Evaluation of Patellar Tendon Structural Changes following Biological Treatments: Secondary Analysis of Double-Blinded Clinical Trial of Bone Marrow Mesenchymal Stromal Cells and Leukocyte-Poor Platelet-Rich Plasma
Abstract
:1. Introduction
2. Materials and Methods
2.1. Design and Participants
2.2. Procedure
2.3. Statistical Analysis
3. Results
3.1. Evaluation of Echo-Types between BM-MSC and Lp-PRP
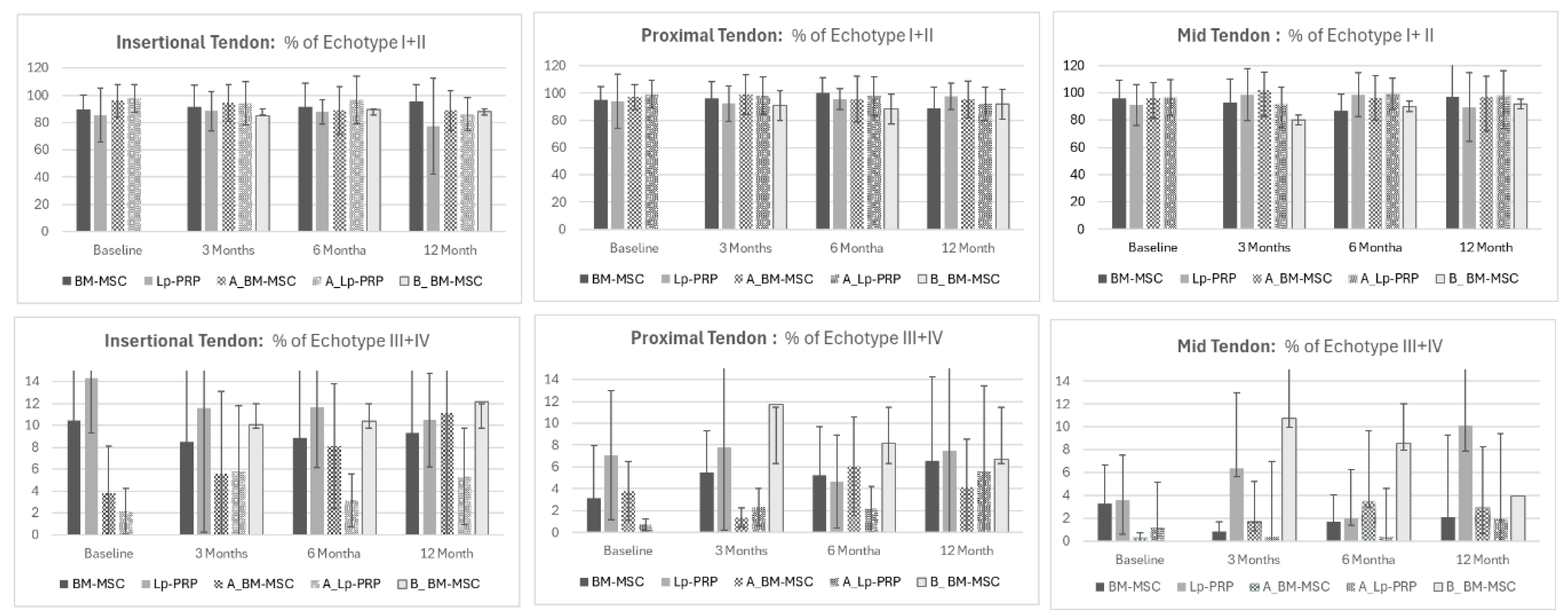
- Comparison between the differences in echo-types between BM-MSC and Lp-PRP showed higher disorganization in the Lp-PRP group after 3 months in echo-types II and III in the mid-tendon (echo-type II: BM-MSC 9.38 ± 14.81; MDC = 12.98; PRP −2.98 ± 8.4; MDC = 0.05; p = 0.04; ES = 1.06) (echo-type III: BM-MSC −0.65 ± 4.38; MDC = 4.85; PRP 9.61 ± 8.84; MDC = 0.29; p = 0.02; ES = −1.47).
- Similar results were seen after six months, with Lp-PRP still showing a higher disorganized tendon structure at echo-type III compared to BM-MSC (BM-MSC = −0.19 ± 2.01; MDC = 2.23; Lp-PRP 4.65 ± 5.17; MDC = 0.17; p = 0.03; ES = −1.4).
- Our results demonstrate that BM-MSC shows greater improvement in tendon organization than Lp-PRP at 3 and 6 months.
3.2. Evaluation of Echo-Types between BM-MSC and B-BM-MSC
- Comparisons of patellar tendon structure between BM-MSC as the first treatment and B-BM-MSC as the second biological treatment showed that BM-MSC presented more organized tendon structures at the proximal tendon for echo-type I after 6 months compared to B-BM-MSC (BM-MSC = 1.13 ± 12.6; MDC = 6.05; B-BM-MSC = −8.83 ± 20.46; MDC = 9.82; p = 0.04; ES = 0.58).
- Interestingly, long-term results showed more organized tendon fibers in the B-BM-MSC group compared to the first BM-MSC treatment for echo-types III and IV at the insertional tendon (echo-type III: BM-MSC = −1.67 ± 2.85; MDC = 2.62; B-BM-MSC 2.15 ± 6.18; MDC = 5.68; p = 0.04; ES = −0.79) (echo-type IV: BM-MSC = −0.89 ± 1.75; MDC = 2.02; B-BM-MSC 1.22 ± 3.32; MDC = 3.91; p = 0.04; ES = −0.79).
3.3. Evaluation of Clinical Outcomes between BM-MSC and Lp-PRP
- Clinical outcomes after 3 months of treatment showed A-BM-MSC and A-Lp-PRP to be significantly stronger compared to the affected tendon (BM-MSC; p = 0.01; ES = 3.92 and Lp-PRP; p = 0.02; ES = 4.36). At 6 months, A-Lp-PRP remained significantly stronger than Lp-PRP. Data for clinical outcomes show improvement in strength levels of the treated tendon and decreased pain levels. See Table 1.
3.4. Evaluation of Echo-Type between Symptomatic and Asymptomatic
- Patellar tendon structural changes between symptomatic and asymptomatic tendons showed minor significant differences. Only the mid-tendon showed significant differences for echo-type I in the BM-MSC group after 3 months. BM-MSC showed a higher amount of organized tendon fibers (6.92% ± 22.77) compared to A-BM-MSC (−2.35 ± 7.93; p = 0.02; ES = 0.1). Additionally, the MDC was 10.93% for BM-MSC and 3.8% for A-BM-MSC, with a large effect size of 0.1.
- The results confirm the positive effect of the treated tendon with BM-MSC compared to the untreated tendon. Conversely, Lp-PRP treatment did not show enough tendon structural change as the comparison between Lp-PRP and the asymptomatic Lp-PRP contralateral tendon showed no significant differences.
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| BM-MSC | A-BM-MSC | Lp-PRP | A-Lp-PRP | BM-MSC-Lp-PRP | B-BM-MSC | BM-MSC-B-BM-MSC | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean (SD) | 95% CI (sup tp Inf) | MDC | Mean (SD) | 95% CI (sup tp Inf) | MDC | p-Value | Cohens d | Mean (SD) | 95% CI (sup tp Inf) | MDC | Mean (SD) | 95% CI (sup tp Inf) | MDC | p-Value | Cohens d | p-Value | Cohens d | Mean (SD) | 95% CI (sup tp Inf) | MDC | p-Value | Cohens d | |||
| 3 months | Insertion | Type I | −12.26 (26.99) | (7.05 to −31.57) | 12.96 | −0.82 (10.45) | (6.65 to −8.29) | 5.01 | 0.66 | −0.26 | −0.25 (13.82) | (12.45 to −12.93) | −0.26 | 0 (13.19) | (12.45 to −12.93) | −0.26 | 0.96 | −0.01 | 0.23 | −0.62 | −15.06 (25.7) | −5.09 to −25.02) | 12.33 | 0.85 | 0.1 |
| Type II | −4.16 (21.14) | (10.96 to −19.28) | 18.53 | −0.9 (8.71) | (5.32 to −7.14) | 7.63 | 0.3 | −0.15 | −3.41 (10.31) | (4.56 to −17) | −0.15 | 2.81 (12.52) | (4.59 to −17.03) | −0.15 | 0.24 | −0.12 | 0.92 | −0.04 | −5.29 (17.57) | 1.51 to −12.11) | 15.4 | 0.92 | 0.05 | ||
| Type III | −1.98 (9.58) | (4.87 to −8.83) | 10.62 | 1.53 (3.83) | (4.27 to −1.21) | 4.25 | 0.18 | 0.09 | 2.52 (5.1) | (11.33 to −0.91) | 0.09 | −2.69 (7.66) | (11.39 to −0.97) | 0.09 | 0.09 | 0.13 | 0.21 | −0.64 | −0.08 (11.06) | 4.2 to −4.37) | 12.26 | 0.65 | −0.18 | ||
| Type IV | −1.67 (4.04) | (1.22 to −4.56) | 3.54 | 0.19 (0.64) | (0.64 to −0.26) | 0.56 | 0.23 | −0.05 | 1.13 (2.13) | (4.82 to −2.44) | −0.05 | −0.05 (5.03) | (4.95 to −2.57) | −0.05 | 0.5 | 0.23 | 0.07 | −0.94 | −0.53 (5.71) | 1.68 to −2.74) | 5 | 0.54 | −0.23 | ||
| Proximal Tendon | Type I | 1.43 (17.96) | (14.28 to −11.42) | 8.62 | −7.16 (12.84) | (2.02 to −16.34) | 6.16 | 0.87 | −0.23 | −12.91 (25.89) | (13.08 to −32.19) | −0.23 | −3.35 (20.12) | (12.81 to −31.92) | −0.23 | 0.37 | −0.4 | 0.16 | 0.91 | −6.52 (21.53) | 1.82 to −14.87) | 10.33 | 0.33 | 0.4 | |
| Type II | 0.76 (11.44) | (8.94 to −7.42) | 10.02 | −0.12 (12.59) | (8.89 to −9.13) | 11.04 | 0.27 | 0.02 | −7.45 (21.69) | (2 to −30.88) | 0.02 | 6.98 (9.05) | (1.84 to −30.72) | 0.02 | 0.07 | −0.15 | 0.3 | 0.85 | 0.68 (11.88) | 5.29 to −3.91) | 10.41 | 0.98 | 0 | ||
| Type III | −0.3 (9.85) | (6.74 to −7.34) | 10.92 | 4.18 (7.85) | (9.79 to −1.43) | 8.7 | 0.14 | 0.25 | 0.19 (2.69) | (13.19 to −7.34) | 0.25 | −2.73 (15.17) | (14.64 to −8.79) | 0.25 | 0.58 | −0.11 | 0.88 | −0.07 | 0.05 (10.57) | 4.15 to −4.04) | 11.72 | 0.82 | −0.03 | ||
| Type IV | −1.3 (4.64) | (2.02 to −4.62) | 4.07 | 3.09 (7.62) | (8.54 to −2.36) | 6.68 | 0.08 | 0.06 | 0.19 (1.5) | (6.89 to −4.07) | 0.06 | −1.22 (8.09) | (7.66 to −4.84) | 0.06 | 0.61 | −0.07 | 0.35 | −0.45 | −1.06 (4.85) | 0.81 to −2.94) | 4.25 | 0.94 | −0.05 | ||
| Mid−tendon | Type I | 6.92 (22.77) | (23.21 to −9.37) | 10.93 | −2.35 (7.93) | (3.32 to −8.02) | 3.8 | 0.02 * | 0.1 | −8.88 (9.43) | (15.02 to −20.66) | 0.1 | −6.06 (15.35) | (29.77 to −35.41) | 0.1 | 0.78 | −0.69 | 0.07 | 0.85 | −0.67 (21.86) | 7.8 to −9.15) | 10.49 | 0.39 | 0.33 | |
| Type II | 9.38 (14.81) | (19.97 to −1.21) | 12.98 | −4.12 (8.02) | (1.61 to −9.85) | 7.03 | 0.05 | 0.05 | −2.98 (8.4) | (9.2 to −33.7) | 0.05 | 9.26 (22.7) | (40.22 to −64.72) | 0.05 | 0.45 | 0.08 | 0.04 * | 1.06 | 6.05 (13.21) | 11.17 to 0.92) | 11.58 | 0.41 | 0.23 | ||
| Type III | −0.65 (4.38) | (2.48 to −3.78) | 4.85 | 4.64 (6.81) | (9.51 to −0.23) | 7.56 | 0.46 | 0.26 | 9.61 (8.84) | (26.95 to −1.05) | 0.26 | −3.33 (8.65) | (29.74 to −3.85) | 0.26 | 0.09 | 0.6 | 0.0 2 * | −1.47 | 0.25 (9.87) | 4.07 to −3.57) | 10.94 | 0.94 | −0.12 | ||
| Type IV | 1.02 (5.94) | (5.27 to −3.23) | 5.2 | 2.67 (3.54) | (5.2 to 0.13) | 3.1 | 0.23 | 0.46 | 3.71 (3.73) | (13.07 to −1.77) | 0.46 | −1.93 (6.72) | (20.22 to −8.92) | 0.46 | 0.27 | 0.38 | 0.26 | −0.52 | 0.45 (5.67) | 2.65 to −1.74) | 4.97 | 0.63 | 0.09 | ||
| 6 months * | Insertion | Type I | −11.42 (28.18) | (8.74 to −31.58) | 13.53 | 2.99 (8.95) | (9.39 to −3.41) | 4.29 | 0.4 | −0.03 | −0.19 (9.71) | (16.53 to −6.47) | −0.03 | −5.23 (14.33) | (16.65 to −6.59) | −0.03 | 0.37 | −0.16 | 0.25 | −0.56 | −17.08 (28.55) | −6.01 to −28.15) | 13.7 | 0.46 | 0.19 |
| Type II | −6.55 (17.06) | (5.65 to −18.75) | 14.95 | −1.29 (9.21) | (5.29 to −7.87) | 8.07 | 0.93 | −0.26 | −0.99 (9.3) | (6.62 to −15.78) | −0.26 | 3.59 (14.05) | (6.74 to −15.9) | −0.26 | 0.4 | 0.04 | 0.38 | −0.46 | −6.15 (16.33) | 0.18 to −12.48) | 14.32 | 0.59 | −0.02 | ||
| Type III | −1.18 (4.71) | (2.18 to −4.54) | 5.22 | −1.05 (2.24) | (0.55 to −2.65) | 2.48 | 0.9 | −0.35 | 0.71 (1.36) | (3.62 to −3.02) | −0.35 | 0.41 (4.81) | (3.8 to −3.2) | −0.35 | 0.85 | 0.24 | 0.24 | −0.56 | 1.33 (6.48) | 3.85 to −1.18) | 7.19 | 0.21 | −0.44 | ||
| Type IV | −0.66 (2.56) | (1.17 to −2.49) | 2.24 | −0.77 (1.46) | (0.28 to −1.82) | 1.28 | 0.25 | −0.39 | 0.48 (0.97) | (1.63 to −3.19) | −0.39 | 1.26 (3.5) | (1.77 to −3.33) | −0.39 | 0.51 | 0.4 | 0.21 | −0.61 | 0.61 (2.78) | 1.69 to −0.46) | 2.44 | 0.09 | −0.47 | ||
| Proximal Tendon | Type I | 1.13 (12.6) | (10.14 to −7.88) | 6.05 | −5.02 (10.73) | (2.65 to −12.69) | 5.15 | 0.46 | −0.18 | 1.07 (8.73) | (19.26 to −9.95) | −0.18 | −3.57 (17.5) | (19.03 to −9.72) | −0.18 | 0.49 | −0.04 | 0.99 | 0 | −8.83 (20.46) | −0.9 to −16.77) | 9.82 | 0.04 * | 0.58 | |
| Type II | −0.37 (8.5) | (5.71 to −6.46) | 7.45 | 3.21 (12.69) | (12.29 to −5.87) | 11.12 | 0.56 | 0.1 | −2.78 (9.11) | (2.02 to −19.13) | 0.1 | 5.76 (11.08) | (1.9 to −19) | 0.1 | 0.1 | 0 | 0.57 | 0.36 | 1.02 (13.12) | 6.11 to −4.06) | 11.5 | 0.4 | −0.12 | ||
| Type III | −0.2 (3.75) | (2.48 to −2.88) | 4.16 | 0.69 (3.04) | (2.86 to −1.48) | 3.37 | 0.37 | 0.08 | 1.11 (1.91) | (9.16 to −3.18) | 0.08 | −1.87 (7.96) | (9.18 to −3.2) | 0.08 | 0.3 | 0.02 | 0.35 | −0.45 | 0.73 (5.34) | 2.8 to −1.33) | 5.92 | 0.47 | −0.2 | ||
| Type IV | −0.19 (1.66) | (1 to −1.38) | 1.45 | 1.11 (4.13) | (4.06 to −1.84) | 3.62 | 0.13 | 0.07 | 0.63 (1.01) | (4.24 to −2.39) | 0.07 | −0.28 (4.28) | (4.25 to −2.4) | 0.07 | 0.54 | 0.15 | 0.21 | −0.62 | 0.18 (2.28) | 1.07 to −0.7) | 2 | 0.47 | −0.18 | ||
| Mid−tendon | Type I | 6.54 (17.94) | (19.37 to −6.29) | 8.61 | −5.84 (11.39) | (2.31 to −13.99) | 5.47 | 0.14 | −0.07 | −17.63 (19.94) | (25.65 to −44.42) | −0.07 | −8.25 (13.08) | (33.66 to −52.43) | −0.07 | 0.49 | −0.82 | 0.01 * | 1.35 | −2.11 (17.19) | 4.55 to −8.78) | 8.25 | 0.05 | 0.49 | |
| Type II | 11.26 (17.95) | (24.1 to −1.58) | 15.74 | 1.15 (9.8) | (8.16 to −5.86) | 8.59 | 0.19 | 0.37 | −1.08 (13.01) | (12.23 to −31.54) | 0.37 | 8.56 (18.07) | (27.95 to −47.26) | 0.37 | 0.46 | 0.14 | 0.11 | 0.91 | 6.66 (14.4) | 12.25 to 1.08) | 12.62 | 0.48 | 0.28 | ||
| Type III | −0.19 (2.01) | (1.24 to −1.62) | 2.23 | 1.4 (3.16) | (3.66 to −0.86) | 3.5 | 0.84 | 0.17 | 4.65 (5.17) | (19.37 to −3.85) | 0.17 | −3.11 (12.84) | (37.37 to −21.85) | 0.17 | 0.4 | 0.26 | 0.03 * | −1.41 | 1.18 (4.66) | 2.99 to −0.62) | 5.17 | 0.31 | −0.38 | ||
| Type IV | 0.47 (2.86) | (2.51 to −1.57) | 2.51 | 0.26 (1.73) | (1.5 to −0.98) | 1.52 | 0.15 | 0.15 | 1.57 (2.01) | (2.89 to −3.28) | 0.15 | 1.76 (2.04) | (3.8 to −4.19) | 0.15 | 0.89 | 0.81 | 0.35 | −0.46 | 0.8 (2.34) | 1.71 to −0.11) | 2.05 | 0.67 | −0.12 | ||
| 12 months | Insertion | Type I | 11.31 (9.94) | (3.55 to −39.29) | 8.75 | 2.18 (8.54) | (8.28 to −3.92) | 8.2 | 0.89 | −0.17 | 3.29 (9.21) | (12.16 to −10.18) | −0.17 | 2.3 (14.07) | (12.29 to −10.31) | −0.17 | 0.85 | 0.27 | 0.04 * | −0.99 | −21.77 (30.56) | −9.92 to −33.63) | 29.34 | 0.81 | 0.12 |
| Type II | −4.28 (16.76) | (7.71 to −16.27) | 20.78 | −3.5 (7.61) | (1.94 to −8.94) | 9.44 | 0.06 | −0.35 | −2.44 (10.39) | (11.53 to −6.25) | −0.35 | −5.08 (8.43) | (11.55 to −6.27) | −0.35 | 0.54 | −0.34 | 0.77 | −0.15 | −4.43 (16.35) | 1.9 to −10.77) | 20.27 | 0.93 | 0 | ||
| Type III | −1.67 (2.85) | (0.36 to −3.7) | 2.62 | 0.85 (2.95) | (2.96 to −1.26) | 2.71 | 0.06 | −0.14 | −0.67 (2.56) | (2.65 to −6.27) | −0.14 | 1.14 (6.21) | (2.82 to −6.44) | −0.14 | 0.41 | −0.01 | 0.42 | −0.48 | 2.15 (6.18) | 4.55 to −0.24) | 5.68 | 0.04 * | −0.79 | ||
| Type IV | −0.89 (1.72) | (0.34 to −2.12) | 2.02 | 0.5 (1.45) | (1.54 to −0.54) | 1.71 | 0.62 | −0.08 | −0.19 (0.97) | (1.22 to −4.88) | −0.08 | 1.64 (4.5) | (1.42 to −5.08) | −0.08 | 0.23 | 0.19 | 0.28 | −0.57 | 1.22 (3.32) | 2.51 to −0.06) | 3.91 | 0.04 * | −0.79 | ||
| Proximal Tendon | Type I | −0.17 (15.16) | (10.66 to −11.02) | 14.55 | −3.17 (11.02) | (4.71 to −11.05) | 10.58 | 0.83 | −0.14 | 2.8 (6.79) | (17.25 to −10.25) | −0.14 | −0.7 (17.03) | (17.11 to −10.11) | −0.14 | 0.58 | 0.15 | 0.58 | −0.25 | −12.61 (23.96) | −3.31 to −21.9) | 23.01 | 0.05 | 0.61 | |
| Type II | 0.93 (12.27) | (9.71 to −7.85) | 15.22 | −0.13 (10.47) | (7.36 to −7.62) | 12.98 | 0.13 | 0.03 | −1.82 (5.88) | (4.93 to −14.8) | 0.03 * | 3.11 (11.82) | (4.77 to −14.64) | 0.03 | 0.28 | −0.02 | 0.54 | 0.29 | 1.7 (13.07) | 6.77 to −3.37) | 16.2 | 0.69 | −0.06 | ||
| Type III | −0.99 (3.47) | (1.49 to −3.47) | 3.19 | 1.45 (3.58) | (4.01 to −1.11) | 3.29 | 0.17 | 0.05 | −0.73 (2.97) | (5.01 to −3.77) | 0.05 | −1.35 (5.09) | (4.93 to −3.69) | 0.05 | 0.76 | −0.25 | 0.87 | −0.09 | 2.53 (8.39) | 5.79 to −0.71) | 7.71 | 0.13 | −0.54 | ||
| Type IV | −0.58 (1.83) | (0.72 to −1.88) | 2.15 | 1.83 (4.96) | (5.37 to −1.71) | 5.83 | 0.16 | 0.02 | −0.25 (1.05) | (2.52 to −2.49) | 0.02 * | −0.26 (3.16) | (2.51 to −2.48) | 0.02 | 0.98 | −0.14 | 0.63 | −0.23 | 1.09 (3.64) | 2.5 to −0.31) | 4.28 | 0.11 | −0.57 | ||
| Mid−tendon | Type I | 7.53 (26.82) | (26.71 to −11.65) | 25.75 | −6.73 (8.1) | (−0.92 to −12.53) | 7.78 | 0.33 | −0.27 | −0.9 (7.47) | (21.47 to −4.77) | −0.27 | −9.25 (0.63) | (15.27 to 1.42) | −0.27 | 0.02 | −0.7 | 0.36 | 0.4 | −0.93 (19.68) | 6.69 to −8.56) | 18.9 | 0.16 | 0.35 | |
| Type II | 8.12 (16.8) | (20.13 to −3.89) | 20.82 | 1.88 (10.51) | (9.4 to −5.64) | 13.03 | 0.14 | 0.33 | 0.48 (6.65) | (23.34 to −12.17) | 0.33 | −5.1 (18.66) | (150.97 to −139.8) | 0.33 | 0.74 | −0.12 | 0.21 | 0.58 | 3.84 (14.03) | 9.28 to −1.59) | 17.4 | 0.43 | 0.27 | ||
| Type III | −0.44 (0.84) | (0.16 to −1.04) | 0.78 | 1.65 (4.08) | (4.57 to −1.27) | 3.75 | 0.55 | −0.05 | 0.27 (4.07) | (2.81 to −17.47) | −0.05 | 7.6 (10.04) | (67.92 to −82.58) | −0.05 | 0.48 | 0.44 | 0.66 | −1.04 | 1.52 (4.78) | 3.37 to −0.32) | 4.39 | 0.11 | −0.57 | ||
| Type IV | 0.45 (1.08) | (1.22 to −0.32) | 1.27 | 1 (2.66) | (2.9 to −0.9) | 3.13 | 0.06 | 0.39 | 0.1 (1.41) | (0.43 to −13.73) | 0.39 | 6.75 (9.26) | (74.3 to −87.6) | 0.39 | 0.49 | 0.57 | 0.59 | 0.41 | 1.32 (3.48) | 2.67 to −0.02) | 4.09 | 0.47 | −0.33 | ||
References
- Maffulli, N.; Khan, K.M.; Puddu, G. Overuse Tendon Conditions: Time to Change a Confusing Terminology. Arthroscopy 1998, 14, 840–843. [Google Scholar] [CrossRef] [PubMed]
- Tsehaie, J.; Poot, D.H.J.; Oei, E.H.G.; Verhaar, J.A.N.; de Vos, R.J. Value of Quantitative MRI Parameters in Predicting and Evaluating Clinical Outcome in Conservatively Treated Patients with Chronic Midportion Achilles Tendinopathy: A Prospective Study. J. Sci. Med. Sport 2017, 20, 633–637. [Google Scholar] [CrossRef] [PubMed]
- Dan, M.J.; McMahon, J.; Parr, W.C.H.; Broe, D.; Lucas, P.; Cross, M.; Walsh, W.R. Evaluation of Intrinsic Biomechanical Risk Factors in Patellar Tendinopathy: A Retrospective Radiographic Case-Control Series. Orthop. J. Sports Med. 2018, 6, 2325967118816038. [Google Scholar] [CrossRef] [PubMed]
- Malliaras, P.; Purdam, C.; Maffulli, N.; Cook, J. Temporal Sequence of Greyscale Ultrasound Changes and Their Relationship with Neovascularity and Pain in the Patellar Tendon. Br. J. Sports Med. 2010, 44, 944–947. [Google Scholar] [CrossRef] [PubMed]
- Docking, S.I.; Cook, J. Pathological Tendons Maintain Sufficient Aligned Fibrillar Structure on Ultrasound Tissue Characterization (UTC). Scand. J. Med. Sci. Sports 2016, 26, 675–683. [Google Scholar] [CrossRef] [PubMed]
- Malliaras, P.; Cook, J.; Purdam, C.; Rio, E. Patellar Tendinopathy: Clinical Diagnosis, Load Management, and Advice for Challenging Case Presentations. J. Orthop. Sports Phys. Ther. 2015, 45, 887–898. [Google Scholar] [CrossRef] [PubMed]
- Rio, E.; Kidgell, D.; Purdam, C.; Gaida, J.; Moseley, G.L.; Pearce, A.J.; Cook, J. Isometric Exercise Induces Analgesia and Reduces Inhibition in Patellar Tendinopathy. Br. J. Sports Med. 2015, 49, 1277–1283. [Google Scholar] [CrossRef] [PubMed]
- Rudavsky, A.; Cook, J.; Docking, S. Quantifying Proximal Patellar Tendon Changes during Adolescence in Elite Ballet Dancers, a 2-Year Study. Scand. J. Med. Sci. Sports 2018, 28, 2369–2374. [Google Scholar] [CrossRef] [PubMed]
- Drew, M.; Cook, J.; Finch, C. Sports-Related Workload and Injury Risk: Simply Knowing the Risks Will Not Prevent Injuries. Br. J. Sports Med. 2016, 50, 1306–1308. [Google Scholar] [CrossRef]
- Malliaras, P.; Kamal, B.; Nowell, A.; Farley, T.; Dhamu, H.; Simpson, V.; Morrissey, D.; Langberg, H.; Maffulli, N.; Reeves, N.D. Patellar Tendon Adaptation in Relation to Load-Intensity and Contraction Type. J. Biomech. 2013, 46, 1893–1899. [Google Scholar] [CrossRef]
- Andersson, G.; Forsgren, S.; Scott, A.; Gaida, J.E.; Stjernfeldt, J.E.; Lorentzon, R.; Alfredson, H.; Backman, C.; Danielson, P. Tenocyte Hypercellularity and Vascular Proliferation in a Rabbit Model of Tendinopathy: Contralateral Effects Suggest the Involvement of Central Neuronal Mechanisms. Br. J. Sports Med. 2011, 45, 399–406. [Google Scholar] [CrossRef] [PubMed]
- Rio, E.; Kidgell, D.; Lorimer Moseley, G.; Gaida, J.; Docking, S.; Purdam, C.; Cook, J. Tendon Neuroplastic Training: Changing the Way We Think about Tendon Rehabilitation: A Narrative Review. Br. J. Sports Med. 2016, 50, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Giombini, A.; Dragoni, S.; Di Cesare, A.; Di Cesare, M.; Del Buono, A.; Maffulli, N. Asymptomatic Achilles, Patellar, and Quadriceps Tendinopathy: A Longitudinal Clinical and Ultrasonographic Study in Elite Fencers. Scand. J. Med. Sci. Sports 2013, 23, 311–316. [Google Scholar] [CrossRef] [PubMed]
- Rabello, L.M.; van den Akker-Scheek, I.; Kuipers, I.F.; Diercks, R.L.; Brink, M.S.; Zwerver, J. Bilateral Changes in Tendon Structure of Patients Diagnosed with Unilateral Insertional or Midportion Achilles Tendinopathy or Patellar Tendinopathy. Knee Surg. Sports Traumatol. Arthrosc. 2020, 28, 1631–1638. [Google Scholar] [CrossRef] [PubMed]
- Docking, S.I.; Rosengarten, S.; Daffy, J.; Cook, J. Structural Integrity Is Decreased in Both Achilles Tendons in People with Unilateral Achilles Tendinopathy. J. Sci. Med. Sport 2014, 18, 383–387. [Google Scholar] [CrossRef] [PubMed]
- Lundgreen, K.; Lian, O.B.; Engebretsen, L.; Scott, A. Tenocyte Apoptosis in the Torn Rotator Cuff: A Primary or Secondary Pathological Event? Br. J. Sports Med. 2011, 45, 1035–1039. [Google Scholar] [CrossRef] [PubMed]
- Manca, A.; Hortobágyi, T.; Carroll, T.J.; Enoka, R.M.; Farthing, J.P.; Gandevia, S.C.; Kidgell, D.J.; Taylor, J.L.; Deriu, F. Contralateral Effects of Unilateral Strength and Skill Training: Modified Delphi Consensus to Establish Key Aspects of Cross-Education. Sports Med. 2021, 51, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Gaida, J.E.; Ashe, M.C.; Bass, S.L.; Cook, J.L. Is Adiposity an Under-Recognized Risk Factor for Tendinopathy? A Systematic Review. Arthritis Rheum. 2009, 61, 840–849. [Google Scholar] [CrossRef] [PubMed]
- Heales, L.J.; Lim, E.C.W.; Hodges, P.W.; Vicenzino, B. Sensory and Motor Deficits Exist on the Non-Injured Side of Patients with Unilateral Tendon Pain and Disability--Implications for Central Nervous System Involvement: A Systematic Review with Meta-Analysis. Br. J. Sports Med. 2014, 48, 1400–1406. [Google Scholar] [CrossRef]
- Van Schie, H.; Docking, S.; Daffy, J.; Praet, S.; Rosengarten, S.; Cook, J.L. Ultrasound Tissue Characterization, an Innovative Technique for Injury-Prevention and Monitoring of Tendinopathy. Br. J. Sports Med. 2013, 47, e2. [Google Scholar] [CrossRef]
- Rabello, L.M.; Zwerver, J.; Stewart, R.E.; Akker-Scheek, I.v.D.; Brink, M.S. Patellar Tendon Structure Responds to Load over a 7-week Preseason in Elite Male Volleyball Players. Scand. J. Med. Sci. Sports 2019, 29, 992–999. [Google Scholar] [CrossRef]
- Paganelli, A.; Contu, L.; Condorelli, A.; Ficarelli, E.; Motolese, A.; Paganelli, R.; Motolese, A. Platelet-Rich Plasma (PRP) and Adipose-Derived Stem Cell (ADSC) Therapy in the Treatment of Genital Lichen Sclerosus: A Comprehensive Review. Int. J. Mol. Sci. 2023, 24, 16107. [Google Scholar] [CrossRef]
- Scott, A.; LaPrade, R.F.; Harmon, K.G.; Filardo, G.; Kon, E.; Della Villa, S.; Bahr, R.; Moksnes, H.; Torgalsen, T.; Lee, J.; et al. Platelet-Rich Plasma for Patellar Tendinopathy: A Randomized Controlled Trial of Leukocyte-Rich PRP or Leukocyte-Poor PRP Versus Saline. Am. J. Sports Med. 2019, 47, 1654–1661. [Google Scholar] [CrossRef]
- Mao, X.-F.; Zhang, X.-Q.; Yao, Z.-Y.; Mao, H.-J. Advances in Mesenchymal Stem Cells Therapy for Tendinopathies. Chin. J. Traumatol. = Zhonghua Chuang Shang Za Zhi 2024, 27, 11–17. [Google Scholar] [CrossRef]
- Soler, R.; Rodas, G.; Rius-Tarruella, J.; Alomar, X.; Balius, R.; Ruíz-Cotorro, Á.; Masci, L.; Maffulli, N.; Orozco, L. Safety and Efficacy of Bone Marrow–Derived Mesenchymal Stem Cells for Chronic Patellar Tendinopathy (with Gap > 3 Mm) in Patients: 12-Month Follow-up Results of a Phase 1/2 Clinical Trial. Orthop. J. Sports Med. 2023, 11, 23259671231184400. [Google Scholar] [CrossRef]
- Rodas, G.; Soler-Rich, R.; Rius-Tarruella, J.; Alomar, X.; Balius, R.; Orozco, L.; Masci, L.; Maffulli, N. Effect of Autologous Expanded Bone Marrow Mesenchymal Stem Cells or Leukocyte-Poor Platelet-Rich Plasma in Chronic Patellar Tendinopathy (with Gap > 3 Mm): Preliminary Outcomes After 6 Months of a Double-Blind, Randomized, Prospective Study. Am. J. Sports Med. 2021, 49, 1492–1504. [Google Scholar] [CrossRef]
- Goldberg, A.J.; Masci, L.; Donnell, P.O.; Green, R.; Brooking, D.; Bassett, P.; Lowdell, M.W.; Smith, R.K.W. Autologous Bone Marrow Derived Mesenchymal Stem Cells Are Safe for the Treatment of Achilles Tendinopathy. Sci. Rep. 2024, 14, 11421. [Google Scholar] [CrossRef]
- Zult, T.; Goodall, S.; Thomas, K.; Hortobágyi, T.; Howatson, G. Mirror Illusion Reduces Motor Cortical Inhibition in the Ipsilateral Primary Motor Cortex during Forceful Unilateral Muscle Contractions. J. Neurophysiol. 2015, 113, 2262–2270. [Google Scholar] [CrossRef]
- Rodas, G.; Soler, R.; Balius, R.; Alomar, X.; Peirau, X.; Alberca, M.; Sánchez, A.; Sancho, J.G.; Rodellar, C.; Romero, A.; et al. Autologous Bone Marrow Expanded Mesenchymal Stem Cells in Patellar Tendinopathy: Protocol for a Phase I/II, Single-Centre, Randomized with Active Control PRP, Double-Blinded Clinical Trial. J. Orthop. Surg. Res. 2019, 14, 441. [Google Scholar] [CrossRef]
- Ark, M.; Rabello, L.; Hoevenaars, D.; Meijerink, J.; Gelderen, N.; Zwerver, J.; Akker-Scheek, I. Inter- and Intra-rater Reliability of Ultrasound Tissue Characterization (UTC) in Patellar Tendons. Scand. J. Med. Sci. Sports 2019, 29, 1205–1211. [Google Scholar] [CrossRef]
- Rabello, M.l.; Dams, O.; Akker-Scheek, I.; Zwerver, J.; O’Neill, S. Substantiating the Use of Ultrasound Tissue Characterization in the Analysis of Tendon Structure: A Systematic Review. Clin. J. Sports Med. 2019, 31, e161–e175. [Google Scholar] [CrossRef]
- Ortega-Cebrián, S.; Navarro, R.; Seda, S.; Salas, S.; Guerra-Balic, M. Patellar Tendon Structural Adaptations Occur during Pre-Season and First Competitive Cycle in Male Professional Handball Players. Int. J. Environ. Res. Public Health 2021, 18, 12156. [Google Scholar] [CrossRef]
- van Schie, H.T.M.; de Vos, R.J.; de Jonge, S.; Bakker, E.M.; Heijboer, M.P.; Verhaar, J.A.N.; Tol, J.L.; Weinans, H. Ultrasonographic Tissue Characterisation of Human Achilles Tendons: Quantification of Tendon Structure through a Novel Non-Invasive Approach. Br. J. Sports Med. 2010, 44, 1153–1159. [Google Scholar] [CrossRef]
- Romero, A.; Barrachina, L.; Ranera, B.; Remacha, A.R.; Moreno, B.; de Blas, I.; Sanz, A.; Vázquez, F.J.; Vitoria, A.; Junquera, C.; et al. Comparison of Autologous Bone Marrow and Adipose Tissue Derived Mesenchymal Stem Cells, and Platelet Rich Plasma, for Treating Surgically Induced Lesions of the Equine Superficial Digital Flexor Tendon. Veter J. 2017, 224, 76–84. [Google Scholar] [CrossRef]
- Beccia, E.; Carbone, A.; Cecchino, L.R.; Pedicillo, M.C.; Annacontini, L.; Lembo, F.; Di Gioia, S.; Parisi, D.; Angiolillo, A.; Pannone, G.; et al. Adipose Stem Cells and Platelet-Rich Plasma Induce Vascular-Like Structures in a Dermal Regeneration Template. Tissue Eng. Part A 2021, 27, 631–641. [Google Scholar] [CrossRef]
- Gentile, P.; Garcovich, S. Advances in Regenerative Stem Cell Therapy in Androgenic Alopecia and Hair Loss: Wnt Pathway, Growth-Factor, and Mesenchymal Stem Cell Signaling Impact Analysis on Cell Growth and Hair Follicle Development. Cells 2019, 8, 466. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, N.; Yang, M.; Sun, T.; Zhang, J.; Zhao, Y.; Huo, N.; Li, Z. Periosteum and Development of the Tissue-Engineered Periosteum for Guided Bone Regeneration. J. Orthop. Transl. 2022, 33, 41–54. [Google Scholar] [CrossRef]
- Lu, J.; Li, H.; Zhang, Z.; Xu, R.; Wang, J.; Jin, H. Platelet-Rich Plasma in the Pathologic Processes of Tendinopathy: A Review of Basic Science Studies. Front. Bioeng. Biotechnol. 2023, 11, 1187974. [Google Scholar] [CrossRef]
- Rabello, L.; Albers, I.; Ark, M.; Diercks, R.; Akker-Scheek, I.; Zwerver, J. Running a Marathon—Its Influence on Achilles Tendon Structure. J. Athl. Train. 2020, 55, 176–180. [Google Scholar] [CrossRef]
- Cook, J.L.; Purdam, C.R. Is Tendon Pathology a Continuum? A Pathology Model to Explain the Clinical Presentation of Load-Induced Tendinopathy. Br. J. Sports Med. 2009, 43, 409–416. [Google Scholar] [CrossRef]
- Maffulli, N.; Longo, U.G.; Denaro, V. Novel Approaches for the Management of Tendinopathy. J. Bone Jt. Surg.—Ser. A 2010, 92, 2604–2613. [Google Scholar] [CrossRef]
- Malliaras, P.; O’Neill, S. Potential Risk Factors Leading to Tendinopathy. Apunt. Med. L’esport 2017, 52, 71–77. [Google Scholar] [CrossRef]
- Shen, H.; Kormpakis, I.; Havlioglu, N.; Linderman, S.W.; Sakiyama-Elbert, S.E.; Erickson, I.E.; Zarembinski, T.; Silva, M.J.; Gelberman, R.H.; Thomopoulos, S. The Effect of Mesenchymal Stromal Cell Sheets on the Inflammatory Stage of Flexor Tendon Healing. Stem Cell Res. Ther. 2016, 7, 144. [Google Scholar] [CrossRef]
- Smith, R.K.W.; Werling, N.J.; Dakin, S.G.; Alam, R.; Goodship, A.E.; Dudhia, J. Beneficial Effects of Autologous Bone Marrow-Derived Mesenchymal Stem Cells in Naturally Occurring Tendinopathy. PLoS ONE 2013, 8, e75697. [Google Scholar] [CrossRef]
- Chalidis, B.; Givissis, P.; Papadopoulos, P.; Pitsilos, C. Molecular and Biologic Effects of Platelet-Rich Plasma (PRP) in Ligament and Tendon Healing and Regeneration: A Systematic Review. Int. J. Mol. Sci. 2023, 24, 2744. [Google Scholar] [CrossRef]
- Fitzpatrick, J.; Bulsara, M.; Zheng, M.H. The Effectiveness of Platelet-Rich Plasma in the Treatment of Tendinopathy. Am. J. Sports Med. 2017, 45, 226–233. [Google Scholar] [CrossRef]
- Vander Doelen, T.; Jelley, W. Non-Surgical Treatment of Patellar Tendinopathy: A Systematic Review of Randomized Controlled Trials. J. Sci. Med. Sport 2020, 23, 118–124. [Google Scholar] [CrossRef]
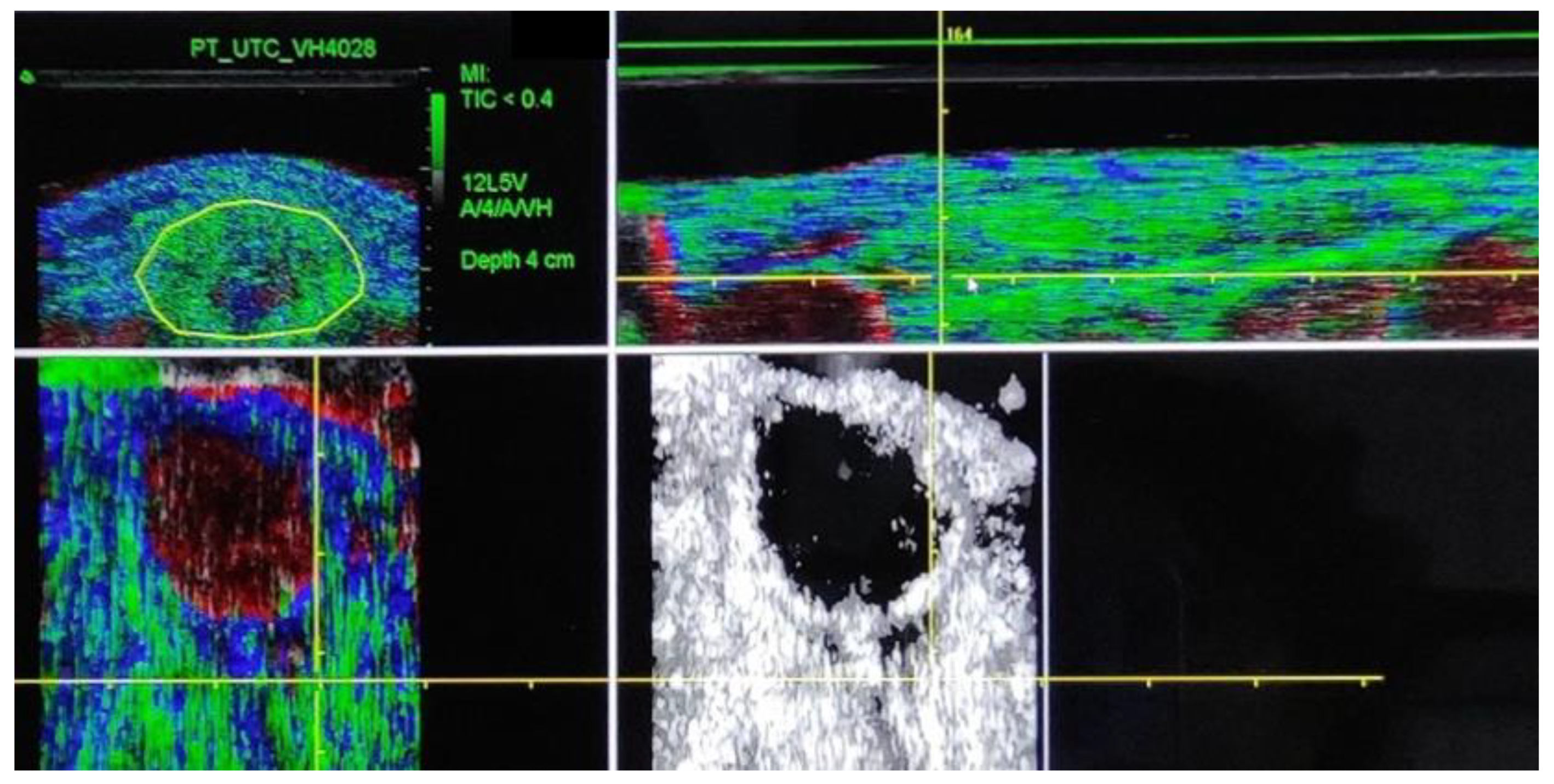
| Descriptive Strength and Pain | N | Media | SD | CI | Effect Size | p-Value | |||
|---|---|---|---|---|---|---|---|---|---|
| 95% sup | 95% inf | ||||||||
| Baseline | Strength | Lp-PRP | 10 | 29.71 | 6.80 | 33.93 | 25.49 | 4.36 | 0.19 |
| A-Lp-PRP | 9 | 31.44 | 3.81 | 6.18 | 3.15 | ||||
| BM-MSC | 10 | 37.19 | 11.01 | 28.72 | 45.65 | 3.37 | 0.06 Ɨ | ||
| A-BM-MSC | 10 | 40.19 | 11.35 | 31.46 | 48.91 | 3.54 | 0.16 ƗƗ | ||
| Pain | Lp-PRP | 9 | 4.67 | 2.45 | 6.18 | 3.15 | 1.90 | 0.25 | |
| BM-MSC | 10 | 3.56 | 3.00 | 1.25 | 5.87 | 1.18 | |||
| 3 Months | Strength | Lp-PRP | 9 | 28.44 | 5.98 | 35.02 | 21.87 | 4.75 | 0.02 |
| A-Lp-PRP | 8 | 31.44 | 9.41 | 37.27 | 25.61 | ||||
| BM-MSC | 10 | 32.91 | 6.92 | 27.59 | 38.23 | 4.75 | 0.09 Ɨ | ||
| A-BM-MSC | 10 | 36.29 | 9.25 | 29.18 | 43.40 | 3.92 | 0.01 ƗƗ | ||
| B-BM-MSC | 9 | 34.83 | 14.04 | 43.53 | 26.12 | 0.85 | 0.07 ƗƗ | ||
| Pain | Lp-PRP | 10 | 1.50 | 2.01 | 2.75 | 0.25 | 0.74 | 0.07 | |
| BM-MSC | 10 | 2.00 | 3.04 | −0.34 | 4.34 | ||||
| B-BM-MSC | 8 | 1.12 | 2.23 | 2.43 | −0.18 | −0.26 | 0.56 ƗƗ | ||
| 6 Months | Strength | Lp-PRP | 8 | 28.91 | 5.23 | 35.61 | 22.21 | 5.53 | 0.03 |
| A-Lp-PRP | 7 | 34.37 | 5.64 | 42.57 | 26.17 | ||||
| BM-MSC | 10 | 33.37 | 9.25 | 26.26 | 40.48 | 3.60 | 0.55 Ɨ | ||
| A-BM-MSC | 10 | 34.24 | 9.33 | 27.07 | 41.41 | 3.67 | 0.41 ƗƗ | ||
| B-BM-MSC | 8 | 0.00 | 0.06 | 1.54 | 0.07 | 0.87 | 0.08 ƗƗ | ||
| Pain | Lp-PRP | 10 | 0.90 | 1.52 | 1.84 | −0.04 | 0.59 | 0.17 | |
| BM-MSC | 10 | 1.89 | 3.06 | −0.46 | 4.24 | ||||
| B-BM-MSC | 8 | 1.51 | 1.04 | 1.21 | −3.19 | −0.70 | 0.13 ƗƗ | ||
| 12 Months | Strength | Lp-PRP | 9 | 33.15 | 9.69 | 39.15 | 27.15 | 3.42 | 0.87 |
| A-Lp-PRP | 9 | 31.55 | 1.48 | 32.47 | 30.63 | ||||
| BM-MSC | 10 | 38.43 | 9.72 | 30.96 | 45.90 | 3.95 | 0.51 Ɨ | ||
| A-BM-MSC | 10 | 40.31 | 11.33 | 31.61 | 49.02 | 3.55 | 0.09 ƗƗ | ||
| B-BM-MSC | 8 | 36.86 | 8.36 | 45.88 | 27.83 | −0.17 | 0.71 ƗƗ | ||
| Pain | Lp-PRP | 9 | 1.22 | 2.54 | −0.73 | 3.17 | 0.00 | 0.21 | |
| BM-MSC | 10 | 0.00 | 0.00 | 0.00 | 0.00 | ||||
| B-BM-MSC | 9 | 1.13 | 1.40 | 14.67 | −4.41 | −0.62 | 0.18 ƗƗ | ||
| Months | BM-MSC and A-BM-MSC | Lp-PRP and A-Lp-PRP | BM-MSC and Lp-PRP | BM-MSC and B-BM-MSC | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Insertional Tendon | Proximal Tendon | Mid-Tendon | Insertional Tendon | Proximal Tendon | Mid-Tendon | Insertional Tendon | Proximal Tendon | Mid-Tendon | Insertional Tendon | Proximal Tendon | Mid-Tendon | |
| 3 | BM-MSC group show greater organization of echo-type I | Lp-PRP group show greater disorganization of echo-type II and III | ||||||||||
| 6 | Lp-PRP group show greater disorganization of echo-type II and III | BM-MSC group show lower disorganized echo-type I | ||||||||||
| 12 | Lp-PRP cohort show greater disorganization of echo-type I | BM-MSC group show lower disorganization of echo-type III and IV | ||||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ortega-Cebrián, S.; Soler-Rich, R.; Orozco, L.; Rodas, G. Evaluation of Patellar Tendon Structural Changes following Biological Treatments: Secondary Analysis of Double-Blinded Clinical Trial of Bone Marrow Mesenchymal Stromal Cells and Leukocyte-Poor Platelet-Rich Plasma. Biomedicines 2024, 12, 1599. https://doi.org/10.3390/biomedicines12071599
Ortega-Cebrián S, Soler-Rich R, Orozco L, Rodas G. Evaluation of Patellar Tendon Structural Changes following Biological Treatments: Secondary Analysis of Double-Blinded Clinical Trial of Bone Marrow Mesenchymal Stromal Cells and Leukocyte-Poor Platelet-Rich Plasma. Biomedicines. 2024; 12(7):1599. https://doi.org/10.3390/biomedicines12071599
Chicago/Turabian StyleOrtega-Cebrián, Silvia, Robert Soler-Rich, Lluis Orozco, and Gil Rodas. 2024. "Evaluation of Patellar Tendon Structural Changes following Biological Treatments: Secondary Analysis of Double-Blinded Clinical Trial of Bone Marrow Mesenchymal Stromal Cells and Leukocyte-Poor Platelet-Rich Plasma" Biomedicines 12, no. 7: 1599. https://doi.org/10.3390/biomedicines12071599







