Decompressive Hemicraniectomy without Evacuation of Acute Intraparenchymal Hemorrhage
Abstract
:1. Introduction
2. Materials and Methods
2.1. Human Cohort
2.2. Patient Selection
2.3. Considerations for Decompressive Craniectomy without Clot Evacuation
2.4. Clot Evacuation
2.5. Variables
2.6. Statistical Analysis
2.7. Ethical Concerns
3. Results
3.1. Descriptive Statistics and Comparison of the Study Groups
3.2. Binary Logistic Regression
4. Discussion
4.1. Pathophysiological Considerations
4.2. Reason for Not Evacuating the Clot
- The vasculature plays an important role in long-term striatal neurogenesis after stroke. For several months, neuroblasts migrate close to the blood vessels through an area exhibiting early vascular remodeling. Optimization and preservation of vascularization should therefore be an important strategy for stimulating neurogenesis after stroke [33]. Studies showing vascular growth factors in the periphery of brain contusions [34] have generated debate as to whether these factors are responsible for trying to repair the damage caused by the stroke, whilst the risk of surgical manipulation in venous and arterial compromise in the brain has not been assessed.
- Another equally important concept is cerebral autoregulation, by which the cerebral vascular resistance is modified to maintain a cerebral blood flow (CBF) that meets the brain’s metabolic demand for oxygen at any particular time [35]. Concepts such as classic autoregulation (CA) [36,37] and dynamic autoregulation (DA) [38] have fostered progress in an area that is contributing enormously to the understanding of brain pathophysiology. CA and DA impairment is described in a number of pathological conditions, such as post-traumatic brain injury syndrome, SAH, acute ischemic stroke, and carotid vascular disease. It has been suggested that both CA and DA have different control mechanisms, and that DA is more susceptible to impairment in pathological situations but is unaffected by aging and the mechanisms underlying autoregulation, such as adrenergic mechanisms [39,40,41,42,43], cholinergic mechanisms [44], and myogenic modulation [45,46].
4.3. Weaknesses and Strengths of the Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rajashekar, D.; Liang, J.W. Intracerebral Hemorrhage; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Rymer, M.M. Hemorrhagic Stroke: Intracerebral Hemorrhage. Mo. Med. 2011, 108, 50. [Google Scholar]
- Ziai, W.C.; Carhuapoma, J.R. Intracerebral Hemorrhage. Continuum 2018, 24, 1603–1622. [Google Scholar] [CrossRef] [PubMed]
- Jakubovic, R.; Aviv, R.I. Intracerebral Hemorrhage: Toward Physiological Imaging of Hemorrhage Risk in Acute and Chronic Bleeding. Front. Neurol. 2012, 3, 00086. [Google Scholar] [CrossRef]
- Schlunk, F.; Greenberg, S.M. The Pathophysiology of Intracerebral Hemorrhage Formation and Expansion. Transl. Stroke Res. 2015, 6, 257–263. [Google Scholar] [CrossRef] [PubMed]
- van Asch, C.J.; Luitse, M.J.; Rinkel, G.J.; van der Tweel, I.; Algra, A.; Klijn, C.J. Incidence, Case Fatality, and Functional Outcome of Intracerebral Haemorrhage over Time, According to Age, Sex, and Ethnic Origin: A Systematic Review and Meta-Analysis. Lancet Neurol. 2010, 9, 167–176. [Google Scholar] [CrossRef]
- Bullock, M.R.; Chesnut, R.; Ghajar, J.; Gordon, D.; Hartl, R.; Newell, D.W.; Servadei, F.; Walters, B.C.; Wilberger, J. Surgical Management of Traumatic Parenchymal Lesions. Neurosurgery 2006, 58, S2–S25. [Google Scholar] [CrossRef]
- Hemphill, J.C.; Greenberg, S.M.; Anderson, C.S.; Becker, K.; Bendok, B.R.; Cushman, M.; Fung, G.L.; Goldstein, J.N.; MacDonald, R.L.; Mitchell, P.H.; et al. Guidelines for the Management of Spontaneous Intracerebral Hemorrhage. Stroke 2015, 46, 2032–2060. [Google Scholar] [CrossRef] [PubMed]
- Sahni, R.; Weinberger, J. Management of Intracerebral Hemorrhage. Vasc. Health Risk Manag. 2007, 3, 701. [Google Scholar] [CrossRef]
- Law, Z.K.; Appleton, J.P.; Bathc, P.M.; Sprigg, N. Management of Acute Intracerebral Haemorrhage—An Update. Clin. Med. 2017, 17, 166. [Google Scholar] [CrossRef]
- Esquenazi, Y.; Savitz, S.I.; Khoury, R.E.; McIntosh, M.A.; Grotta, J.C.; Tandon, N. Decompressive Hemicraniectomy with or without Clot Evacuation for Large Spontaneous Supratentorial Intracerebral Hemorrhages. Clin. Neurol. Neurosurg. 2015, 128, 117–122. [Google Scholar] [CrossRef]
- Beck, J.; Fung, C.; Strbian, D.; Bütikofer, L.; Z’Graggen, W.J.; Lang, M.F.; Beyeler, S.; Gralla, J.; Ringel, F.; Schaller, K.; et al. Decompressive Craniectomy plus Best Medical Treatment versus Best Medical Treatment Alone for Spontaneous Severe Deep Supratentorial Intracerebral Haemorrhage: A Randomised Controlled Clinical Trial. Lancet 2024, 403, 2395–2404. [Google Scholar] [CrossRef]
- Fung, C.; Murek, M.; Z’Graggen, W.J.; Krähenbühl, A.K.; Gautschi, O.P.; Schucht, P.; Gralla, J.; Schaller, K.; Arnold, M.; Fischer, U.; et al. Decompressive Hemicraniectomy in Patients with Supratentorial Intracerebral Hemorrhage. Stroke 2012, 43, 3207–3211. [Google Scholar] [CrossRef] [PubMed]
- Mendelow, A.D.; Unterberg, A. Surgical Treatment of Intracerebral Haemorrhage. Curr. Opin. Crit. Care 2007, 13, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Helweg-Larsen, S.; Sommer, W.; Strange, P.; Lester, J.; Boysen, G. Prognosis for Patients Treated Conservatively for Spontaneous Intracerebral Hematomas. Stroke 1984, 15, 1045–1048. [Google Scholar] [CrossRef] [PubMed]
- Morgenstern, L.B.; Hemphill, J.C.; Anderson, C.; Becker, K.; Broderick, J.P.; Connolly, E.S.; Greenberg, S.M.; Huang, J.N.; MacDonald, R.L.; Messé, S.R.; et al. Guidelines for the Management of Spontaneous Intracerebral Hemorrhage. Stroke 2010, 41, 2108–2129. [Google Scholar] [CrossRef] [PubMed]
- Kothari, R.U.; Brott, T.; Broderick, J.P.; Barsan, W.G.; Sauerbeck, L.R.; Zuccarello, M.; Khoury, J. The ABCs of Measuring Intracerebral Hemorrhage Volumes. Stroke 1996, 27, 1304–1305. [Google Scholar] [CrossRef] [PubMed]
- Dierssen, G.; Carda, R.; Coca, J.M. The Influence of Large Decompressive Craniectomy on the Outcome of Surgical Treatment in Spontaneous Intracerebral Haematomas. Acta Neurochir. 1983, 69, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Kazui, S.; Naritomi, H.; Yamamoto, H.; Sawada, T.; Yamaguchi, T. Enlargement of Spontaneous Intracerebral Hemorrhage. Incidence and Time Course. Stroke 1996, 27, 1783–1787. [Google Scholar] [CrossRef]
- Zazulia, A.R.; Diringer, M.N.; Derdeyn, C.P.; Powers, W.J. Progression of Mass Effect after Intracerebral Hemorrhage. Stroke 1999, 30, 1167–1173. [Google Scholar] [CrossRef]
- Cheng Mei, S.; Alvord, E.C.; Berry, R.G. Swelling of the Brain Following Ischemic Infarction with Arterial Occlusion. Arch. Neurol. 1959, 1, 161–177. [Google Scholar] [CrossRef]
- Clasen, R.; Huckman, M.; Von Roenn, K.; Pandolfi, S.; Laing, I.; Clasen, J. Time Course of Cerebral Swelling in Stroke: A Correlative Autopsy and CT Study-PubMed. Adv. Neurol. 1980, 28, 395–412. [Google Scholar]
- Mayer, S.; Brun, N.; Begtrup, K.; Broderick, J.; Davis, S.; Diringer, M.; Skolnick, B.; Steiner, T. Recombinant Activated Factor VII for Acute Intracerebral Hemorrhage. N. Engl. J. Med. 2005, 352, 280. [Google Scholar] [CrossRef]
- Gebel, J.M.; Jauch, E.C.; Brott, T.G.; Khoury, J.; Sauerbeck, L.; Salisbury, S.; Spilker, J.; Tomsick, T.A.; Duldner, J.; Broderick, J.P. Natural History of Perihematomal Edema in Patients with Hyperacute Spontaneous Intracerebral Hemorrhage. Stroke 2002, 33, 2631–2635. [Google Scholar] [CrossRef] [PubMed]
- Dolinskas, C.A.; Bilaniuk, L.T.; Zimmerman, R.A.; Kuhl, D.E. Computed Tomography of Intracerebral Hematomas. I. Transmission CT Observations on Hematoma Resolution. Am. J. Roentgenol. 1977, 129, 681–688. [Google Scholar] [CrossRef] [PubMed]
- Mutlu, N.; Berry, R.G.; Alpers, B.J. Massive Cerebral Hemorrhage. Clinical and Pathological Correlations. Arch. Neurol. 1963, 8, 644–661. [Google Scholar] [CrossRef] [PubMed]
- Mendelow, A.D.; Gregson, B.A.; Fernandes, H.M.; Murray, G.D.; Teasdale, G.M.; Hope, D.T.; Karimi, A.; Shaw, M.D.M.; Barer, D.H. Early Surgery versus Initial Conservative Treatment in Patients with Spontaneous Supratentorial Intracerebral Haematomas in the International Surgical Trial in Intracerebral Haemorrhage (STICH): A Randomised Trial. Lancet 2005, 365, 387–397. [Google Scholar] [CrossRef] [PubMed]
- Smith, E.R.; Carter, B.S.; Ogilvy, C.S.; Giannotta, S.L.; Lawton, M.T.; Mocco, J.; Connolly, E.S.; Solomon, R.A. Proposed Use of Prophylactic Decompressive Craniectomy in Poor-Grade Aneurysmal Subarachnoid Hemorrhage Patients Presenting with Associated Large Sylvian Hematomas. Neurosurgery 2002, 51, 117–124. [Google Scholar] [CrossRef]
- Weiner, G.M.; Lacey, M.R.; MacKenzie, L.; Shah, D.P.; Frangos, S.G.; Grady, M.S.; Kofke, A.; Levine, J.; Schuster, J.; Le Roux, P.D. Decompressive Craniectomy for Elevated Intracranial Pressure and Its Effect on the Cumulative Ischemic Burden and Therapeutic Intensity Levels after Severe Traumatic Brain Injury. Neurosurgery 2010, 66, 1111–1118. [Google Scholar] [CrossRef]
- Marinkovic, I.; Strbian, D.; Pedrono, E.; Vekovischeva, O.Y.; Shekhar, S.; Durukan, A.; Korpi, E.R.; Abo-Ramadan, U.; Tatlisumak, T. Decompressive Craniectomy for Intracerebral Hemorrhage. Neurosurgery 2009, 65, 780–786. [Google Scholar] [CrossRef]
- Arvidsson, A.; Collin, T.; Kirik, D.; Kokaia, Z.; Lindvall, O. Neuronal Replacement from Endogenous Precursors in the Adult Brain after Stroke. Nat. Med. 2002, 8, 963–970. [Google Scholar] [CrossRef]
- Jin, K.; Minami, M.; Lan, J.Q.; Mao, X.O.; Batteur, S.; Simon, R.P.; Greenberg, D.A. Neurogenesis in Dentate Subgranular Zone and Rostral Subventricular Zone after Focal Cerebral Ischemia in the Rat. Proc. Natl. Acad. Sci. USA 2001, 98, 4710–4715. [Google Scholar] [CrossRef] [PubMed]
- Thored, P.; Wood, J.; Arvidsson, A.; Cammenga, J.; Kokaia, Z.; Lindvall, O. Long-Term Neuroblast Migration along Blood Vessels in an Area with Transient Angiogenesis and Increased Vascularization after Stroke. Stroke 2007, 38, 3032–3039. [Google Scholar] [CrossRef]
- Vilalta, A.; Sahuquillo, J.; Poca, M.A.; De Los Rios, J.; Cuadrado, E.; Ortega-Aznar, A.; Riveiro, M.; Montaner, J. Brain Contusions Induce a Strong Local Overexpression of MMP-9. Results of a Pilot Study. Acta Neurochir. Suppl. 2008, 102, 415–419. [Google Scholar] [CrossRef]
- Lassen, N.A. Cerebral Blood Flow and Oxygen Consumption in Man. Physiol. Rev. 1959, 39, 183–238. [Google Scholar] [CrossRef]
- Zhang, R.; Zuckerman, J.H.; Giller, C.A.; Levine, B.D. Transfer Function Analysis of Dynamic Cerebral Autoregulation in Humans. Am. J. Physiol. 1998, 274, H233–H241. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Czosnyka, M.; Donnelly, J.; Budohoski, K.P.; Varsos, G.V.; Nasr, N.; Brady, K.M.; Reinhard, M.; Hutchinson, P.J.; Smielewski, P. Comparison of Frequency and Time Domain Methods of Assessment of Cerebral Autoregulation in Traumatic Brain Injury. J. Cereb. Blood Flow Metab. 2015, 35, 248–256. [Google Scholar] [CrossRef]
- Czosnyka, M.; Miller, C.; Le Roux, P.; Menon, D.K.; Vespa, P.; Citerio, G.; Bader, M.K.; Brophy, G.M.; Diringer, M.N.; Stocchetti, N.; et al. Monitoring of Cerebral Autoregulation. Neurocrit. Care 2014, 21 (Suppl. 2), 95–102. [Google Scholar] [CrossRef]
- Zhang, R.; Zuckerman, J.H.; Iwasaki, K.; Wilson, T.E.; Crandall, C.G.; Levine, B.D. Autonomic Neural Control of Dynamic Cerebral Autoregulation in Humans. Circulation 2002, 106, 1814–1820. [Google Scholar] [CrossRef] [PubMed]
- Ogoh, S. Autonomic Control of Cerebral Circulation: Exercise. Med. Sci. Sports Exerc. 2008, 40, 2046–2054. [Google Scholar] [CrossRef]
- Hamner, J.W.; Tan, C.O.; Lee, K.; Cohen, M.A.; Taylor, J.A. Sympathetic Control of the Cerebral Vasculature in Humans. Stroke 2010, 41, 102–109. [Google Scholar] [CrossRef]
- Peebles, K.C.; Ball, O.G.; MacRae, B.A.; Horsman, H.M.; Tzeng, Y.C. Sympathetic Regulation of the Human Cerebrovascular Response to Carbon Dioxide. J. Appl. Physiol. 2012, 113, 700–706. [Google Scholar] [CrossRef] [PubMed]
- Purkayastha, S.; Saxena, A.; Eubank, W.L.; Hoxha, B.; Raven, P.B. A1-Adrenergic Receptor Control of the Cerebral Vasculature in Humans at Rest and during Exercise. Exp. Physiol. 2013, 98, 451–461. [Google Scholar] [CrossRef] [PubMed]
- Hamner, J.W.; Tan, C.O.; Tzeng, Y.C.; Taylor, J.A. Cholinergic Control of the Cerebral Vasculature in Humans. J. Physiol. 2012, 590, 6343. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, S.; Wada, K.; Nagatani, K.; Otani, N.; Mori, K. Decompressive Hemicraniectomy for Spontaneous Intracerebral Hemorrhage. Neurosurg. Focus 2013, 34, E5. [Google Scholar] [CrossRef]
- Tzeng, Y.C.; MacRae, B.A. Interindividual Relationships between Blood Pressure and Cerebral Blood Flow Variability with Intact and Blunted Cerebrovascular Control. J. Appl. Physiol. 2013, 114, 888–895. [Google Scholar] [CrossRef]
- Kim, K.T.; Park, J.K.; Kang, S.G.; Cho, K.S.; Yoo, D.S.; Jang, D.K.; Huh, P.W.; Kim, D.S. Comparison of the Effect of Decompressive Craniectomy on Different Neurosurgical Diseases. Acta Neurochir. 2009, 151, 21–30. [Google Scholar] [CrossRef]
- Ramnarayan, R.; Anto, D.; Anilkumar, T.V.; Nayar, R. Decompressive Hemicraniectomy in Large Putaminal Hematomas: An Indian Experience. J. Stroke Cerebrovasc. Dis. 2009, 18, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Heuts, S.G.; Bruce, S.S.; Zacharia, B.E.; Hickman, Z.L.; Kellner, C.P.; Sussman, E.S.; McDowell, M.M.; Bruce, R.A.; Connolly, E.S. Decompressive Hemicraniectomy without Clot Evacuation in Dominant-Sided Intracerebral Hemorrhage with ICP Crisis. Neurosurg. Focus 2013, 34, E4. [Google Scholar] [CrossRef]
- Sun, W.; Sun, W.; Pan, W.; Kranz, P.G.; Hailey, C.E.; Williamson, R.A.; Laskowitz, D.T.; James, M.L. Predictors of Late Neurological Deterioration after Spontaneous Intracerebral Hemorrhage. Neurocrit. Care 2013, 19, 299–305. [Google Scholar] [CrossRef]
- Pedro, K.M.; Chua, A.E.; Lapitan, M.C.M. Decompressive Hemicraniectomy without Clot Evacuation in Spontaneous Intracranial Hemorrhage: A Systematic Review. Clin. Neurol. Neurosurg. 2020, 192, 105730. [Google Scholar] [CrossRef]
- Rasras, S.; Safari, H.; Zeinali, M.; Jahangiri, M. Decompressive Hemicraniectomy without Clot Evacuation in Supratentorial Deep-Seated Intracerebral Hemorrhage. Clin. Neurol. Neurosurg. 2018, 174, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Molian, V.A.; Seaman, S.C.; Zanaty, M.; Howard, M.A.; Greenlee, J.D.; Hasan, D.M.; Leira, E.C. Impact of Intracerebral Hematoma Evacuation During Decompressive Hemicraniectomy on Functional Outcomes. Stroke 2021, 52, 1105–1108. [Google Scholar] [CrossRef] [PubMed]

| DC w/o CE | CE | Total | p-Value | |
|---|---|---|---|---|
| Number of patients | 11 | 18 | 29 | |
| Intervention | ||||
| Clot evacuation | 0 (0%) | 13 (100%) | 13 (59%) | <0.001 |
| Decompressive craniectomy | 9 (100%) | 6 (46%) | 15 (68%) | <0.05 |
| Baseline characteristics | ||||
| Age (years) | 49 ± 16 (1) | 57 ± 14 | 54 ± 15 | 0.238 |
| Sex, male | 60% | 62% | 59% | 0.779 |
| Hematoma characteristics | ||||
| Spontaneous etiology vs. trauma | 78% | 85% | 82% | 1.000 |
| Aetiology by group | 0.452 | |||
| Spontaneous | 11% | 31% | 23% | |
| HT (2) | 33% | 15% | 23% | |
| SAH (3) | 11% | 31% | 23% | |
| Amyloidosis | 11% | 8% | 9% | |
| Anticoagulation | 11% | 0% | 5% | |
| Reperfusion (4) | 22% | 15% | 18% | |
| Head injury | 78% | 46% | 59% | 0.297 |
| Left laterality | 89% | 77% | 82% | 0.878 |
| Subarachnoid component | 11% | 15% | 14% | 1.000 |
| Open to ventricles | 67% | 54% | 59% | 0.873 |
| Superficial location (5) | 33% | 54% | 46% | 0.607 |
| Sylvian location | ||||
| Capsule involvement | 78% | 54% | 64% | 0.486 |
| External/extreme | 33% | 8% | 18% | 0.332 |
| Putamen involvement | 11% | 15% | 14% | 1.000 |
| Internal capsule involvement | 11% | 0% | 5% | 0.850 |
| Caudate nucleus involvement | 42 ± 15 (1) | 46 ± 18 | 44 ± 16 | 0.634 |
| Volume ([A × B × C]/2, mL) | 7.2 ± 1.6 (1) | 7.4 ± 1.7 | 7.3 ± 1.6 | 0.823 |
| Midline shift (mm) | ||||
| Clinical characteristics | ||||
| GCS on admission | 8 ± 1 (1) | 8 ± 2 | 8 ± 2 | 0.920 |
| Surgery in <24 h (6) | 100% | 85% | 91% | 0.631 |
| Outcome | ||||
| Follow-up time (m) | 8 (4–65) (7) | 24 (1–120) | 18 (1–120) | 0.337 |
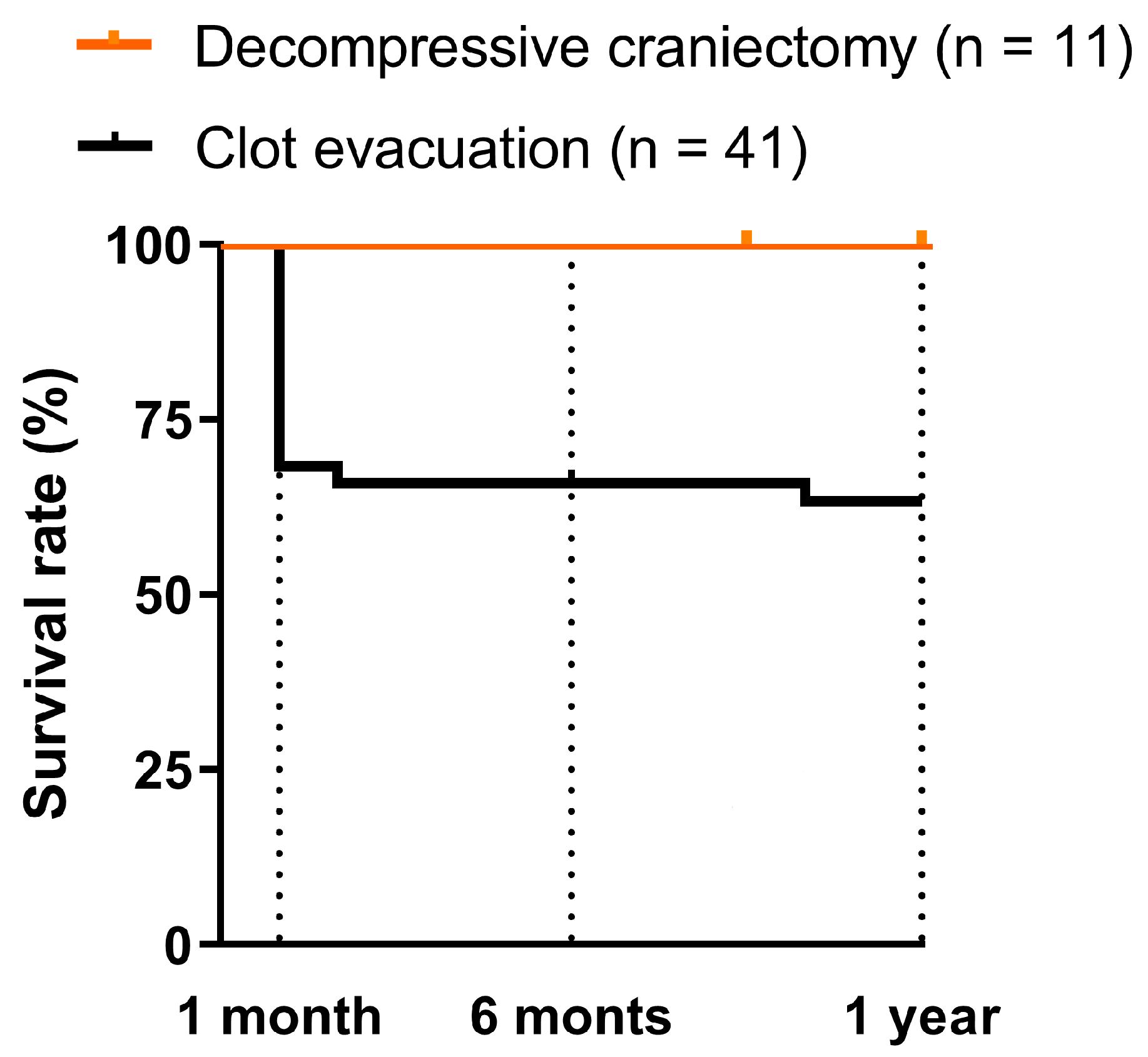
| Mortality | 0% | 23% | 14% | 0.358 |
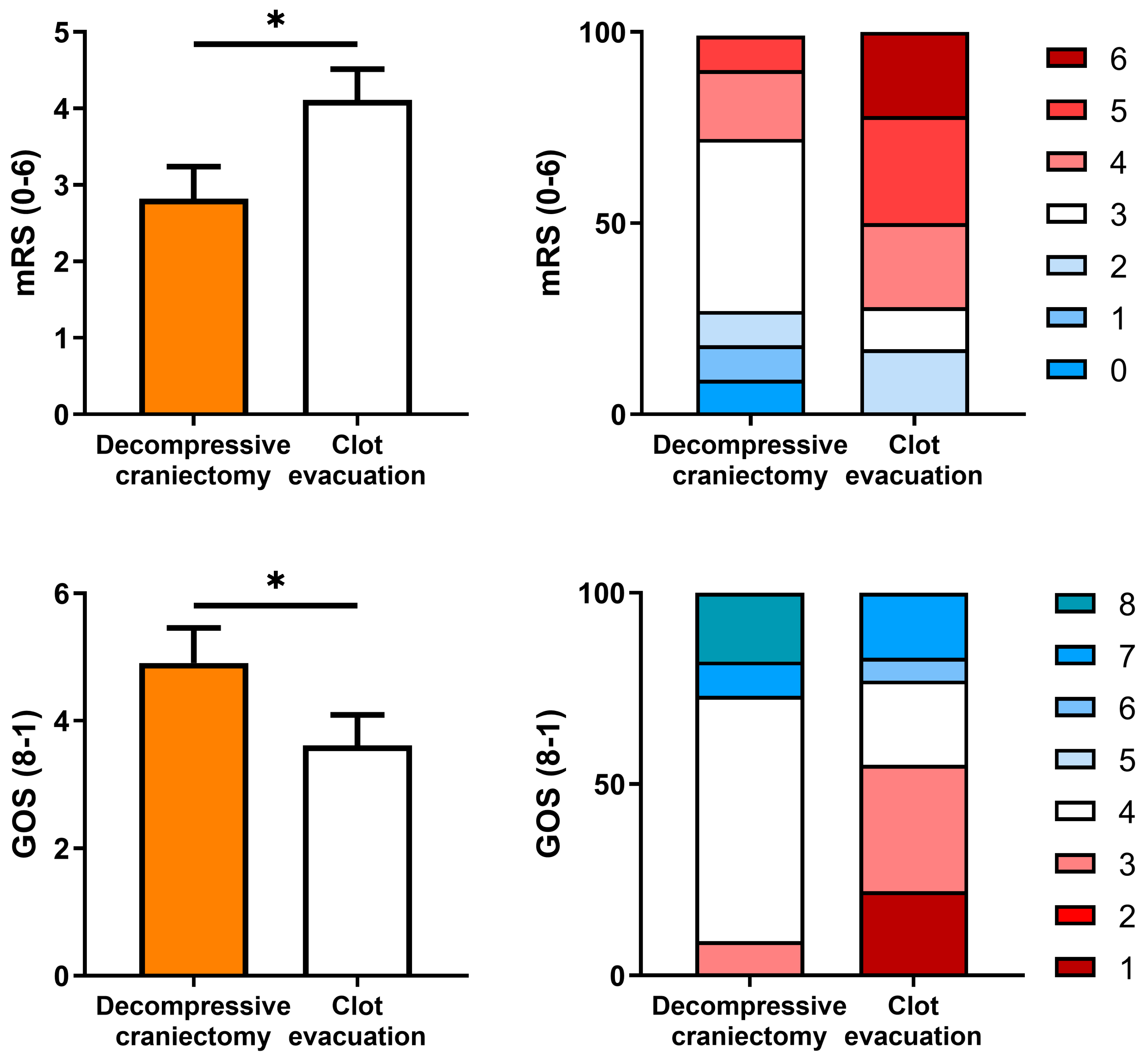
| GOS 4 + GOS 5 | 78% | 15% | 41% | <0.05 |
| mRS 0, 1 or 2 | 78% | 15% | 41% | <0.05 |
| Factor | OR (95% CI) a | p-Value | Adjusted OR (95% CI) b | p-Value |
|---|---|---|---|---|
| Decompressive craniectomy without clot evacuation | 0.052 (0.01–0.46) | 0.008 | 0.050 (0.04–0.61) | 0.019 |
| Age > 55 years | 7.88 (1.11–56.12) | 0.039 | 8.4 (0.7–105) | 0.100 |
| Sex, male | 0.58 (0.10–3.40) | 0.549 | ||
| Spontaneous etiology | 0.80 (0.49–1.31) | 0.378 | ||
| Left laterality | 0.25 (0.04–1.66) | 0.149 | ||
| Superficial location | 0.58 (0.10–3.40) | 0.549 | ||
| Volume (mL) | 1.06 (0.99–1.14) | 0.109 | ||
| Midline shift (mm) | 1.25 (0.72–2.18) | 0.436 | ||
| Glasgow Coma Scale on admission | 0.74 (0.43–1.26) | 0.264 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blanco-Acevedo, C.; Aguera-Morales, E.; Fuentes-Fayos, A.C.; Pelaez-Viña, N.; Diaz-Pernalete, R.; Infante-Santos, N.; Muñoz-Jurado, A.; Porras-Pantojo, M.F.; Ibáñez-Costa, A.; Luque, R.M.; et al. Decompressive Hemicraniectomy without Evacuation of Acute Intraparenchymal Hemorrhage. Biomedicines 2024, 12, 1666. https://doi.org/10.3390/biomedicines12081666
Blanco-Acevedo C, Aguera-Morales E, Fuentes-Fayos AC, Pelaez-Viña N, Diaz-Pernalete R, Infante-Santos N, Muñoz-Jurado A, Porras-Pantojo MF, Ibáñez-Costa A, Luque RM, et al. Decompressive Hemicraniectomy without Evacuation of Acute Intraparenchymal Hemorrhage. Biomedicines. 2024; 12(8):1666. https://doi.org/10.3390/biomedicines12081666
Chicago/Turabian StyleBlanco-Acevedo, Cristóbal, Eduardo Aguera-Morales, Antonio C. Fuentes-Fayos, Nazareth Pelaez-Viña, Rosa Diaz-Pernalete, Nazaret Infante-Santos, Ana Muñoz-Jurado, Manuel F. Porras-Pantojo, Alejandro Ibáñez-Costa, Raúl M. Luque, and et al. 2024. "Decompressive Hemicraniectomy without Evacuation of Acute Intraparenchymal Hemorrhage" Biomedicines 12, no. 8: 1666. https://doi.org/10.3390/biomedicines12081666







