Dopamine Receptors and TAAR1 Functional Interaction Patterns in the Duodenum Are Impaired in Gastrointestinal Disorders
Abstract
:1. Introduction
2. Materials and Methods
2.1. Public Resources and Databases
2.2. Data Normalization and Statistical Analysis
2.3. Measurement of Co-Expression and GO Enrichment Analysis
2.4. Analysis of Functional Semantic Similarity between Genes
3. Results
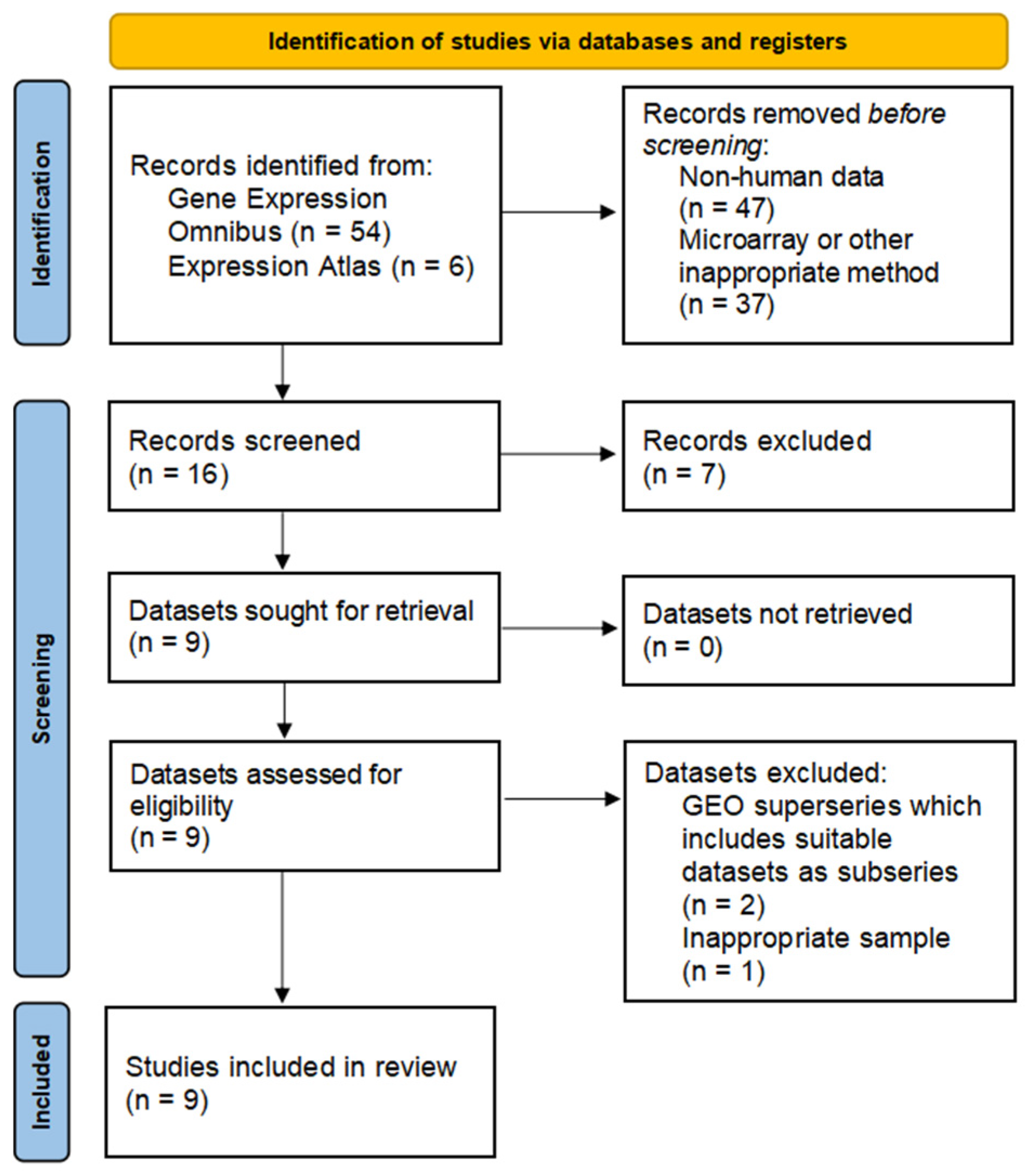
3.1. Data Search, Selection, and Inclusion
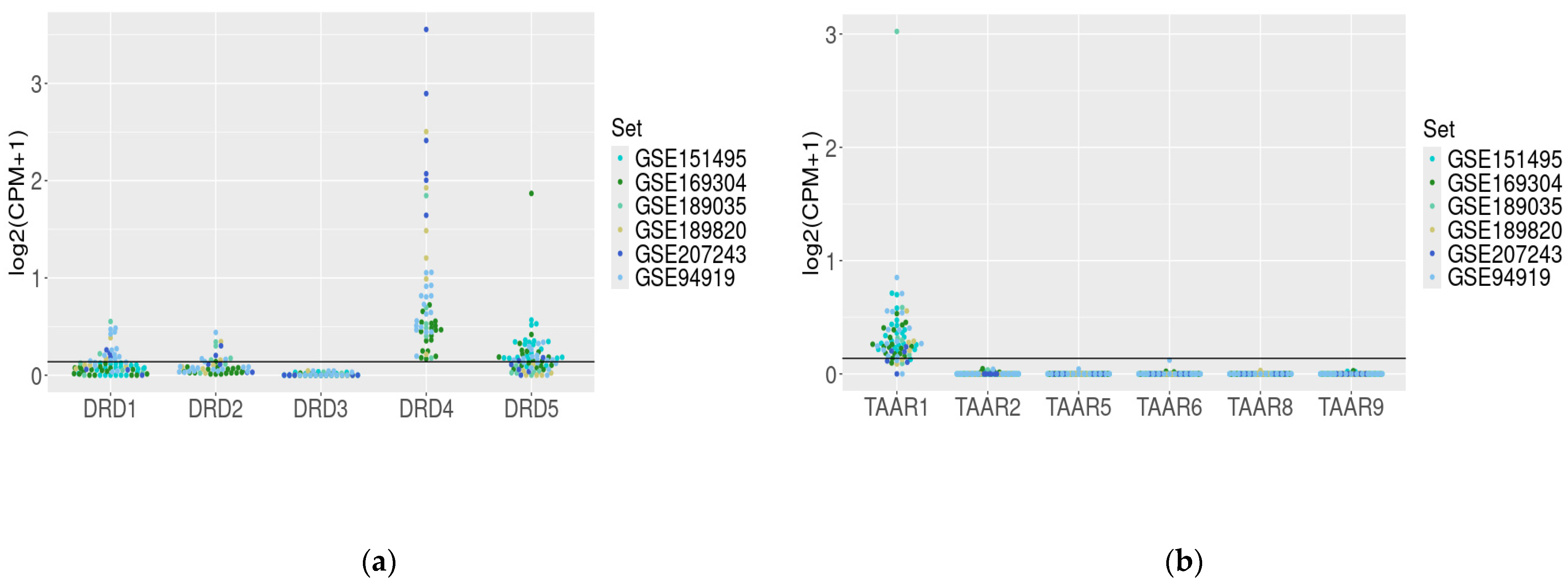
3.2. Dopamine Receptor Genes and TAAR mRNA Expression in the Healthy Duodenum
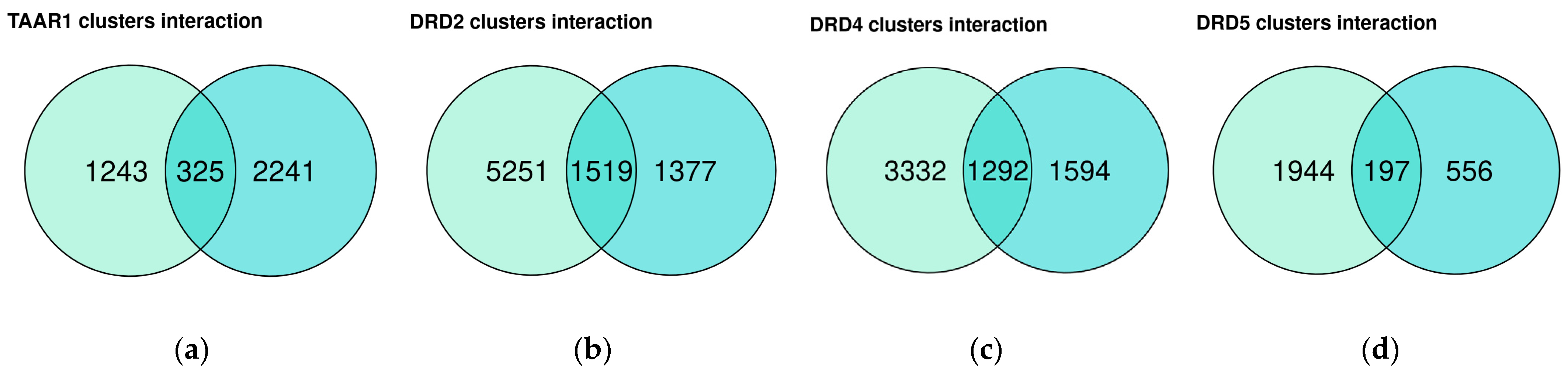
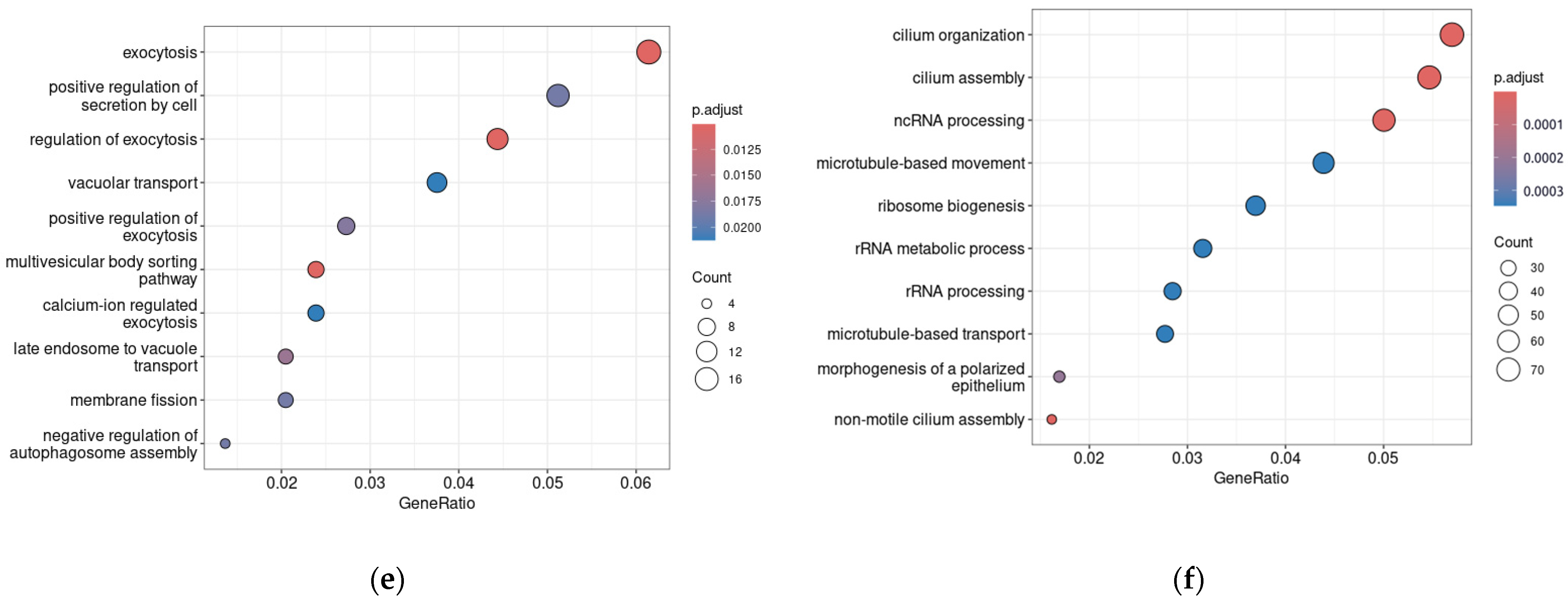
3.3. Dopamine Receptor Genes and TAAR1 mRNA Co-Expressed Clusters in Healthy Duodenum Demonstrate High Heterogeneity
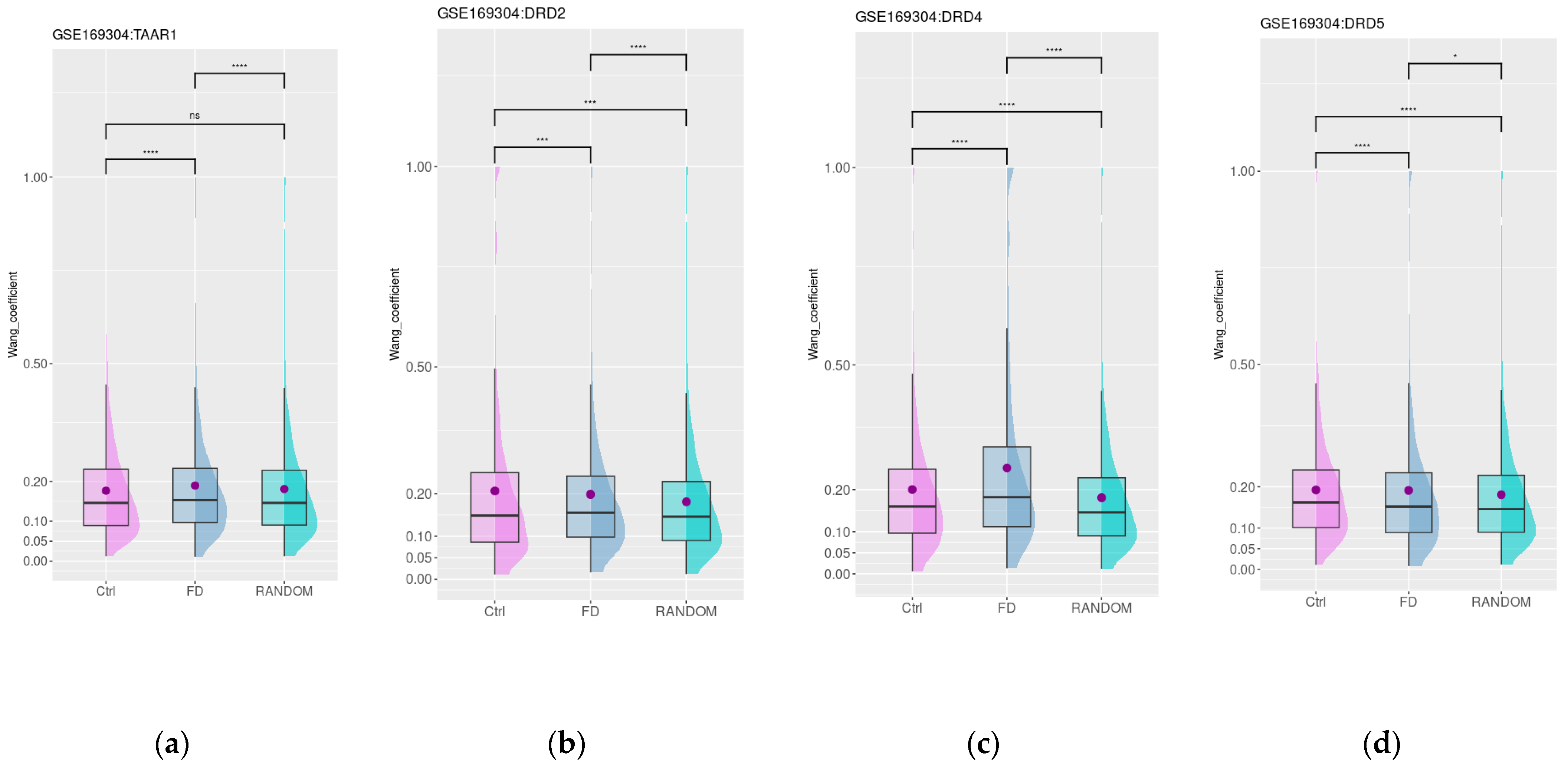
3.4. Dopamine and Trace Amine Co-Expression Patterns in the Duodenum Are Disrupted in Patients with Functional Dyspepsia
3.5. Dopamine and Trace Amine Co-Expression Patterns in the Duodenum Are Impaired in Patients with Gastrointestinal Disorders Associated with DM
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bucolo, C.; Leggio, G.M.; Drago, F.; Salomone, S. Dopamine Outside the Brain: The Eye, Cardiovascular System and Endocrine Pancreas. Pharmacol. Ther. 2019, 203, 107392. [Google Scholar] [CrossRef] [PubMed]
- Chaudhry, S.; Bernardes, M.; Harris, P.E.; Maffei, A. Gastrointestinal Dopamine as an Anti-Incretin and Its Possible Role in Bypass Surgery as Therapy for Type 2 Diabetes with Associated Obesity. Minerva Endocrinol. 2016, 41, 43–56. [Google Scholar] [PubMed]
- Liu, C.-Z.; Feng, X.-Y.; Liu, S.; Zhang, X.-L.; Zhu, J.-X. Synthesis and Metabolism of Gut Dopamine. In Dopamine in the Gut; Zhu, J.-X., Ed.; Springer: Singapore, 2021; pp. 25–51. ISBN 978-981-336-586-5. [Google Scholar]
- Serio, R.; Zizzo, M.G. The Multiple Roles of Dopamine Receptor Activation in the Modulation of Gastrointestinal Motility and Mucosal Function. Auton. Neurosci. 2023, 244, 103041. [Google Scholar] [CrossRef] [PubMed]
- Kurnik-Łucka, M.; Pasieka, P.; Łączak, P.; Wojnarski, M.; Jurczyk, M.; Gil, K. Gastrointestinal Dopamine in Inflammatory Bowel Diseases: A Systematic Review. Int. J. Mol. Sci. 2021, 22, 12932. [Google Scholar] [CrossRef] [PubMed]
- Montoro-Huguet, M.A.; Belloc, B.; Domínguez-Cajal, M. Small and Large Intestine (I): Malabsorption of Nutrients. Nutrients 2021, 13, 1254. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Yan, J.; Li, G.; Liu, J.; Fan, R.; Li, S.; Zheng, L.; Zhang, Y.; Zhu, J. Source of Dopamine in Gastric Juice and Luminal Dopamine-induced Duodenal Bicarbonate Secretion via Apical Dopamine D2 Receptors. Br. J. Pharmacol. 2020, 177, 3258–3272. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.-Y.; Zhang, D.-N.; Wang, Y.-A.; Fan, R.-F.; Hong, F.; Zhang, Y.; Li, Y.; Zhu, J.-X. Dopamine Enhances Duodenal Epithelial Permeability via the Dopamine D5 Receptor in Rodent. Acta Physiol. 2017, 220, 113–123. [Google Scholar] [CrossRef] [PubMed]
- Chojnacki, C.; Popławski, T.; Błasiak, J.; Fila, M.; Konrad, P.; Chojnacki, J. Altered Dopamine Signalling in Chronic Epigastric Pain Syndrome. J. Physiol. Pharmacol. 2020, 71, 6. [Google Scholar] [CrossRef]
- Tolstanova, G.; Deng, X.; Ahluwalia, A.; Paunovic, B.; Prysiazhniuk, A.; Ostapchenko, L.; Tarnawski, A.; Sandor, Z.; Szabo, S. Role of Dopamine and D2 Dopamine Receptor in the Pathogenesis of Inflammatory Bowel Disease. Dig. Dis. Sci. 2015, 60, 2963–2975. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-L.; Sun, Q.; Quan, Z.-S.; Wu, L.; Liu, Z.-M.; Xia, Y.-Q.; Wang, Q.-Y.; Zhang, Y.; Zhu, J.-X. Dopamine Regulates Colonic Glial Cell-Derived Neurotrophic Factor Secretion through Cholinergic Dependent and Independent Pathways. Br. J. Pharmacol. 2024, 181, 413–428. [Google Scholar] [CrossRef]
- Liu, X.-Y.; Zheng, L.-F.; Fan, Y.-Y.; Shen, Q.-Y.; Qi, Y.; Li, G.-W.; Sun, Q.; Zhang, Y.; Feng, X.-Y.; Zhu, J.-X. Activation of Dopamine D2 Receptor Promotes Pepsinogen Secretion by Suppressing Somatostatin Release from the Mouse Gastric Mucosa. Am. J. Physiol.-Cell Physiol. 2022, 322, C327–C337. [Google Scholar] [CrossRef] [PubMed]
- Cerantola, S.; Caputi, V.; Contarini, G.; Mereu, M.; Bertazzo, A.; Bosi, A.; Banfi, D.; Mantini, D.; Giaroni, C.; Giron, M.C. Dopamine Transporter Genetic Reduction Induces Morpho-Functional Changes in the Enteric Nervous System. Biomedicines 2021, 9, 465. [Google Scholar] [CrossRef] [PubMed]
- Cerantola, S.; Faggin, S.; Caputi, V.; Bosi, A.; Banfi, D.; Rambaldo, A.; Porzionato, A.; Di Liddo, R.; De Caro, R.; Savarino, E.V.; et al. Small Intestine Neuromuscular Dysfunction in a Mouse Model of Dextran Sulfate Sodium-Induced Ileitis: Involvement of Dopaminergic Neurotransmission. Life Sci. 2022, 301, 120562. [Google Scholar] [CrossRef] [PubMed]
- Bauer, E.E.; Reed, C.H.; Lyte, M.; Clark, P.J. An Evaluation of the Rat Intestinal Monoamine Biogeography Days Following Exposure to Acute Stress. Front. Physiol. 2022, 13, 1021985. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, M.; Iaccarino, C.; Saiardi, A.; Heidt, V.; Bozzi, Y.; Picetti, R.; Vitale, C.; Westphal, H.; Drago, J.; Borrelli, E. Simultaneous Absence of Dopamine D1 and D2 Receptor-Mediated Signaling Is Lethal in Mice. Proc. Natl. Acad. Sci. USA 2004, 101, 11465–11470. [Google Scholar] [CrossRef] [PubMed]
- Gainetdinov, R.R.; Hoener, M.C.; Berry, M.D. Trace Amines and Their Receptors. Pharmacol. Rev. 2018, 70, 549–620. [Google Scholar] [CrossRef] [PubMed]
- Espinoza, S.; Salahpour, A.; Masri, B.; Sotnikova, T.D.; Messa, M.; Barak, L.S.; Caron, M.G.; Gainetdinov, R.R. Functional Interaction between Trace Amine-Associated Receptor 1 and Dopamine D2 Receptor. Mol. Pharmacol. 2011, 80, 416–425. [Google Scholar] [CrossRef]
- Correll, C.U.; Koblan, K.S.; Hopkins, S.C.; Li, Y.; Dworak, H.; Goldman, R.; Loebel, A. Safety and Effectiveness of Ulotaront (SEP-363856) in Schizophrenia: Results of a 6-Month, Open-Label Extension Study. NPJ Schizophr. 2021, 7, 63. [Google Scholar] [CrossRef]
- Heffernan, M.L.R.; Herman, L.W.; Brown, S.; Jones, P.G.; Shao, L.; Hewitt, M.C.; Campbell, J.E.; Dedic, N.; Hopkins, S.C.; Koblan, K.S.; et al. Ulotaront: A TAAR1 Agonist for the Treatment of Schizophrenia. ACS Med. Chem. Lett. 2021, 13, 92–98. [Google Scholar] [CrossRef]
- Hopkins, S.C.; Ogirala, A.; Worden, M.; Koblan, K.S. Depicting Safety Profile of TAAR1 Agonist Ulotaront Relative to Reactions Anticipated for a Dopamine D2-Based Pharmacological Class in FAERS. Clin. Drug Investig. 2021, 41, 1067–1073. [Google Scholar] [CrossRef]
- Xu, Z.; Guo, L.; Yu, J.; Shen, S.; Wu, C.; Zhang, W.; Zhao, C.; Deng, Y.; Tian, X.; Feng, Y.; et al. Ligand Recognition and G-Protein Coupling of Trace Amine Receptor TAAR1. Nature 2023, 624, 672–681. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zheng, Y.; Wang, Y.; Wang, Y.; He, X.; Xu, P.; Huang, S.; Yuan, Q.; Zhang, X.; Wang, L.; et al. Recognition of Methamphetamine and Other Amines by Trace Amine Receptor TAAR1. Nature 2023, 624, 663–671. [Google Scholar] [CrossRef] [PubMed]
- Raab, S.; Wang, H.; Uhles, S.; Cole, N.; Alvarez-Sanchez, R.; Künnecke, B.; Ullmer, C.; Matile, H.; Bedoucha, M.; Norcross, R.D.; et al. Incretin-like Effects of Small Molecule Trace Amine-Associated Receptor 1 Agonists. Mol. Metab. 2016, 5, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Dedic, N.; Wang, L.; Hajos-Korcsok, E.; Hecksher-Sørensen, J.; Roostalu, U.; Vickers, S.P.; Wu, S.; Anacker, C.; Synan, C.; Jones, P.G.; et al. TAAR1 Agonists Improve Glycemic Control, Reduce Body Weight and Modulate Neurocircuits Governing Energy Balance and Feeding. Mol. Metab. 2024, 80, 101883. [Google Scholar] [CrossRef] [PubMed]
- Ito, J.; Ito, M.; Nambu, H.; Fujikawa, T.; Tanaka, K.; Iwaasa, H.; Tokita, S. Anatomical and Histological Profiling of Orphan G-Protein-Coupled Receptor Expression in Gastrointestinal Tract of C57BL/6J Mice. Cell Tissue Res. 2009, 338, 257–269. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, D.M.N.; Oliveira-Silva, C.A.; Pinheiro, C.G.; de Carvalho, E.F.; Gadelha, K.K.L.; Lima-Silva, K.; Cavalcante, A.K.M.; de Oliveira Belém, M.; Paula, S.M.; dos Santos, A.A.; et al. Differential Effects of β-Methylphenylethylamine and Octopamine on Contractile Parameters of the Rat Gastrointestinal Tract. Eur. J. Pharmacol. 2021, 908, 174339. [Google Scholar] [CrossRef] [PubMed]
- Zhai, L.; Huang, C.; Ning, Z.; Zhang, Y.; Zhuang, M.; Yang, W.; Wang, X.; Wang, J.; Zhang, L.; Xiao, H.; et al. Ruminococcus Gnavus Plays a Pathogenic Role in Diarrhea-Predominant Irritable Bowel Syndrome by Increasing Serotonin Biosynthesis. Cell Host Microbe 2023, 31, 33–44.e5. [Google Scholar] [CrossRef] [PubMed]
- Bharucha, A.E.; Kudva, Y.C.; Prichard, D.O. Diabetic Gastroparesis. Endocr. Rev. 2019, 40, 1318–1352. [Google Scholar] [CrossRef] [PubMed]
- Concepción Zavaleta, M.J.; Gonzáles Yovera, J.G.; Moreno Marreros, D.M.; Rafael Robles, L.d.P.; Palomino Taype, K.R.; Soto Gálvez, K.N.; Arriola Torres, L.F.; Coronado Arroyo, J.C.; Concepción Urteaga, L.A. Diabetic Gastroenteropathy: An Underdiagnosed Complication. World J. Diabetes 2021, 12, 794–809. [Google Scholar] [CrossRef]
- Kornum, D.S.; Terkelsen, A.J.; Bertoli, D.; Klinge, M.W.; Høyer, K.L.; Kufaishi, H.H.A.; Borghammer, P.; Drewes, A.M.; Brock, C.; Krogh, K. Assessment of Gastrointestinal Autonomic Dysfunction: Present and Future Perspectives. J. Clin. Med. 2021, 10, 1392. [Google Scholar] [CrossRef]
- Tack, J.; Masuy, I.; Van Den Houte, K.; Wauters, L.; Schol, J.; Vanuytsel, T.; Vandenberghe, A.; Carbone, F. Drugs under Development for the Treatment of Functional Dyspepsia and Related Disorders. Expert Opin. Investig. Drugs 2019, 28, 871–889. [Google Scholar] [CrossRef] [PubMed]
- Talley, N.J. Functional Dyspepsia: New Insights into Pathogenesis and Therapy. Korean J. Intern. Med. 2016, 31, 444–456. [Google Scholar] [CrossRef] [PubMed]
- Aro, P.; Talley, N.J.; Johansson, S.-E.; Agréus, L.; Ronkainen, J. Anxiety Is Linked to New-Onset Dyspepsia in the Swedish Population: A 10-Year Follow-up Study. Gastroenterology 2015, 148, 928–937. [Google Scholar] [CrossRef] [PubMed]
- Halff, E.F.; Rutigliano, G.; Garcia-Hidalgo, A.; Howes, O.D. Trace Amine-Associated Receptor 1 (TAAR1) Agonism as a New Treatment Strategy for Schizophrenia and Related Disorders. Trends Neurosci. 2023, 46, 60–74. [Google Scholar] [CrossRef] [PubMed]
- Barrett, T.; Wilhite, S.E.; Ledoux, P.; Evangelista, C.; Kim, I.F.; Tomashevsky, M.; Marshall, K.A.; Phillippy, K.H.; Sherman, P.M.; Holko, M.; et al. NCBI GEO: Archive for Functional Genomics Data Sets-Update. Nucleic Acids Res. 2013, 41, D991–D995. [Google Scholar] [CrossRef] [PubMed]
- Moreno, P.; Fexova, S.; George, N.; Manning, J.R.; Miao, Z.; Mohammed, S.; Muñoz-Pomer, A.; Fullgrabe, A.; Bi, Y.; Bush, N.; et al. Expression Atlas update: Gene and protein expression in multiple species. Nucleic Acids Res. 2022, 50, D129–D140. [Google Scholar] [CrossRef] [PubMed]
- Robinson, M.D.; McCarthy, D.J.; Smyth, G.K. edgeR: A Bioconductor Package for Differential Expression Analysis of Digital Gene Expression Data. Bioinformatics 2010, 26, 139–140. [Google Scholar] [CrossRef] [PubMed]
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016; ISBN 978-3-319-24277-4. [Google Scholar]
- Wu, T.; Hu, E.; Xu, S.; Chen, M.; Guo, P.; Dai, Z.; Feng, T.; Zhou, L.; Tang, W.; Zhan, L.; et al. clusterProfiler 4.0: A Universal Enrichment Tool for Interpreting Omics Data. Innov. Camb. Mass 2021, 2, 100141. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.Z.; Du, Z.; Payattakool, R.; Yu, P.S.; Chen, C.-F. A New Method to Measure the Semantic Similarity of GO Terms. Bioinformatics 2007, 23, 1274–1281. [Google Scholar] [CrossRef]
- Yu, G.; Li, F.; Qin, Y.; Bo, X.; Wu, Y.; Wang, S. GOSemSim: An R Package for Measuring Semantic Similarity among GO Terms and Gene Products. Bioinformatics 2010, 26, 976–978. [Google Scholar] [CrossRef]
- Gene Ontology Consortium; Aleksander, S.A.; Balhoff, J.; Carbon, S.; Cherry, J.M.; Drabkin, H.J.; Ebert, D.; Feuermann, M.; Gaudet, P.; Harris, N.L.; et al. The Gene Ontology Knowledgebase in 2023. Genetics 2023, 224, iyad031. [Google Scholar] [CrossRef] [PubMed]
- Ashburner, M.; Ball, C.A.; Blake, J.A.; Botstein, D.; Butler, H.; Cherry, J.M.; Davis, A.P.; Dolinski, K.; Dwight, S.S.; Eppig, J.T.; et al. Gene Ontology: Tool for the Unification of Biology. The Gene Ontology Consortium. Nat. Genet. 2000, 25, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, S.P.; O’Brien, D.R.; Sharma, M.; Smyrk, T.C.; Graham, R.P.; Grover, M.; Bharucha, A.E. Duodenal Mucosal Barrier in Functional Dyspepsia. Clin. Gastroenterol. Hepatol. 2022, 20, 1019–1028.e3. [Google Scholar] [CrossRef] [PubMed]
- Puthanmadhom Narayanan, S.; O’Brien, D.; Sharma, M.; Miller, K.; Adams, P.; Passos, J.F.; Eirin, A.; Ordog, T.; Bharucha, A.E. Duodenal Mucosal Mitochondrial Gene Expression Is Associated with Delayed Gastric Emptying in Diabetic Gastroenteropathy. JCI Insight 2024, 6, e143596. [Google Scholar] [CrossRef]
- Sikiric, P.; Boban Blagaic, A.; Krezic, I.; Zizek, H.; Kalogjera, L.; Smoday, I.M.; Vukovic, V.; Oroz, K.; Chiddenton, H.M.; Buric, S.; et al. From Selye’s and Szabo’s Cysteamine-Duodenal Ulcer in Rats to Dopamine in the Stomach: Therapy Significance and Possibilities. Pharmaceuticals 2023, 16, 1699. [Google Scholar] [CrossRef]
- Luesma, M.J.; Cantarero, I.; Castiella, T.; Soriano, M.; Garcia–Verdugo, J.M.; Junquera, C. Enteric Neurons Show a Primary Cilium. J. Cell. Mol. Med. 2013, 17, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, V.T.T.; Brücker, L.; Volz, A.-K.; Baumgärtner, J.C.; dos Santos Guilherme, M.; Valeri, F.; May-Simera, H.; Endres, K. Primary Cilia Structure Is Prolonged in Enteric Neurons of 5xFAD Alzheimer’s Disease Model Mice. Int. J. Mol. Sci. 2021, 22, 13564. [Google Scholar] [CrossRef]
- Adriaenssens, A.; Lam, B.Y.H.; Billing, L.; Skeffington, K.; Sewing, S.; Reimann, F.; Gribble, F. A Transcriptome-Led Exploration of Molecular Mechanisms Regulating Somatostatin-Producing D-Cells in the Gastric Epithelium. Endocrinology 2015, 156, 3924–3936. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Z.; Ma, H.; Mou, Y.; Xu, C.F. Association between Polymorphism of Dopamine D2 Receptor Genes and Therapeutic Effect of Domperidone in Functional Dyspepsia. Turk. J. Gastroenterol. 2015, 26, 1–5. [Google Scholar] [CrossRef]
- Auteri, M.; Zizzo, M.G.; Serio, R. GABA and GABA Receptors in the Gastrointestinal Tract: From Motility to Inflammation. Pharmacol. Res. 2015, 93, 11–21. [Google Scholar] [CrossRef]
- Lee, K.J.; Tack, J. Duodenal Implications in the Pathophysiology of Functional Dyspepsia. J. Neurogastroenterol. Motil. 2010, 16, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Mamenko, M.; Drokh, H. Influence of pharmacological correction on the quality of life of children with functional dyspepsia. EUREKA Health Sci. 2020, 6, 46–51. [Google Scholar] [CrossRef]
- Hojo, M.; Miwa, H.; Yokoyama, T.; Ohkusa, T.; Nagahara, A.; Kawabe, M.; Asaoka, D.; Izumi, Y.; Sato, N. Treatment of Functional Dyspepsia with Antianxiety or Antidepressive Agents: Systematic Review. J. Gastroenterol. 2005, 40, 1036–1042. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Jiang, X.; Perwaiz, I.; Yu, P.; Wang, J.; Wang, Y.; Hüttemann, M.; Felder, R.A.; Sibley, D.R.; Polster, B.M.; et al. Dopamine D5 Receptor-Mediated Decreases in Mitochondrial Reactive Oxygen Species Production Are cAMP and Autophagy Dependent. Hypertens. Res. 2021, 44, 628–641. [Google Scholar] [CrossRef] [PubMed]
- Amatya, B.; Yang, S.; Yu, P.; Vaz de Castro, P.A.S.; Armando, I.; Zeng, C.; Felder, R.A.; Asico, L.D.; Jose, P.A.; Lee, H. Peroxiredoxin-4 and Dopamine D5 Receptor Interact to Reduce Oxidative Stress and Inflammation in the Kidney. Antioxid. Redox Signal. 2023, 38, 1150–1166. [Google Scholar] [CrossRef] [PubMed]
- Sumithra, B.; Saxena, U.; Das, A.B. A Comprehensive Study on Genome-Wide Coexpression Network of KHDRBS1/Sam68 Reveals Its Cancer and Patient-Specific Association. Sci. Rep. 2019, 9, 11083. [Google Scholar] [CrossRef]
- Chen, Y.; Li, H.; Li, Y.-Y.; Li, Y. Pan-Cancer Analysis of Head-to-Head Gene Pairs in Terms of Transcriptional Activity, Co-Expression and Regulation. Front. Genet. 2021, 11, 560997. [Google Scholar] [CrossRef]
- Marcon, E.; Ni, Z.; Pu, S.; Turinsky, A.L.; Trimble, S.S.; Olsen, J.B.; Silverman-Gavrila, R.; Silverman-Gavrila, L.; Phanse, S.; Guo, H.; et al. Human-Chromatin-Related Protein Interactions Identify a Demethylase Complex Required for Chromosome Segregation. Cell Rep. 2014, 8, 297–310. [Google Scholar] [CrossRef]

| Dataset ID | Title | n | Samples Characteristics |
|---|---|---|---|
| GSE94919 | Chemoprevention with COX2 and EGFR inhibition in FAP patients: mRNA signatures of duodenal neoplasia | 17 1 | Uninvolved duodenal tissue from familial adenomatous polyposis patients |
| GSE151495 | Altered duodenal mucosal mitochondrial gene expression is associated with delayed gastric emptying in diabetic gastroenteropathy [mRNA-Seq] | 21 | Duodenal mucosa from healthy subjects |
| 39 | Duodenal mucosa from diabetes mellitus patients | ||
| GSE169304 | Duodenal mucosal barrier in functional dyspepsia [mRNA] | 18 | Duodenal mucosa from healthy subjects |
| 37 | Duodenal mucosa from patients with functional dyspepsia | ||
| GSE189035 | PIGA mutation and glycosylphosphatidylinositol anchor dysregulation in polyposis-associated duodenal tumorigenesis | 10 1 | Uninvolved duodenal tissue from familial adenomatous polyposis patients |
| GSE189820 | Gene expression in duodenal biopsy samples from CVID enteropathy patients and healthy controls | 6 1 | Duodenal mucosa from healthy subjects |
| GSE207243 | Duodenal inflammation in common variable immunodeficiency has altered transcriptional response to viruses | 5 1 | Duodenal mucosa from healthy subjects |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vaganova, A.N.; Markina, A.A.; Belousov, A.M.; Lenskaia, K.V.; Gainetdinov, R.R. Dopamine Receptors and TAAR1 Functional Interaction Patterns in the Duodenum Are Impaired in Gastrointestinal Disorders. Biomedicines 2024, 12, 1590. https://doi.org/10.3390/biomedicines12071590
Vaganova AN, Markina AA, Belousov AM, Lenskaia KV, Gainetdinov RR. Dopamine Receptors and TAAR1 Functional Interaction Patterns in the Duodenum Are Impaired in Gastrointestinal Disorders. Biomedicines. 2024; 12(7):1590. https://doi.org/10.3390/biomedicines12071590
Chicago/Turabian StyleVaganova, Anastasia N., Alisa A. Markina, Aleksandr M. Belousov, Karina V. Lenskaia, and Raul R. Gainetdinov. 2024. "Dopamine Receptors and TAAR1 Functional Interaction Patterns in the Duodenum Are Impaired in Gastrointestinal Disorders" Biomedicines 12, no. 7: 1590. https://doi.org/10.3390/biomedicines12071590






