Unveiling the Potential of Migrasomes: A Machine-Learning-Driven Signature for Diagnosing Acute Myocardial Infarction
Abstract
1. Introduction
2. Materials and Methods
2.1. Acquisition and Processing of Data
2.2. Development and Validation of a Migrasome-Related Signature (MS)
2.3. Comparison of the MS and Other Published Signatures
2.4. Pre-Processing of scRNA-Seq Data
2.5. Cell Type Annotation and Pseudo-Time Analysis
2.6. Mendelian Randomization (MR) Analysis
2.7. Candidate Drug Prediction and Molecular Docking
3. Results
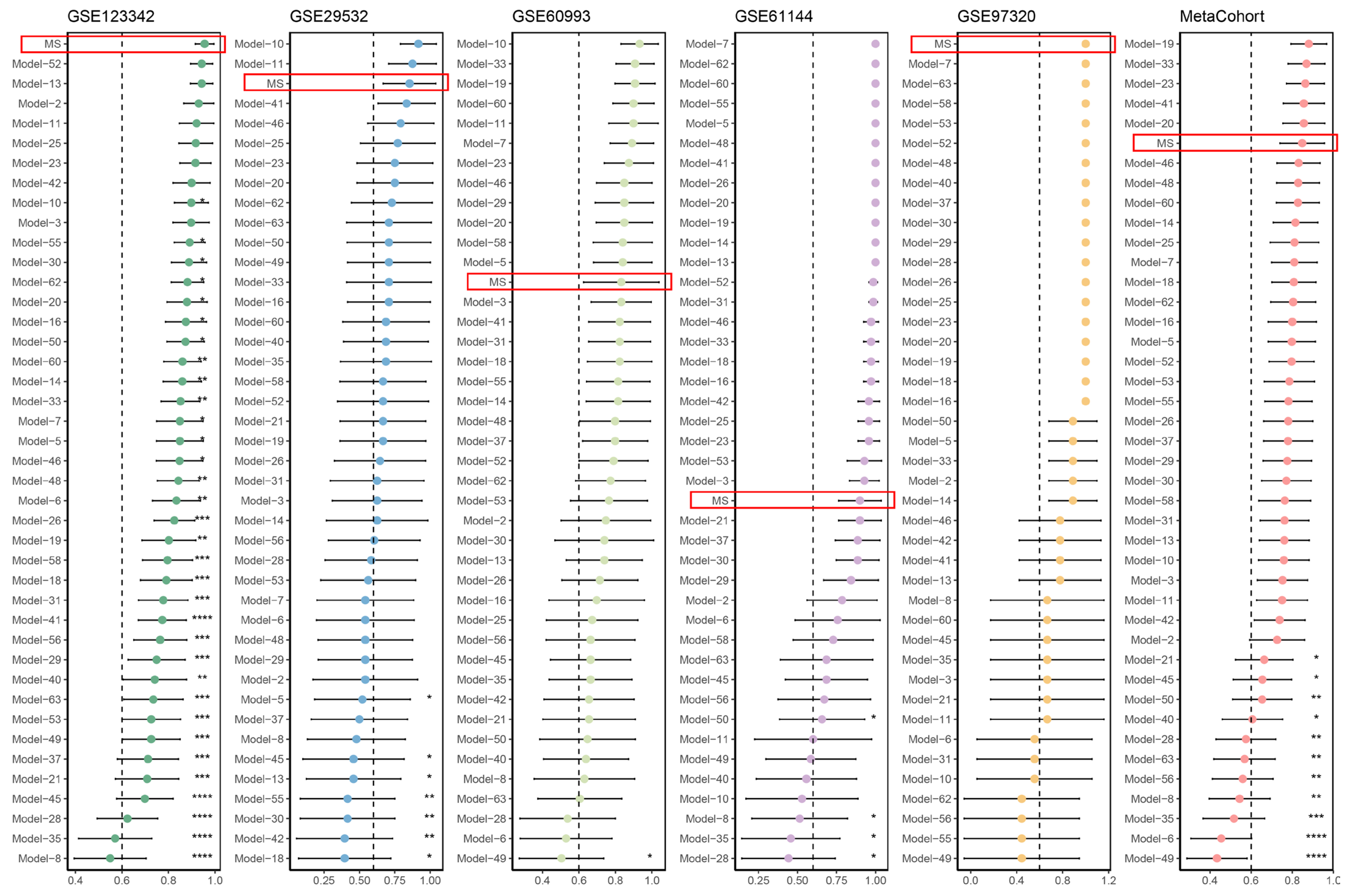
3.1. An Integrative ML-Based Framework Develops a Roust Migrasome-Related Signature (MS) to Predict AMI
3.2. Comparison of Predictive Performance between the MS and Published Signatures
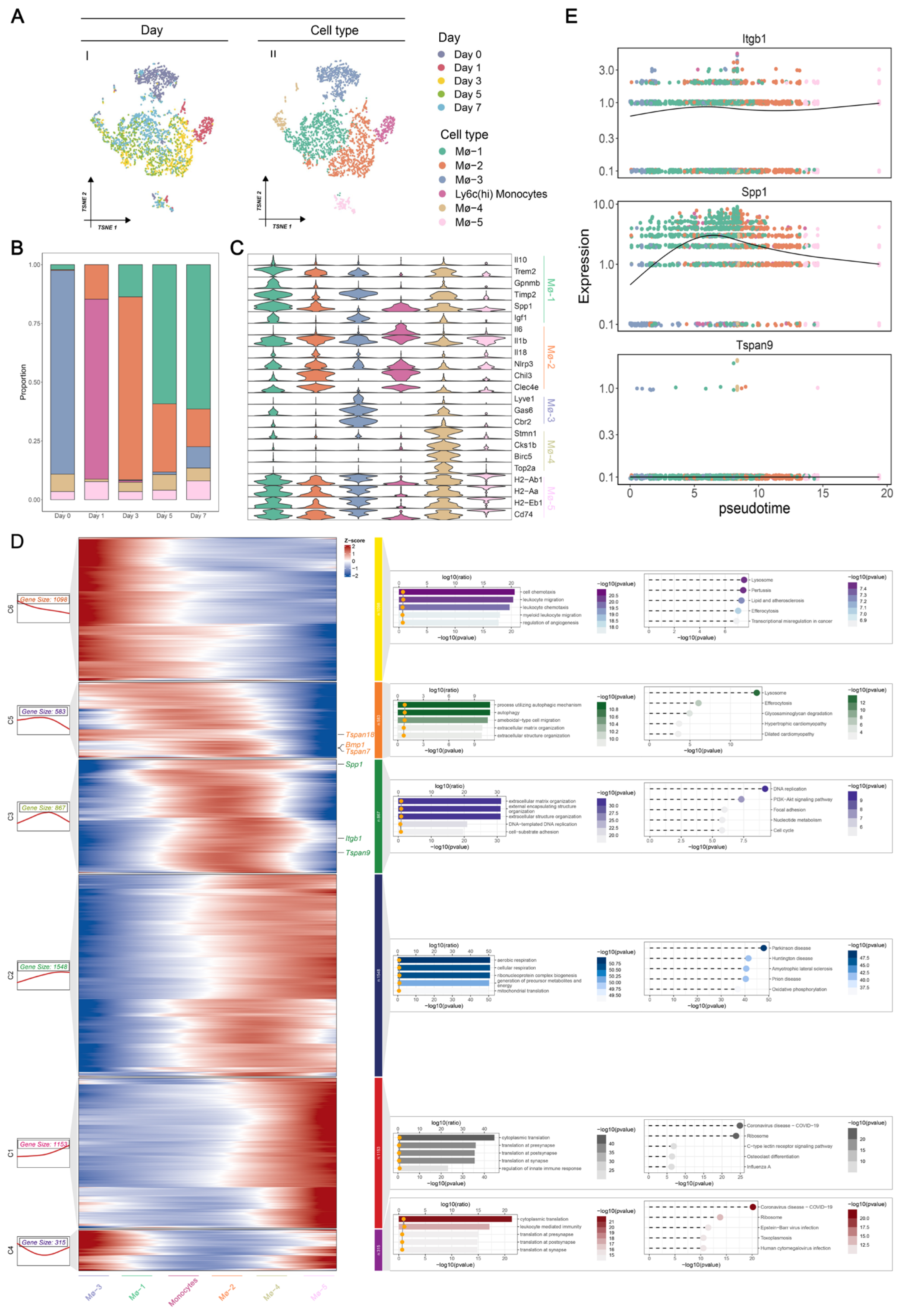
3.3. Macrophage-Specific Expression Patterns of MS in AMI at Single-Cell Resolution
3.4. Identification of Dynamic MS Genes during the Transition of Macrophages
3.5. Causal Effect and Druggability of ITGB1 on AMI
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xu, W.; Wang, L.; Zhang, R.; Sun, X.; Huang, L.; Su, H.; Wei, X.; Chen, C.-C.; Lou, J.; Dai, H.; et al. Diagnosis and prognosis of myocardial infarction on a plasmonic chip. Nat. Commun. 2020, 11, 1654. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, L.; Wang, S.; Cheng, H.; Xu, L.; Pei, G.; Wang, Y.; Fu, C.; Jiang, Y.; He, C.; et al. Signaling pathways and targeted therapy for myocardial infarction. Signal Transduct. Target. Ther. 2022, 7, 78. [Google Scholar] [CrossRef] [PubMed]
- Hasselbalch, R.; Sindet-Pedersen, C.; Pries-Heje, M.; Strandkjaer, N.; Kristensen, J.; Porsborg Andersen, M.; Torp-Pedersen, C.; Bundgaard, H.; Iversen, K. The specificity of cardiac troponin elevations for myocardial infarction declines with age. Eur. Heart J. 2023, 44, ehad655.1453. [Google Scholar] [CrossRef]
- Duque-Ossa, L.; García-Ferrera, B.; Reyes-Retana, J. Troponin I as a biomarker for early detection of acute myocardial infarction. Curr. Probl. Cardiol. 2023, 48, 101067. [Google Scholar] [CrossRef] [PubMed]
- Thygesen, K.; Alpert, J.; Jaffe, A.; Chaitman, B.; Bax, J.; Morrow, D.; White, H.; on behalf of the Joint European Society of Cardiology (ESC)/American College of Cardiology (ACC)/American Heart Association (AHA)/World Heart Federation (WHF) Task Force for the Universal Definition of Myocardial Infarction. Fourth Universal Definition of Myocardial Infarction (2018). Circulation 2018, 138, 97. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Li, Y.; Peng, J.; Wu, D.; Zhao, X.; Cui, Y.; Chen, L.; Yan, X.; Du, Y.; Yu, L. Discovery of the migrasome, an organelle mediating release of cytoplasmic contents during cell migration. Cell Res. 2015, 25, 24–38. [Google Scholar] [CrossRef]
- Zhang, Y.; Guo, W.; Bi, M.; Liu, W.; Zhou, L.; Liu, H.; Yan, F.; Guan, L.; Zhang, J.; Xu, J. Migrasomes: From biogenesis, release, uptake, rupture to homeostasis and diseases. Oxidative Med. Cell. Longev. 2022, 2022, 4525778. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.; Jiang, Z.; Lu, D.; Wang, X.; Liang, H.; Zhang, J.; Meng, Y.; Li, Y.; Wu, D.; Huang, Y.; et al. Migrasomes provide regional cues for organ morphogenesis during zebrafish gastrulation. Nat. Cell Biol. 2019, 21, 966–977. [Google Scholar] [CrossRef] [PubMed]
- Jiao, H.; Jiang, D.; Hu, X.; Du, W.; Ji, L.; Yang, Y.; Li, X.; Sho, T.; Wang, X.; Li, Y.; et al. Mitocytosis, a migrasome-mediated mitochondrial quality-control process. Cell 2021, 184, 2896–2910.e13. [Google Scholar] [CrossRef]
- Zhu, M.; Zou, Q.; Huang, R.; Li, Y.; Xing, X.; Fang, J.; Ma, L.; Li, L.; Yang, X.; Yu, L. Lateral transfer of mRNA and protein by migrasomes modifies the recipient cells. Cell Res. 2021, 31, 237–240. [Google Scholar] [CrossRef]
- Zhang, F.; Kotha, J.; Jennings, L.K.; Zhang, X.A. Tetraspanins and vascular functions. Cardiovasc. Res. 2009, 83, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, J.; Ding, Y.; Zhang, J.; Xu, Y.; Xu, J.; Zheng, S.; Yang, H. Migrasome and tetraspanins in vascular homeostasis: Concept, present, and future. Front. Cell Dev. Biol. 2020, 8, 438. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, T.; Takeda, Y.; Maruyama, K.; Yokosaki, Y.; Tsujino, K.; Tetsumoto, S.; Kuhara, H.; Nakanishi, K.; Otani, Y.; Jin, Y.; et al. Deletion of tetraspanin CD9 diminishes lymphangiogenesis in vivo and in vitro. J. Biol. Chem. 2013, 288, 2118–2131. [Google Scholar] [CrossRef] [PubMed]
- Ushikoshi, H.; Takahashi, T.; Takemura, G.; Li, Y.; Esaki, M.; Khai, N.C.; Kawai, T.; Minatoguchi, S.; Fujiwara, T.; Fujiwara, H.; et al. 929. CD9 Gene Therapy Inhibits Cardiac Hypertrophy and Tachycardia, and Attenuates the Remodeling after Myocardial Infarction in Mice. Mol. Ther. 2005, 11, S359. [Google Scholar]
- Clough, E.; Barrett, T. The gene expression omnibus database. Stat. Genom. Methods Protoc. 2016, 1418, 93–110. [Google Scholar]
- Vanhaverbeke, M.; Vausort, M.; Veltman, D.; Zhang, L.; Wu, M.; Laenen, G.; Gillijns, H.; Moreau, Y.; Bartunek, J.; Van De Werf, F.; et al. Peripheral blood RNA levels of QSOX1 and PLBD1 are new independent predictors of left ventricular dysfunction after acute myocardial infarction. Circ. Genom. Precis. Med. 2019, 12, e002656. [Google Scholar] [CrossRef]
- Veltman, D.; Wu, M.; Pokreisz, P.; Claus, P.; Gillijns, H.; Caluwé, E.; Vanhaverbeke, M.; Gsell, W.; Himmelreich, U.; Sinnaeve, P.R.; et al. Clec4e-receptor signaling in myocardial repair after ischemia-reperfusion injury. Basic Transl. Sci. 2021, 6, 631–646. [Google Scholar] [CrossRef]
- Silbiger, V.N.; Luchessi, A.D.; Hirata, R.D.; Lima-Neto, L.G.; Cavichioli, D.; Carracedo, A.; Brión, M.; Dopazo, J.; García-García, F.; dos Santos, E.S.; et al. Novel genes detected by transcriptional profiling from whole-blood cells in patients with early onset of acute coronary syndrome. Clin. Chim. Acta 2013, 421, 184–190. [Google Scholar] [CrossRef]
- Park, H.-J.; Noh, J.H.; Eun, J.W.; Koh, Y.-S.; Seo, S.M.; Park, W.S.; Lee, J.Y.; Chang, K.; Seung, K.B.; Kim, P.-J.; et al. Assessment and diagnostic relevance of novel serum biomarkers for early decision of ST-elevation myocardial infarction. Oncotarget 2015, 6, 12970. [Google Scholar] [CrossRef]
- Allison, D.B.; Cui, X.; Page, G.P.; Sabripour, M. Microarray data analysis: From disarray to consolidation and consensus. Nat. Rev. Genet. 2006, 7, 55–65. [Google Scholar] [CrossRef]
- Jin, K.; Gao, S.; Yang, P.; Guo, R.; Li, D.; Zhang, Y.; Lu, X.; Fan, G.; Fan, X. Single-cell RNA sequencing reveals the temporal diversity and dynamics of cardiac immunity after myocardial infarction. Small Methods 2022, 6, 2100752. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Guo, C.; Dang, Q.; Wang, L.; Liu, L.; Weng, S.; Xu, H.; Lu, T.; Sun, Z.; Han, X. Integrative analysis from multi-center studies identities a consensus machine learning-derived lncRNA signature for stage II/III colorectal cancer. EBioMedicine 2022, 75, 103750. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Chen, B.; Zu, Y. Identifying OGN as a Biomarker Covering Multiple Pathogenic Pathways for Diagnosing Heart Failure: From Machine Learning to Mechanism Interpretation. Biomolecules 2024, 14, 179. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Hao, S.; Andersen-Nissen, E.; Mauck, W.M.; Zheng, S.; Butler, A.; Lee, M.J.; Wilk, A.J.; Darby, C.; Zager, M.; et al. Integrated analysis of multimodal single-cell data. Cell 2021, 184, 3573–3587.e29. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Spielmann, M.; Qiu, X.; Huang, X.; Ibrahim, D.M.; Hill, A.J.; Zhang, F.; Mundlos, S.; Christiansen, L.; Steemers, F.J.; et al. The single-cell transcriptional landscape of mammalian organogenesis. Nature 2019, 566, 496–502. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, Y.; Min, Q.; Zhang, W.; Teng, H.; Li, C.; Zhang, K.; Shi, L.; Wang, B.; Zhan, Q. Comparative transcriptome characterization of esophageal squamous cell carcinoma and adenocarcinoma. Comput. Struct. Biotechnol. J. 2023, 21, 3841–3853. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Wang, L.-G.; Han, Y.; He, Q.-Y. clusterProfiler: An R package for comparing biological themes among gene clusters. Omics J. Integr. Biol. 2012, 16, 284–287. [Google Scholar] [CrossRef]
- Jacobs, B.M.; Noyce, A.J.; Giovannoni, G.; Dobson, R. BMI and low vitamin D are causal factors for multiple sclerosis: A Mendelian Randomization study. Neurol. Neuroimmunol. Neuroinflamm. 2020, 7, e662. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Jiang, X.; Xiao, Y.; Zhang, Y.; Zhang, W.; Doherty, M.; Nestor, J.; Li, C.; Ye, J.; Sha, T.; et al. Combining single-cell RNA sequencing and population-based studies reveals hand osteoarthritis-associated chondrocyte subpopulations and pathways. Bone Res. 2023, 11, 58. [Google Scholar] [CrossRef]
- Li, J.; Wang, J.; Yang, M.; Wang, G.; Xu, P. The relationship between major depression and delirium: A two-sample Mendelian randomization analysis. J. Affect. Disord. 2023, 338, 69–73. [Google Scholar] [CrossRef]
- Cao, Y.; Yang, Y.; Hu, Q.; Wei, G. Identification of potential drug targets for rheumatoid arthritis from genetic insights: A Mendelian randomization study. J. Transl. Med. 2023, 21, 616. [Google Scholar] [CrossRef] [PubMed]
- Yoo, M.; Shin, J.; Kim, J.; Ryall, K.A.; Lee, K.; Lee, S.; Jeon, M.; Kang, J.; Tan, A.C. DSigDB: Drug signatures database for gene set analysis. Bioinformatics 2015, 31, 3069–3071. [Google Scholar] [CrossRef] [PubMed]
- Kontoyianni, M. Docking and virtual screening in drug discovery. Proteom. Drug Discov. Methods Protoc. 2017, 1647, 255–266. [Google Scholar]
- Shen, S.; Wei, J.; Kang, W.; Wang, T. Elucidating shared biomarkers and pathways in kidney stones and diabetes: Insights into novel therapeutic targets and the role of resveratrol. J. Transl. Med. 2023, 21, 491. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yao, L.; Meng, Y.; Li, B.; Yang, Y.; Gao, F. Migrasome: A new functional extracellular vesicle. Cell Death Discov. 2023, 9, 381. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Liu, Z.; Liang, R.; Wang, W.; Zhu, R.; Li, J.; Xing, Z.; Weng, S.; Han, X.; Sun, Y.-l. Comprehensive machine-learning survival framework develops a consensus model in large-scale multicenter cohorts for pancreatic cancer. Elife 2022, 11, e80150. [Google Scholar] [CrossRef] [PubMed]
- Xiang, J.; Shen, J.; Zhang, L.; Tang, B. Identification and validation of senescence-related genes in circulating endothelial cells of patients with acute myocardial infarction. Front. Cardiovasc. Med. 2022, 9, 1057985. [Google Scholar] [CrossRef] [PubMed]
- Tamura, T.; Lu, W.; Akutsu, T. Computational methods for modification of metabolic networks. Comput. Struct. Biotechnol. J. 2015, 13, 376–381. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Szklarczyk, D.; Kirsch, R.; Koutrouli, M.; Nastou, K.; Mehryary, F.; Hachilif, R.; Gable, A.L.; Fang, T.; Doncheva, N.T.; Pyysalo, S.; et al. The STRING database in 2023: Protein–protein association networks and functional enrichment analyses for any sequenced genome of interest. Nucleic Acids Res. 2023, 51, D638–D646. [Google Scholar] [CrossRef]
- Li, Y.; Lu, F.; Yin, Y. Applying logistic LASSO regression for the diagnosis of atypical Crohn’s disease. Sci. Rep. 2022, 12, 11340. [Google Scholar] [CrossRef]
- Peet, C.; Ivetic, A.; Bromage, D.I.; Shah, A.M. Cardiac monocytes and macrophages after myocardial infarction. Cardiovasc. Res. 2020, 116, 1101–1112. [Google Scholar] [CrossRef] [PubMed]
- Kain, V.; Halade, G.V. Big eater macrophages dominate inflammation resolution following myocardial infarction. J. Mol. Cell. Cardiol. 2015, 100, 225–227. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Chen, B.; Kubota, A.; Hanna, A.; Humeres, C.; Hernandez, S.C.; Liu, Y.; Ma, R.; Tuleta, I.; Huang, S.; et al. Protective effects of macrophage-specific integrin α5 in myocardial infarction are associated with accentuated angiogenesis. Nat. Commun. 2023, 14, 7555. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.-Q.; Li, H.; Oliver, T.; Glogauer, M.; Guo, J.; He, Y.-W. Integrin β1 regulates phagosome maturation in macrophages through Rac expression. J. Immunol. 2008, 180, 2419–2428. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Lv, L.; Shao, D.; Xie, Y.; Cao, Y.; Zheng, X. Engineering nanopatterned structures to orchestrate macrophage phenotype by cell shape. J. Funct. Biomater. 2022, 13, 31. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Frangogiannis, N.G. Integrins in cardiac fibrosis. J. Mol. Cell. Cardiol. 2022, 172, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Tian, X.; Cao, Y.; Ma, X.; Shang, L.; Li, H.; Zhang, X.; Deng, F.; Li, S.; Guo, T.; et al. Cardiac shock wave therapy improves ventricular function by relieving fibrosis through PI3K/Akt signaling pathway: Evidence from a rat model of post-infarction heart failure. Front. Cardiovasc. Med. 2021, 8, 693875. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Zhang, C.; Tang, S.; Wu, X.; Peng, X. Network pharmacology analyses of the pharmacological targets and therapeutic mechanisms of salvianolic acid A in myocardial infarction. Evid.-Based Complement. Altern. Med. 2022, 2022, 8954035. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.; Ma, X.; Zhang, Y.; Liu, Y.; Liu, N.; Zhang, W.; Chen, J.; Liu, B.; Du, W.; Liu, X.; et al. The formation of migrasomes is initiated by the assembly of sphingomyelin synthase 2 foci at the leading edge of migrating cells. Nat. Cell Biol. 2023, 25, 1173–1184. [Google Scholar] [CrossRef]
- Huang, Y.; Zucker, B.; Zhang, S.; Elias, S.; Zhu, Y.; Chen, H.; Ding, T.; Li, Y.; Sun, Y.; Lou, J.; et al. Migrasome formation is mediated by assembly of micron-scale tetraspanin macrodomains. Nat. Cell Biol. 2019, 21, 991–1002. [Google Scholar] [CrossRef]
- Yu, S.; Yu, L. Migrasome biogenesis and functions. FEBS J. 2022, 289, 7246–7254. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Mayea, Y.; Mir, C.; Carballo, L.; Sánchez-García, A.; Bataller, M.; Lleonart, M.E. TSPAN1, a novel tetraspanin member highly involved in carcinogenesis and chemoresistance. Biochim. Biophys. Acta (BBA) Rev. Cancer 2022, 1877, 188674. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Wu, W.; Zhang, W.; Lu, Y.W.; Tou, E.; Ye, J.; Gao, P.; Jourd’heuil, D.; Singer, H.A.; Wu, M.; et al. Selective expression of TSPAN2 in vascular smooth muscle is independently regulated by TGF-β1/SMAD and myocardin/serum response factor. FASEB J. 2017, 31, 2576. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.Y.; Bajaj, J.; Ito, T.; Blevins, A.; Konuma, T.; Weeks, J.; Lytle, N.K.; Koechlein, C.S.; Rizzieri, D.; Chuah, C.; et al. Tetraspanin 3 is required for the development and propagation of acute myelogenous leukemia. Cell Stem Cell 2015, 17, 152–164. [Google Scholar] [CrossRef] [PubMed]
- Sun, G.; Chen, J.; Ding, Y.; Wren, J.D.; Xu, F.; Lu, L.; Wang, Y.; Wang, D.-W.; Zhang, X.A. A Bioinformatics Perspective on the Links Between Tetraspanin-Enriched Microdomains and Cardiovascular Pathophysiology. Front. Cardiovasc. Med. 2021, 8, 630471. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Du, F.-H.; Wang, R.-X.; Han, W.-B.; Lv, X.; Zeng, L.; Chen, G.-Q. TSPAN6 reinforces the malignant progression of glioblastoma via interacting with CDK5RAP3 and regulating STAT3 signaling pathway. Int. J. Biol. Sci. 2024, 20, 2440–2453. [Google Scholar] [CrossRef] [PubMed]
- Gavin, R.L.; Koo, C.Z.; Tomlinson, M.G. Tspan18 is a novel regulator of thrombo-inflammation. Med. Microbiol. Immunol. 2020, 209, 553–564. [Google Scholar] [CrossRef] [PubMed]
- Wei, Q.; Zhang, F.; Richardson, M.M.; Roy, N.H.; Rodgers, W.; Liu, Y.; Zhao, W.; Fu, C.; Ding, Y.; Huang, C.; et al. CD82 Restrains Pathological Angiogenesis by Altering Lipid Raft Clustering and CD44 Trafficking in Endothelial Cells. Circulation 2014, 130, 1493–1504. [Google Scholar] [CrossRef] [PubMed]
- Su, M.; Hou, Y.; Cai, S.; Li, W.; Wei, Y.; Wang, R.; Wu, M.; Liu, M.; Chang, J.; Yang, K.; et al. Elevated ITGA1 levels in type 2 diabetes: Implications for cardiac function impairment. Diabetologia 2024, 67, 850–863. [Google Scholar] [CrossRef]
- Xing, L.; Xue, Y.; Yang, Y.; Wu, P.; Wong, C.C.L.; Wang, H.; Song, Z.; Shi, D.; Tong, C.; Yao, C.; et al. TMT-Based Quantitative Proteomic Analysis Identification of Integrin Alpha 3 and Integrin Alpha 5 as Novel Biomarkers in Pathogenesis of Acute Aortic Dissection. BioMed Res. Int. 2020, 2020, 068402. [Google Scholar] [CrossRef]
- Zhao, X.; Lei, Y.; Zheng, J.; Peng, J.; Li, Y.; Yu, L.; Chen, Y. Identification of markers for migrasome detection. Cell Discov. 2019, 5, 27. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, N.; Basi, D.L.; Townsend, D.; Rusch, M.; Mariash, A.; Mullegama, S.; Watson, A.; Larson, J.; Tan, S.; Lerman, B.; et al. Heparan sulfate Ndst1 regulates vascular smooth muscle cell proliferation, vessel size and vascular remodeling. J. Mol. Cell. Cardiol. 2010, 49, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.T.M.; Murakami, Y.; Mobilio, S.; Niceta, M.; Zampino, G.; Philippe, C.; Moutton, S.; Zaki, M.S.; James, K.N.; Musaev, D.; et al. Bi-allelic Variants in the GPI Transamidase Subunit PIGK Cause a Neurodevelopmental Syndrome with Hypotonia, Cerebellar Atrophy, and Epilepsy. Am. J. Hum. Genet. 2020, 106, 484–495. [Google Scholar] [CrossRef] [PubMed]
- Alam, S.M.D.; Tsukamoto, Y.; Ogawa, M.; Senoo, Y.; Ikeda, K.; Tashima, Y.; Takeuchi, H.; Okajima, T. N-Glycans on EGF domain-specific O-GlcNAc transferase (EOGT) facilitate EOGT maturation and peripheral endoplasmic reticulum localization. J. Biol. Chem. 2020, 295, 8560–8574. [Google Scholar] [CrossRef] [PubMed]
- Lu, P.; Liu, R.; Lu, D.; Xu, Y.; Yang, X.; Jiang, Z.; Yang, C.; Yu, L.; Lei, X.; Chen, Y. Chemical screening identifies ROCK1 as a regulator of migrasome formation. Cell Discov. 2020, 6, 51. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Li, T.; Yin, S.; Gao, M.; He, H.; Li, Y.; Jiang, D.; Shi, M.; Wang, J.; Yu, L. Monocytes deposit migrasomes to promote embryonic angiogenesis. Nat. Cell Biol. 2022, 24, 1726–1738. [Google Scholar] [CrossRef]
- Frangogiannis, N.G. Transforming growth factor-β in myocardial disease. Nat. Rev. Cardiol. 2022, 19, 435–455. [Google Scholar] [CrossRef] [PubMed]
- Good, E.; De Muinck, E. Targeting Systemic Inflammation in Atherosclerosis: Who Will Benefit? SAGE Publications: London, UK, 2018; pp. 921–922. [Google Scholar]
- Kim, H.-J.; Cheng, P.; Travisano, S.; Weldy, C.; Monteiro, J.P.; Kundu, R.; Nguyen, T.; Sharma, D.; Shi, H.; Lin, Y.; et al. Molecular mechanisms of coronary artery disease risk at the PDGFD locus. Nat. Commun. 2023, 14, 847. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Gong, M.; Zuo, S.; Xu, J.; Paul, C.; Li, H.; Liu, M.; Wang, Y.-G.; Ashraf, M.; Xu, M. WNT11-Conditioned Medium Promotes Angiogenesis through the Activation of Non-Canonical WNT-PKC-JNK Signaling Pathway. Genes 2020, 11, 1277. [Google Scholar] [CrossRef] [PubMed]
- Korf-Klingebiel, M.; Reboll, M.R.; Klede, S.; Brod, T.; Pich, A.; Polten, F.; Napp, L.C.; Bauersachs, J.; Ganser, A.; Brinkmann, E.; et al. Myeloid-derived growth factor (C19orf10) mediates cardiac repair following myocardial infarction. Nat. Med. 2015, 21, 140–149. [Google Scholar] [CrossRef]
- Merino, D.; Villar, A.V.; García, R.; Tramullas, M.; Ruiz, L.; Ribas, C.; Cabezudo, S.; Nistal, J.F.; Hurlé, M.A. BMP-7 attenuates left ventricular remodelling under pressure overload and facilitates reverse remodelling and functional recovery. Cardiovasc. Res. 2016, 110, 331–345. [Google Scholar] [CrossRef] [PubMed]
- Li, C.Y.; Zhang, J.R.; Li, X.X.; Zhao, L.; Xi, H.; Hu, W.N.; Li, S.N. Lefty1 ameliorates post-infarction fibrosis by suppressing p-Smad2 and p-ERK1/2 signaling pathways. J. Cardiovasc. Transl. Res. 2021, 14, 636–646. [Google Scholar] [CrossRef] [PubMed]




| GSE Accession | Specimen Source | Platform | Control Cases | AMI Cases | Usage |
|---|---|---|---|---|---|
| GSE123342 | PB | GPL17586 | 22 | 65 | Training cohort |
| GSE29532 | PB | GPL5175 | 6 | 8 | Testing cohort |
| GSE60993 | PB | GPL6884 | 7 | 17 | Testing cohort |
| GSE61144 | PB | GPL6106 | 10 | 7 | Testing cohort |
| GSE97320 | PB | GPL570 | 3 | 3 | Testing cohort |
| GSE163465 | Cd45+ cells | GPL24247 | 1 | 3 | scRNA-seq cohort |
| Model No. | AUC Performance in | ML Algorithms/ Bioinformatics Approaches | |||||
|---|---|---|---|---|---|---|---|
| GSE123342 | GSE29532 | GSE60993 | GSE61144 | GSE97320 | Meta- Cohort | ||
| MS | 0.955 | 0.854 | 0.832 | 0.900 | 1.000 | 0.848 | Stepglm, LASSO |
| 2 | 0.930 | 0.542 | 0.748 | 0.786 | 0.889 | 0.726 | Univariate Regression, LASSO |
| 3 | 0.897 | 0.625 | 0.832 | 0.929 | 0.667 | 0.753 | RF, Artificial Neural Network (ANN) |
| 5 | 0.849 | 0.521 | 0.840 | 1.000 | 0.889 | 0.798 | Monte Carlo feature selection, incremental feature selection, SVM |
| 6 | 0.834 | 0.542 | 0.529 | 0.757 | 0.556 | 0.455 | Logistic Regression |
| 7 | 0.849 | 0.542 | 0.891 | 1.000 | 1.000 | 0.810 | Protein-protein interaction (PPI) |
| 8 | 0.550 | 0.479 | 0.630 | 0.514 | 0.667 | 0.544 | LASSO |
| 10 | 0.898 | 0.917 | 0.933 | 0.529 | 0.556 | 0.759 | PPI |
| 11 | 0.921 | 0.875 | 0.899 | 0.600 | 0.667 | 0.751 | PPI |
| 13 | 0.943 | 0.458 | 0.739 | 1.000 | 0.778 | 0.762 | PPI |
| 14 | 0.859 | 0.625 | 0.815 | 1.000 | 0.889 | 0.815 | PPI |
| 16 | 0.875 | 0.708 | 0.697 | 0.971 | 1.000 | 0.800 | PPI |
| 18 | 0.791 | 0.396 | 0.824 | 0.971 | 1.000 | 0.808 | Weighted Gene Co-expression Network Analysis (WGCNA) |
| 19 | 0.801 | 0.667 | 0.908 | 1.000 | 1.000 | 0.880 | SVM |
| 20 | 0.880 | 0.750 | 0.849 | 1.000 | 1.000 | 0.856 | PPI |
| 21 | 0.708 | 0.667 | 0.655 | 0.900 | 0.667 | 0.664 | NetworkAnalyst, PPI |
| 23 | 0.916 | 0.750 | 0.874 | 0.957 | 1.000 | 0.864 | WGCNA, PPI |
| 25 | 0.917 | 0.771 | 0.672 | 0.957 | 1.000 | 0.811 | PPI |
| 26 | 0.825 | 0.646 | 0.714 | 1.000 | 1.000 | 0.780 | Differentially-Expressed Genes (DEGs) analysis |
| 28 | 0.624 | 0.583 | 0.538 | 0.443 | 1.000 | 0.575 | PPI |
| 29 | 0.749 | 0.542 | 0.849 | 0.843 | 1.000 | 0.776 | PPI, Transcription Regulatory Network (TRN) |
| 30 | 0.888 | 0.417 | 0.739 | 0.886 | 1.000 | 0.771 | ANN |
| 31 | 0.778 | 0.625 | 0.824 | 0.986 | 0.556 | 0.763 | RF, SVM |
| 33 | 0.852 | 0.708 | 0.908 | 0.971 | 0.889 | 0.869 | PPI |
| 35 | 0.571 | 0.688 | 0.664 | 0.457 | 0.667 | 0.516 | LASSO, RF |
| 37 | 0.712 | 0.500 | 0.798 | 0.886 | 1.000 | 0.779 | DEGs, WGCNA |
| 40 | 0.741 | 0.688 | 0.639 | 0.557 | 1.000 | 0.607 | PPI |
| 41 | 0.773 | 0.833 | 0.824 | 1.000 | 0.778 | 0.856 | PPI |
| 42 | 0.899 | 0.396 | 0.655 | 0.957 | 0.778 | 0.738 | PPI, TRN |
| 45 | 0.699 | 0.458 | 0.664 | 0.686 | 0.667 | 0.655 | PPI |
| 46 | 0.848 | 0.792 | 0.849 | 0.971 | 0.778 | 0.831 | PPI |
| 48 | 0.843 | 0.542 | 0.798 | 1.000 | 1.000 | 0.829 | DEGs analysis |
| 49 | 0.726 | 0.708 | 0.504 | 0.586 | 0.444 | 0.434 | PPI, Fuzzy C-Means Clustering (FCM) |
| 50 | 0.873 | 0.708 | 0.647 | 0.657 | 0.889 | 0.654 | PPI |
| 52 | 0.943 | 0.667 | 0.790 | 0.986 | 1.000 | 0.797 | PPI |
| 53 | 0.726 | 0.563 | 0.765 | 0.929 | 1.000 | 0.786 | WGCNA |
| 55 | 0.891 | 0.417 | 0.815 | 1.000 | 0.444 | 0.781 | WGCNA, PPI |
| 56 | 0.764 | 0.604 | 0.664 | 0.671 | 0.444 | 0.559 | LASSO, Support Vector Machine Recursive Feature Elimination (SVM-RFE) |
| 58 | 0.796 | 0.667 | 0.840 | 0.729 | 1.000 | 0.764 | LASSO, SVM-RFE |
| 60 | 0.860 | 0.688 | 0.899 | 1.000 | 0.667 | 0.827 | LASSO, SVM-REF, RF |
| 62 | 0.882 | 0.729 | 0.773 | 1.000 | 0.444 | 0.804 | WGCNA |
| 63 | 0.735 | 0.708 | 0.605 | 0.686 | 1.000 | 0.568 | Traditional AMI markers |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, Y.; Chen, Y.; Xu, J.; Zu, Y. Unveiling the Potential of Migrasomes: A Machine-Learning-Driven Signature for Diagnosing Acute Myocardial Infarction. Biomedicines 2024, 12, 1626. https://doi.org/10.3390/biomedicines12071626
Zhu Y, Chen Y, Xu J, Zu Y. Unveiling the Potential of Migrasomes: A Machine-Learning-Driven Signature for Diagnosing Acute Myocardial Infarction. Biomedicines. 2024; 12(7):1626. https://doi.org/10.3390/biomedicines12071626
Chicago/Turabian StyleZhu, Yihao, Yuxi Chen, Jiajin Xu, and Yao Zu. 2024. "Unveiling the Potential of Migrasomes: A Machine-Learning-Driven Signature for Diagnosing Acute Myocardial Infarction" Biomedicines 12, no. 7: 1626. https://doi.org/10.3390/biomedicines12071626
APA StyleZhu, Y., Chen, Y., Xu, J., & Zu, Y. (2024). Unveiling the Potential of Migrasomes: A Machine-Learning-Driven Signature for Diagnosing Acute Myocardial Infarction. Biomedicines, 12(7), 1626. https://doi.org/10.3390/biomedicines12071626







