % (% relative to P1) | 100.00 | 162.36 (±46.53) | 109.70 (±40.36) | 0.0005 | 0.0065 | 0.0094 | >0.9999 |
| cOCN % (% relative to P1) | 100.00 | 161.56 (±46.73) | 111.52 (±42.90) | 0.0007 | 0.0062 | 0.0080 | 0.6836 |
| unOCN % (% relative to P1) | 100.00 | 176.67 (±66.66) | 87.11 (±21.43) | 0.0028 | 0.0135 | 0.0076 | 0.2476 |
| Leptin (ng/mL) | 4.50 (±3.67) | 15.08 (±8.00) | 4.57 (±3.48) | <0.0001 | 0.0010 | 0.0052 | >0.9999 |
| Cortisol (ng/mL) | 126.20 (±52.67) | 112.70 (±58.46) | 131.90 (±52.18) | 0.4362 | >0.9999 | 0.5391 | >0.9999 |
| Serotonin (ng/mL) | 21.05 (±22.83) | 18.38 (±16.03) | 21.77 (±16.80) | 0.6013 | 0.7907 | >0.9999 | >0.9999 |
| GLP-1 (pg/mL) | 1383.18 (±911.36) | 576.72 (±452.43) | 1471.85 (±1066.75) | 0.0075 | 0.0209 | 0.0219 | >0.9999 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cooper, I.D.; Kyriakidou, Y.; Petagine, L.; Edwards, K.; Soto-Mota, A.; Brookler, K.; Elliott, B.T. Ketosis Suppression and Ageing (KetoSAge) Part 2: The Effect of Suppressing Ketosis on Biomarkers Associated with Ageing, HOMA-IR, Leptin, Osteocalcin, and GLP-1, in Healthy Females. Biomedicines 2024, 12, 1553. https://doi.org/10.3390/biomedicines12071553
Cooper ID, Kyriakidou Y, Petagine L, Edwards K, Soto-Mota A, Brookler K, Elliott BT. Ketosis Suppression and Ageing (KetoSAge) Part 2: The Effect of Suppressing Ketosis on Biomarkers Associated with Ageing, HOMA-IR, Leptin, Osteocalcin, and GLP-1, in Healthy Females. Biomedicines. 2024; 12(7):1553. https://doi.org/10.3390/biomedicines12071553
Chicago/Turabian StyleCooper, Isabella D., Yvoni Kyriakidou, Lucy Petagine, Kurtis Edwards, Adrian Soto-Mota, Kenneth Brookler, and Bradley T. Elliott. 2024. "Ketosis Suppression and Ageing (KetoSAge) Part 2: The Effect of Suppressing Ketosis on Biomarkers Associated with Ageing, HOMA-IR, Leptin, Osteocalcin, and GLP-1, in Healthy Females" Biomedicines 12, no. 7: 1553. https://doi.org/10.3390/biomedicines12071553
APA StyleCooper, I. D., Kyriakidou, Y., Petagine, L., Edwards, K., Soto-Mota, A., Brookler, K., & Elliott, B. T. (2024). Ketosis Suppression and Ageing (KetoSAge) Part 2: The Effect of Suppressing Ketosis on Biomarkers Associated with Ageing, HOMA-IR, Leptin, Osteocalcin, and GLP-1, in Healthy Females. Biomedicines, 12(7), 1553. https://doi.org/10.3390/biomedicines12071553








