Dynamics of Leukocyte Telomere Length in Patients with Fabry Disease
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Participants
2.2. Sample Collection and Isolation of Genomic DNA
2.3. Measurement of Telomere Length by Quantitative PCR
2.4. Statistical Analysis
3. Results
3.1. Patient’s Characteristics
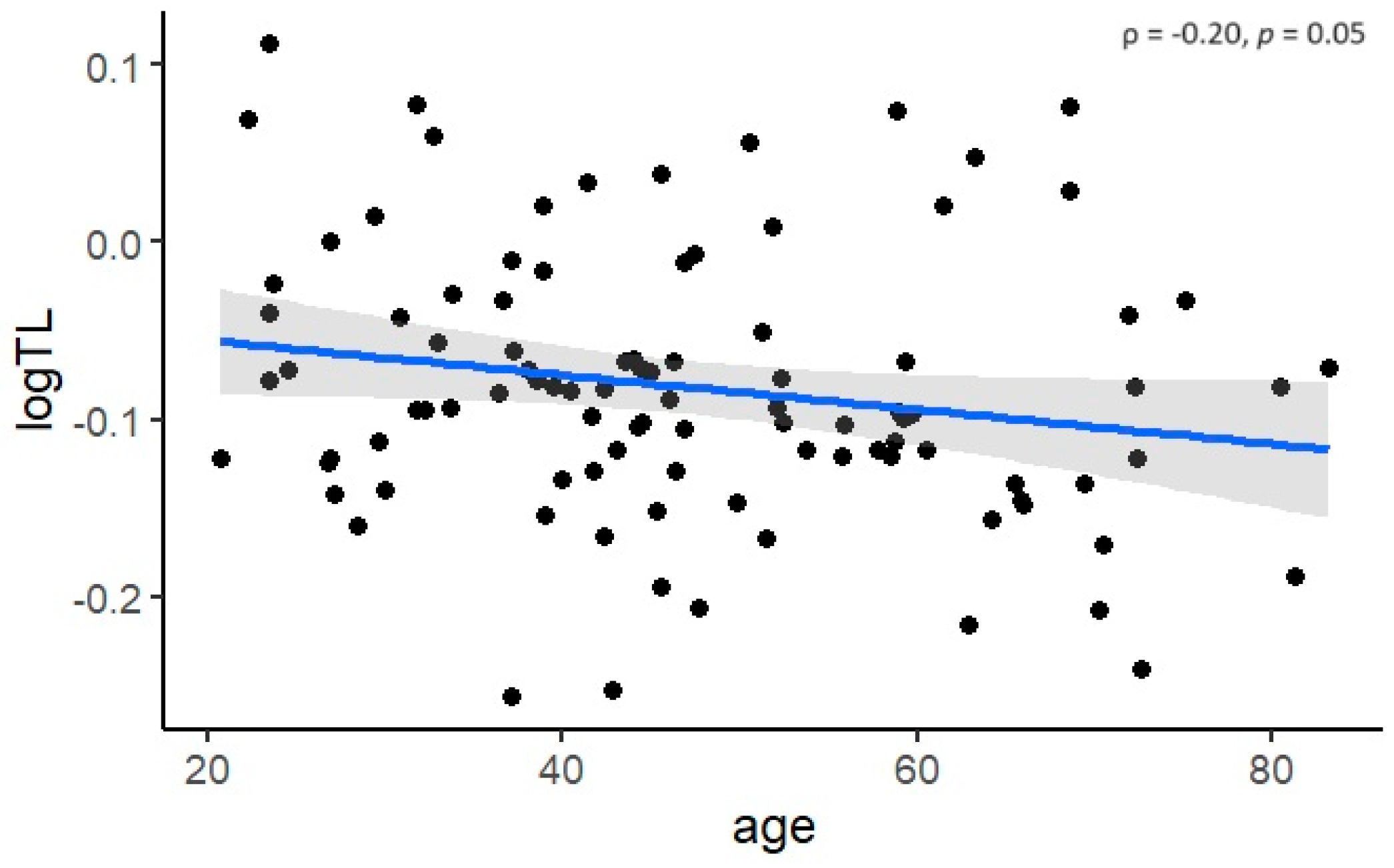
3.2. Cross-Sectional Analysis of Telomere Length in Fabry Patients
3.3. Longitudinal Analysis of Telomere Length Dynamics in Fabry Patients
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brady, R.O.; Gal, A.E.; Bradley, R.M.; Martensson, E.; Warshaw, A.L.; Laster, L. Enzymatic defect in Fabry’s disease. Ceramidetrihexosidase deficiency. N. Engl. J. Med. 1967, 276, 1163–1167. [Google Scholar] [CrossRef] [PubMed]
- Aerts, J.M.; Groener, J.E.; Kuiper, S.; Donker-Koopman, W.E.; Strijland, A.; Ottenhoff, R.; Van Roomen, C.; Mirzaian, M.; Wijburg, F.A.; Linthorst, G.E.; et al. Elevated globotriaosylsphingosine is a hallmark of Fabry disease. Proc. Natl. Acad. Sci. USA 2008, 105, 2812–2817. [Google Scholar] [CrossRef] [PubMed]
- Askari, H.; Kaneski, C.R.; Semino-Mora, C.; Desai, P.; Ang, A.; Kleiner, D.E.; Perlee, L.T.; Quezado, M.; Spollen, L.E.; Wustman, B.A.; et al. Cellular and tissue localization of globotriaosylceramide in Fabry disease. Virchows Arch. 2007, 451, 823–834. [Google Scholar] [CrossRef] [PubMed]
- Germain, D.P. Fabry disease. Orphanet J. Rare Dis. 2010, 5, 30. [Google Scholar] [CrossRef] [PubMed]
- Redonnet-Vernhet, I.; Ploos Van Amstel, J.K.; Jansen, R.P.M.; Wevers, R.A.; Salvayre, R.; Levade, T. Uneven X inactivation in a female monozygotic twin pair with Fabry disease and discordant expression of a novel mutation in the α-galactosidase A gene. J. Med. Genet. 1996, 33, 682–688. [Google Scholar] [CrossRef]
- Izhar, R.; Borriello, M.; La Russa, A.; Di Paola, R.; De, A.; Capasso, G.; Ingrosso, D.; Perna, A.F.; Simeoni, M. Fabry disease in women: Genetic basis, available biomarkers, and clinical manifestations. Genes 2023, 15, 37. [Google Scholar] [CrossRef] [PubMed]
- Wagenhauser, L.; Rickert, V.; Sommer, C.; Wanner, C.; Nordbeck, P.; Rost, S.; Uceyler, N. X-chromosomal inactivation patterns in women with Fabry disease. Mol. Genet. Genom. Med. 2022, 10, e2029. [Google Scholar] [CrossRef] [PubMed]
- Umer, M.; Kalra, D.K. Treatment of Fabry disease: Established and emerging therapies. Pharmaceuticals 2023, 16, 320. [Google Scholar] [CrossRef]
- Waldek, S.; Patel, M.R.; Banikazemi, M.; Lemay, R.; Lee, P. Life expectancy and cause of death in males and females with Fabry disease: Findings from the Fabry Registry. Genet. Med. 2009, 11, 790–796. [Google Scholar] [CrossRef]
- Kooman, J.P.; Stenvinkel, P.; Shiels, P.G. Fabry disease: A new model of premature ageing? Nephron 2020, 144, 1–4. [Google Scholar] [CrossRef]
- Lopez-Otin, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The hallmarks of aging. Cell 2013, 153, 1194–1217. [Google Scholar] [CrossRef]
- de Lange, T. Shelterin: The protein complex that shapes and safeguards human telomeres. Genes Dev. 2005, 19, 2100–2110. [Google Scholar] [CrossRef]
- Blackburn, E.H. The molecular structure of centromeres and telomeres. Annu. Rev. Biochem 1984, 53, 163–194. [Google Scholar] [CrossRef]
- Griffith, J.D.; Comeau, L.; Rosenfield, S.; Stansel, R.M.; Bianchi, A.; Moss, H.; de Lange, T. Mammalian telomeres end in a large duplex loop. Cell 1999, 97, 503–514. [Google Scholar] [CrossRef] [PubMed]
- Hayflick, L. The limited in vitro lifetime of human diploid cell strains. Exp. Cell Res. 1965, 37, 614–636. [Google Scholar] [CrossRef] [PubMed]
- Rossiello, F.; Jurk, D.; Passos, J.F.; d’Adda di Fagagna, F. Telomere dysfunction in ageing and age-related diseases. Nat. Cell Biol. 2022, 24, 135–147. [Google Scholar] [CrossRef] [PubMed]
- Ehrlenbach, S.; Willeit, P.; Kiechl, S.; Willeit, J.; Reindl, M.; Schanda, K.; Kronenberg, F.; Brandstatter, A. Influences on the reduction of relative telomere length over 10 years in the population-based Bruneck Study: Introduction of a well-controlled high-throughput assay. Int. J. Epidemiol. 2009, 38, 1725–1734. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zhan, Y.; Pedersen, N.L.; Fang, F.; Hagg, S. Telomere length and all-cause mortality: A meta-analysis. Aging Res. Rev. 2018, 48, 11–20. [Google Scholar] [CrossRef]
- Levstek, T.; Trebušak Podkrajšek, K. Telomere attrition in chronic kidney diseases. Antioxidants 2023, 12, 579. [Google Scholar] [CrossRef]
- Oliveira, J.P.; Ferreira, S. Multiple phenotypic domains of Fabry disease and their relevance for establishing genotype-phenotype correlations. Appl. Clin. Genet. 2019, 12, 35–50. [Google Scholar] [CrossRef]
- Cokan Vujkovac, A.; Novaković, S.; Vujkovac, B.; Števanec, M.; Škerl, P.; Šabovič, M. Aging in Fabry disease: Role of telomere length, telomerase activity, and kidney disease. Nephron 2020, 144, 5–13. [Google Scholar] [CrossRef] [PubMed]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 233–270. [Google Scholar] [CrossRef] [PubMed]
- Wanner, C.; Arad, M.; Baron, R.; Burlina, A.; Elliott, P.M.; Feldt-Rasmussen, U.; Fomin, V.V.; Germain, D.P.; Hughes, D.A.; Jovanovic, A.; et al. European expert consensus statement on therapeutic goals in Fabry disease. Mol. Genet Metab. 2018, 124, 189–203. [Google Scholar] [CrossRef] [PubMed]
- Levey, A.S.; Stevens, L.A.; Schmid, C.H.; Zhang, Y.; Castro, A.F.; Feldman, H.I.; Kusek, J.W.; Eggers, P.; Van Lente, F.; Greene, T.; et al. A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 2009, 150, 604–612. [Google Scholar] [CrossRef] [PubMed]
- Cawthon, R.M. Telomere length measurement by a novel monochrome multiplex quantitative PCR method. Nucleic Acids Res. 2009, 37, e21. [Google Scholar] [CrossRef] [PubMed]
- Levstek, T.; Redensek, S.; Trost, M.; Dolzan, V.; Podkrajsek, K.T. Assessment of the telomere length and its effect on the symptomatology of Parkinson’s disease. Antioxidants 2021, 10, 137. [Google Scholar] [CrossRef] [PubMed]
- Okuda, K. Telomere length in the newborn. Pediatr. Res. 2002, 52, 377–381. [Google Scholar] [CrossRef] [PubMed]
- Hunt, S.C.; Chen, W.; Gardner, J.P.; Kimura, M.; Srinivasan, S.R.; Eckfeldt, J.H.; Berenson, G.S.; Aviv, A. Leukocyte telomeres are longer in African Americans than in whites: The National Heart, Lung, and Blood Institute Family Heart Study and the Bogalusa Heart Study. Aging Cell 2008, 7, 451–458. [Google Scholar] [CrossRef] [PubMed]
- Gardner, M.; Bann, D.; Wiley, L.; Cooper, R.; Hardy, R.; Nitsch, D.; Martin-Ruiz, C.; Shiels, P.; Sayer, A.A.; Barbieri, M.; et al. Gender and telomere length: Systematic review and meta-analysis. Exp. Gerontol. 2014, 51, 15–27. [Google Scholar] [CrossRef]
- Ramaswami, U.; Beck, M.; Hughes, D.; Kampmann, C.; Botha, J.; Pintos-Morell, G.; West, M.L.; Niu, D.M.; Nicholls, K.; Giugliani, R. Cardio-renal outcomes with long-term agalsidase alfa enzyme replacement therapy: A 10-year Fabry Outcome Survey (FOS) analysis. Drug Des. Dev. Ther. 2019, 13, 3705–3715. [Google Scholar] [CrossRef]
- Wanner, C.; Feldt-Rasmussen, U.; Jovanovic, A.; Linhart, A.; Yang, M.; Ponce, E.; Brand, E.; Germain, D.P.; Hughes, D.A.; Jefferies, J.L.; et al. Cardiomyopathy and kidney function in agalsidase beta-treated female Fabry patients: A pre-treatment vs. post-treatment analysis. ESC Heart Fail. 2020, 7, 825–834. [Google Scholar] [CrossRef] [PubMed]
- Smith, L.; Luchini, C.; Demurtas, J.; Soysal, P.; Stubbs, B.; Hamer, M.; Nottegar, A.; Lawlor, R.T.; Lopez-Sanchez, G.F.; Firth, J.; et al. Telomere length and health outcomes: An umbrella review of systematic reviews and meta-analyses of observational studies. Ageing Res. Rev. 2019, 51, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Martin-Ruiz, C.M.; Gussekloo, J.; van Heemst, D.; von Zglinicki, T.; Westendorp, R.G. Telomere length in white blood cells is not associated with morbidity or mortality in the oldest old: A population-based study. Aging Cell 2005, 4, 287–290. [Google Scholar] [CrossRef] [PubMed]
- Harris, S.E.; Marioni, R.E.; Martin-Ruiz, C.; Pattie, A.; Gow, A.J.; Cox, S.R.; Corley, J.; von Zglinicki, T.; Starr, J.M.; Deary, I.J. Longitudinal telomere length shortening and cognitive and physical decline in later life: The Lothian Birth Cohorts 1936 and 1921. Mech. Ageing Dev. 2016, 154, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Martin-Ruiz, C.; Jagger, C.; Kingston, A.; Collerton, J.; Catt, M.; Davies, K.; Dunn, M.; Hilkens, C.; Keavney, B.; Pearce, S.H.; et al. Assessment of a large panel of candidate biomarkers of ageing in the Newcastle 85+ study. Mech. Ageing Dev. 2011, 132, 496–502. [Google Scholar] [CrossRef] [PubMed]
- Aviv, A.; Chen, W.; Gardner, J.P.; Kimura, M.; Brimacombe, M.; Cao, X.; Srinivasan, S.R.; Berenson, G.S. Leukocyte telomere dynamics: Longitudinal findings among young adults in the Bogalusa Heart Study. Am. J. Epidemiol. 2009, 169, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Nordfjall, K.; Svenson, U.; Norrback, K.F.; Adolfsson, R.; Lenner, P.; Roos, G. The individual blood cell telomere attrition rate is telomere length dependent. PLoS Genet. 2009, 5, e1000375. [Google Scholar] [CrossRef] [PubMed]
- Njajou, O.T.; Cawthon, R.M.; Damcott, C.M.; Wu, S.-H.; Ott, S.; Garant, M.J.; Blackburn, E.H.M.; Braxton, D.; Shuldiner, A.R.; Hsueh, W.-C. Telomere length is paternally inherited andis associated with parental lifespan. Proc. Natl. Acad. Sci. USA 2007, 104, 12135–12139. [Google Scholar] [CrossRef]
- Andrew, T.; Aviv, A.; Falchi, M.; Surdulescu, G.L.; Gardner, J.P.; Lu, X.; Kimura, M.; Kato, B.S.; Valdes, A.M.; Spector, T.D. Mapping genetic loci that determine leukocyte telomere length in a large sample of unselected female sibling pairs. Am. J. Hum. Genet. 2006, 78, 480–486. [Google Scholar] [CrossRef]
- Slagboom, P.; Droog, S.; Boomsma, D. Genetic determination of telomere size in humans: A twin study of three age groups. Am. J. Hum. Genet. 1994, 55, 876–882. [Google Scholar]
- Hjelmborg, J.B.; Dalgard, C.; Moller, S.; Steenstrup, T.; Kimura, M.; Christensen, K.; Kyvik, K.O.; Aviv, A. The heritability of leucocyte telomere length dynamics. J. Med. Genet. 2015, 52, 297–302. [Google Scholar] [CrossRef] [PubMed]
- Benetos, A.; Kark, J.D.; Susser, E.; Kimura, M.; Sinnreich, R.; Chen, W.; Steenstrup, T.; Christensen, K.; Herbig, U.; von Bornemann Hjelmborg, J.; et al. Tracking and fixed ranking of leukocyte telomere length across the adult life course. Aging Cell 2013, 12, 615–621. [Google Scholar] [CrossRef] [PubMed]
- Popli, S.; Leehey, D.J.; Molnar, Z.V.; Nawab, Z.M.; Ing, T.S. Demonstration of Fabry’s disease deposits in placenta. Am. J. Obstet. Gynecol. 1990, 162, 464–465. [Google Scholar] [CrossRef] [PubMed]
- Horvath, S.; Raj, K. DNA methylation-based biomarkers and the epigenetic clock theory of ageing. Nat. Rev. Genet. 2018, 19, 371–384. [Google Scholar] [CrossRef]
- Wang, M.; Lemos, B. Ribosomal DNA harbors an evolutionarily conserved clock of biological aging. Genome Res. 2019, 29, 325–333. [Google Scholar] [CrossRef]
- Lin, J.; Smith, D.L.; Esteves, K.; Drury, S. Telomere length measurement by qPCR—Summary of critical factors and recommendations for assay design. Psychoneuroendocrinology 2019, 99, 271–278. [Google Scholar] [CrossRef]

| Parameter | Cross-Sectional Analysis (n = 99) | Longitudinal Analysis (n = 50) |
|---|---|---|
| Males (%) | 32 (32.2) | 16 (32.0) |
| Age (years) | 47.3 ± 15.3 | 43.1 ± 15.1 |
| DST | 66 (66.7) | 29 (58.0) |
| HCMP | 37 (37.4) | 20 (40.0) |
| Progressive nephropathy | 15 (15.2) | 8 (16.0) |
| Stroke | 8 (8.1) | 4 (8.0) |
| Estimate | St. Error | p Value | |
|---|---|---|---|
| Model 1 | |||
| Years | −0.0030 | 0.0016 | 0.07 |
| Baseline LTL | 0.2006 | 0.0539 | <0.01 |
| Age | −0.0004 | 0.0005 | 0.40 |
| Group 1 vs. group 2 | −0.0266 | 0.0154 | 0.09 |
| Group 1 vs. group 3 | −0.0058 | 0.0196 | 0.77 |
| Model 2 | |||
| Years | −0.0030 | 0.0016 | 0.05 |
| Baseline LTL | 0.2060 | 0.0535 | <0.01 |
| Age | −0.0003 | 0.0005 | 0.49 |
| Group 1 vs. group 4 | −0.0199 | 0.0139 | 0.16 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Levstek, T.; Breznik, N.; Vujkovac, B.; Nowak, A.; Trebušak Podkrajšek, K. Dynamics of Leukocyte Telomere Length in Patients with Fabry Disease. Biomedicines 2024, 12, 1724. https://doi.org/10.3390/biomedicines12081724
Levstek T, Breznik N, Vujkovac B, Nowak A, Trebušak Podkrajšek K. Dynamics of Leukocyte Telomere Length in Patients with Fabry Disease. Biomedicines. 2024; 12(8):1724. https://doi.org/10.3390/biomedicines12081724
Chicago/Turabian StyleLevstek, Tina, Nika Breznik, Bojan Vujkovac, Albina Nowak, and Katarina Trebušak Podkrajšek. 2024. "Dynamics of Leukocyte Telomere Length in Patients with Fabry Disease" Biomedicines 12, no. 8: 1724. https://doi.org/10.3390/biomedicines12081724







