Exploring Synergistic Effects of Bioprinted Extracellular Vesicles for Skin Regeneration
Abstract
1. Introduction
2. Extracellular Vesicles (EVs)
3. Skin Regeneration
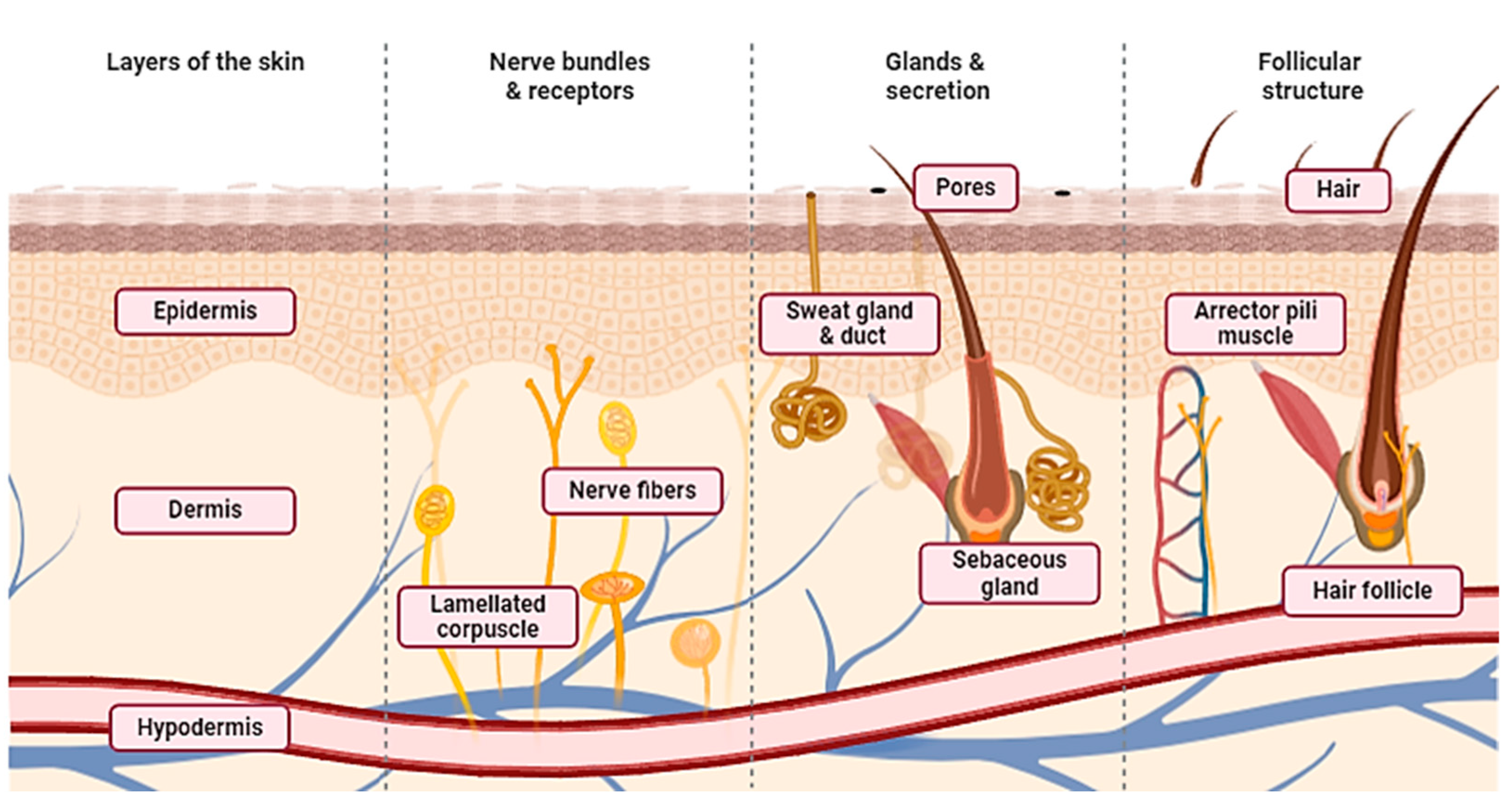
3.1. Skin
3.2. Wound Healing
3.3. Role of EVs from Different Cellular Origins for Wound Healing
3.4. Role of GF in EVs for Wound Healing
4. 3D Bioprinting
4.1. Uses of 3D Bioprinting for Skin Regeneration
4.2. Combining Imaging Techniques and AI with 3D Bioprinting
5. Future Direction and Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Carter, M.J.; DaVanzo, J.; Haught, R.; Nusgart, M.; Cartwright, D.; Fife, C.E. Chronic Wound Prevalence and the Associated Cost of Treatment in Medicare Beneficiaries: Changes between 2014 and 2019. J. Med. Econ. 2023, 26, 894–901. [Google Scholar] [CrossRef] [PubMed]
- Raziyeva, K.; Kim, Y.; Zharkinbekov, Z.; Kassymbek, K.; Jimi, S.; Saparov, A. Immunology of Acute and Chronic Wound Healing. Biomolecules 2021, 11, 700. [Google Scholar] [CrossRef]
- Sen, C.K. Human Wound and Its Burden: Updated 2020 Compendium of Estimates. Adv. Wound Care 2021, 10, 281–292. [Google Scholar] [CrossRef] [PubMed]
- Hama, R.; Reinhardt, J.W.; Ulziibayar, A.; Watanabe, T.; Kelly, J.; Shinoka, T. Recent Tissue Engineering Approaches to Mimicking the Extracellular Matrix Structure for Skin Regeneration. Biomimetics 2023, 8, 130. [Google Scholar] [CrossRef]
- Saini, G.; Segaran, N.; Mayer, J.L.; Saini, A.; Albadawi, H.; Oklu, R. Applications of 3D Bioprinting in Tissue Engineering and Regenerative Medicine. J. Clin. Med. 2021, 10, 4966. [Google Scholar] [CrossRef]
- Schäfer, N.; Grässel, S. New Refinements Aim to Optimize Articular Cartilage Tissue Engineering. Nat. Rev. Rheumatol. 2023, 19, 66–67. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.Y.; Xiang, Y.; Tang, M.; Chen, S. 3D Printing Approaches to Engineer Cardiac Tissue. Curr. Cardiol. Rep. 2023, 25, 505–514. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, S.; Mandal, S.S.; Bauri, S.; Maiti, P. 3D Bioprinting and Its Innovative Approach for Biomedical Applications. MedComm 2022, 4, e194. [Google Scholar] [CrossRef] [PubMed]
- Umur, E.; Bayrak, E.; Arslan, F.; Bulut, S.B.; Baysoy, E.; Kaleli-Can, G.; Ayan, B. Advances in Three Dimensional Bioprinting for Wound Healing: A Comprehensive Review. Appl. Sci. 2023, 13, 10269. [Google Scholar] [CrossRef]
- Wang, S.; Lin, Y.; Zhang, Y.; Qiu, X.; Pan, Y.; Yeung, S.-C.J.; Zhang, H. Complex RNA World in Small Extracellular Vesicles for Liquid Biopsy in Cancer Management. Extracell. Vesicle 2022, 1, 100015. [Google Scholar] [CrossRef]
- Murphy, S.V.; De Coppi, P.; Atala, A. Opportunities and Challenges of Translational 3D Bioprinting. Nat. Biomed. Eng. 2020, 4, 370–380. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, I.K.; Wood, M.J.A.; Fuhrmann, G. Extracellular Vesicles as a Next-Generation Drug Delivery Platform. Nat. Nanotechnol. 2021, 16, 748–759. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Zhang, B.; Zhai, D.; Wu, C. Three-Dimensional Printing of Bioceramic-Induced Macrophage Exosomes: Immunomodulation and Osteogenesis/Angiogenesis. NPG Asia Mater. 2021, 13, 72. [Google Scholar] [CrossRef]
- Doyle, L.M.; Wang, M.Z. Overview of Extracellular Vesicles, Their Origin, Composition, Purpose, and Methods for Exosome Isolation and Analysis. Cells 2019, 8, 727. [Google Scholar] [CrossRef]
- Jiao, K.; Walsh, L.J.; Ivanovski, S.; Han, P. The Emerging Regulatory Role of Circular RNAs in Periodontal Tissues and Cells. Int. J. Mol. Sci. 2021, 22, 4636. [Google Scholar] [CrossRef] [PubMed]
- Kowal, J.; Tkach, M.; Théry, C. Biogenesis and Secretion of Exosomes. Curr. Opin. Cell Biol. 2014, 29, 116–125. [Google Scholar] [CrossRef] [PubMed]
- Carnino, J.M.; Lee, H.; Jin, Y. Isolation and Characterization of Extracellular Vesicles from Broncho-Alveolar Lavage Fluid: A Review and Comparison of Different Methods. Respir. Res. 2019, 20, 240. [Google Scholar] [CrossRef] [PubMed]
- De Sousa, K.P.; Rossi, I.; Abdullahi, M.; Ramirez, M.I.; Stratton, D.; Inal, J.M. Isolation and Characterization of Extracellular Vesicles and Future Directions in Diagnosis and Therapy. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2023, 15, e1835. [Google Scholar] [CrossRef] [PubMed]
- Sidhom, K.; Obi, P.O.; Saleem, A. A Review of Exosomal Isolation Methods: Is Size Exclusion Chromatography the Best Option? Int. J. Mol. Sci. 2020, 21, 6466. [Google Scholar] [CrossRef]
- Davies, R.; Allen, S.; Mennan, C.; Platt, M.; Wright, K.; Kehoe, O. Extracellular Vesicle Depletion Protocols of Foetal Bovine Serum Influence Umbilical Cord Mesenchymal Stromal Cell Phenotype, Immunomodulation, and Particle Release. Int. J. Mol. Sci. 2023, 24, 9242. [Google Scholar] [CrossRef]
- Lehrich, B.M.; Liang, Y.; Khosravi, P.; Federoff, H.J.; Fiandaca, M.S. Fetal Bovine Serum-Derived Extracellular Vesicles Persist within Vesicle-Depleted Culture Media. Int. J. Mol. Sci. 2018, 19, 3538. [Google Scholar] [CrossRef] [PubMed]
- Welsh, J.A.; Goberdhan, D.C.I.; O’Driscoll, L.; Buzas, E.I.; Blenkiron, C.; Bussolati, B.; Cai, H.; Di Vizio, D.; Driedonks, T.A.P.; Erdbrügger, U.; et al. Minimal Information for Studies of Extracellular Vesicles (MISEV2023): From Basic to Advanced Approaches. J. Extracell. Vesicles 2024, 13, e12404. [Google Scholar] [CrossRef] [PubMed]
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal Information for Studies of Extracellular Vesicles 2018 (MISEV2018): A Position Statement of the International Society for Extracellular Vesicles and Update of the MISEV2014 Guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Koehler, K.R. Skin Organoids: A New Human Model for Developmental and Translational Research. Exp. Dermatol. 2021, 30, 613–620. [Google Scholar] [CrossRef] [PubMed]
- Tavakoli, S.; Klar, A.S. Advanced Hydrogels as Wound Dressings. Biomolecules 2020, 10, 1169. [Google Scholar] [CrossRef] [PubMed]
- Okur, M.E.; Karantas, I.D.; Şenyiğit, Z.; Üstündağ Okur, N.; Siafaka, P.I. Recent Trends on Wound Management: New Therapeutic Choices Based on Polymeric Carriers. Asian J. Pharm. Sci. 2020, 15, 661–684. [Google Scholar] [CrossRef] [PubMed]
- Chhabra, S.; Chhabra, N.; Kaur, A.; Gupta, N. Wound Healing Concepts in Clinical Practice of OMFS. J. Maxillofac. Oral Surg. 2017, 16, 403–423. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.; Russo, J.; Fiegel, J.; Brogden, N. Antibiotic Delivery Strategies to Treat Skin Infections When Innate Antimicrobial Defense Fails. Antibiotics 2020, 9, 56. [Google Scholar] [CrossRef] [PubMed]
- Scopelliti, F.; Cattani, C.; Dimartino, V.; Mirisola, C.; Cavani, A. Platelet Derivatives and the Immunomodulation of Wound Healing. Int. J. Mol. Sci. 2022, 23, 8370. [Google Scholar] [CrossRef]
- Kendall, R.T.; Feghali-Bostwick, C.A. Fibroblasts in Fibrosis: Novel Roles and Mediators. Front. Pharmacol. 2014, 5, 123. [Google Scholar] [CrossRef]
- Hofmann, E.; Fink, J.; Pignet, A.L.; Schwarz, A.; Schellnegger, M.; Nischwitz, S.P.; Holzer-Geissler, J.C.J.; Kamolz, L.P.; Kotzbeck, P. Human In Vitro Skin Models for Wound Healing and Wound Healing Disorders. Biomedicines 2023, 11, 1056. [Google Scholar] [CrossRef] [PubMed]
- Schilrreff, P.; Alexiev, U. Chronic Inflammation in Non-Healing Skin Wounds and Promising Natural Bioactive Compounds Treatment. Int. J. Mol. Sci. 2022, 23, 4928. [Google Scholar] [CrossRef]
- Zhang, W.; Ling, Y.; Sun, Y.; Xiao, F.; Wang, L. Extracellular Vesicles Derived from Mesenchymal Stem Cells Promote Wound Healing and Skin Regeneration by Modulating Multiple Cellular Changes: A Brief Review. Genes 2023, 14, 1516. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Wang, T.; Tian, H.; Wei, G.; Zhao, L.; Shi, Y. Macrophage-Derived Exosomes Accelerate Wound Healing through Their Anti-Inflammation Effects in a Diabetic Rat Model. Artif. Cells Nanomed. Biotechnol. 2019, 47, 3793–3803. [Google Scholar] [CrossRef] [PubMed]
- Cao, W.; Meng, X.; Cao, F.; Wang, J.; Yang, M. Exosomes Derived from Platelet-Rich Plasma Promote Diabetic Wound Healing via the JAK2/STAT3 Pathway. iScience 2023, 26, 108236. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Lin, Y.; Huang, Y.; Zhu, N.; Shen, Y.-Q. Extracellular vesicles modulate key signalling pathways in refractory wound healing. Burn. Trauma 2023, 11, tkad039. [Google Scholar] [CrossRef] [PubMed]
- Poniatowski, L.A.; Wojdasiewicz, P.; Gasik, R.; Szukiewicz, D. Transforming Growth Factor Beta Family: Insight into the Role of Growth Factors in Regulation of Fracture Healing Biology and Potential Clinical Applications. Mediators Inflamm. 2015, 2015, 137823. [Google Scholar] [CrossRef] [PubMed]
- Miricescu, D.; Badoiu, S.C.; Stanescu-Spinu, I.I.; Ripszky Totan, A.; Stefani, C.; Greabu, M. Growth Factors, Reactive Oxygen Species, and Metformin-Promoters of the Wound Healing Process in Burns? Int. J. Mol. Sci. 2021, 22, 9512. [Google Scholar] [CrossRef]
- Chen, K.; Rao, Z.; Dong, S.; Chen, Y.; Wang, X.; Luo, Y.; Gong, F.; Li, X. Roles of the Fibroblast Growth Factor Signal Transduction System in Tissue Injury Repair. Burn. Trauma 2022, 10, tkac005. [Google Scholar] [CrossRef]
- Liu, Z.L.; Chen, H.H.; Zheng, L.L.; Sun, L.P.; Shi, L. Angiogenic Signaling Pathways and Anti-Angiogenic Therapy for Cancer. Signal Transduct. Target. Ther. 2023, 8, 198. [Google Scholar] [CrossRef]
- Garoufalia, Z.; Papadopetraki, A.; Karatza, E.; Vardakostas, D.; Philippou, A.; Kouraklis, G.; Mantas, D. Insulin-like Growth Factor-I and Wound Healing, a Potential Answer to Non-Healing Wounds: A Systematic Review of the Literature and Future Perspectives. Biomed. Rep. 2021, 15, 66. [Google Scholar] [CrossRef] [PubMed]
- Ji, S.; Guvendiren, M. Recent Advances in Bioink Design for 3D Bioprinting of Tissues and Organs. Front. Bioeng. Biotechnol. 2017, 5, 23. [Google Scholar] [CrossRef] [PubMed]
- Kačarević, Ž.P.; Rider, P.M.; Alkildani, S.; Retnasingh, S.; Smeets, R.; Jung, O.; Ivanišević, Z.; Barbeck, M. An Introduction to 3D Bioprinting: Possibilities, Challenges and Future Aspects. Materials 2018, 11, 2199. [Google Scholar] [CrossRef] [PubMed]
- Murphy, S.V.; Atala, A. 3D Bioprinting of Tissues and Organs. Nat. Biotechnol. 2014, 32, 773–785. [Google Scholar] [CrossRef]
- Ramiah, P.; du Toit, L.C.; Choonara, Y.E.; Kondiah, P.P.D.; Pillay, V. Hydrogel-Based Bioinks for 3D Bioprinting in Tissue Regeneration. Front. Mater. 2020, 7, 506968. [Google Scholar] [CrossRef]
- Benwood, C.; Chrenek, J.; Kirsch, R.L.; Masri, N.Z.; Richards, H.; Teetzen, K.; Willerth, S.M. Natural Biomaterials and Their Use as Bioinks for Printing Tissues. Bioengineering 2021, 8, 27. [Google Scholar] [CrossRef] [PubMed]
- Ratnamani, M.P.; Zhang, X.; Wang, H. A Comprehensive Assessment on the Pivotal Role of Hydrogels in Scaffold-Based Bioprinting. Gels 2022, 8, 239. [Google Scholar] [CrossRef] [PubMed]
- Xie, M.; Su, J.; Zhou, S.; Li, J.; Zhang, K. Application of Hydrogels as Three-Dimensional Bioprinting Ink for Tissue Engineering. Gels 2023, 9, 88. [Google Scholar] [CrossRef]
- Bercea, M. Rheology as a Tool for Fine-Tuning the Properties of Printable Bioinspired Gels. Molecules 2023, 28, 2766. [Google Scholar] [CrossRef]
- Bom, S.; Ribeiro, R.; Ribeiro, H.M.; Santos, C.; Marto, J. On the Progress of Hydrogel-Based 3D Printing: Correlating Rheological Properties with Printing Behaviour. Int. J. Pharm. 2022, 615, 121506. [Google Scholar] [CrossRef]
- Jiao, K.; Liu, C.; Basu, S.; Raveendran, N.; Nakano, T.; Ivanovski, S.; Han, P. Bioprinting Extracellular Vesicles as a “Cell-Free” Regenerative Medicine Approach. Extracell Vesicle Circ. Nucleic Acids 2023, 4, 218–239. [Google Scholar] [CrossRef]
- Shafei, S.; Khanmohammadi, M.; Heidari, R.; Ghanbari, H.; Taghdiri Nooshabadi, V.; Farzamfar, S.; Akbariqomi, M.; Sanikhani, N.S.; Absalan, M.; Tavoosidana, G. Exosome Loaded Alginate Hydrogel Promotes Tissue Regeneration in Full-Thickness Skin Wounds: An in Vivo Study. J. Biomed. Mater. Res. Part A 2020, 108, 545–556. [Google Scholar] [CrossRef]
- Lim, W.; Shin, S.Y.; Cha, J.M.; Bae, H. Optimization of Polysaccharide Hydrocolloid for the Development of Bioink with High Printability/Biocompatibility for Coextrusion 3D Bioprinting. Polymers 2021, 13, 1773. [Google Scholar] [CrossRef] [PubMed]
- Ribezzi, D.; Pinos, R.; Bonetti, L.; Cellani, M.; Barbaglio, F.; Scielzo, C.; Farè, S. Design of a Novel Bioink Suitable for the 3D Printing of Lymphoid Cells. Front. Biomater. Sci. 2023, 2, 1081065. [Google Scholar] [CrossRef]
- Lima, T.D.P.; Canelas, C.A.D.A.; Concha, V.O.; Costa, F.A.D.; Passos, M.F. 3D Bioprinting Technology and Hydrogels Used in the Process. J. Funct. Biomater. 2022, 13, 214. [Google Scholar] [CrossRef] [PubMed]
- Song, D.; Xu, Y.; Liu, S.; Wen, L.; Wang, X. Progress of 3D Bioprinting in Organ Manufacturing. Polymers 2021, 13, 3178. [Google Scholar] [CrossRef]
- Liu, N.; Zhang, X.; Guo, Q.; Wu, T.; Wang, Y. 3D Bioprinted Scaffolds for Tissue Repair and Regeneration. Front. Mater. 2022, 9, 925321. [Google Scholar] [CrossRef]
- Palmara, G.; Frascella, F.; Roppolo, I.; Chiappone, A.; Chiadò, A. Functional 3D Printing: Approaches and Bioapplications. Biosens. Bioelectron. 2021, 175, 112849. [Google Scholar] [CrossRef] [PubMed]
- Kammona, O.; Tsanaktsidou, E.; Kiparissides, C. Recent Developments in 3D-(Bio)Printed Hydrogels as Wound Dressings. Gels 2024, 10, 147. [Google Scholar] [CrossRef]
- Tsegay, F.; Elsherif, M.; Butt, H. Smart 3D Printed Hydrogel Skin Wound Bandages: A Review. Polymers 2022, 14, 1012. [Google Scholar] [CrossRef]
- Li, F.X.Z.; Lin, X.; Xu, F.; Shan, S.K.; Guo, B.; Lei, L.M.; Zheng, M.H.; Wang, Y.; Xu, Q.S.; Yuan, L.Q. The Role of Mesenchymal Stromal Cells-Derived Small Extracellular Vesicles in Diabetes and Its Chronic Complications. Front. Endocrinol. 2021, 12, 780974. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.; Chen, P.; Zhang, H.; Weng, H.; Fang, Z.; Chen, C.; Peng, G.; Gao, H.; Hu, K.; Chen, J.; et al. Comparison of Curative Effect of Human Umbilical Cord-Derived Mesenchymal Stem Cells and Their Small Extracellular Vesicles in Treating Osteoarthritis. Int. J. Nanomed. 2021, 16, 8185–8202. [Google Scholar] [CrossRef] [PubMed]
- Han, P.; Raveendran, N.; Liu, C.; Basu, S.; Jiao, K.; Johnson, N.; Moran, C.S.; Ivanovski, S. 3D Bioprinted Small Extracellular Vesicles from Periodontal Cells Enhance Mesenchymal Stromal Cell Function. Biomater. Adv. 2024, 158, 213770. [Google Scholar] [CrossRef] [PubMed]
- Gu, Z.; Fu, J.; Lin, H.; He, Y. Development of 3D Bioprinting: From Printing Methods to Biomedical Applications. Asian J. Pharm. Sci. 2020, 15, 529–557. [Google Scholar] [CrossRef]
- Hernandez, J.L.; Woodrow, K.A. Medical Applications of Porous Biomaterials: Features of Porosity and Tissue-Specific Implications for Biocompatibility. Adv. Healthc. Mater. 2022, 11, 2102087. [Google Scholar] [CrossRef]
- Agarwal, S.; Saha, S.; Balla, V.K.; Pal, A.; Barui, A.; Bodhak, S. Current Developments in 3D Bioprinting for Tissue and Organ Regeneration—A Review. Front. Mech. Eng. 2020, 6, 589171. [Google Scholar] [CrossRef]
- Jain, P.; Kathuria, H.; Dubey, N. Advances in 3D Bioprinting of Tissues/Organs for Regenerative Medicine and in-Vitro Models. Biomaterials 2022, 287, 121639. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.J.; Kim, L.; Kwon, O.S. Application of 3D Bioprinting Technology for Tissue Regeneration, Drug Evaluation, and Drug Delivery. Appl. Sci. Converg. Technol. 2023, 32, 1–6. [Google Scholar] [CrossRef]
- Mani, M.P.; Sadia, M.; Jaganathan, S.K.; Khudzari, A.Z.; Supriyanto, E.; Saidin, S.; Ramakrishna, S.; Ismail, A.F.; Faudzi, A.A.M. A Review on 3D Printing in Tissue Engineering Applications. J. Polym. Eng. 2022, 42, 243–265. [Google Scholar] [CrossRef]
- Xue, J.; Qin, C.; Wu, C. 3D Printing of Cell-Delivery Scaffolds for Tissue Regeneration. Regen. Biomater. 2023, 10, rbad032. [Google Scholar] [CrossRef]
- Bari, E.; Scocozza, F.; Perteghella, S.; Sorlini, M.; Auricchio, F.; Torre, M.L.; Conti, M. 3D Bioprinted Scaffolds Containing Mesenchymal Stem/Stromal Lyosecretome: Next Generation Controlled Release Device for Bone Regenerative Medicine. Pharmaceutics 2021, 13, 515. [Google Scholar] [CrossRef] [PubMed]
- Ferroni, L.; D’Amora, U.; Gardin, C.; Leo, S.; Dalla Paola, L.; Tremoli, E.; Giuliani, A.; Calzà, L.; Ronca, A.; Ambrosio, L.; et al. Stem Cell-Derived Small Extracellular Vesicles Embedded into Methacrylated Hyaluronic Acid Wound Dressings Accelerate Wound Repair in a Pressure Model of Diabetic Ulcer. J. Nanobiotechnol. 2023, 21, 469. [Google Scholar] [CrossRef]
- Zhong, Y.; Ma, H.; Lu, Y.; Cao, L.; Cheng, Y.Y.; Tang, X.; Sun, H.; Song, K. Investigation on Repairing Diabetic Foot Ulcer Based on 3D Bio-Printing Gel/DECM/Qcs Composite Scaffolds. Tissue Cell 2023, 85, 102213. [Google Scholar] [CrossRef] [PubMed]
- Bari, E.; Di Gravina, G.M.; Scocozza, F.; Perteghella, S.; Frongia, B.; Tengattini, S.; Segale, L.; Torre, M.L.; Conti, M. Silk Fibroin Bioink for 3D Printing in Tissue Regeneration: Controlled Release of MSC Extracellular Vesicles. Pharmaceutics 2023, 15, 383. [Google Scholar] [CrossRef] [PubMed]
- Bar, A.; Kryukov, O.; Cohen, S. Three-Dimensional Bio-Printed Cardiac Patch for Sustained Delivery of Extracellular Vesicles from the Interface. Gels 2022, 8, 769. [Google Scholar] [CrossRef] [PubMed]
- Bar, A.; Kryukov, O.; Etzion, S.; Cohen, S. Engineered Extracellular Vesicle-Mediated Delivery of MiR-199a-3p Increases the Viability of 3D-Printed Cardiac Patches. Int. J. Bioprinting 2023, 9, 316–330. [Google Scholar] [CrossRef] [PubMed]
- Born, L.J.; McLoughlin, S.T.; Dutta, D.; Mahadik, B.; Jia, X.; Fisher, J.P.; Jay, S.M. Sustained Released of Bioactive Mesenchymal Stromal Cell-Derived Extracellular Vesicles from 3D-Printed Gelatin Methacrylate Hydrogels. J. Biomed. Mater. Res. A 2022, 110, 1190–1198. [Google Scholar] [CrossRef] [PubMed]
- Maiullari, F.; Chirivì, M.; Costantini, M.; Ferretti, A.M.; Recchia, S.; Maiullari, S.; Milan, M.; Presutti, D.; Pace, V.; Raspa, M.; et al. In Vivo Organized Neovascularization Induced by 3D Bioprinted Endothelial-Derived Extracellular Vesicles. Biofabrication 2021, 13, 035014. [Google Scholar] [CrossRef] [PubMed]
- Lindner, N.; Blaeser, A. Scalable Biofabrication: A Perspective on the Current State and Future Potentials of Process Automation in 3D-Bioprinting Applications. Front. Bioeng. Biotechnol. 2022, 10, 855042. [Google Scholar] [CrossRef]
- Anisuzzaman, D.M.; Wang, C.; Rostami, B.; Gopalakrishnan, S.; Niezgoda, J.; Yu, Z. Image-Based Artificial Intelligence in Wound Assessment: A Systematic Review. Adv. Wound Care 2022, 11, 687–709. [Google Scholar] [CrossRef]
- Chairat, S.; Chaichulee, S.; Dissaneewate, T.; Wangkulangkul, P.; Kongpanichakul, L. AI-Assisted Assessment of Wound Tissue with Automatic Color and Measurement Calibration on Images Taken with a Smartphone. Healthcare 2023, 11, 273. [Google Scholar] [CrossRef]
- Reifs, D.; Casanova-Lozano, L.; Reig-Bolaño, R.; Grau-Carrion, S. Clinical Validation of Computer Vision and Artificial Intelligence Algorithms for Wound Measurement and Tissue Classification in Wound Care. Inform. Med. Unlocked 2023, 37, 101185. [Google Scholar] [CrossRef]



| Source | Origin | Role in Wound Healing |
|---|---|---|
| [33] | MSCs | Induce anti-inflammatory response in macrophages, promote angiogenesis, support epithelial recovery, regulate collagen production, and scar formation. |
| [34] | Macrophages | Reduce inflammation, enhance wound healing through angiogenesis and re-epithelialization, and modulate collagen synthesis to regulate scar formation. |
| [10] | Skin-related cells | Promote cell growth, angiogenesis, collagen synthesis, tissue remodeling, and wound contraction to facilitate effective wound healing. |
| [35] | Blood-derived | Enhance cell proliferation, migration, and tissue regeneration, expedite re-epithelialization and angiogenesis, and promote collagen synthesis to accelerate wound healing. |
| [36] | Endothelial cells | Influence endothelial cell activities, enhance microvascular density, regulate collagen deposition, and modulate angiogenesis, thereby impacting wound healing positively or negatively. |
| Growth Factors | Function |
|---|---|
| FGF Family (FGF-2, FGF-7, FGF-10) | Regulates fibroblast cell migration, angiogenesis, and wound repair signaling pathways |
| EGF | Stimulates keratinocyte migration, fibroblast function, and granulation tissue formation |
| TGF-β Family (TGF-β1) | Regulates mesenchymal cell functions, ECM production, and remodeling during wound healing |
| VEGF Family | Promotes angiogenesis and endothelial cell proliferation |
| IGF Family (IGF-1) | Mends wounds and empowers tissue fixing by empowering fibroblast and keratinocyte movement and improvement |
| KGF | Advances keratinocyte relocation and multiplication, which supports tissue recovery and reepithelization |
| GM-CSF | Controls inflammatory responses and cell movement to animate the development of fresh blood vessels and rush the mending of wounds |
| PDGF Family (PDGF-AA, PDGF-BB) | Advances fibroblast expansion, ECM blend, and myofibroblast differentiation |
| Study | Material | Improvement |
|---|---|---|
| [71] | Poly(ε-caprolactone) (PCL) and freeze-dried lyosecretome from MSC. | Homogeneous loading of protein and EVs and a controlled slow release. |
| [78] | GelMA bioink with EV from HUVEC cells. | The implant demonstrated in situ retention and formation of functional vasculature. |
| [75] | A cardiac patch composed of alginate sulfate (AlgS) and EVs. | Superior integration and sustained release of EVs. Better assimilation of patch into cardiac tissue. |
| [77] | GelMA bioinks with MSC EVs. | Sustained release of bioactive EVs and promoting angiogenesis. |
| [74] | Silk–alginate (SA-SF) hydrogel. | Improved shape fidelity, mechanical properties, and controlled release of bioactive molecules. |
| [72] | Hyaluronic acid derivatives (HA) and small EVs from human MSC. | Improved mechanical properties and residence time for wound dressings. |
| [76] | Alginate (LVG) and THP-1-derived activated macrophages. | Inclusion of EVs yielded superior cell viability and lower ratio of apoptotic CM. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taghdi, M.H.; Muttiah, B.; Chan, A.M.L.; Fauzi, M.B.; Law, J.X.; Lokanathan, Y. Exploring Synergistic Effects of Bioprinted Extracellular Vesicles for Skin Regeneration. Biomedicines 2024, 12, 1605. https://doi.org/10.3390/biomedicines12071605
Taghdi MH, Muttiah B, Chan AML, Fauzi MB, Law JX, Lokanathan Y. Exploring Synergistic Effects of Bioprinted Extracellular Vesicles for Skin Regeneration. Biomedicines. 2024; 12(7):1605. https://doi.org/10.3390/biomedicines12071605
Chicago/Turabian StyleTaghdi, Manal Hussein, Barathan Muttiah, Alvin Man Lung Chan, Mh Busra Fauzi, Jia Xian Law, and Yogeswaran Lokanathan. 2024. "Exploring Synergistic Effects of Bioprinted Extracellular Vesicles for Skin Regeneration" Biomedicines 12, no. 7: 1605. https://doi.org/10.3390/biomedicines12071605
APA StyleTaghdi, M. H., Muttiah, B., Chan, A. M. L., Fauzi, M. B., Law, J. X., & Lokanathan, Y. (2024). Exploring Synergistic Effects of Bioprinted Extracellular Vesicles for Skin Regeneration. Biomedicines, 12(7), 1605. https://doi.org/10.3390/biomedicines12071605









