A Highly Standardized Pre-Clinical Porcine Wound Healing Model Powered by Semi-Automated Histological Analysis
Abstract
1. Introduction
2. Material and Methods
2.1. Animal Procedure
2.2. Statistical Analysis
3. Results
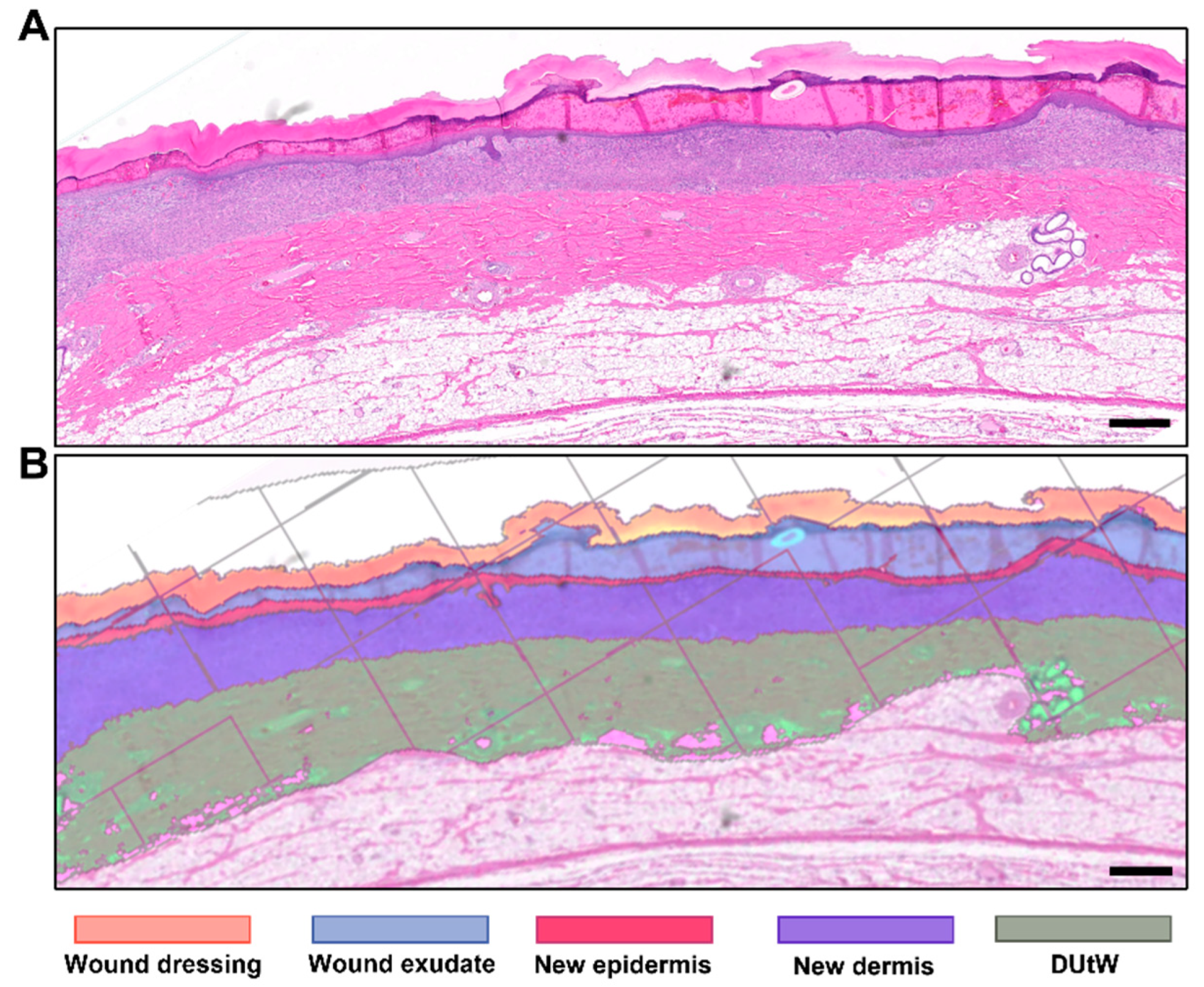
3.1. Morphometry Measured Using Semi-Automated Software
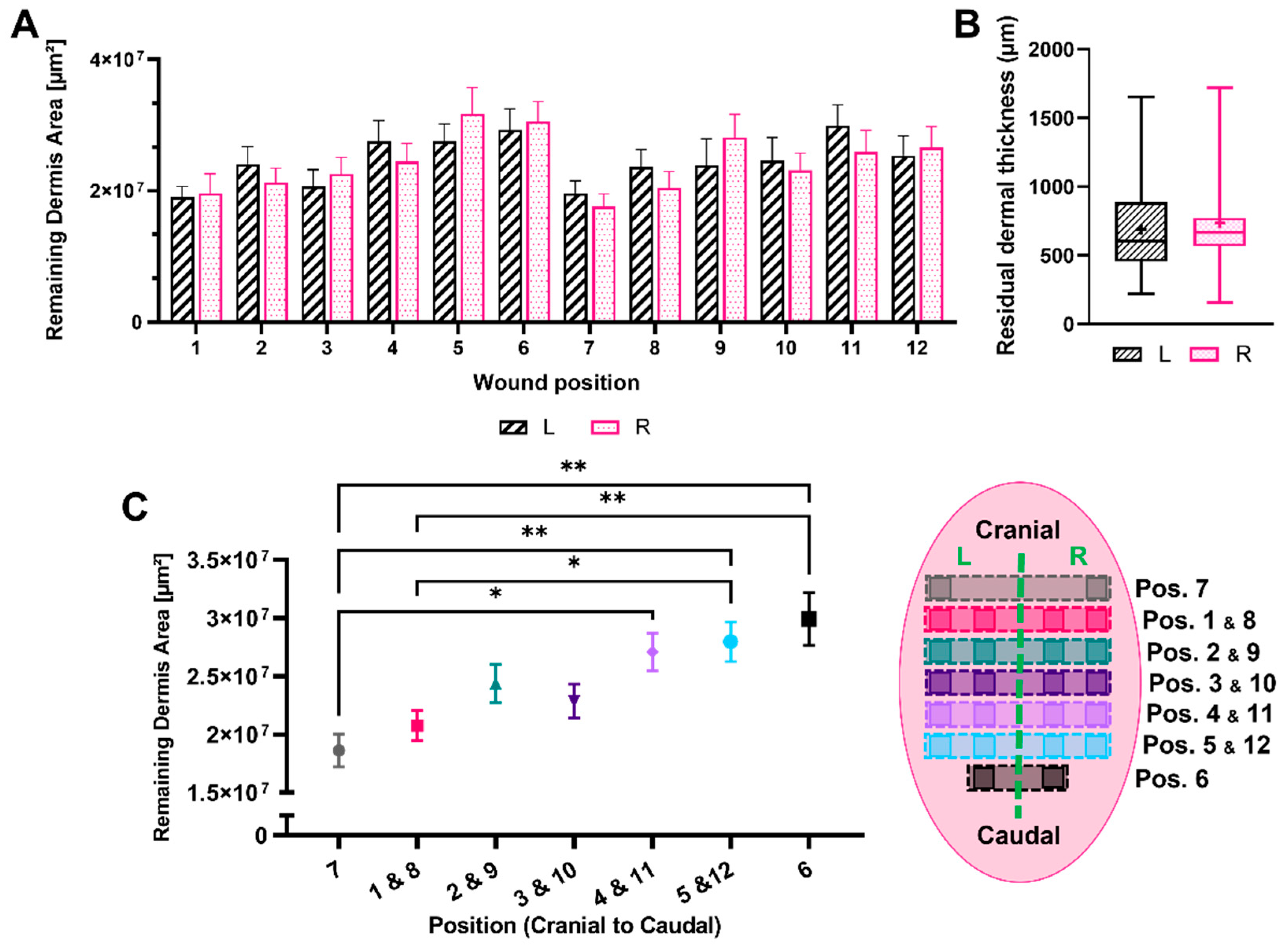
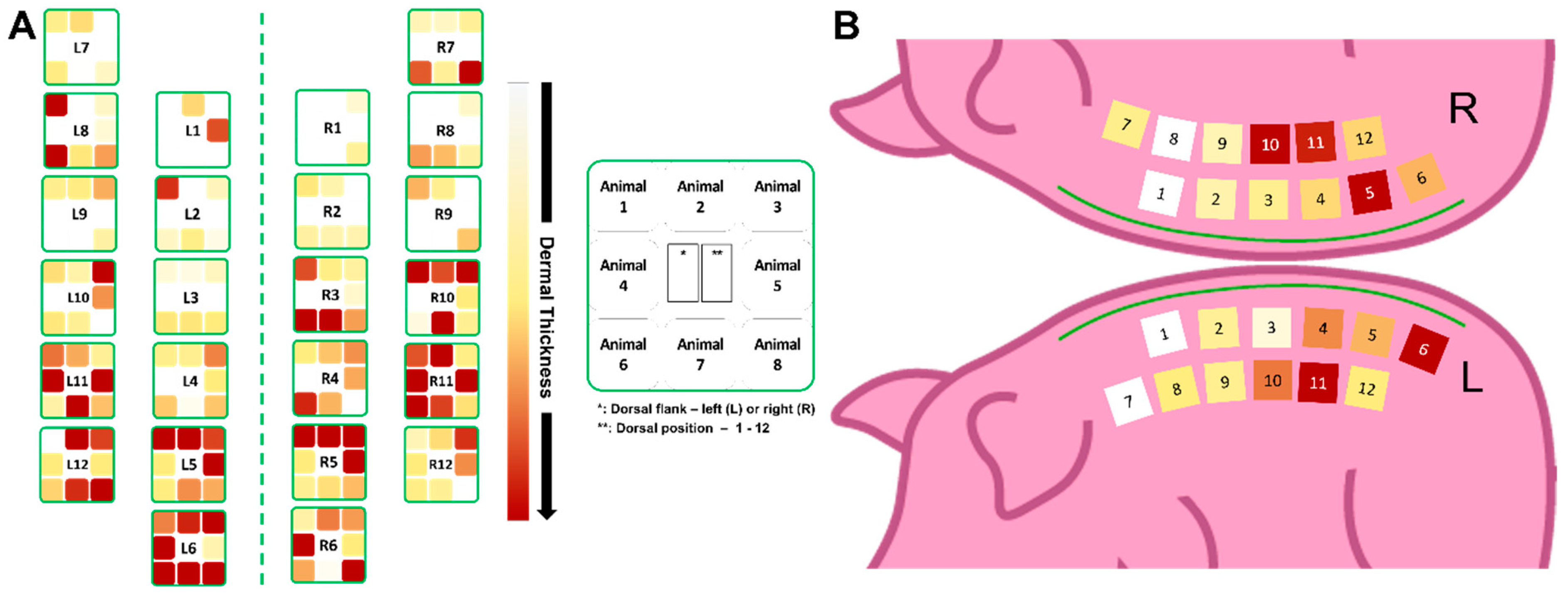
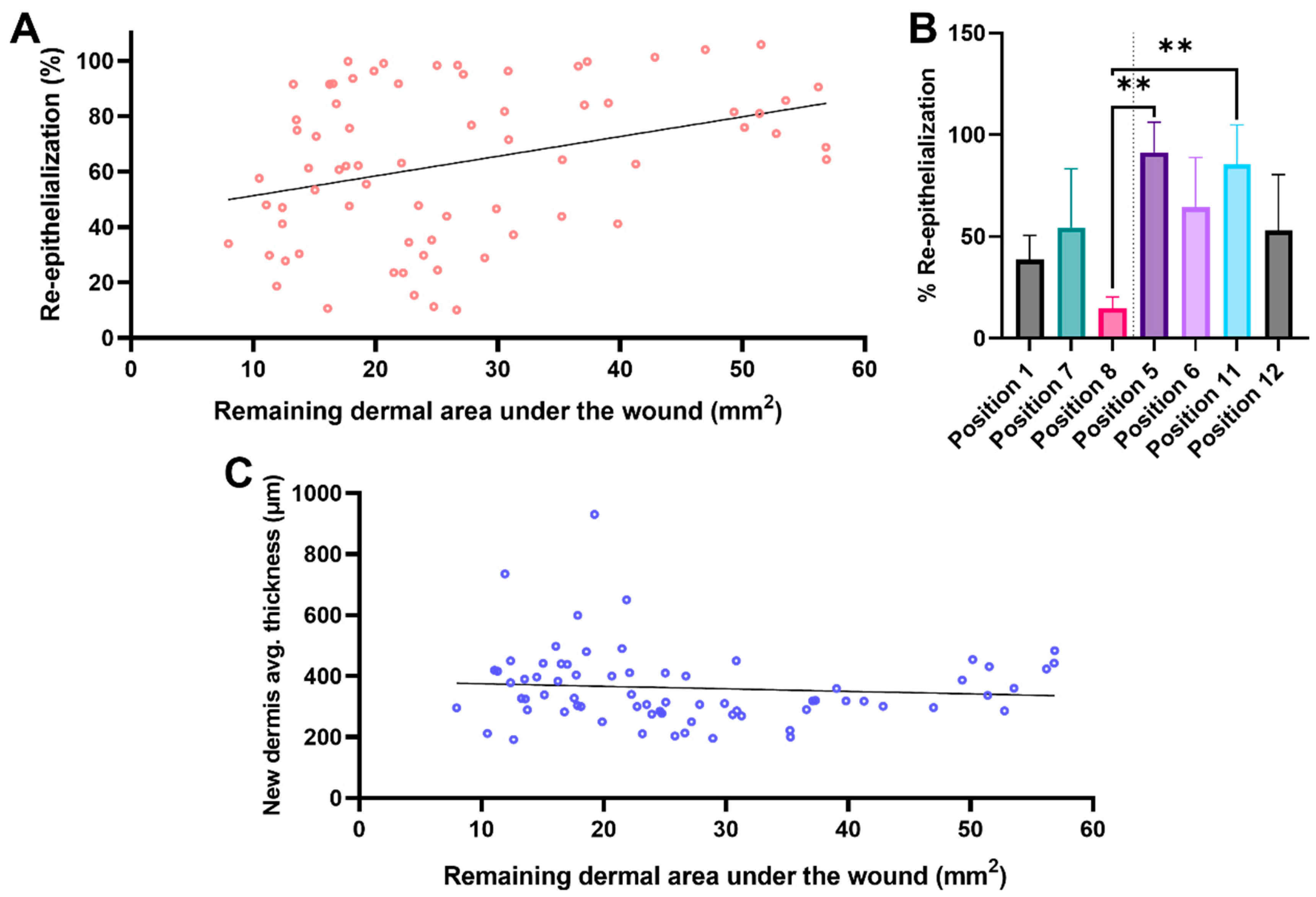
3.2. Remaining Dermis under the Wounded Area
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Singer, A.J.; Clark, R.A. Cutaneous wound healing. N. Engl. J. Med. 1999, 341, 738–746. [Google Scholar] [CrossRef] [PubMed]
- Tottoli, E.M.; Dorati, R.; Genta, I.; Chiesa, E.; Pisani, S.; Conti, B. Skin wound healing process and new emerging technologies for skin wound care and regeneration. Pharmaceutics 2020, 12, 735. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, M.; Kosaric, N.; Bonham, C.A.; Gurtner, G.C. Wound healing: A cellular perspective. Physiol. Rev. 2019, 99, 665–706. [Google Scholar] [CrossRef] [PubMed]
- Gurtner, G.C.; Werner, S.; Barrandon, Y.; Longaker, M.T. Wound repair and regeneration. Nature 2008, 453, 314–321. [Google Scholar] [CrossRef] [PubMed]
- Reinke, J.M.; Sorg, H. Wound repair and regeneration. Eur. Surg. Res. 2012, 49, 35–43. [Google Scholar] [CrossRef]
- Velnar, T.; Bailey, T.; Smrkolj, V. The wound healing process: An overview of the cellular and molecular mechanisms. J. Int. Med. Res. 2009, 37, 1528–1542. [Google Scholar] [CrossRef] [PubMed]
- Larouche, J.; Sheoran, S.; Maruyama, K.; Martino, M.M. Immune regulation of skin wound healing: Mechanisms and novel therapeutic targets. Adv. Wound Care 2018, 7, 209–231. [Google Scholar] [CrossRef] [PubMed]
- Tuca, A.C.; Bernardelli de Mattos, I.; Funk, M.; Winter, R.; Palackic, A.; Groeber-Becker, F.; Kruse, D.; Kukla, F.; Lemarchand, T.; Kamolz, L.P. Orchestrating the dermal/epidermal tissue ratio during wound healing by controlling the moisture content. Biomedicines 2022, 10, 1286. [Google Scholar] [CrossRef] [PubMed]
- Russell, W.M.S.; Burch, R.L. The Principles of Humane Experimental Technique; Methuen: London, UK, 1959. [Google Scholar]
- Groeber, F.; Schober, L.; Schmid, F.F.; Traube, A.; Kolbus-Hernandez, S.; Daton, K.; Hoffmann, S.; Petersohn, D.; Schäfer-Korting, M.; Walles, H.; et al. Catch-up validation study of an in vitro skin irritation test method based on an open source reconstructed epidermis (phase II). Toxicol. Vitr. Int. J. Publ. Assoc. BIBRA 2016, 36, 254–261. [Google Scholar] [CrossRef]
- Kiesewetter, L.; Littau, L.; Walles, H.; Boccaccini, A.R.; Groeber-Becker, F. Reepithelialization in focus: Non-invasive monitoring of epidermal wound healing in vitro. Biosens. Bioelectron. 2019, 142, 111555. [Google Scholar] [CrossRef]
- Schneider, V.; Kruse, D.; de Mattos, I.B.; Zophel, S.; Tiltmann, K.K.; Reigl, A.; Khan, S.; Funk, M.; Bodenschatz, K.; Groeber-Becker, F. A 3D in vitro model for burn wounds: Monitoring of regeneration on the epidermal level. Biomedicines 2021, 9, 1153. [Google Scholar] [CrossRef] [PubMed]
- Stamm, A.; Reimers, K.; Strauß, S.; Vogt, P.; Scheper, T.; Pepelanova, I. In vitro wound healing assays–state of the art. BioNanoMaterials 2016, 17, 79–87. [Google Scholar] [CrossRef]
- Zhang, Y.; Enhejirigala; Yao, B.; Li, Z.; Song, W.; Li, J.; Zhu, D.; Wang, Y.; Duan, X.; Yuan, X.; et al. Using bioprinting and spheroid culture to create a skin model with sweat glands and hair follicles. Burn. Trauma 2021, 9, tkab013. [Google Scholar] [CrossRef]
- Krafts, K.P. Tissue repair: The hidden drama. Organogenesis 2010, 6, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Ragol, S.; Remer, I.; Shoham, Y.; Hazan, S.; Willenz, U.; Sinelnikov, I.; Dronov, V.; Rosenberg, L.; Bilenca, A. Static laser speckle contrast analysis for noninvasive burn diagnosis using a camera-phone imager. J. Biomed. Opt. 2015, 20, 86009. [Google Scholar] [CrossRef] [PubMed]
- Cattelaens, J.; Turco, L.; Berclaz, L.M.; Huelsse, B.; Hitzl, W.; Vollkommer, T.; Bodenschatz, K.J. The impact of a nanocellulose-based wound dressing in the management of thermal injuries in children: Results of a retrospective evaluation. Life 2020, 10, 212. [Google Scholar] [CrossRef] [PubMed]
- Luca-Pozner, V.; Nischwitz, S.P.; Conti, E.; Lipa, G.; Ghezal, S.; Luze, H.; Funk, M.; Remy, H.; Qassemyar, Q. The use of a novel burn dressing out of bacterial nanocellulose compared to the French standard of care in paediatric 2nd degree burns—A retrospective analysis. Burn. J. Int. Soc. Burn. Inj. 2021, 48, 1472–1480. [Google Scholar] [CrossRef] [PubMed]
- Schober, P.; Boer, C.; Schwarte, L.A. Correlation coefficients: Appropriate use and interpretation. Anesth. Analg. 2018, 126, 1763–1768. [Google Scholar] [CrossRef] [PubMed]
- Percie du Sert, N.; Hurst, V.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U.; et al. The ARRIVE guidelines 2.0: Updated guidelines for reporting animal research. PLoS Biol. 2020, 18, e3000410. [Google Scholar]
- Singer, A.J.; McClain, S.A. Development of a porcine excisional wound model. Acad. Emerg. Med. Off. J. Soc. Acad. Emerg. Med. 2003, 10, 1029–1033. [Google Scholar] [CrossRef]
- Wlaschin, K.F.; Ninkovic, J.; Griesgraber, G.W.; Colak Atan, S.; Young, A.J.; Pereira, J.M.; Solberg, M.J.; Smith, G.; Parks, P.J.; McNulty, A.K.; et al. The impact of first-aid dressing design on healing of porcine partial thickness wounds. Wound Repair Regen. 2019, 27, 622–633. [Google Scholar] [CrossRef] [PubMed]
- Schiefer, J.L.; Rath, R.; Held, M.; Werner, J.-O.; Petersen, W.; Schaller, H.-E.; Rahmanian-Schwarz, A. Gelatin-collagen nonwoven scaffold provides an alternative to suprathel for treatment of superficial skin defects. Adv. Ski. Wound Care 2019, 32, 329–332. [Google Scholar] [CrossRef] [PubMed]
- Pirone, L.A.; Bolton, L.L.; Monte, K.A.; Shannon, R.J. Effect of calcium alginate dressings on partial-thickness wounds in swine. J. Investig. Surg. 1992, 5, 149–153. [Google Scholar] [CrossRef] [PubMed]
- Connolly, J.M.; Stevenson, R.S.; Railer, R.F.; Clark, O.E.; Whitten, K.A.; Lee-Stubbs, R.B.; Anderson, D.R. Impairment of wound healing by reactive skin decontamination lotion (RSDL((R))) in a Gottingen minipig((R)) model. Cutan. Ocul. Toxicol. 2020, 39, 143–157. [Google Scholar] [CrossRef] [PubMed]
- Bergmann, J.; Hackl, F.; Koyama, T.; Aflaki, P.; Smith, C.A.; Robson, M.C.; Eriksson, E. The effect of amnion-derived cellular cytokine solution on the epithelialization of partial-thickness donor site wounds in normal and streptozotocin-induced diabetic swine. Eplasty 2009, 9, e49. [Google Scholar] [PubMed]
- Gaines, C.; Poranki, D.; Du, W.; Clark, R.A.F.; Van Dyke, M. Development of a porcine deep partial thickness burn model. Burn. J. Int. Soc. Burn. Inj. 2013, 39, 311–319. [Google Scholar] [CrossRef] [PubMed]
- Olson, M.E.; Wright, J.B.; Lam, K.; Burrell, R.E. Healing of porcine donor sites covered with silver-coated dressings. Eur. J. Surg. 2000, 166, 486–489. [Google Scholar] [PubMed]
- Mauskar, N.A.; Sood, S.; Travis, T.E.; Matt, S.E.; Mino, M.J.; Burnett, M.-S.; Moffatt, L.T.; Fidler, P.; Epstein, S.E.; Jordan, M.H.; et al. Donor site healing dynamics. J. Burn. Care Res. 2013, 34, 549–562. [Google Scholar] [CrossRef]
- Masella, P.C.; Balent, E.M.; Carlson, T.L.; Lee, K.W.; Pierce, L.M. Evaluation of six split-thickness skin graft donor-site dressing materials in a swine model. Plast. Reconstr. Surg. Glob. Open 2013, 1, e84. [Google Scholar] [CrossRef]
- Zhu, K.Q.; Engrav, L.H.; Tamura, R.N.; Cole, J.A.; Muangman, P.; Carrougher, G.J.; Gibran, N.S. Further similarities between cutaneous scarring in the female, red Duroc pig and human hypertrophic scarring. Burn. J. Int. Soc. Burn. Inj. 2004, 30, 518–530. [Google Scholar] [CrossRef]
- Peura, M.; Kaartinen, I.; Suomela, S.; Hukkanen, M.; Bizik, J.; Harjula, A.; Kankuri, E.; Vuola, J. Improved skin wound epithelialization by topical delivery of soluble factors from fibroblast aggregates. Burn. J. Int. Soc. Burn. Inj. 2012, 38, 541–550. [Google Scholar] [CrossRef]
- Kuo, T.Y.; Huang, C.C.; Shieh, S.J.; Wang, Y.B.; Lin, M.J.; Wu, M.C.; Huang, L.L.H. Skin wound healing assessment via an optimized wound array model in miniature pigs. Sci. Rep. 2022, 12, 445. [Google Scholar] [CrossRef] [PubMed]
- Kiwanuka, E.; Hackl, F.; Philip, J.; Caterson, E.J.; Junker, J.P.; Eriksson, E. Comparison of healing parameters in porcine full-thickness wounds transplanted with skin micrografts, split-thickness skin grafts, and cultured keratinocytes. J. Am. Coll. Surg. 2011, 213, 728–735. [Google Scholar] [CrossRef] [PubMed]
- Yao, F.; Visovatti, S.; Johnson, C.S.; Chen, M.; Slama, J.; Wenger, A.; Eriksson, E. Age and growth factors in porcine full-thickness wound healing. Wound Repair Regen. 2001, 9, 371–377. [Google Scholar] [CrossRef]
- Gallant-Behm, C.L.; Hart, D.A. Genetic analysis of skin wound healing and scarring in a porcine model. Wound Repair Regen. 2006, 14, 46–54. [Google Scholar] [CrossRef]
- Branski, L.K.; Mittermayr, R.; Herndon, D.N.; Norbury, W.B.; Masters, O.E.; Hofmann, M.; Traber, D.L.; Redl, H.; Jeschke, M.G. A porcine model of full-thickness burn, excision and skin autografting. Burn. J. Int. Soc. Burn. Inj. 2008, 34, 1119–1127. [Google Scholar] [CrossRef]
- Stone, R.; Saathoff, E.C.; Larson, D.A.; Wall, J.T.; Wienandt, N.A.; Magnusson, S.; Kjartansson, H.; Natesan, S.; Christy, R.J. Accelerated wound closure of deep partial thickness burns with acellular fish skin graft. Int. J. Mol. Sci. 2021, 22, 1590. [Google Scholar] [CrossRef]
- Wang, J.; Wang, C.Z.; Salsbury, J.R.; Zhang, J.; Enkhbaatar, P.; Herndon, D.N.; El Ayadi, A.; Ansari, N.H. Thermal injury induces early blood vessel occlusion in a porcine model of brass comb burn. Sci. Rep. 2021, 11, 12457. [Google Scholar] [CrossRef]
- Bolton, L. Burn debridement: Are we optimizing outcomes? Wounds 2019, 31, 298–300. [Google Scholar]
- Falabella, A.F. Debridement and wound bed preparation. Dermatol. Ther. 2006, 19, 317–325. [Google Scholar] [CrossRef]
- Singer, A.J.; Wang, Z.; McClain, S.A.; Pan, Y. Optical coherence tomography: A noninvasive method to assess wound reepithelialization. Acad. Emerg. Med. Off. J. Soc. Acad. Emerg. Med. 2007, 14, 387–391. [Google Scholar] [CrossRef] [PubMed]
- Travis, T.E.; Mauskar, N.A.; Mino, M.J.; Prindeze, N.; Moffatt, L.T.; Fidler, P.E.; Jordan, M.H.; Shupp, J.W. Commercially available topical platelet-derived growth factor as a novel agent to accelerate burn-related wound healing. J. Burn. Care Res. 2014, 35, e321–e329. [Google Scholar] [CrossRef] [PubMed]
- Kelley, K.W.; Curtis, S.E.; Marzan, G.T.; Karara, H.M.; Anderson, C.R. Body surface area of female swine. J. Anim. Sci. 1973, 36, 927–930. [Google Scholar] [CrossRef] [PubMed]


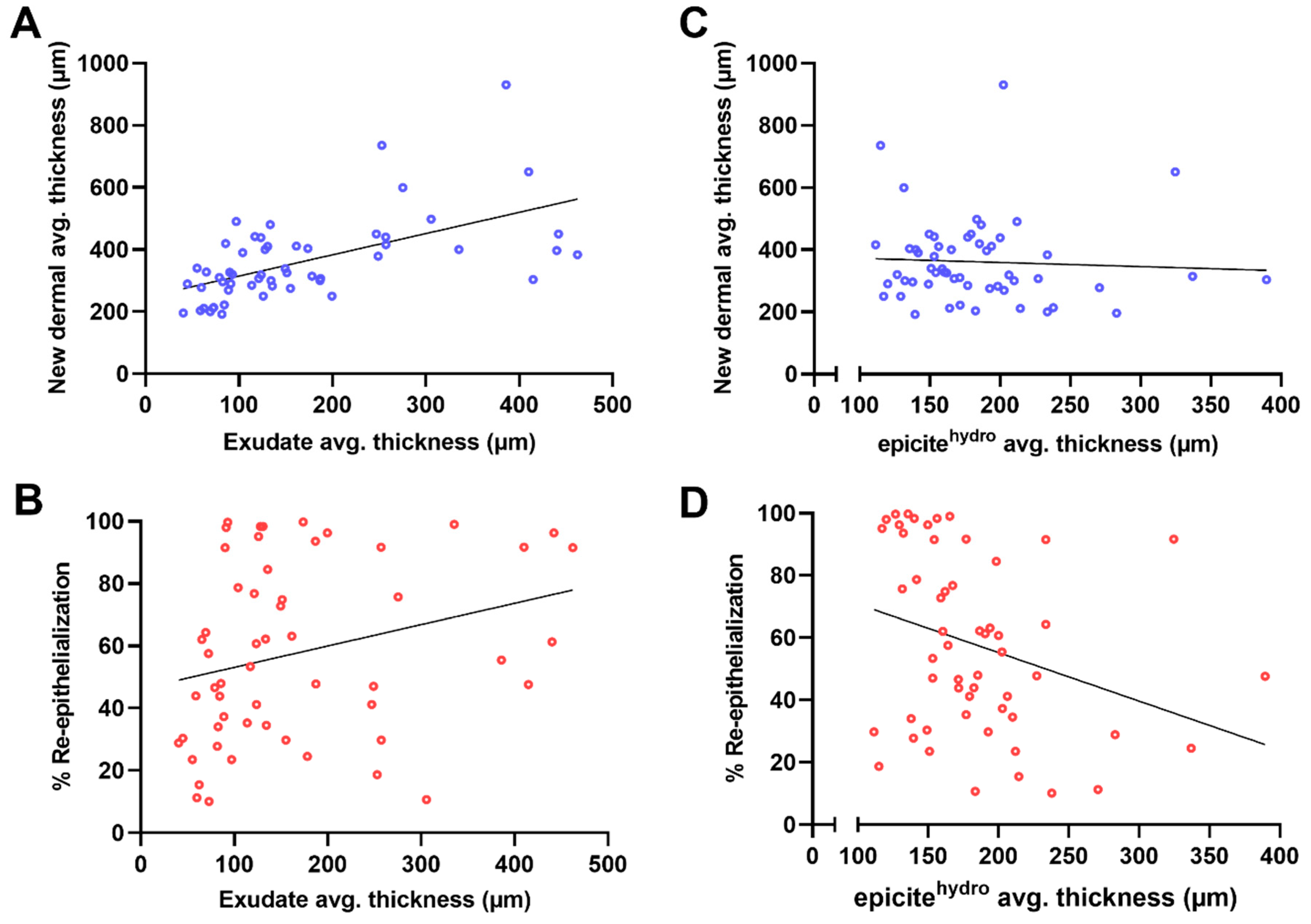

| Re-Epithelialization (%) | Thickness of the Regenerated Dermal Tissue (µm) | |
|---|---|---|
| remaining dermal tissue (mm2) | 0.34 * 0.12 to 0.53 | −0.09 −0.32 to 0.15 |
| thickness of the regenerated dermal tissue (µm) | 0.09 −0.15 to 0.32 | - |
| thickness of the exudate at the wound surface (µm) | 0.28 * −0.01 to 0.50 | 0.57 * 0.37 to 0.73 |
| thickness of the wound dressing (residual moisture) (µm) | −0.30 * −0.53 to −0.04 | −0.05 −0.32 to 0.21 |
| No. of Animals | No. of Wounds per Animal | Pig | Weight Range (kg) | Wounded Area | Wound Depth | Healing Parameters | Histological Evaluation | |
|---|---|---|---|---|---|---|---|---|
| Singer et al. (2003) [21] | 3 | Avg.: 38.3 (total 115) | Sus scrofa domestica | 20–30 | 2.5 × 2.5 cm (6.25 cm2) | 0.6 mm (SPT) | Wound closure and thickness of scar | Manual morphometric analysis |
| Singer et al. (2007) [42] | 2 | 10 | Sus scrofa domestica | 40 | 2.5 × 2.5 cm (6.25 cm2) Ventral area | 0.6 mm (SPT) | Wound closure | Manual morphometric analysis and optical coherence tomography |
| Wlaschin et al. (2019) [34] | 9 | 4 | Sus scrofa domestica Yorkshire Chester White crossed swine | 28–32 | 2.5 × 2.5 cm (6.25 cm2) | 0.5 mm (SPT) | Wound closure, serocellular crust, and epidermal thickness | Manual morphometric analysis |
| Schiefer et al. (2019) [24] | 6 | 3 | Minipigs | 25.8 (±2.5) | 2.4 × 2.4 cm (5.76 cm2) | 0.5 mm (SPT) | Epidermal thickness and cutometer analysis | Manual morphometric analysis |
| Pirone et al. (1992) [25] | 6 | 12 | Five-way Cross swine | 15–20 | 2.2 × 2.2 cm (4.84 cm2) | 0.5 mm (SPT) | Epidermal wound healing and moisture vapor transmission rates | None |
| Travis et al. (2014) [43] | 2 | 3 | Male Sus scrofa domestica Duroc swine | 30–55 | 7.6 × 7.6 cm (57.76 cm2) | ~1.5 mm (DPT) | Wound closure and perfusion units | Manual morphometric analysis and laser doppler imaging |
| Connolly et al. (2020) [42] | 16 | 6 | Sus scrofa domestica | 10–15 | 5 × 5 cm (25.00 cm2) Ventral area | 0.1 mm (SPT) | Wound closure, epidermal hyperplasia, epidermal/dermal separation, inflammatory cells, hair follicles, glands, elastic fibers, smooth muscles, collagen orientation, fibroplasia, vascular proliferation, and hemorrhage | High-frequency ultrasound and manual subjective analysis |
| Kuo et al. (2022) [33] | Not described | 20/19 | Lanyu pigs (Minipig) | 25 | 2.5 × 2.5 cm (6.25 cm2) | 2.3 (DPT) and 6 mm (FT) | Wound closure, region-based variation on epidermis and dermis thickness, region-based variation on wound closure and contraction, tissue maturation after 2 and 6 months, blood flow, and collagen content | Manual morphometry, imaging software analysis, and laser doppler imaging |
| Current approach | 8 | 24 | Sus domesticus: hybrid from Deutsche Landrasse and Deutsches Edelschwein | 34–47 | 3 × 3 cm (9.00 cm2) | 1.2 mm (DPT) | Wound closure, epidermal area, epidermal thickness, regenerated dermis area and thickness, residual dermal area and thickness, area and thickness of exudate at day 7, and area and thickness of wound dressing at day 7 | Semi-automated analysis |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bernardelli de Mattos, I.; Tuca, A.C.; Kukla, F.; Lemarchand, T.; Markovic, D.; Kamolz, L.P.; Funk, M. A Highly Standardized Pre-Clinical Porcine Wound Healing Model Powered by Semi-Automated Histological Analysis. Biomedicines 2024, 12, 1697. https://doi.org/10.3390/biomedicines12081697
Bernardelli de Mattos I, Tuca AC, Kukla F, Lemarchand T, Markovic D, Kamolz LP, Funk M. A Highly Standardized Pre-Clinical Porcine Wound Healing Model Powered by Semi-Automated Histological Analysis. Biomedicines. 2024; 12(8):1697. https://doi.org/10.3390/biomedicines12081697
Chicago/Turabian StyleBernardelli de Mattos, Ives, Alexandru C. Tuca, Fabian Kukla, Thomas Lemarchand, Danijel Markovic, Lars P. Kamolz, and Martin Funk. 2024. "A Highly Standardized Pre-Clinical Porcine Wound Healing Model Powered by Semi-Automated Histological Analysis" Biomedicines 12, no. 8: 1697. https://doi.org/10.3390/biomedicines12081697
APA StyleBernardelli de Mattos, I., Tuca, A. C., Kukla, F., Lemarchand, T., Markovic, D., Kamolz, L. P., & Funk, M. (2024). A Highly Standardized Pre-Clinical Porcine Wound Healing Model Powered by Semi-Automated Histological Analysis. Biomedicines, 12(8), 1697. https://doi.org/10.3390/biomedicines12081697







