Anti-Microbial Drug Metronidazole Promotes Fracture Healing: Enhancement in the Bone Regenerative Efficacy of the Drug by a Biodegradable Sustained-Release In Situ Gel Formulation
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Chemicals
2.2. Animal Experiments
2.3. Femur Osteotomy
2.4. Ovariectomy
2.5. Culture of Rat Calvarial Osteoblasts (RCOs)
2.6. ALP Assay
2.7. BrdU Assay
2.8. Nodule Mineralization and Alizarin Staining
2.9. Quantitative Real-Time Polymerase Chain Reaction
2.10. Wnt/β-Catenin Reporter Assay
2.11. Immunoflorescence
2.12. Western Blotting
2.13. Formulation of PLGA-Based Biodegradable In Situ Gel of MTZ
2.14. Determination of Drug Content
2.15. Morphological Evaluation
2.16. Fourier Transform Infrared Spectroscopy (FTIR)
2.17. Evaluation of MTZ Release and Its Kinetics
2.18. Bioanalytical Method Development Using LC-MS/MS
2.19. Pharmacokinetic (PK) Studies
2.20. Micro-CT Analysis
2.21. Bone Strength Test
2.22. Bone Histomorphometry
2.23. Bone Turnover Markers
2.24. Statistical Analysis
3. Results
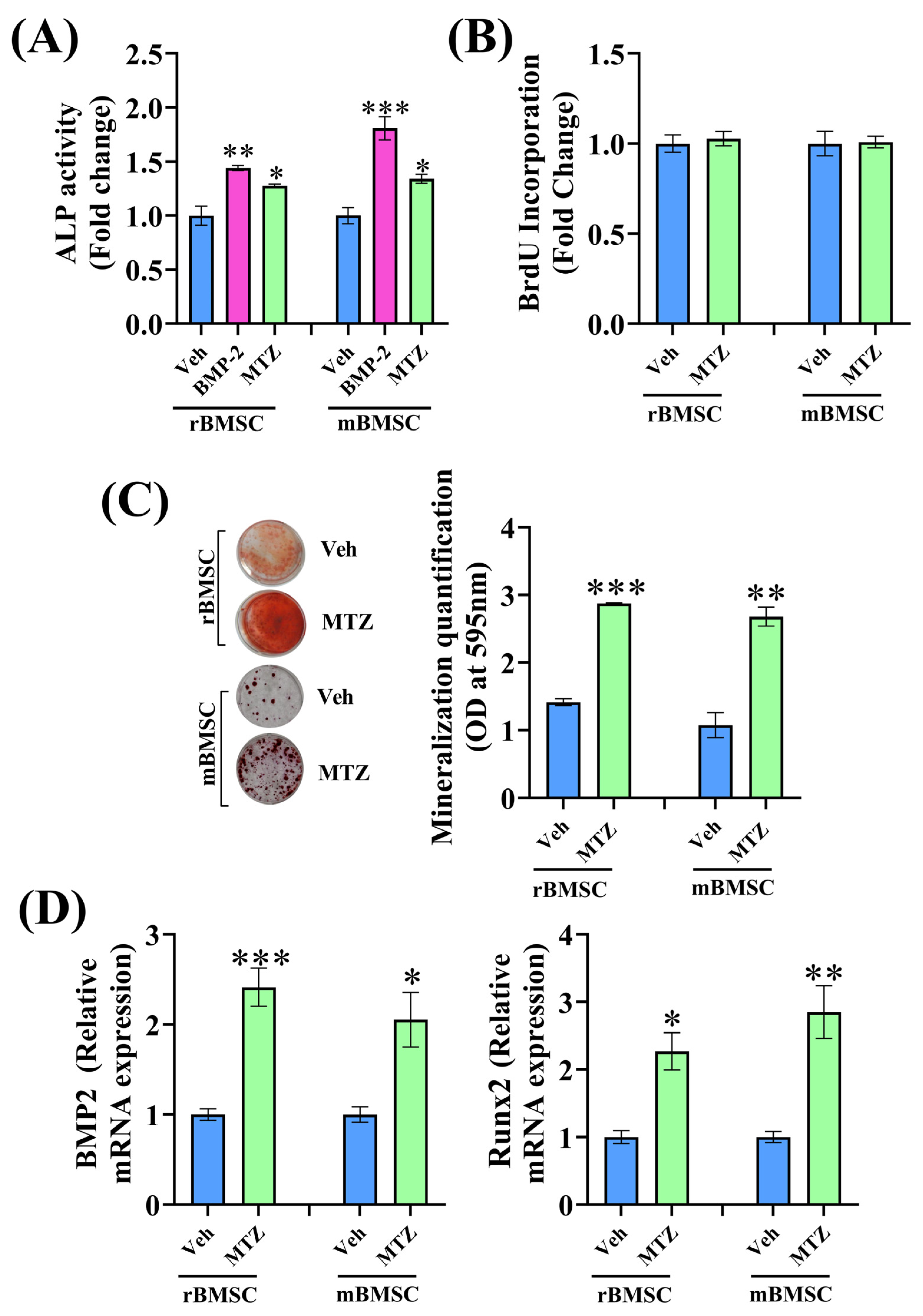
3.1. Effect of Nitroimidazole Drugs on ALP Activity
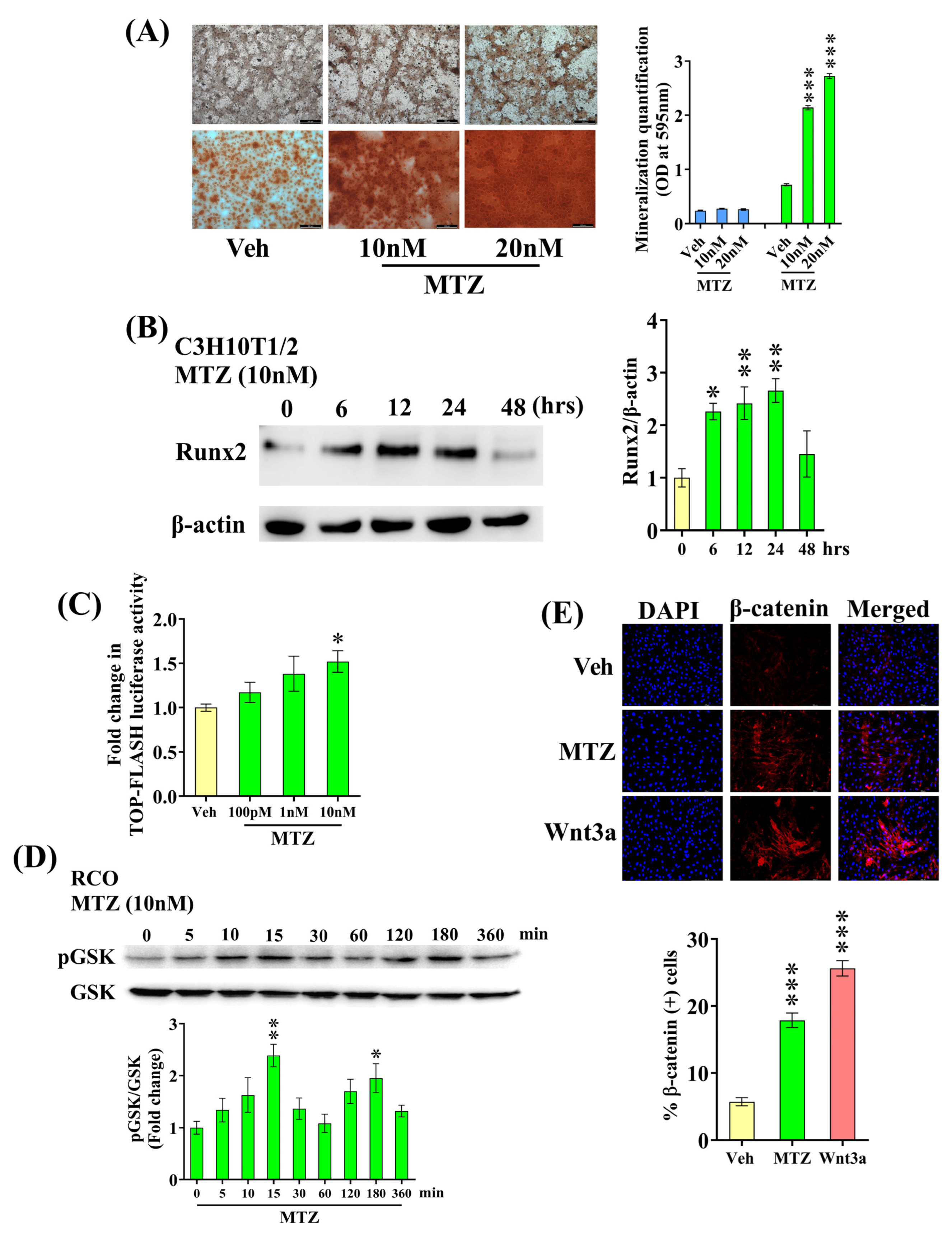
3.2. MTZ Increases Osteoblast Differentiation
3.3. MTZ Stimulates Wnt Pathway in Osteoblasts
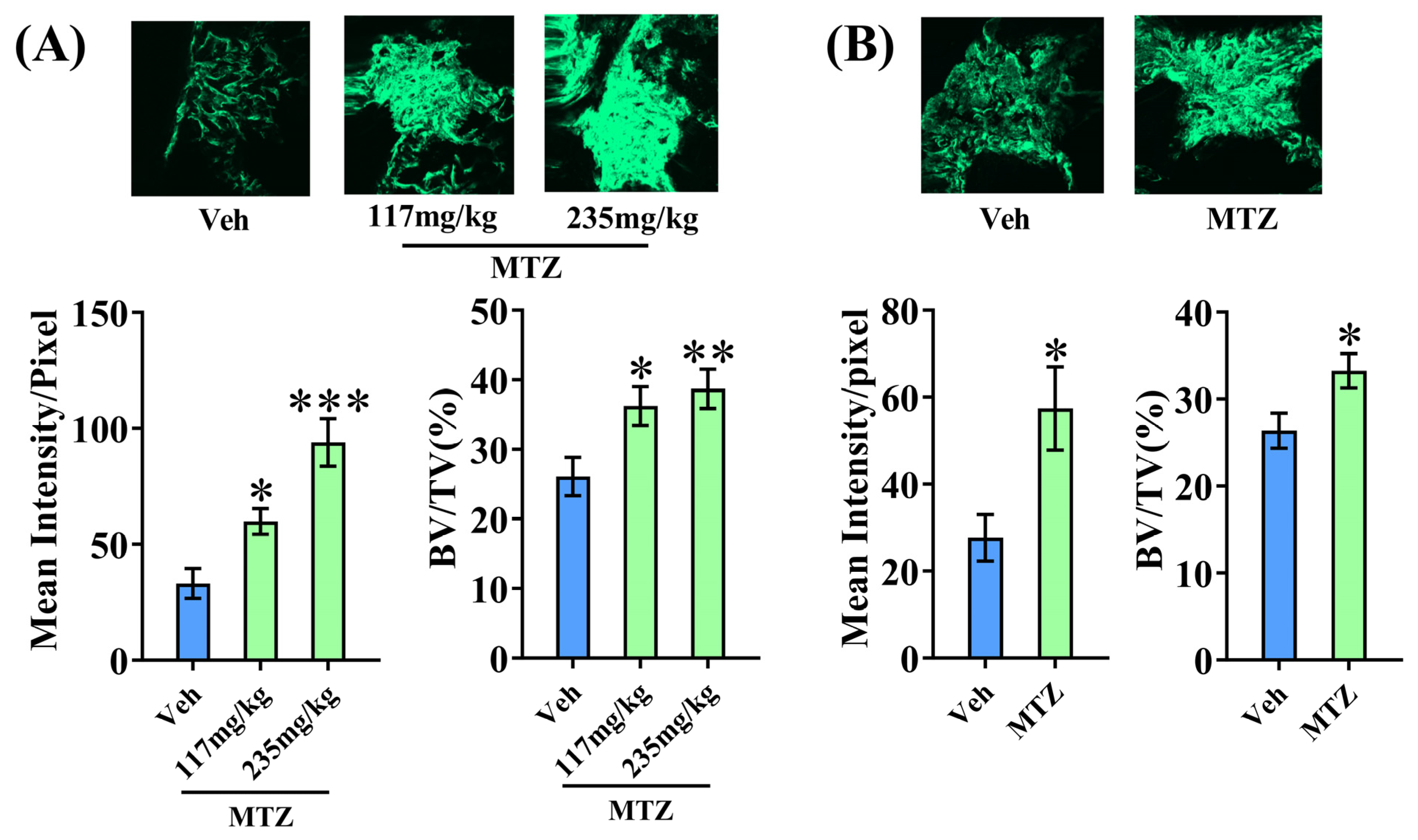
3.4. MTZ Enhances Bone Regeneration at the Osteotomy Site
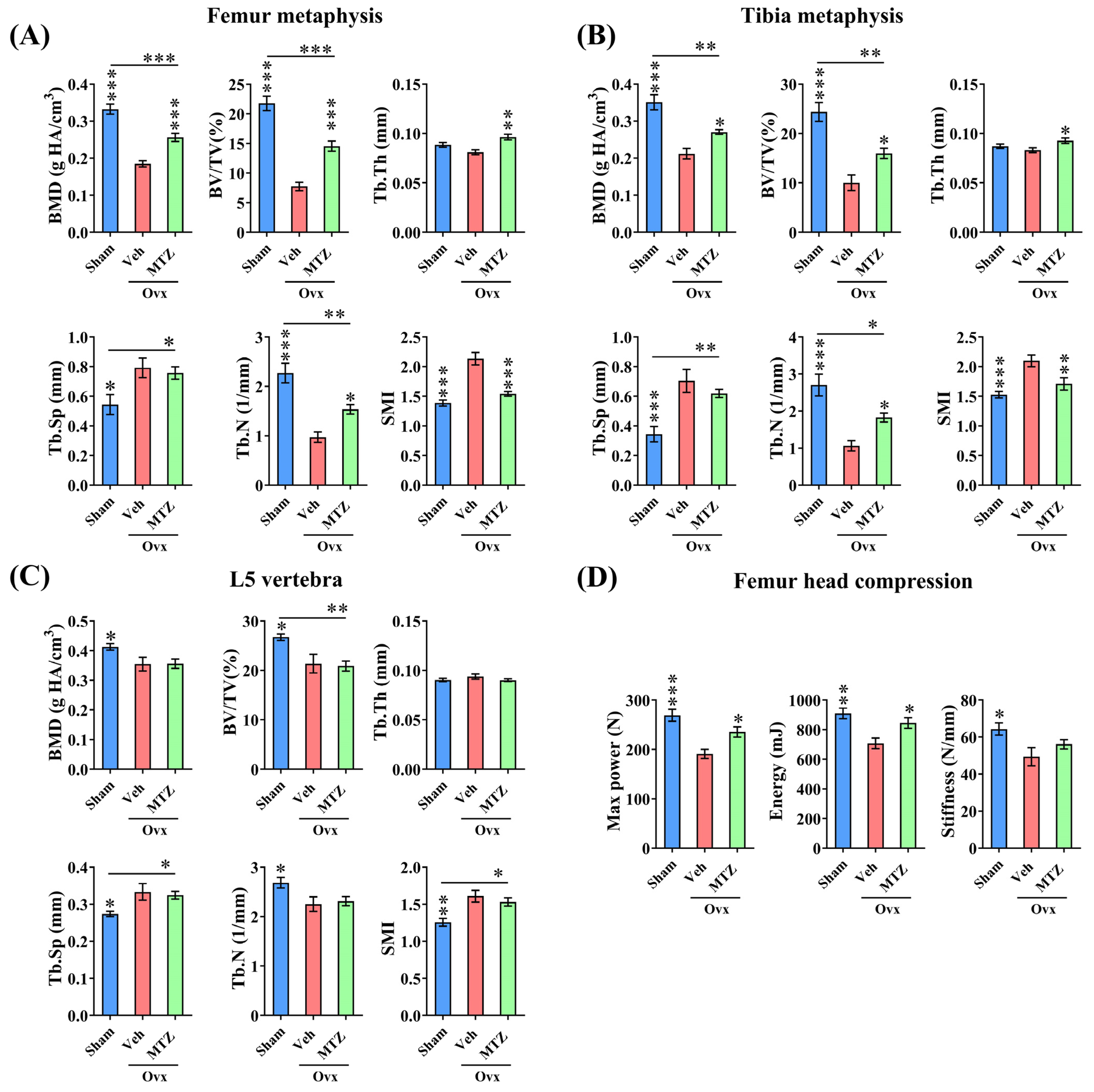
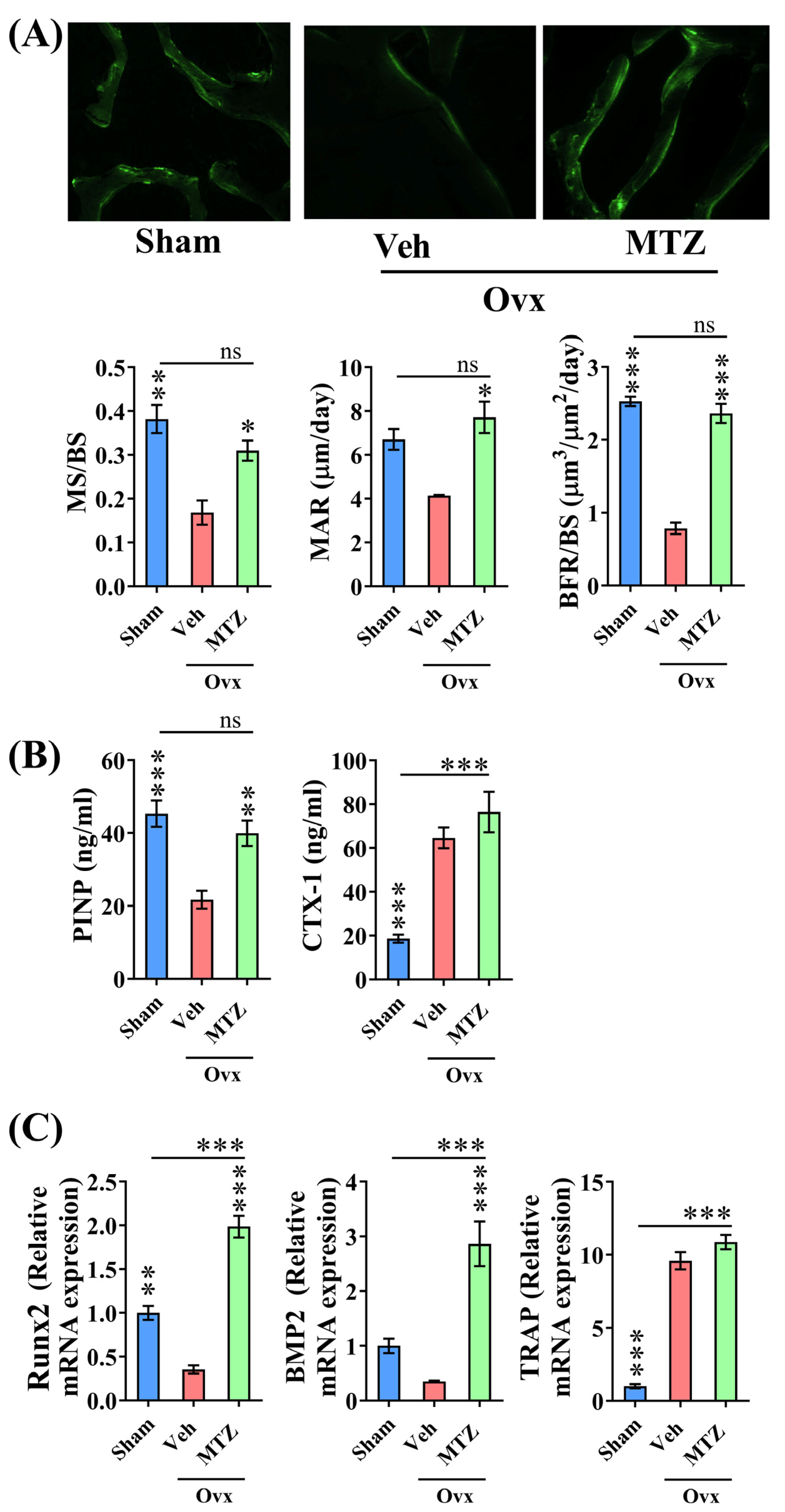
3.5. MTZ Restores OVX-Induced Bone Loss and Strength
3.6. In Vivo Osteogenic Effect of MTZ
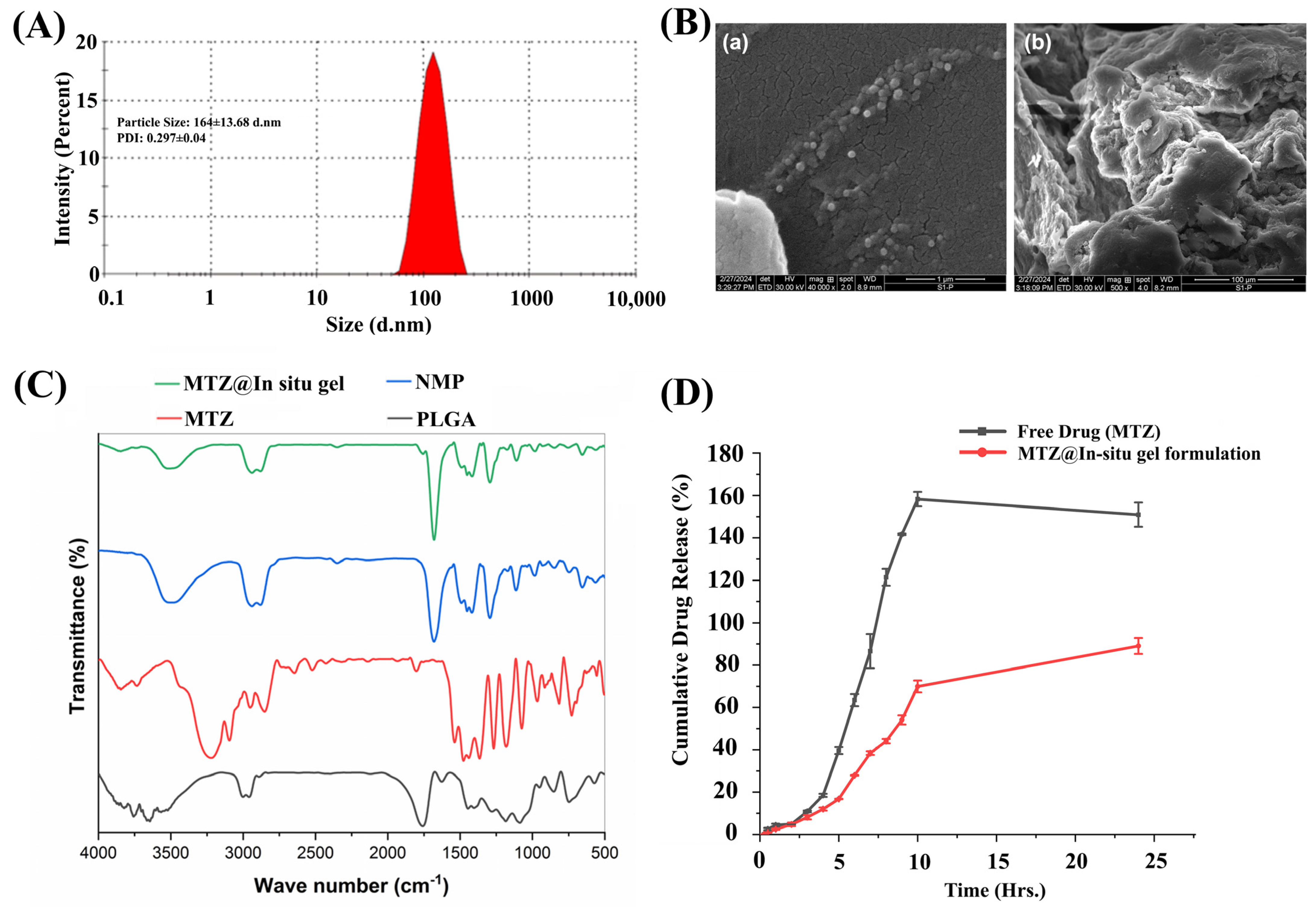
3.7. Development of Biodegradable-Based In Situ Gel (MTZ@In Situ Gel)
3.8. Morphological and Functional Characterization by Scanning Electron Microscopy (SEM) Fourier Transform Infrared Spectroscopy (FTIR)
3.9. Evaluation of MTZ Release and Its Kinetics
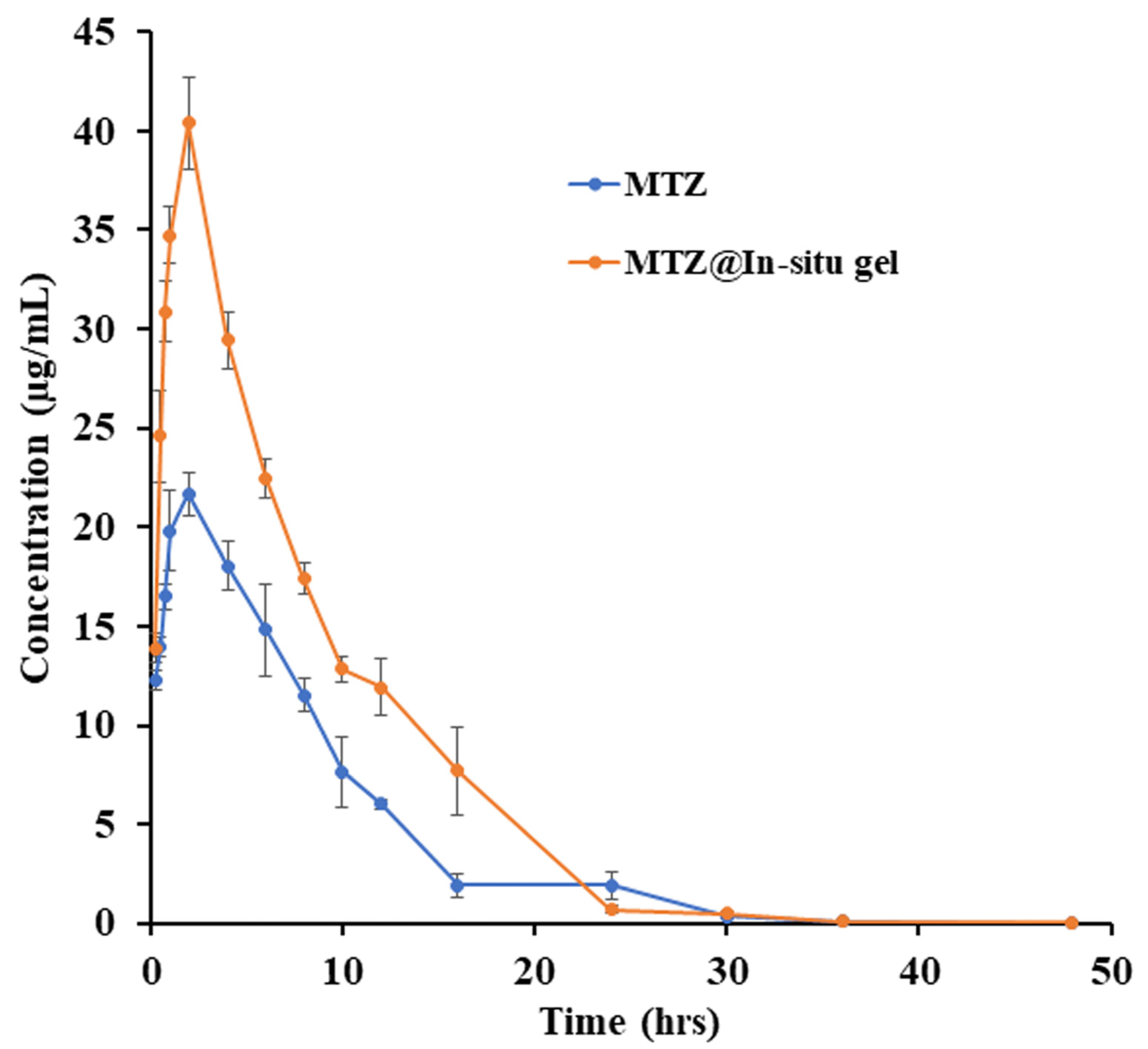
3.10. PK Profile of MTZ and MTZ@In Situ gel
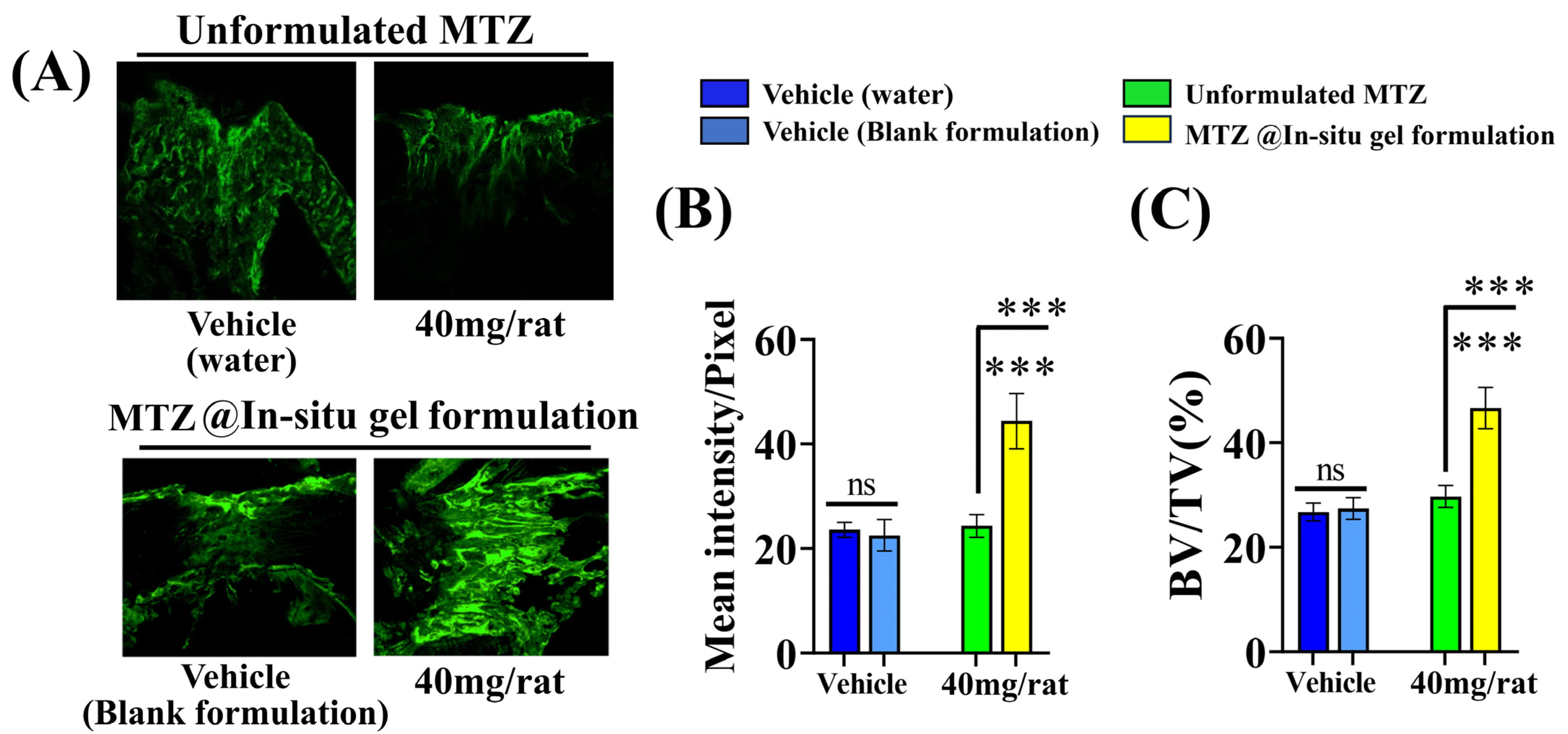
3.11. Effect of MTZ@In Situ Gel Formulation on Bone Regeneration at the Osteotomy Site
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Makaram, N.S.; Leow, J.M.; Clement, N.D.; Oliver, W.M.; Ng, Z.H.; Simpson, C.; Keating, J.F. Risk Factors Associated with Delayed and Aseptic Nonunion Following Tibial Diaphyseal Fractures Managed with Intramedullary Nailing. Bone Jt. Open 2021, 2, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Hackl, S.; Keppler, L.; von Rüden, C.; Friederichs, J.; Perl, M.; Hierholzer, C. The Role of Low-Grade Infection in the Pathogenesis of Apparently Aseptic Tibial Shaft Nonunion. Injury 2021, 52, 3498–3504. [Google Scholar] [CrossRef] [PubMed]
- Stewart, S.K. Fracture Non-Union: A Review of Clinical Challenges and Future Research Needs. Malaysian Orthop. J. 2019, 13, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Gillespie, W.J.; Walenkamp, G.H. Antibiotic Prophylaxis for Surgery for Proximal Femoral and Other Closed Long Bone Fractures. Cochrane Database Syst. Rev. 2010, 3, CD000244. [Google Scholar] [CrossRef] [PubMed]
- Flores, M.J.; Brown, K.E.; Morshed, S.; Shearer, D.W. Evidence for Local Antibiotics in the Prevention of Infection in Orthopaedic Trauma. J. Clin. Med. 2022, 11, 7461. [Google Scholar] [CrossRef] [PubMed]
- Hall, M.M.; Finnoff, J.T.; Smith, J. Musculoskeletal Complications of Fluoroquinolones: Guidelines and Precautions for Usage in the Athletic Population. PM R 2011, 3, 132–142. [Google Scholar] [CrossRef] [PubMed]
- Barnhill, A.E.; Brewer, M.T.; Carlson, S.A. Adverse Effects of Antimicrobials via Predictable or Idiosyncratic Inhibition of Host Mitochondrial Components. Antimicrob. Agents Chemother. 2012, 56, 4046–4051. [Google Scholar] [CrossRef] [PubMed]
- Warner, A.J.; Hathaway-Schrader, J.D.; Lubker, R.; Davies, C.; Novince, C.M. Tetracyclines and Bone: Unclear Actions with Potentially Lasting Effects. Bone 2022, 159, 116377. [Google Scholar] [CrossRef] [PubMed]
- Boyce, B.; Yao, Z.; Xing, L. Osteoclasts Have Multiple Roles in Bone in Addition to Bone Resorption. Crit. Rev. Eukaryot. Gene Expr. 2009, 19, 171–180. [Google Scholar] [CrossRef]
- Bahney, C.S.; Zondervan, R.L.; Allison, P.; Theologis, A.; Ashley, J.W.; Ahn, J.; Miclau, T.; Marcucio, R.S.; Hankenson, K.D. Cellular Biology of Fracture Healing. J. Orthop. Res. 2019, 37, 35–50. [Google Scholar]
- Beuttel, E.; Bormann, N.; Pobloth, A.M.; Duda, G.N.; Wildemann, B. Impact of Gentamicin-Loaded Bone Graft on Defect Healing in a Sheep Model. Materials 2019, 12, 1116. [Google Scholar] [CrossRef] [PubMed]
- Ince, A.; Schütze, N.; Karl, N.; Löhr, J.F.; Eulert, J. Gentamicin Negatively Influenced Osteogenic Function in Vitro. Int. Orthop. 2007, 31, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Liu, H.; Tian, Y.; Fan, Y.; Li, S.; Wang, G.; Wang, Y.; Peng, C.; Wu, D. Dual-Functional Composite Scaffolds for Inhibiting Infection and Promoting Bone Regeneration. Mater. Today Bio 2022, 16, 100409. [Google Scholar] [CrossRef] [PubMed]
- Croes, M.; van der Wal, B.C.H.; Vogely, H.C. Impact of Bacterial Infections on Osteogenesis: Evidence From In Vivo Studies. J. Orthop. Res. 2019, 37, 2067–2076. [Google Scholar] [CrossRef] [PubMed]
- Weir, C.B.; Le, J.K. Metronidazole; StatPearls: St. Petersburg, FL, USA, 2023. [Google Scholar]
- Fraimow, H. Systemic Antimicrobial Therapy in Osteomyelitis. Semin. Plast. Surg. 2009, 23, 090–099. [Google Scholar] [CrossRef] [PubMed]
- Metronidazole (Oral Route) Proper Use-Mayo Clinic. Available online: https://www.mayoclinic.org/drugs-supplements/metronidazole-oral-route/proper-use/drg-20064745 (accessed on 12 May 2024).
- Martinez, V.; Caumes, E. [Metronidazole]—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/11590342/ (accessed on 12 May 2024).
- Alsaeed, O.M.; Bukhari, A.A.; Alshehri, A.A.; Alsumairi, F.A.; Alnami, A.M.; Elsheikh, H.A. The Use of Antibiotics for the Prevention of Surgical Site Infections in Two Government Hospitals in Taif, Saudi Arabia: A Retrospective Study. Cureus 2022, 14, e26731. [Google Scholar] [CrossRef] [PubMed]
- Pal, S.; Sharma, S.; Porwal, K.; Riyazuddin, M.; Kulkarni, C.; Chattopadhyay, S.; Sanyal, S.; Gayen, J.R.; Chattopadhyay, N. Oral Administration of Isovitexin, a Naturally Occurring Apigenin Derivative Showed Osteoanabolic Effect in Ovariectomized Mice: A Comparative Study with Teriparatide. Calcif. Tissue Int. 2022, 111, 196–210. [Google Scholar] [CrossRef] [PubMed]
- Pal, S.; Maurya, S.K.; Chattopadhyay, S.; Pal China, S.; Porwal, K.; Kulkarni, C.; Sanyal, S.; Sinha, R.A.; Chattopadhyay, N. The Osteogenic Effect of Liraglutide Involves Enhanced Mitochondrial Biogenesis in Osteoblasts. Biochem. Pharmacol. 2019, 164, 34–44. [Google Scholar] [CrossRef] [PubMed]
- Pal, S.; Porwal, K.; Singh, H.; Malik, M.Y.; Rashid, M.; Kulkarni, C.; Khan, Y.; Jagavelu, K.; Wahajuddin, M.; Chattopadhyay, N. Reversal of Osteopenia in Ovariectomized Rats by Pentoxifylline: Evidence of Osteogenic and Osteo-Angiogenic Roles of the Drug. Calcif. Tissue Int. 2019, 105, 294–307. [Google Scholar] [CrossRef]
- Tripathi, J.K.; Pal, S.; Awasthi, B.; Kumar, A.; Tandon, A.; Mitra, K.; Chattopadhyay, N.; Ghosh, J.K. Variants of Self-Assembling Peptide, KLD-12 That Show Both Rapid Fracture Healing and Antimicrobial Properties. Biomaterials 2015, 56, 92–103. [Google Scholar] [CrossRef]
- Pal, S.; Sayeed, M.; Kumar, A.; Verma, D.P.; Harioudh, M.K.; Verma, N.K.; Porwal, K.; Sharma, S.; Kulkarni, C.; Bandyopadhyay, A.; et al. Self-Assembling Nano-Globular Peptide from Human Lactoferrin Acts as a Systemic Enhancer of Bone Regeneration: A Novel Peptide for Orthopedic Application. ACS Appl. Mater. Interfaces 2021, 13, 17300–17315. [Google Scholar] [CrossRef]
- Pal, S.; Kumar, P.; Ramakrishna, E.; Kumar, S.; Porwal, K.; Kumar, B.; Arya, K.R.; Maurya, R.; Chattopadhyay, N. Extract and Fraction of Cassia occidentalis L. (a Synonym of Senna occidentalis) Have Osteogenic Effect and Prevent Glucocorticoid-Induced Osteopenia. J. Ethnopharmacol. 2019, 235, 8–18. [Google Scholar] [CrossRef] [PubMed]
- Porwal, K.; Pal, S.; Dev, K.; China, S.P.; Kumar, Y.; Singh, C.; Barbhuyan, T.; Sinha, N.; Sanyal, S.; Trivedi, A.K.; et al. Guava Fruit Extract and Its Triterpene Constituents Have Osteoanabolic Effect: Stimulation of Osteoblast Differentiation by Activation of Mitochondrial Respiration via the Wnt/β-Catenin Signaling. J. Nutr. Biochem. 2017, 44, 22–34. [Google Scholar] [CrossRef]
- Pal, S.; Singh, M.; Porwal, K.; Rajak, S.; Das, N.; Rajput, S.; Trivedi, A.K.; Maurya, R.; Sinha, R.A.; Siddiqi, M.I.; et al. Adiponectin Receptors by Increasing Mitochondrial Biogenesis and Respiration Promote Osteoblast Differentiation: Discovery of Isovitexin as a New Class of Small Molecule Adiponectin Receptor Modulator with Potential Osteoanabolic Function. Eur. J. Pharmacol. 2021, 913, 174634. [Google Scholar] [CrossRef]
- Sherafudeen, S.P.; Vasantha, P.V. Development and Evaluation of in Situ Nasal Gel Formulations of Loratadine. Res. Pharm. Sci. 2015, 10, 466–476. [Google Scholar] [PubMed]
- Pal, S.; Mittapelly, N.; Husain, A.; Kushwaha, S.; Chattopadhyay, S.; Kumar, P.; Ramakrishna, E.; Kumar, S.; Maurya, R.; Sanyal, S.; et al. A Butanolic Fraction from the Standardized Stem Extract of Cassia occidentalis L. Delivered by a Self-Emulsifying Drug Delivery System Protects Rats from Glucocorticoid-Induced Osteopenia and Muscle Atrophy. Sci. Rep. 2020, 10, 195. [Google Scholar] [CrossRef]
- Pal, S.; Sharma, S.; Porwal, K.; Tiwari, M.C.; Khan, Y.A.; Kumar, S.; Kumar, N.; Chattopadhyay, N. The Role of Osteogenic Effect and Vascular Function in Bone Health in Hypertensive Rats: A Study of Anti-Hypertensive and Hemorheologic Drugs. Calcif. Tissue Int. 2024, 114, 295–309. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Wei, C.; Chen, H.; Qin, J.; Liang, J.; Kong, D.; Liu, T.; Lv, F. Real-Time Imaging Tracking of a Dual Fluorescent Drug Delivery System Based on Zinc Phthalocyanine-Incorporated Hydrogel. ACS Biomater. Sci. Eng. 2016, 2, 2001–2010. [Google Scholar] [CrossRef]
- Khaing, E.M.; Mahadlek, J.; Okonogi, S.; Phaechamud, T. Lime Peel Oil–Incorporated Rosin-Based Antimicrobial In Situ Forming Gel. Gels 2022, 8, 169. [Google Scholar] [CrossRef]
- Hosny, K.M.; Rizg, W.Y.; Khallaf, R.A. Preparation and Optimization of in Situ Gel Loaded with Rosuvastatin-Ellagic Acid Nanotransfersomes to Enhance the Anti-Proliferative Activity. Pharmaceutics 2020, 12, 263. [Google Scholar] [CrossRef]
- Haider, M.G. Ways to Prevent Infection after Open Fracture of the Lower Limb. Clujul Med. 2013, 86, 240–244. [Google Scholar] [PubMed]
- Hellwinkel, J.E.; Working, Z.M.; Certain, L.; García, A.J.; Wenke, J.C.; Bahney, C.S. The Intersection of Fracture Healing and Infection: Orthopaedics Research Society Workshop 2021. J. Orthop. Res. 2022, 40, 541–552. [Google Scholar] [CrossRef] [PubMed]
- Kumar, G.; Roger, P.M. From Crosstalk between Immune and Bone Cells to Bone Erosion in Infection. Int. J. Mol. Sci. 2019, 20, 5154. [Google Scholar] [CrossRef]
- Terkawi, M.A.; Matsumae, G.; Shimizu, T.; Takahashi, D.; Kadoya, K.; Iwasaki, N. Interplay between Inflammation and Pathological Bone Resorption: Insights into Recent Mechanisms and Pathways in Related Diseases for Future Perspectives. Int. J. Mol. Sci. 2022, 23, 1786. [Google Scholar] [CrossRef]
- Golestani, S.; Golestaneh, A.; Gohari, A.A. Comparative Effects of Systemic Administration of Levofloxacin and Cephalexin on Fracture Healing in Rats. J. Korean Assoc. Oral Maxillofac. Surg. 2022, 48, 94–100. [Google Scholar] [CrossRef]
- Ihn, H.J.; Lee, T.; Lee, D.; Bae, J.S.; Kim, S.H.; Jang, I.H.; Bae, Y.C.; Shin, H.I.; Park, E.K. Inhibitory Effect of KP-A038 on Osteoclastogenesis and Inflammatory Bone Loss Is Associated with Downregulation of BLIMP1. Front. Pharmacol. 2019, 10, 367. [Google Scholar] [CrossRef]
- Heersche, J.N.M.; Jez, D.H. The Effect of Imidazole and Imidazole-Analogues on Bone Resorption in Vitro: A Suggested Role for Thromboxane A2. Prostaglandins 1981, 21, 401–411. [Google Scholar] [CrossRef]
- Chen, T.; Knapp, A.C.; Wu, Y.; Huang, J.; Lynch, J.S.; Dickson, J.K.; Lawrence, R.M.; Feyen, J.H.M.; Agler, M.L. High Throughput Screening Identified a Substituted Imidazole as a Novel RANK Pathway-Selective Osteoclastogenesis Inhibitor. Assay Drug Dev. Technol. 2006, 4, 387–396. [Google Scholar] [CrossRef] [PubMed]
- Bryer, H.P.; Isserow, J.A.; Armstrong, E.C.; Mann, G.N.; Rucinski, B.; Buchinsky, F.J.; Romero, D.F.; Epstein, S. AzaThioprine Alone Is Bone Sparing and Does Not Alter Cyclosporin A-induced Osteopenia in the Rat. J. Bone Miner. Res. 1995, 10, 132–138. [Google Scholar] [CrossRef]
- Morgan, S.; Hooper, K.M.; Milne, E.M.; Farquharson, C.; Stevens, C.; Staines, K.A. Azathioprine Has a Deleterious Effect on the Bone Health of Mice with DSS-Induced Inflammatory Bowel Disease. Int. J. Mol. Sci. 2019, 20, 6085. [Google Scholar] [CrossRef]
- Nelson, A.L.; Fontana, G.L.; Miclau, E.; Rongstad, M.; Murphy, W.; Huard, J.; Ehrhart, N.; Bahney, C. Therapeutic Approaches to Activate the Canonical Wnt Pathway for Bone Regeneration. J. Tissue Eng. Regen. Med. 2022, 16, 961–976. [Google Scholar] [CrossRef] [PubMed]
- Leanza, G.; Fontana, F.; Lee, S.Y.; Remedi, M.S.; Schott, C.; Ferron, M.; Hamilton-Hall, M.; Alippe, Y.; Strollo, R.; Napoli, N.; et al. Gain-of-Function Lrp5 Mutation Improves Bone Mass and Strength and Delays Hyperglycemia in a Mouse Model of Insulin-Deficient Diabetes. J. Bone Miner. Res. 2021, 36, 1403–1415. [Google Scholar] [CrossRef] [PubMed]
- Joiner, D.M.; Ke, J.; Zhong, Z.; Xu, H.E.; Williams, B.O. LRP5 and LRP6 in Development and Disease. Trends Endocrinol. Metab. 2013, 24, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Arya, P.N.; Saranya, I.; Selvamurugan, N. Crosstalk between Wnt and Bone Morphogenetic Protein Signaling during Osteogenic Differentiation. World J. Stem Cells 2024, 16, 102–113. [Google Scholar] [CrossRef] [PubMed]
- Phimphilai, M.; Zhao, Z.; Boules, H.; Roca, H.; Franceschi, R.T. BMP Signaling Is Required for RUNX2-Dependent Induction of the Osteoblast Phenotype. J. Bone Miner. Res. 2006, 21, 637–646. [Google Scholar] [CrossRef]
- Bellei, B.; Pitisci, A.; Izzo, E.; Picardo, M. Inhibition of Melanogenesis by the Pyridinyl Imidazole Class of Compounds: Possible Involvement of the Wnt/β-Catenin Signaling Pathway. PLoS ONE 2012, 7, e33021. [Google Scholar] [CrossRef] [PubMed]
- Garg, A.; Agrawal, R.; Singh Chauhan, C.; Deshmukh, R. In-Situ Gel: A Smart Carrier for Drug Delivery. Int. J. Pharm. 2024, 652, 123819. [Google Scholar] [CrossRef]
- Madan, M.; Bajaj, A.; Lewis, S.; Udupa, N.; Baig, J.A. In Situ Forming Polymeric Drug Delivery Systems. Indian J. Pharm. Sci. 2009, 71, 242–251. [Google Scholar] [CrossRef]
- Dash, S.; Murthy, P.N.; Nath, L.; Chowdhury, P. Kinetic Modeling on Drug Release from Controlled Drug Delivery Systems. Acta Pol. Pharm. Drug Res. 2010, 67, 217–223. [Google Scholar]
- Mircioiu, C.; Voicu, V.; Anuta, V.; Tudose, A.; Celia, C.; Paolino, D.; Fresta, M.; Sandulovici, R.; Mircioiu, I. Mathematical Modeling of Release Kinetics from Supramolecular Drug Delivery Systems. Pharmaceutics 2019, 11, 140. [Google Scholar] [CrossRef]
- Tian, Y.; Shen, Y.; Jv, M. Synthesis, Characterization and Evaluation of Tinidazole-Loaded MPEG–PDLLA (10/90) in Situ Gel Forming System for Periodontitis Treatment. Drug Deliv. 2016, 23, 2726–2735. [Google Scholar] [CrossRef] [PubMed]
- Chu, B.; Zhang, L.; Qu, Y.; Chen, X.; Peng, J.; Huang, Y.; Qian, Z. Synthesis, Characterization and Drug Loading Property of Monomethoxy-Poly(Ethylene Glycol)-Poly(ϵ-Caprolactone)-Poly(D,L-Lactide) (MPEG-PCLA) Copolymers. Sci. Rep. 2016, 6, 34069. [Google Scholar] [CrossRef] [PubMed]
| Gene Name | Primer Sequence |
|---|---|
| Species: Rat | |
| Runx2 | F-CCACAGAGCTATTAAAGTGACAGTG |
| R-AACAAACTAGGTTTAGAGTCATCAAGC | |
| BMP2 | F-CGGACTGCGGTCTCCTAA |
| R-GGGGAAGCAGCAACACTAGA | |
| TRAP | F-CAGGGACGGGAGAGATTGG |
| R-GCTGTACAGTGAGCCAGGA | |
| GAPDH | F-TGGGAAGCTGGTCATCAAC |
| R-GCATCACCCCATTTGATGTT | |
| Species: Mouse | |
| Runx2 | F-TTGACCTTTGTCCCAATGC |
| R-AGGTTGGAGGCACACATAGG | |
| BMP2 | F-AGATCTGTACCGCAGGCACT |
| R-GTTCCTCCACGGCTTCTTC | |
| GAPDH | F-AGGGATGCTGCCCTTACC |
| R-TCTACGGGACGAGGAAACAC | |
| Compounds | Activity in ALP Assay | Structure |
|---|---|---|
| Etanidazole | Positive (EC50: 400 nM) |  |
| Secnidazole | Negative (EC50: >10 μM) | 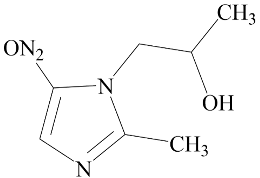 |
| Ronidazole | Negative (EC50: >10 μM) | 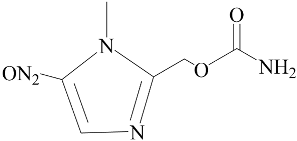 |
| Metronidazole | Positive (EC50: 1.2 nM) | 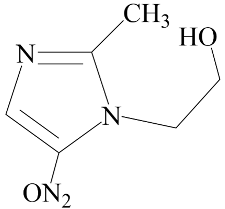 |
| Tinidazole | Negative (EC50: >10 μM) | 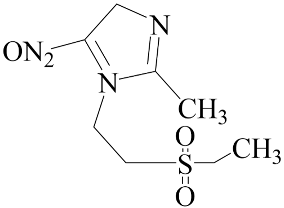 |
| Ornidazole | Negative (EC50: >10 μM) | 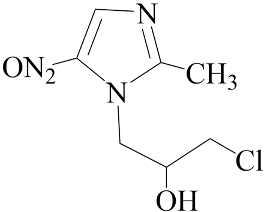 |
| Release Kinetics | Parameters | MTZ in PBS | MTZ@In Situ Gel in PBS |
|---|---|---|---|
| Zero-order | K0 | 4.77 ± 0.05 | 2.43 ± 0.04 |
| R2 | 0.76 ± 0.01 | 0.83 ± 0.04 | |
| First-order | K1 | 0.08 ± 0.00 | 0.03 ± 0.00 |
| R2 | 0.84 ± 0.00 | 0.90 ± 0.03 | |
| Higuchi | kH | 16.34 ± 0.12 | 8.27 ± 0.18 |
| R2 | 0.71 ± 0.00 | 0.76 ± 0.01 | |
| Korsmeyer–Peppas | kKP | 8.64 ± 0.11 | 4.15 ± 0.46 |
| n | 0.78 ± 0.01 | 0.80 ± 0.04 | |
| R2 | 0.81 ± 0.01 | 0.87 ± 0.03 | |
| Hixson–Crowell | kHC | 0.02 ± 0.00 | 0.01 ± 0.00 |
| R2 | 0.86 ± 0.01 | 0.88 ± 0.03 |
| PK Parameter | MTZ | MTZ@In Situ Gel |
|---|---|---|
| t1/2 (h) | 4.39 ± 0.26 | 7.34 ± 1.37 |
| Tmax (h) | 1.75 ± 0.25 | 2.00 ± 0.00 |
| Cmax (µg/mL) | 22.99 ± 0.87 | 40.40 ± 2.33 *** |
| AUC 0–∞ (h µg/mL) | 205.84 ± 7.05 | 355.71 ± 22.10 *** |
| Vd (L/kg) | 4.91 ± 0.15 | 4.95 ± 1.11 |
| Cl (L/h/kg) | 0.78 ± 0.03 | 0.45 ± 0.03 *** |
| MRT (h) | 7.63 ± 0.30 | 7.44 ± 0.27 |
| Relative bioavailability (%F) | 100 | 172.62 ± 7.29 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duggal, S.; Sharma, S.; Rai, N.; Chauhan, D.; Upadhyay, V.; Srivastava, S.; Porwal, K.; Kulkarni, C.; Trivedi, A.K.; Gayen, J.R.; et al. Anti-Microbial Drug Metronidazole Promotes Fracture Healing: Enhancement in the Bone Regenerative Efficacy of the Drug by a Biodegradable Sustained-Release In Situ Gel Formulation. Biomedicines 2024, 12, 1603. https://doi.org/10.3390/biomedicines12071603
Duggal S, Sharma S, Rai N, Chauhan D, Upadhyay V, Srivastava S, Porwal K, Kulkarni C, Trivedi AK, Gayen JR, et al. Anti-Microbial Drug Metronidazole Promotes Fracture Healing: Enhancement in the Bone Regenerative Efficacy of the Drug by a Biodegradable Sustained-Release In Situ Gel Formulation. Biomedicines. 2024; 12(7):1603. https://doi.org/10.3390/biomedicines12071603
Chicago/Turabian StyleDuggal, Shivali, Shivani Sharma, Nikhil Rai, Divya Chauhan, Vishal Upadhyay, Swati Srivastava, Konica Porwal, Chirag Kulkarni, Arun K. Trivedi, Jiaur R. Gayen, and et al. 2024. "Anti-Microbial Drug Metronidazole Promotes Fracture Healing: Enhancement in the Bone Regenerative Efficacy of the Drug by a Biodegradable Sustained-Release In Situ Gel Formulation" Biomedicines 12, no. 7: 1603. https://doi.org/10.3390/biomedicines12071603
APA StyleDuggal, S., Sharma, S., Rai, N., Chauhan, D., Upadhyay, V., Srivastava, S., Porwal, K., Kulkarni, C., Trivedi, A. K., Gayen, J. R., Mishra, P. R., Chattopadhyay, N., & Pal, S. (2024). Anti-Microbial Drug Metronidazole Promotes Fracture Healing: Enhancement in the Bone Regenerative Efficacy of the Drug by a Biodegradable Sustained-Release In Situ Gel Formulation. Biomedicines, 12(7), 1603. https://doi.org/10.3390/biomedicines12071603






