Advanced Glycation End-Products Acting as Immunomodulators for Chronic Inflammation, Inflammaging and Carcinogenesis in Patients with Diabetes and Immune-Related Diseases
Abstract
:1. Introduction
2. High Blood Sugar as a Modifiable Environmental Factor Inducing Persistent Epigenetic Changes in Patients with Glycemic Trait for Insulin Resistance
3. Mechanisms of AGEs as a Major Source of Oxidants Implicating in Diabetic Nephropathy, Tissue Inflammation and Aging
3.1. The Link of Circulating AGEs to Oxidative Stress, Inflammatory Response, Aging, and Age-Related Diseases
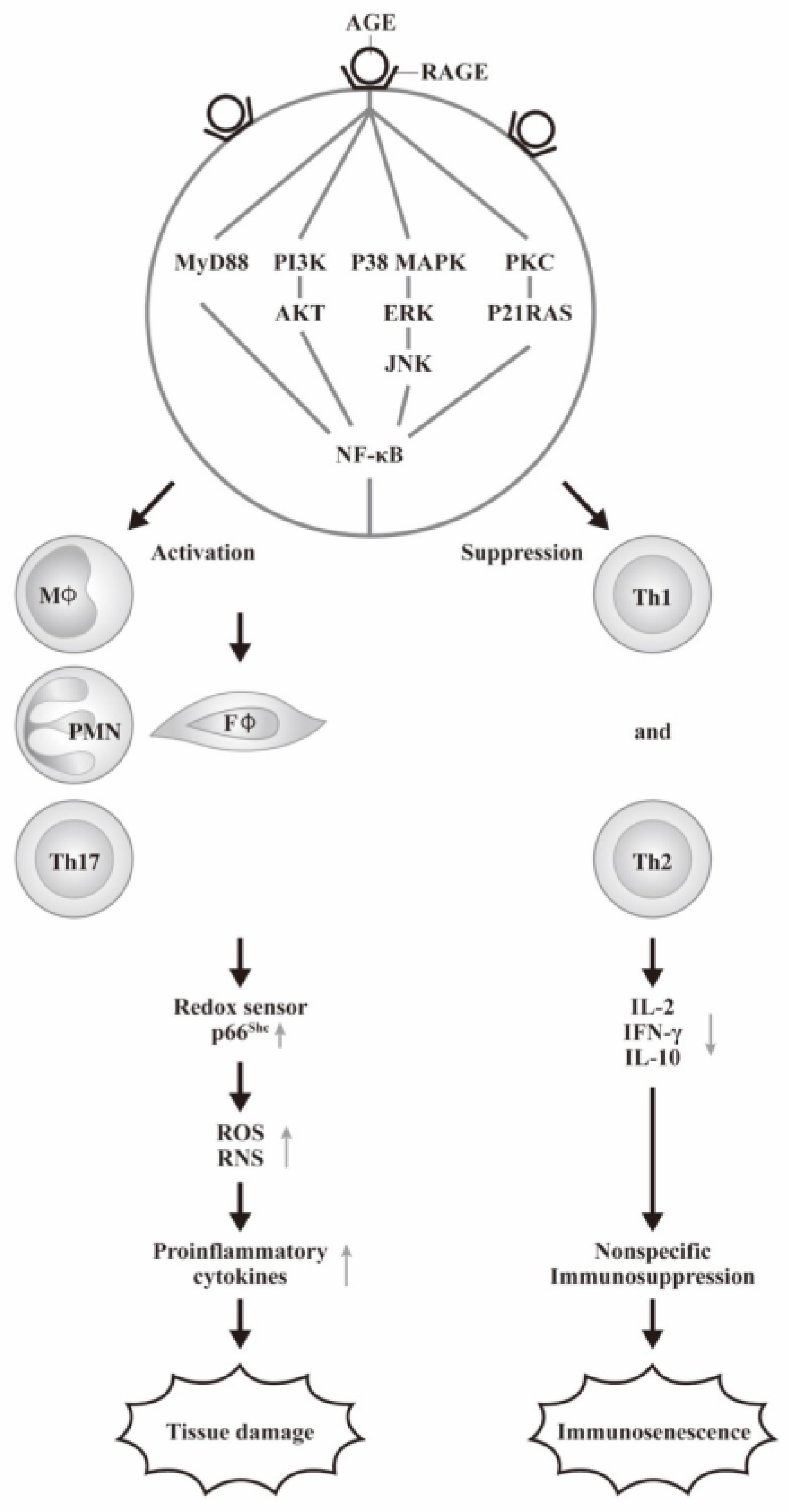
3.2. The Interaction of AGEs with Its Receptor RAGE in Inducing Inflammation via ROS Formation as Reflected by Increasing p66shc Protein Expression
3.3. The Cellular Basis of AGE-RAGE Interactions in the Inflammatory Responses of Diabetic Patients
4. AGE-RAGE Signaling in Mediating the Pathogenesis of Inflamm-Aging
4.1. Effects of AGE and RAGE Interaction in the Inflamm-Aging of Various Immune-Related Rheumatic and Autoimmune Diseases
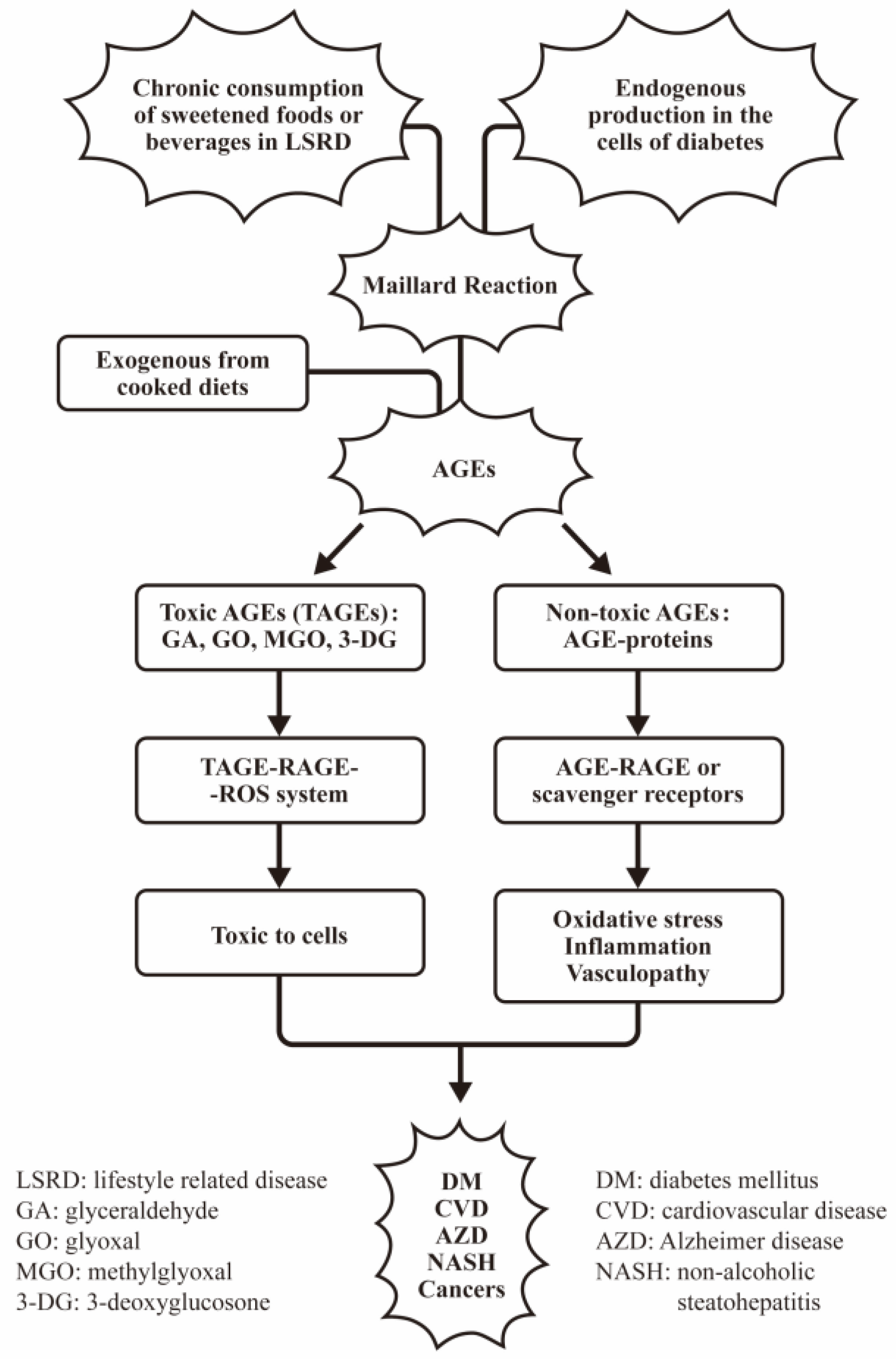
4.2. The Factors Involving in the Exogenous AGEs Formation and the Interventions for Patients with LSRD
5. The Roles of AGE-RAGE Axis in Cancer Risk
5.1. Molecular Basis of Adipokine and Hyperinsulinemia in Obesity and T2DM Association with Cancer Risk
5.2. The Roles of AGE-RAGE Axis on Cancer Metabolic and Apoptotic Signaling Pathways for Promoting Cancer Progression
5.3. The Roles of Dietary Processed Food-Related AGEs on Cancer Risk
5.4. Implications of RAGE on Predicting the Cancer Incidence in Obesity
6. The Pathological Effects of Other Reducing Sugar-Related Glycation End-Products in Human Diseases
6.1. The Pathological Roles of Fructose-Related AGEs on Human Disease
6.2. The Molecular Basis of Fructose-Related AGE Involving in the Different Degenerative Diseases
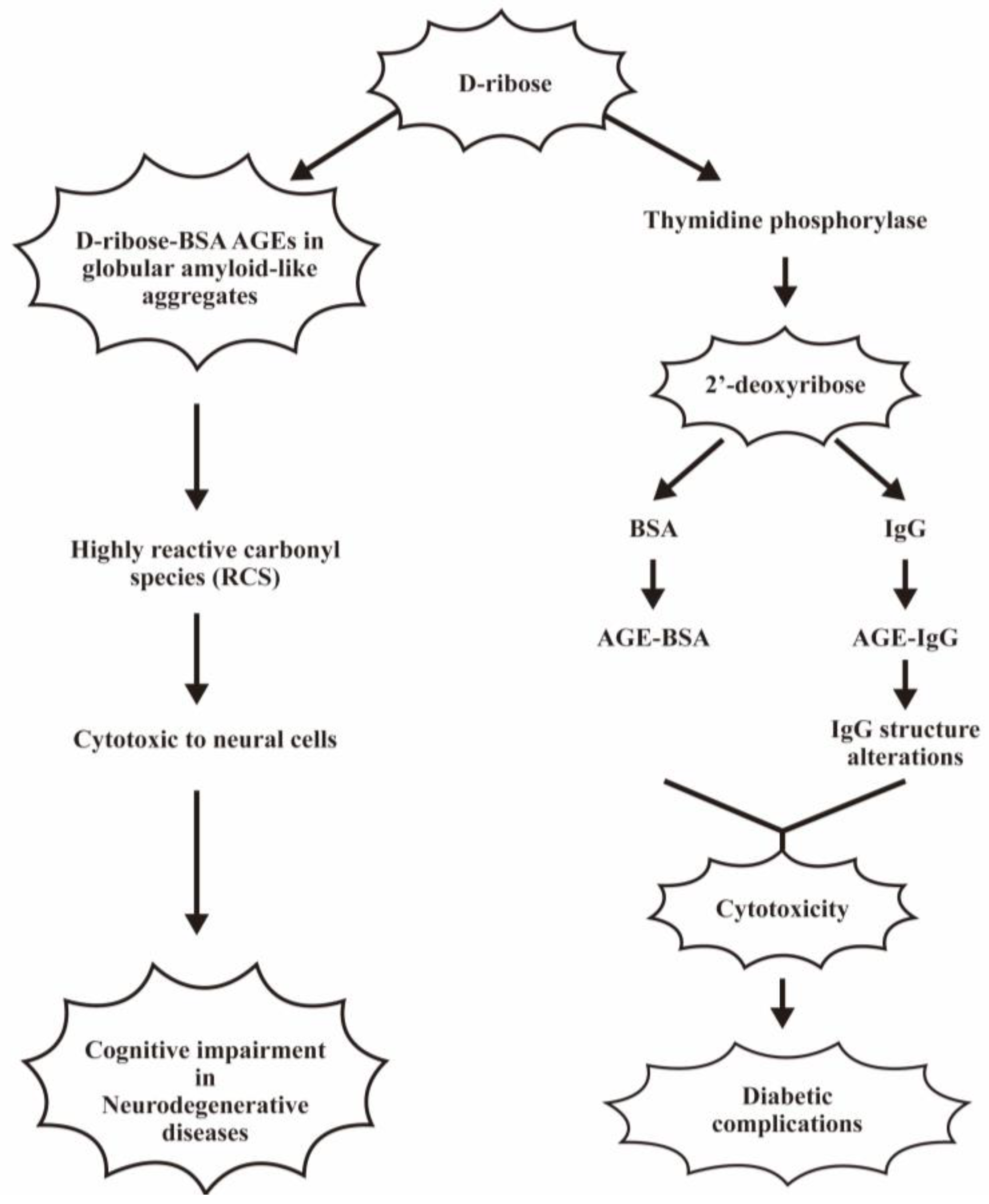
6.3. Pathological Effects of D-Ribose- and 2′-Deoxyribose-Induced Protein Glycations on Human Diseases
6.4. D-Galacose-Related Protein Glycation in Daily Clinical Practice
6.5. Pathological Effects of Glycosylated-Low Density Lipoprotein (LDL) on Human Diseases
6.6. Increased DNA Glycation in Patients with T2DM
7. Unveiling the Molecular Basis of Inflamm-Aging, Vasculopathy and Carcinogenesis Induced by AGE-Albumin
8. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wang, M.; Yu, M.; Fang, L.; Hu, R.Y. Association between sugar-sweetened beverages and type 2 diabetes: A meta-analysis. J. Diabetes Investig. 2015, 6, 360–366. [Google Scholar] [CrossRef]
- Malik, V.S. Sugar sweetened beverages and cardiometabolic health. Curr. Opin. Cardiol. 2017, 32, 572–579. [Google Scholar] [CrossRef]
- Takeuchi, M.; Sakasai-Sakai, A.; Takata, T.; Takino, J.I.; Koriyama, Y. Effects of Toxic AGEs (TAGE) on Human Health. Cells 2022, 11, 2178. [Google Scholar] [CrossRef]
- Watanabe, M.; Kawai, Y.; Kitayama, M.; Akao, H.; Motoyama, A.; Wakasa, M.; Saito, R.; Aoki, H.; Fujibayashi, K.; Tsuchiya, T.; et al. Diurnal glycemic fluctuation is associated with severity of coronary artery disease in prediabetic patients: Possible role of nitrotyrosine and glyceraldehyde-derived advanced glycation end products. J. Cardiol. 2017, 69, 625–631. [Google Scholar] [CrossRef]
- Takeuchi, M.; Bucala, R.; Suzuki, T.; Ohkubo, T.; Yamazaki, M.; Koike, T.; Kameda, Y.; Makita, Z. Neurotoxicity of advanced glycation end-products for cultured cortical neurons. J. Neuropathol. Exp. Neurol. 2000, 59, 1094–1105. [Google Scholar] [CrossRef]
- Takeuchi, M.; Yamagishi, S. Involvement of toxic AGEs (TAGE) in the pathogenesis of diabetic vascular complications and Alzheimer’s disease. J. Alzheimers Dis. 2009, 16, 845–858. [Google Scholar] [CrossRef]
- Hyogo, H.; Yamagishi, S.; Iwamoto, K.; Arihiro, K.; Takeuchi, M.; Sato, T.; Ochi, H.; Nonaka, M.; Nabeshima, Y.; Inoue, M.; et al. Elevated levels of serum advanced glycation end products in patients with non-alcoholic steatohepatitis. J. Gastroenterol. Hepatol. 2007, 22, 1112–1119. [Google Scholar] [CrossRef]
- Takeuchi, M.; Takino, J.I.; Sakasai-Sakai, A.; Takata, T.; Tsutsumi, M. Toxic AGE (TAGE) Theory for the Pathophysiology of the Onset/Progression of NAFLD and ALD. Nutrients 2017, 9, 634. [Google Scholar] [CrossRef]
- Takino, J.; Yamagishi, S.; Takeuchi, M. Cancer malignancy is enhanced by glyceraldehyde-derived advanced glycation end-products. J. Oncol. 2010, 2010, 739852. [Google Scholar] [CrossRef]
- Kong, S.Y.; Takeuchi, M.; Hyogo, H.; McKeown-Eyssen, G.; Yamagishi, S.; Chayama, K.; O’Brien, P.J.; Ferrari, P.; Overvad, K.; Olsen, A.; et al. The Association between Glyceraldehyde-Derived Advanced Glycation End-Products and Colorectal Cancer Risk. Cancer Epidemiol. Biomark. Prev. 2015, 24, 1855–1863. [Google Scholar] [CrossRef]
- Takeuchi, M.; Makita, Z. Alternative routes for the formation of immunochemically distinct advanced glycation end-products in vivo. Curr. Mol. Med. 2001, 1, 305–315. [Google Scholar] [CrossRef]
- Brings, S.; Fleming, T.; Freichel, M.; Muckenthaler, M.U.; Herzig, S.; Nawroth, P.P. Dicarbonyls and Advanced Glycation End-Products in the Development of Diabetic Complications and Targets for Intervention. Int. J. Mol. Sci. 2017, 18, 634. [Google Scholar] [CrossRef]
- Takeuchi, M.; Yamagishi, S. TAGE (toxic AGEs) hypothesis in various chronic diseases. Med. Hypotheses 2004, 63, 449–452. [Google Scholar] [CrossRef]
- Takeuchi, M.; Takino, J.; Yamagishi, S. Involvement of the toxic AGEs (TAGE)-RAGE system in the pathogenesis of diabetic vascular complications: A novel therapeutic strategy. Curr. Drug Targets 2010, 11, 1468–1482. [Google Scholar] [CrossRef]
- Takeuchi, M.; Sakasai-Sakai, A.; Takata, T.; Takino, J.I.; Koriyama, Y.; Kikuchi, C.; Furukawa, A.; Nagamine, K.; Hori, T.; Matsunaga, T. Intracellular Toxic AGEs (TAGE) Triggers Numerous Types of Cell Damage. Biomolecules 2021, 11, 387. [Google Scholar] [CrossRef]
- Sakai-Sakasai, A.; Takeda, K.; Suzuki, H.; Takeuchi, M. Structures of Toxic Advanced Glycation End-Products Derived from Glyceraldehyde, A Sugar Metabolite. Biomolecules 2024, 14, 202. [Google Scholar] [CrossRef]
- Maillard, L.C. Formation d’humus et de combustibles mineraux sans intervention de l’oxygene atmospherique, des microorganismes des hautes temperatures ou des fortes pressions. CR Acad. Sci. 1912, 154, 66–68. [Google Scholar]
- Federico, G.; Gori, M.; Randazzo, E.; Vierucci, F. Skin advanced glycation end-products evaluation in infants according to the type of feeding and mother’s smoking habits. SAGE Open Med. 2016, 4, 2050312116682126. [Google Scholar] [CrossRef]
- Wu, Y.; Zong, M.; Wu, H.; He, D.; Li, L.; Zhang, X.; Zhao, D.; Li, B. Dietary advanced glycation end-products affect the progression of early diabetes by intervening in carbohydrate and lipid metabolism. Mol. Nutr. Food Res. 2022, 66, 2200046. [Google Scholar] [CrossRef]
- Takata, T.; Sakasai-Sakai, A.; Takeuchi, M. Intracellular toxic advanced glycation end-products may induce cell death and suppress cardiac fibroblasts. Metabolites 2022, 12, 615. [Google Scholar] [CrossRef]
- Ooi, H.; Nasu, R.; Furukawa, A.; Takeuchi, M.; Koriyama, Y. Pyridoxamine and aminoguanidine attenuate the abnormal aggregation of β-tubulin and suppression of neurite outgrowth by glyceraldehyde-derived toxic advanced glycation end-products. Front. Pharmocol. 2022, 13, 921611. [Google Scholar] [CrossRef]
- Pan, S.; Guan, Y.; Ma, Y.; Cui, Q.; Tang, Z.; Li, J.; Zu, C.; Zhang, Y.; Zhu, L.; Liu, Z. Advanced glycation end products correlate with breast cancer metastasis by activating RAGE/TLR4 signaling. BMJ Open Diab Res. Care 2022, 10, e002697. [Google Scholar] [CrossRef]
- Yan, S.D.; Schmidt, A.M.; Mark Anderson, G.; Zhang, J.; Brett, J.; Zou, Y.S.; Pinsky, D.; Stern, D. Enhanced cellular oxidant stress by interaction of advanced glycation end products with their receptors/binding proteins. J. Biol. Chem. 1994, 269, 9889–9897. [Google Scholar] [CrossRef]
- Schmidt, A.M.; Weidman, E.; Lalla, E.; Yan, S.D.; Hori, O.; Cao, R.; Brett, J.G.; Lamster, I.B. Advanced glycation endproducts (AGEs) induce oxidant stress in the gingiva: A potential mechanism underlying accelerated periodontal disease associated with diabetes. J. Periodont. Res. 1996, 31, 508–515. [Google Scholar] [CrossRef]
- Xiao, Z.-L.; Ma, L.-P.; Yang, D.-F.; Yang, M.; Li, Z.-Y.; Chen, M.-F. Profilin-1 is involved in macroangiopathy induced by advanced glycation end products via vascular remodeling and inflammation. World J. Diabetes 2021, 12, 1875–1893. [Google Scholar] [CrossRef]
- Xie, Q.; Ma, L.; Xiao, Z.; Yang, M.; Chen, M. Role of profilin-1 in vasculopathy induced by advanced glycation end products (AGEs). J. Diabetes Its Comlications 2023, 37, 108415. [Google Scholar] [CrossRef]
- Ottosson, F.; Smith, E.; Melander, O.; Fernandez, C. Altered Asparagine and Glutamate Homeostasis Precede Coronary Artery Disease and Type 2 Diabetes. J. Clin. Endocrinol. Metab. 2018, 103, 3060–3069. [Google Scholar] [CrossRef]
- Shen, Y.; Si, Y.; Lu, J.; Ma, X.; Zhang, L.; Mo, Y.; Lu, W.; Zhu, W.; Bao, Y.; Hu, G.; et al. Association between 1,5-Anhydroglucitol and Acute C Peptide Response to Arginine among Patients with Type 2 Diabetes. J. Diabetes Res. 2020, 2020, 4243053. [Google Scholar] [CrossRef]
- Gall, W.E.; Beebe, K.; Lawton, K.A.; Adam, K.P.; Mitchell, M.W.; Nakhle, P.J.; Ryals, J.A.; Milburn, M.V.; Nannipieri, M.; Camastra, S.; et al. alpha-hydroxybutyrate is an early biomarker of insulin resistance and glucose intolerance in a nondiabetic population. PLoS ONE 2010, 5, e10883. [Google Scholar] [CrossRef]
- Bi, X.; Henry, C.J. Plasma-free amino acid profiles are predictors of cancer and diabetes development. Nutr. Diabetes 2017, 7, e249. [Google Scholar] [CrossRef]
- Sun, Y.; Lu, Y.K.; Gao, H.Y.; Yan, Y.X. Effect of Metabolite Levels on Type 2 Diabetes Mellitus and Glycemic Traits: A Mendelian Randomization Study. J. Clin. Endocrinol. Metab. 2021, 106, 3439–3447. [Google Scholar] [CrossRef]
- Chou, J.; Liu, R.; Yu, J.; Liu, X.; Zhao, X.; Li, Y.; Liu, L.; Sun, C. Fasting serum alpha-hydroxybutyrate and pyroglutamic acid as important metabolites for detecting isolated post-challenge diabetes based on organic acid profiles. J. Chromatogr. B Analyt Technol. Biomed. Life Sci. 2018, 1100–1101, 6–16. [Google Scholar] [CrossRef]
- El-Osta, A.; Brasacchio, D.; Yao, D.; Pocai, A.; Jones, P.L.; Roeder, R.G.; Cooper, M.E.; Brownlee, M. Transient high glucose causes persistent epigenetic changes and altered gene expression during subsequent normoglycemia. J. Exp. Med. 2008, 205, 2409–2417. [Google Scholar] [CrossRef]
- Perrone, A.; Giovino, A.; Benny, J.; Martinelli, F. Advanced Glycation End Products (AGEs): Biochemistry, Signaling, Analytical Methods, and Epigenetic Effects. Oxid. Med. Cell. Longev. 2020, 2020, 3818196. [Google Scholar] [CrossRef]
- Ottum, M.S.; Mistry, A.M. Advanced glycation end-products: Modifiable environmental factors profoundly mediate insulin resistance. J. Clin. Biochem. Nutr. 2015, 57, 1–12. [Google Scholar] [CrossRef]
- Shen, C.Y.; Wu, C.H.; Lu, C.H.; Kuo, Y.M.; Li, K.J.; Hsieh, S.C.; Yu, C.L. Advanced Glycation End Products of Bovine Serum Albumin Suppressed Th1/Th2 Cytokine but Enhanced Monocyte IL-6 Gene Expression via MAPK-ERK and MyD88 Transduced NF-kappaB p50 Signaling Pathways. Molecules 2019, 24, 2461. [Google Scholar] [CrossRef]
- Finkel, T.; Holbrook, N.J. Oxidants, oxidative stress and the biology of ageing. Nature 2000, 408, 239–247. [Google Scholar] [CrossRef]
- Vlassara, H.; Uribarri, J.; Ferrucci, L.; Cai, W.; Torreggiani, M.; Post, J.B.; Zheng, F.; Striker, G.E. Identifying advanced glycation end products as a major source of oxidants in aging: Implications for the management and/or prevention of reduced renal function in elderly persons. Semin. Nephrol. 2009, 29, 594–603. [Google Scholar] [CrossRef]
- Moldogazieva, N.T.; Mokhosoev, I.M.; Mel’nikova, T.I.; Porozov, Y.B.; Terentiev, A.A. Oxidative Stress and Advanced Lipoxidation and Glycation End Products (ALEs and AGEs) in Aging and Age-Related Diseases. Oxid. Med. Cell. Longev. 2019, 2019, 3085756. [Google Scholar] [CrossRef]
- Yuan, Y.; Sun, H.; Sun, Z. Advanced glycation end products (AGEs) increase renal lipid accumulation: A pathogenic factor of diabetic nephropathy (DN). Lipids Health Dis. 2017, 16, 126. [Google Scholar] [CrossRef]
- Dai, L.; Schurgers, L.J.; Shiels, P.G.; Stenvinkel, P. Early vascular ageing in chronic kidney disease: Impact of inflammation, vitamin K, senescence and genomic damage. Nephrol. Dial. Transplant. 2020, 35, ii31–ii37. [Google Scholar] [CrossRef]
- Wada, Y.; Umeno, R.; Nagasu, H.; Kondo, M.; Tokuyama, A.; Kadoya, H.; Kidokoro, K.; Taniguchi, S.; Takahashi, M.; Sasaki, T.; et al. Endothelial Dysfunction Accelerates Impairment of Mitochondrial Function in Ageing Kidneys via Inflammasome Activation. Int. J. Mol. Sci. 2021, 22, 9269. [Google Scholar] [CrossRef]
- Uribarri, J.; Cai, W.; Peppa, M.; Goodman, S.; Ferrucci, L.; Striker, G.; Vlassara, H. Circulating glycotoxins and dietary advanced glycation endproducts: Two links to inflammatory response, oxidative stress, and aging. J. Gerontol. A Biol. Sci. Med. Sci. 2007, 62, 427–433. [Google Scholar] [CrossRef]
- Dozio, E.; Caldiroli, L.; Molinari, P.; Castellano, G.; Delfrate, N.W.; Romanelli, M.M.C.; Vettoretti, S. Accelerated AGEing: The Impact of Advanced Glycation End Products on the Prognosis of Chronic Kidney Disease. Antioxidants 2023, 12, 584. [Google Scholar] [CrossRef]
- Subramanian, S.; Pallati, P.K.; Sharma, P.; Agrawal, D.K.; Nandipati, K.C. Significant association of TREM-1 with HMGB1, TLRs and RAGE in the pathogenesis of insulin resistance in obese diabetic population. Am. J. Transl. Res. 2017, 9, 3224–3244. [Google Scholar]
- Hofmann, M.A.; Drury, S.; Fu, C.; Qu, W.; Taguchi, A.; Lu, Y.; Avila, C.; Kambham, N.; Bierhaus, A.; Nawroth, P.; et al. RAGE mediates a novel proinflammatory axis: A central cell surface receptor for S100/calgranulin polypeptides. Cell 1999, 97, 889–901. [Google Scholar] [CrossRef]
- Lander, H.M.; Tauras, J.M.; Ogiste, J.S.; Hori, O.; Moss, R.A.; Schmidt, A.M. Activation of the receptor for advanced glycation end products triggers a p21(ras)-dependent mitogen-activated protein kinase pathway regulated by oxidant stress. J. Biol. Chem. 1997, 272, 17810–17814. [Google Scholar] [CrossRef]
- Higai, K.; Shimamura, A.; Matsumoto, K. Amadori-modified glycated albumin predominantly induces E-selectin expression on human umbilical vein endothelial cells through NADPH oxidase activation. Clin. Chim. Acta 2006, 367, 137–143. [Google Scholar] [CrossRef]
- Wautier, M.P.; Chappey, O.; Corda, S.; Stern, D.M.; Schmidt, A.M.; Wautier, J.L. Activation of NADPH oxidase by AGE links oxidant stress to altered gene expression via RAGE. Am. J. Physiol. Endocrinol. Metab. 2001, 280, E685–E694. [Google Scholar] [CrossRef]
- Nevado, J.; Peiro, C.; Vallejo, S.; El-Assar, M.; Lafuente, N.; Matesanz, N.; Azcutia, V.; Cercas, E.; Sanchez-Ferrer, C.F.; Rodriguez-Manas, L. Amadori adducts activate nuclear factor-kappaB-related proinflammatory genes in cultured human peritoneal mesothelial cells. Br. J. Pharmacol. 2005, 146, 268–279. [Google Scholar] [CrossRef]
- Yeh, C.H.; Sturgis, L.; Haidacher, J.; Zhang, X.N.; Sherwood, S.J.; Bjercke, R.J.; Juhasz, O.; Crow, M.T.; Tilton, R.G.; Denner, L. Requirement for p38 and p44/p42 mitogen-activated protein kinases in RAGE-mediated nuclear factor-kappaB transcriptional activation and cytokine secretion. Diabetes 2001, 50, 1495–1504. [Google Scholar] [CrossRef]
- Biondi, G.; Marrano, N.; Borrelli, A.; Rella, M.; D’Oria, R.; Genchi, V.A.; Caccioppoli, C.; Cignarelli, A.; Perrini, S.; Laviola, L.; et al. The p66(Shc) Redox Protein and the Emerging Complications of Diabetes. Int. J. Mol. Sci. 2023, 25, 108. [Google Scholar] [CrossRef]
- Gupta, A.; Tripathi, A.K.; Tripathi, R.L.; Madhu, S.V.; Banerjee, B.D. Advanced glycosylated end products-mediated activation of polymorphonuclear neutrophils in diabetes mellitus and associated oxidative stress. Indian J. Biochem. Biophys. 2007, 44, 373–378. [Google Scholar]
- Bansal, S.; Siddarth, M.; Chawla, D.; Banerjee, B.D.; Madhu, S.V.; Tripathi, A.K. Advanced glycation end products enhance reactive oxygen and nitrogen species generation in neutrophils in vitro. Mol. Cell Biochem. 2012, 361, 289–296. [Google Scholar] [CrossRef]
- Lu, H.; Xu, S.; Liang, X.; Dai, Y.; Huang, Z.; Ren, Y.; Lin, J.; Liu, X. Advanced Glycated End Products Alter Neutrophil Effect on Regulation of CD(4)+ T Cell Differentiation Through Induction of Myeloperoxidase and Neutrophil Elastase Activities. Inflammation 2019, 42, 559–571. [Google Scholar] [CrossRef]
- Byun, K.; Yoo, Y.; Son, M.; Lee, J.; Jeong, G.B.; Park, Y.M.; Salekdeh, G.H.; Lee, B. Advanced glycation end-products produced systemically and by macrophages: A common contributor to inflammation and degenerative diseases. Pharmacol. Ther. 2017, 177, 44–55. [Google Scholar] [CrossRef]
- van der Lugt, T.; Weseler, A.R.; Gebbink, W.A.; Vrolijk, M.F.; Opperhuizen, A.; Bast, A. Dietary Advanced Glycation Endproducts Induce an Inflammatory Response in Human Macrophages in Vitro. Nutrients 2018, 10, 1868. [Google Scholar] [CrossRef]
- Nonaka, K.; Kajiura, Y.; Bando, M.; Sakamoto, E.; Inagaki, Y.; Lew, J.H.; Naruishi, K.; Ikuta, T.; Yoshida, K.; Kobayashi, T.; et al. Advanced glycation end-products increase IL-6 and ICAM-1 expression via RAGE, MAPK and NF-κB pathways in human gingival fibroblasts. J. Periodontal Res. 2018, 53, 334–344. [Google Scholar] [CrossRef]
- Dai, J.Z.; Chen, H.; Chai, Y.M. Advanced Glycation End Products (AGES) Induce Apoptosis of Fibroblasts by Activation of NLRP3 Inflammasome via Reactive Oxygen Species (ROS) Signaling Pathway. Med. Sci. Monit. 2019, 25, 7499–7508. [Google Scholar] [CrossRef]
- Leerach, N.; Harashima, A.; Munesue, S.; Kimura, K.; Oshima, Y.; Goto, H.; Yamamoto, H.; Higashida, H.; Yamamoto, Y. Glycation reaction and the role of the receptor for advanced glycation end-products in immunity and social behavior. Glycoconj. J. 2021, 38, 303–310. [Google Scholar] [CrossRef]
- Franceschi, C.; Bonafe, M.; Valensin, S.; Olivieri, F.; De Luca, M.; Ottaviani, E.; De Benedictis, G. Inflamm-aging. An evolutionary perspective on immunosenescence. Ann. N. Y Acad. Sci. 2000, 908, 244–254. [Google Scholar] [CrossRef]
- Frasca, D.; Blomberg, B.B. Inflammaging decreases adaptive and innate immune responses in mice and humans. Biogerontology 2016, 17, 7–19. [Google Scholar] [CrossRef] [PubMed]
- Puzianowska-Kuznicka, M.; Owczarz, M.; Wieczorowska-Tobis, K.; Nadrowski, P.; Chudek, J.; Slusarczyk, P.; Skalska, A.; Jonas, M.; Franek, E.; Mossakowska, M. Interleukin-6 and C-reactive protein, successful aging, and mortality: The PolSenior study. Immun. Ageing 2016, 13, 21. [Google Scholar] [CrossRef] [PubMed]
- Stowe, R.P.; Peek, M.K.; Cutchin, M.P.; Goodwin, J.S. Plasma cytokine levels in a population-based study: Relation to age and ethnicity. J. Gerontol. A Biol. Sci. Med. Sci. 2010, 65, 429–433. [Google Scholar] [CrossRef] [PubMed]
- Ciabattini, A.; Nardini, C.; Santoro, F.; Garagnani, P.; Franceschi, C.; Medaglini, D. Vaccination in the elderly: The challenge of immune changes with aging. Semin. Immunol. 2018, 40, 83–94. [Google Scholar] [CrossRef] [PubMed]
- Osterholm, M.T.; Kelley, N.S.; Sommer, A.; Belongia, E.A. Efficacy and effectiveness of influenza vaccines: A systematic review and meta-analysis (vol 12, pg 36, 2012). Lancet Infect. Dis. 2012, 12, 655. [Google Scholar] [CrossRef] [PubMed]
- Negrean, M.; Stirban, A.; Stratmann, B.; Gawlowski, T.; Horstmann, T.; Gotting, C.; Kleesiek, K.; Mueller-Roesel, M.; Koschinsky, T.; Uribarri, J.; et al. Effects of low- and high-advanced glycation endproduct meals on macro- and microvascular endothelial function and oxidative stress in patients with type 2 diabetes mellitus. Am. J. Clin. Nutr. 2007, 85, 1236–1243. [Google Scholar] [CrossRef] [PubMed]
- Koschinsky, T.; He, C.J.; Mitsuhashi, T.; Bucala, R.; Liu, C.; Buenting, C.; Heitmann, K.; Vlassara, H. Orally absorbed reactive glycation products (glycotoxins): An environmental risk factor in diabetic nephropathy. Proc. Natl. Acad. Sci. USA 1997, 94, 6474–6479. [Google Scholar] [CrossRef]
- Schmidt, A.M.; Yan, S.D.; Yan, S.F.; Stern, D.M. The multiligand receptor RAGE as a progression factor amplifying immune and inflammatory responses. J. Clin. Investig. 2001, 108, 949–955. [Google Scholar] [CrossRef]
- Teissier, T.; Boulanger, E. The receptor for advanced glycation end-products (RAGE) is an important pattern recognition receptor (PRR) for inflammaging. Biogerontology 2019, 20, 279–301. [Google Scholar] [CrossRef]
- Van Puyvelde, K.; Mets, T.; Njemini, R.; Beyer, I.; Bautmans, I. Effect of advanced glycation end product intake on inflammation and aging: A systematic review. Nutr. Rev. 2014, 72, 638–650. [Google Scholar] [CrossRef]
- Tsai, C.Y.; Shen, C.Y.; Liao, H.T.; Li, K.J.; Lee, H.T.; Lu, C.S.; Wu, C.H.; Kuo, Y.M.; Hsieh, S.C.; Yu, C.L. Molecular and Cellular Bases of Immunosenescence, Inflammation, and Cardiovascular Complications Mimicking “Inflammaging” in Patients with Systemic Lupus Erythematosus. Int. J. Mol. Sci. 2019, 20, 3878. [Google Scholar] [CrossRef]
- Nowak, A.; Przywara-Chowaniec, B.; Damasiewicz-Bodzek, A.; Blachut, D.; Nowalany-Kozielska, E.; Tyrpien-Golder, K. Advanced Glycation End-Products (AGEs) and Their Soluble Receptor (sRAGE) in Women Suffering from Systemic Lupus Erythematosus (SLE). Cells 2021, 10, 3523. [Google Scholar] [CrossRef]
- Carrion-Barbera, I.; Triginer, L.; Tio, L.; Perez-Garcia, C.; Ribes, A.; Abad, V.; Pros, A.; Monfort, J.; Salman-Monte, T.C. Serum Advanced Glycation End Products and Their Soluble Receptor as New Biomarkers in Systemic Lupus Erythematosus. Biomedicines 2024, 12, 610. [Google Scholar] [CrossRef]
- Carrion-Barbera, I.; Triginer, L.; Tio, L.; Perez-Garcia, C.; Ribes, A.; Abad, V.; Pros, A.; Bermudez-Lopez, M.; Castro-Boque, E.; Lecube, A.; et al. Role of Advanced Glycation End Products as New Biomarkers in Systemic Lupus Erythematosus. Int. J. Mol. Sci. 2024, 25, 3022. [Google Scholar] [CrossRef]
- Shen, C.Y.; Lu, C.H.; Wu, C.H.; Li, K.J.; Kuo, Y.M.; Hsieh, S.C.; Yu, C.L. Molecular Basis of Accelerated Aging with Immune Dysfunction-Mediated Inflammation (Inflamm-Aging) in Patients with Systemic Sclerosis. Cells 2021, 10, 3402. [Google Scholar] [CrossRef]
- Omarjee, L.; Perrot, F.; Meilhac, O.; Mahe, G.; Bousquet, G.; Janin, A. Immunometabolism at the cornerstone of inflammaging, immunosenescence, and autoimmunity in COVID-19. Aging 2020, 12, 26263–26278. [Google Scholar] [CrossRef]
- Delrue, C.; Speeckaert, R.; Delanghe, J.R.; Speeckaert, M.M. The Potential Influence of Advanced Glycation End Products and (s)RAGE in Rheumatic Diseases. Int. J. Mol. Sci. 2023, 24, 2894. [Google Scholar] [CrossRef]
- Shen, C.Y.; Li, K.J.; Wu, C.H.; Lu, C.H.; Kuo, Y.M.; Hsieh, S.C.; Yu, C.L. Unveiling the molecular basis of inflamm-aging induced by advanced glycation end products (AGEs)-modified human serum albumin (AGE-HSA) in patients with different immune-mediated diseases. Clin. Immunol. 2023, 252, 109655. [Google Scholar] [CrossRef] [PubMed]
- Babtan, A.M.; Ilea, A.; Bosca, B.A.; Crisan, M.; Petrescu, N.B.; Collino, M.; Sainz, R.M.; Gerlach, J.Q.; Campian, R.S. Advanced glycation end products as biomarkers in systemic diseases: Premises and perspectives of salivary advanced glycation end products. Biomark. Med. 2019, 13, 479–495. [Google Scholar] [CrossRef] [PubMed]
- Zgutka, K.; Tkacz, M.; Tomasiak, P.; Tarnowski, M. A Role for Advanced Glycation End Products in Molecular Ageing. Int. J. Mol. Sci. 2023, 24, 9881. [Google Scholar] [CrossRef]
- Azrad, M.; Blair, C.K.; Rock, C.L.; Sedjo, R.L.; Wolin, K.Y.; Demark-Wahnefried, W. Adult weight gain accelerates the onset of breast cancer. Breast Cancer Res. Treat. 2019, 176, 649–656. [Google Scholar] [CrossRef]
- Pearson-Stuttard, J.; Zhou, B.; Kontis, V.; Bentham, J.; Gunter, M.J.; Ezzati, M. Worldwide burden of cancer attributable to diabetes and high body-mass index: A comparative risk assessment. Lancet Diabetes Endocrinol. 2018, 6, e6–e15. [Google Scholar] [CrossRef]
- Kallamadi, P.R.; Esari, D.; Addi, U.R.; Kesavan, R.; Putcha, U.K.; Nagini, S.; Reddy, G.B. Obesity Associated with Prediabetes Increases the Risk of Breast Cancer Development and Progression-A Study on an Obese Rat Model with Impaired Glucose Tolerance. Int. J. Mol. Sci. 2023, 24, 11441. [Google Scholar] [CrossRef]
- Divella, R.; De Luca, R.; Abbate, I.; Naglieri, E.; Daniele, A. Obesity and cancer: The role of adipose tissue and adipo-cytokines-induced chronic inflammation. J. Cancer 2016, 7, 2346–2359. [Google Scholar] [CrossRef]
- Nieman, K.M.; Kenny, H.A.; Penicka, C.V.; Ladanyi, A.; Buell-Gutbrod, R.; Zillhardt, M.R.; Romero, I.L.; Carey, M.S.; Mills, G.B.; Hotamisligil, G.S.; et al. Adipocytes promote ovarian cancer metastasis and provide energy for rapid tumor growth. Nat. Med. 2011, 17, 1498–1503. [Google Scholar] [CrossRef]
- Hoy, A.J.; Balaban, S.; Saunders, D.N. Adipocyte-Tumor Cell Metabolic Crosstalk in Breast Cancer. Trends Mol. Med. 2017, 23, 381–392. [Google Scholar] [CrossRef]
- Gallagher, E.J.; LeRoith, D. The proliferating role of insulin and insulin-like growth factors in cancer. Trends Endocrinol. Metab. 2010, 21, 610–618. [Google Scholar] [CrossRef]
- Novosyadlyy, R.; Lann, D.E.; Vijayakumar, A.; Rowzee, A.; Lazzarino, D.A.; Fierz, Y.; Carboni, J.M.; Gottardis, M.M.; Pennisi, P.A.; Molinolo, A.A.; et al. Insulin-mediated acceleration of breast cancer development and progression in a nonobese model of type 2 diabetes. Cancer Res. 2010, 70, 741–751. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Jimenez, C.; Garcia-Martinez, J.M.; Chocarro-Calvo, A.; De la Vieja, A. A new link between diabetes and cancer: Enhanced WNT/beta-catenin signaling by high glucose. J. Mol. Endocrinol. 2014, 52, R51–R66. [Google Scholar] [CrossRef] [PubMed]
- Clavel, J. Progress in the epidemiological understanding of gene-environment interactions in major diseases: Cancer. Comptes Rendus Biol. 2007, 330, 306–317. [Google Scholar] [CrossRef]
- Landskron, G.; De la Fuente, M.; Thuwajit, P.; Thuwajit, C.; Hermoso, M.A. Chronic inflammation and cytokines in the tumor microenvironment. J. Immunol. Res. 2014, 2014, 149185. [Google Scholar] [CrossRef]
- Blaylock, R.L. Cancer microenvironment, inflammation and cancer stem cells: A hypothesis for a paradigm change and new targets in cancer control. Surg. Neurol. Int. 2015, 6, 92. [Google Scholar] [CrossRef]
- Liou, G.Y.; Storz, P. Reactive oxygen species in cancer. Free Radic. Res. 2010, 44, 479–496. [Google Scholar] [CrossRef]
- Grivennikov, S.I.; Greten, F.R.; Karin, M. Immunity, inflammation, and cancer. Cell 2010, 140, 883–899. [Google Scholar] [CrossRef]
- Multhoff, G.; Molls, M.; Radons, J. Chronic inflammation in cancer development. Front. Immunol. 2012, 2, 98. [Google Scholar] [CrossRef]
- Kang, R.; Tang, D.; Schapiro, N.E.; Livesey, K.M.; Farkas, A.; Loughran, P.; Bierhaus, A.; Lotze, M.T.; Zeh, H.J. The receptor for advanced glycation end products (RAGE) sustains autophagy and limits apoptosis, promoting pancreatic tumor cell survival. Cell Death Differ. 2010, 17, 666–676. [Google Scholar] [CrossRef]
- El-Far, A.H.; Sroga, G.; Jaouni, S.K.A.; Mousa, S.A. Role and Mechanisms of RAGE-Ligand Complexes and RAGE-Inhibitors in Cancer Progression. Int. J. Mol. Sci. 2020, 21, 3613. [Google Scholar] [CrossRef]
- Waghela, B.N.; Vaidya, F.U.; Ranjan, K.; Chhipa, A.S.; Tiwari, B.S.; Pathak, C. AGE-RAGE synergy influences programmed cell death signaling to promote cancer. Mol. Cell. Biochem. 2021, 476, 585–598. [Google Scholar] [CrossRef]
- Palanissami, G.; Paul, S.F.D. AGEs and RAGE: Metabolic and molecular signatures of the glycation-inflammation axis in malignant or metastatic cancers. Explor. Target. Antitumor Ther. 2023, 4, 812–849. [Google Scholar] [CrossRef]
- Eva, T.A.; Barua, N.; Chowdhury, M.M.; Yeasmin, S.; Rakib, A.; Islam, M.R.; Emran, T.B.; Simal-Gandara, J. Perspectives on signaling for biological- and processed food-related advanced glycation end-products and its role in cancer progression. Crit. Rev. Food Sci. Nutr. 2022, 62, 2655–2672. [Google Scholar] [CrossRef] [PubMed]
- Wada, K.; Nakashima, Y.; Yamakawa, M.; Hori, A.; Seishima, M.; Tanabashi, S.; Matsushita, S.; Tokimitsu, N.; Nagata, C. Dietary advanced glycation end products and cancer risk in Japan: From the Takayama study. Cancer Sci. 2022, 113, 2839–2848. [Google Scholar] [CrossRef] [PubMed]
- Renehan, A.G.; Tyson, M.; Egger, M.; Heller, R.F.; Zwahlen, M. Body-Mass Index and Incidence of Cancer: A Systematic Review and Meta-Analysis of Prospective Observational Studies. Am. J. Health Promot. 2008, 23, 153. [Google Scholar] [CrossRef] [PubMed]
- Roberts, D.L.; Dive, C.; Renehan, A.G. Biological mechanisms linking obesity and cancer risk: New perspectives. Annu. Rev. Med. 2010, 61, 301–316. [Google Scholar] [CrossRef] [PubMed]
- Bierhaus, A.; Nawroth, P.P. Multiple levels of regulation determine the role of the receptor for AGE (RAGE) as common soil in inflammation, immune responses and diabetes mellitus and its complications. Diabetologia 2009, 52, 2251–2263. [Google Scholar] [CrossRef] [PubMed]
- Chuah, Y.K.; Basir, R.; Talib, H.; Tie, T.H.; Nordin, N. Receptor for advanced glycation end products and its involvement in inflammatory diseases. Int. J. Inflam. 2013, 2013, 403460. [Google Scholar] [CrossRef] [PubMed]
- Garza-Campos, A.; Prieto-Correa, J.R.; Dominguez-Rosales, J.A.; Hernandez-Nazara, Z.H. Implications of receptor for advanced glycation end products for progression from obesity to diabetes and from diabetes to cancer. World J. Diabetes 2023, 14, 977–994. [Google Scholar] [CrossRef] [PubMed]
- Gugliucci, A. Fructose surges damage hepatic adenosyl-monophosphate-dependent kinase and lead to increased lipogenesis and hepatic insulin resistance. Med. Hypotheses 2016, 93, 87–92. [Google Scholar] [CrossRef]
- Oimomi, M.; Nakamichi, T.; Ohara, T.; Sakai, M.; Igaki, N.; Hata, F.; Baba, S. Fructose-related glycation. Diabetes Res. Clin. Pract. 1989, 7, 137–139. [Google Scholar] [CrossRef]
- Schalkwijk, C.G.; Stehouwer, C.D.; van Hinsbergh, V.W. Fructose-mediated non-enzymatic glycation: Sweet coupling or bad modification. Diabetes Metab. Res. Rev. 2004, 20, 369–382. [Google Scholar] [CrossRef]
- Amani, S.; Fatima, S. Glycation With Fructose: The Bitter Side of Nature’s Own Sweetener. Curr. Diabetes Rev. 2020, 16, 962–970. [Google Scholar] [CrossRef] [PubMed]
- Gugliucci, A. Formation of Fructose-Mediated Advanced Glycation End Products and Their Roles in Metabolic and Inflammatory Diseases. Adv. Nutr. 2017, 8, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Mastrocola, R.; Nigro, D.; Cento, A.S.; Chiazza, F.; Collino, M.; Aragno, M. High-fructose intake as risk factor for neurodegeneration: Key role for carboxy methyllysine accumulation in mice hippocampal neurons. Neurobiol. Dis. 2016, 89, 65–75. [Google Scholar] [CrossRef] [PubMed]
- Mastrocola, R.; Nigro, D.; Chiazza, F.; Medana, C.; Dal Bello, F.; Boccuzzi, G.; Collino, M.; Aragno, M. Fructose-derived advanced glycation end-products drive lipogenesis and skeletal muscle reprogramming via SREBP-1c dysregulation in mice. Free Radic. Biol. Med. 2016, 91, 224–235. [Google Scholar] [CrossRef] [PubMed]
- Rai, A.K.; Jaiswal, N.; Maurya, C.K.; Sharma, A.; Ahmad, I.; Ahmad, S.; Gupta, A.P.; Gayen, J.R.; Tamrakar, A.K. Fructose-induced AGEs-RAGE signaling in skeletal muscle contributes to impairment of glucose homeostasis. J. Nutr. Biochem. 2019, 71, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Faisal, M.; Alatar, A.A.; Ahmad, S. Immunoglobulin-G Glycation by Fructose Leads to Structural Perturbations and Drop Off in Free Lysine and Arginine Residues. Protein Pept. Lett. 2017, 24, 241–244. [Google Scholar] [CrossRef] [PubMed]
- Sotokawauchi, A.; Matsui, T.; Higashimoto, Y.; Yamagishi, S.I. Fructose causes endothelial cell damage via activation of advanced glycation end products-receptor system. Diabetes Vasc. Dis. Res. 2019, 16, 556–561. [Google Scholar] [CrossRef] [PubMed]
- Muraoka, M.Y.; Justino, A.B.; Caixeta, D.C.; Queiroz, J.S.; Sabino-Silva, R.; Salmen Espindola, F. Fructose and methylglyoxal-induced glycation alters structural and functional properties of salivary proteins, albumin and lysozyme. PLoS ONE 2022, 17, e0262369. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Chen, L.; Chen, J.; Ge, L.; He, R.Q. Rapid glycation with D-ribose induces globular amyloid-like aggregations of BSA with high cytotoxicity to SH-SY5Y cells. BMC Cell Biol. 2009, 10, 10. [Google Scholar] [CrossRef]
- Wei, Y.; Han, C.S.; Zhou, J.; Liu, Y.; Chen, L.; He, R.Q. D-ribose in glycation and protein aggregation. Biochim. Biophys. Acta 2012, 1820, 488–494. [Google Scholar] [CrossRef]
- Mou, L.; Hu, P.; Cao, X.; Chen, Y.; Xu, Y.; He, T.; Wei, Y.; He, R. Comparison of bovine serum albumin glycation by ribose and fructose in vitro and in vivo. Biochim. Biophys. Acta Mol. Basis Dis. 2022, 1868, 166283. [Google Scholar] [CrossRef]
- Ahmad, S.; Al-Shaghdali, K.; Rehman, S.; Khan, M.Y.; Rafi, Z.; Faisal, M.; Alatar, A.A.; Tahir, I.K.; Khan, S.; Ahmad, S.; et al. Nonenzymatic glycosylation of isolated human immunoglobulin-G by D-ribose. Cell Biochem. Funct. 2022, 40, 526–534. [Google Scholar] [CrossRef]
- Rafi, Z.; Alouffi, S.; Khan, M.S.; Ahmad, S. 2’-Deoxyribose Mediated Glycation Leads to Alterations in BSA Structure Via Generation of Carbonyl Species. Curr. Protein Pept. Sci. 2020, 21, 924–935. [Google Scholar] [CrossRef]
- Alenazi, F.; Saleem, M.; Syed Khaja, A.S.; Zafar, M.; Alharbi, M.S.; Hagbani, T.A.; Khan, M.Y.; Ahmad, S. Glycation of Immunoglobulin-G from Pentose Sugar: A Cause for Structural Perturbations. Curr. Protein Pept. Sci. 2022, 23, 773–781. [Google Scholar] [CrossRef]
- Bunn, H.F.; Higgins, P.J. Reaction of monosaccharides with proteins: Possible evolutionary significance. Science 1981, 213, 222–224. [Google Scholar] [CrossRef]
- Delwing-de Lima, D.; Hennrich, S.B.; Delwing-Dal Magro, D.; Aurelio, J.G.; Serpa, A.P.; Augusto, T.W.; Pereira, N.R. The effect of d-galactose induced oxidative stress on in vitro redox homeostasis in rat plasma and erythrocytes. Biomed. Pharmacother. 2017, 86, 686–693. [Google Scholar] [CrossRef]
- Berry, G.T.; Nissim, I.; Lin, Z.; Mazur, A.T.; Gibson, J.B.; Segal, S. Endogenous synthesis of galactose in normal men and patients with hereditary galactosaemia. Lancet 1995, 346, 1073–1074. [Google Scholar] [CrossRef]
- Umbayev, B.; Askarova, S.; Almabayeva, A.; Saliev, T.; Masoud, A.R.; Bulanin, D. Galactose-Induced Skin Aging: The Role of Oxidative Stress. Oxid. Med. Cell. Longev. 2020, 2020, 7145656. [Google Scholar] [CrossRef]
- Schleicher, E.; Deufel, T.; Wieland, O.H. Non-enzymatic glycosylation of human serum lipoproteins. Elevated epsilon-lysine glycosylated low density lipoprotein in diabetic patients. FEBS Lett. 1981, 129, 1–4. [Google Scholar] [CrossRef]
- Lyons, T.J. Lipoprotein glycation and its metabolic consequences. Diabetes 1992, 41 (Suppl. 2), 67–73. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.Y.; Alouffi, S.; Ahmad, S. Immunochemical studies on native and glycated LDL-An approach to uncover the structural perturbations. Int. J. Biol. Macromol. 2018, 115, 287–299. [Google Scholar] [CrossRef]
- Ahmad, S.; Akhter, F.; Moinuddin; Shahab, U.; Khan, M.S. Studies on glycation of human low density lipoprotein: A functional insight into physico-chemical analysis. Int. J. Biol. Macromol. 2013, 62, 167–171. [Google Scholar] [CrossRef] [PubMed]
- Abidi, M.; Khan, M.S.; Ahmad, S.; Kausar, T.; Nayeem, S.M.; Islam, S.; Ali, A.; Alam, K. Biophysical and biochemical studies on glycoxidatively modified human low density lipoprotein. Arch. Biochem. Biophys. 2018, 645, 87–99. [Google Scholar] [CrossRef] [PubMed]
- Alyahyawi, A.R.; Khan, M.Y.; Alouffi, S.; Maarfi, F.; Akasha, R.; Khan, S.; Rafi, Z.; Alharazi, T.; Shahab, U.; Ahmad, S. Identification of Glycoxidative Lesion in Isolated Low-Density Lipoproteins from Diabetes Mellitus Subjects. Life 2023, 13, 1986. [Google Scholar] [CrossRef]
- Dutta, U.; Cohenford, M.A.; Dain, J.A. Nonenzymatic glycation of DNA nucleosides with reducing sugars. Anal. Biochem. 2005, 345, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Cohenford, M.A.; Dutta, U.; Dain, J.A. The structural modification of DNA nucleosides by nonenzymatic glycation: An in vitro study based on the reactions of glyoxal and methylglyoxal with 2’-deoxyguanosine. Anal. Bioanal. Chem. 2008, 390, 679–688. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.; Moinuddin; Dixit, K.; Shahab, U.; Alam, K.; Ali, A. Genotoxicity and immunogenicity of DNA-advanced glycation end products formed by methylglyoxal and lysine in presence of Cu2+. Biochem. Biophys. Res. Commun. 2011, 407, 568–574. [Google Scholar] [CrossRef] [PubMed]
- Akhter, F.; Salman Khan, M.; Shahab, U.; Moinuddin; Ahmad, S. Bio-physical characterization of ribose induced glycation: A mechanistic study on DNA perturbations. Int. J. Biol. Macromol. 2013, 58, 206–210. [Google Scholar] [CrossRef] [PubMed]
- Akhter, F.; Khan, M.S.; Ahmad, S. Acquired immunogenicity of calf thymus DNA and LDL modified by D-ribose: A comparative study. Int. J. Biol. Macromol. 2015, 72, 1222–1227. [Google Scholar] [CrossRef]
- Polizzi, F.C.; Andican, G.; Cetin, E.; Civelek, S.; Yumuk, V.; Burcak, G. Increased DNA-glycation in type 2 diabetic patients: The effect of thiamine and pyridoxine therapy. Exp. Clin. Endocrinol. Diabetes 2012, 120, 329–334. [Google Scholar] [CrossRef]
- Jaramillo, R.; Shuck, S.C.; Chan, Y.S.; Liu, X.; Bates, S.E.; Lim, P.P.; Tamae, D.; Lacoste, S.; O’Connor, T.R.; Termini, J. DNA Advanced Glycation End Products (DNA-AGEs) Are Elevated in Urine and Tissue in an Animal Model of Type 2 Diabetes. Chem. Res. Toxicol. 2017, 30, 689–698. [Google Scholar] [CrossRef] [PubMed]
- Thornalley, P.J.; Waris, S.; Fleming, T.; Santarius, T.; Larkin, S.J.; Winklhofer-Roob, B.M.; Stratton, M.R.; Rabbani, N. Imidazopurinones are markers of physiological genomic damage linked to DNA instability and glyoxalase 1-associated tumour multidrug resistance. Nucleic Acids Res. 2010, 38, 5432–5442. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Nakamura, S.; Miyazaki, S.; Morita, T.; Suzuki, M.; Pischetsrieder, M.; Niwa, T. N2-carboxyethyl-2’-deoxyguanosine, a DNA glycation marker, in kidneys and aortas of diabetic and uremic patients. Kidney Int. 2006, 69, 388–392. [Google Scholar] [CrossRef] [PubMed]
- Eichhorst, A.; Daniel, C.; Rzepka, R.; Sehnert, B.; Nimmerjahn, F.; Voll, R.E.; Chevalier, N. Relevance of Receptor for Advanced Glycation end Products (RAGE) in Murine Antibody-Mediated Autoimmune Diseases. Int. J. Mol. Sci. 2019, 20, 3234. [Google Scholar] [CrossRef]
- Shen, C.Y.; Lu, C.H.; Wu, C.H.; Li, K.J.; Kuo, Y.M.; Hsieh, S.C.; Yu, C.L. The Development of Maillard Reaction, and Advanced Glycation End Product (AGE)-Receptor for AGE (RAGE) Signaling Inhibitors as Novel Therapeutic Strategies for Patients with AGE-Related Diseases. Molecules 2020, 25, 5591. [Google Scholar] [CrossRef]








Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, C.-Y.; Lu, C.-H.; Cheng, C.-F.; Li, K.-J.; Kuo, Y.-M.; Wu, C.-H.; Liu, C.-H.; Hsieh, S.-C.; Tsai, C.-Y.; Yu, C.-L. Advanced Glycation End-Products Acting as Immunomodulators for Chronic Inflammation, Inflammaging and Carcinogenesis in Patients with Diabetes and Immune-Related Diseases. Biomedicines 2024, 12, 1699. https://doi.org/10.3390/biomedicines12081699
Shen C-Y, Lu C-H, Cheng C-F, Li K-J, Kuo Y-M, Wu C-H, Liu C-H, Hsieh S-C, Tsai C-Y, Yu C-L. Advanced Glycation End-Products Acting as Immunomodulators for Chronic Inflammation, Inflammaging and Carcinogenesis in Patients with Diabetes and Immune-Related Diseases. Biomedicines. 2024; 12(8):1699. https://doi.org/10.3390/biomedicines12081699
Chicago/Turabian StyleShen, Chieh-Yu, Cheng-Hsun Lu, Chiao-Feng Cheng, Ko-Jen Li, Yu-Min Kuo, Cheng-Han Wu, Chin-Hsiu Liu, Song-Chou Hsieh, Chang-Youh Tsai, and Chia-Li Yu. 2024. "Advanced Glycation End-Products Acting as Immunomodulators for Chronic Inflammation, Inflammaging and Carcinogenesis in Patients with Diabetes and Immune-Related Diseases" Biomedicines 12, no. 8: 1699. https://doi.org/10.3390/biomedicines12081699
APA StyleShen, C.-Y., Lu, C.-H., Cheng, C.-F., Li, K.-J., Kuo, Y.-M., Wu, C.-H., Liu, C.-H., Hsieh, S.-C., Tsai, C.-Y., & Yu, C.-L. (2024). Advanced Glycation End-Products Acting as Immunomodulators for Chronic Inflammation, Inflammaging and Carcinogenesis in Patients with Diabetes and Immune-Related Diseases. Biomedicines, 12(8), 1699. https://doi.org/10.3390/biomedicines12081699





