Ultrasound-Compatible Electrode for Functional Electrical Stimulation
Abstract
1. Introduction
2. Materials and Methods
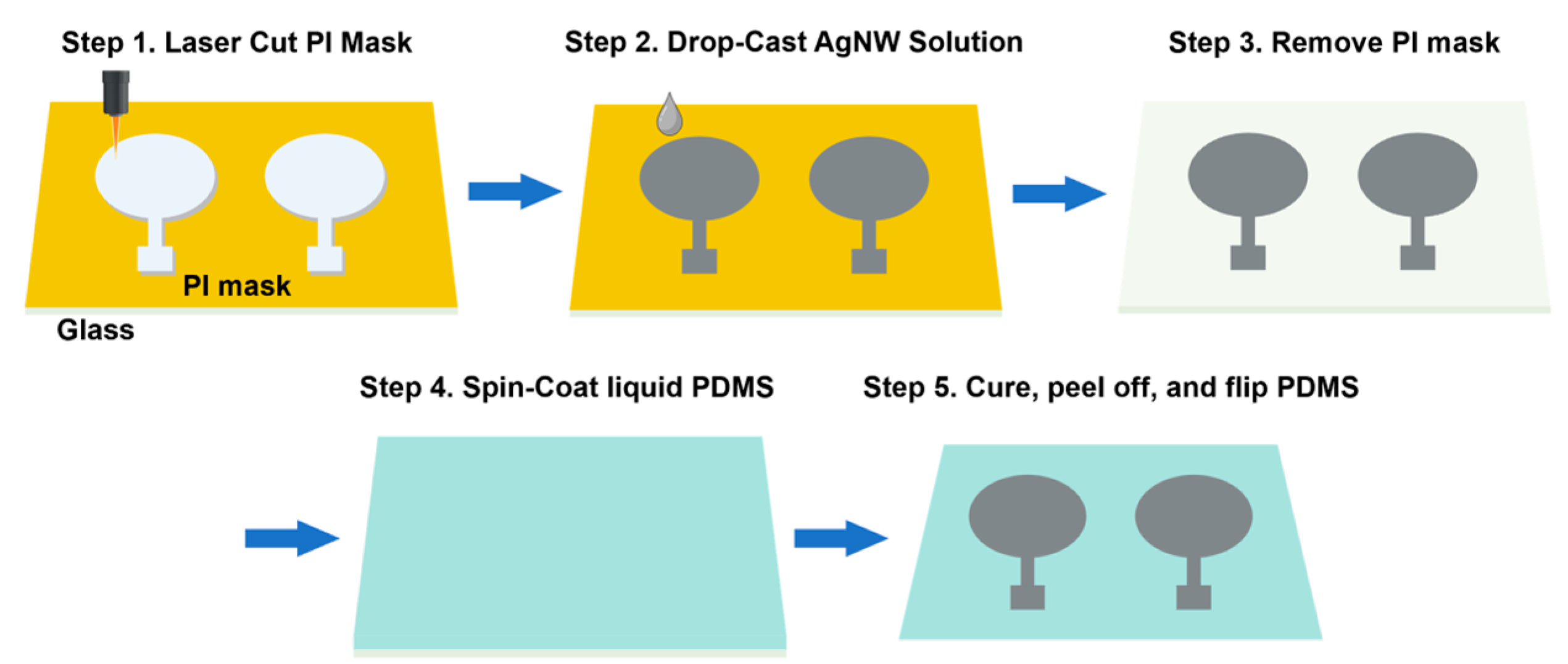
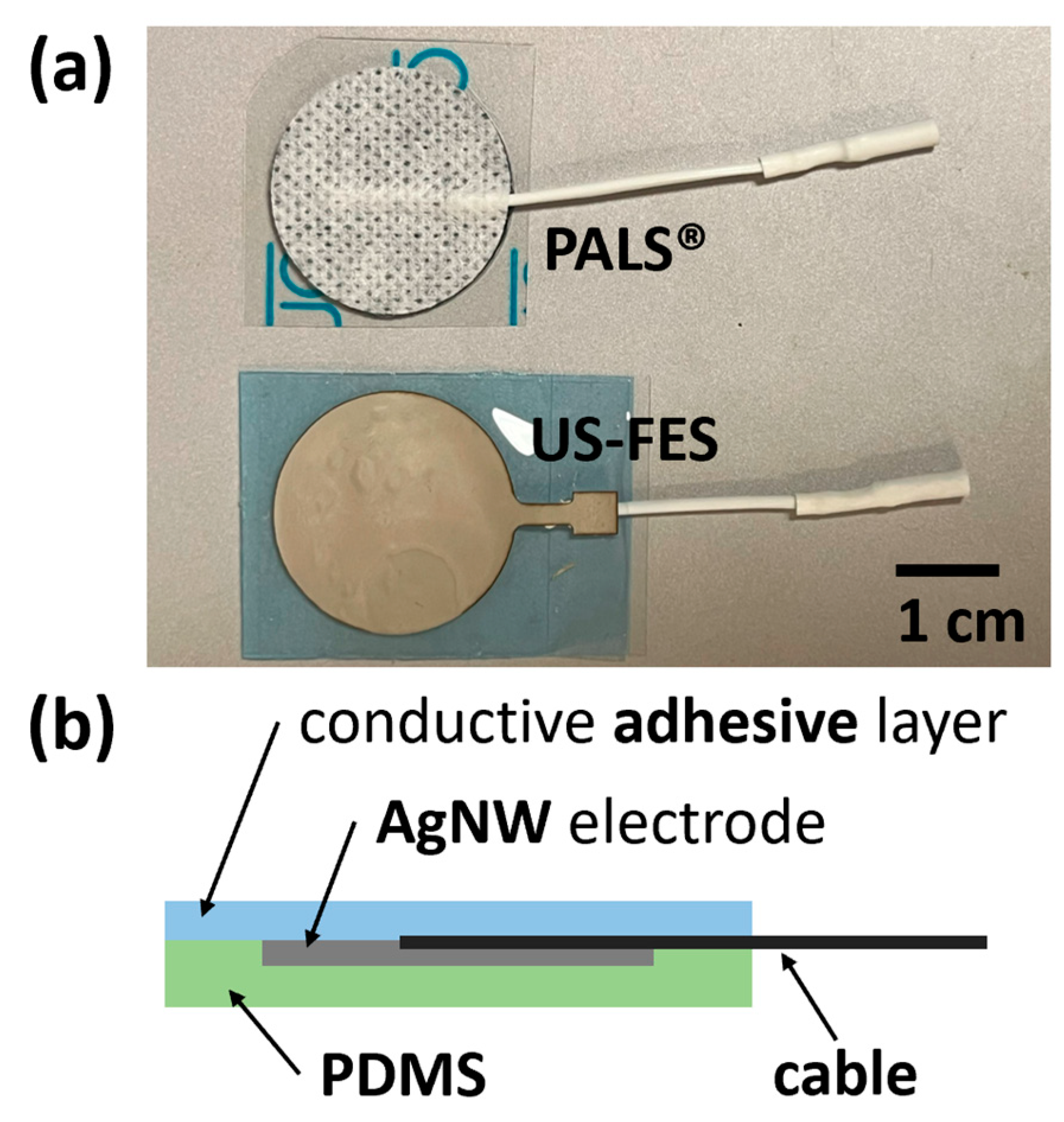
2.1. Ultrasound-Compatible FES (US-FES) Electrode Design, Fabrication, and Characterization
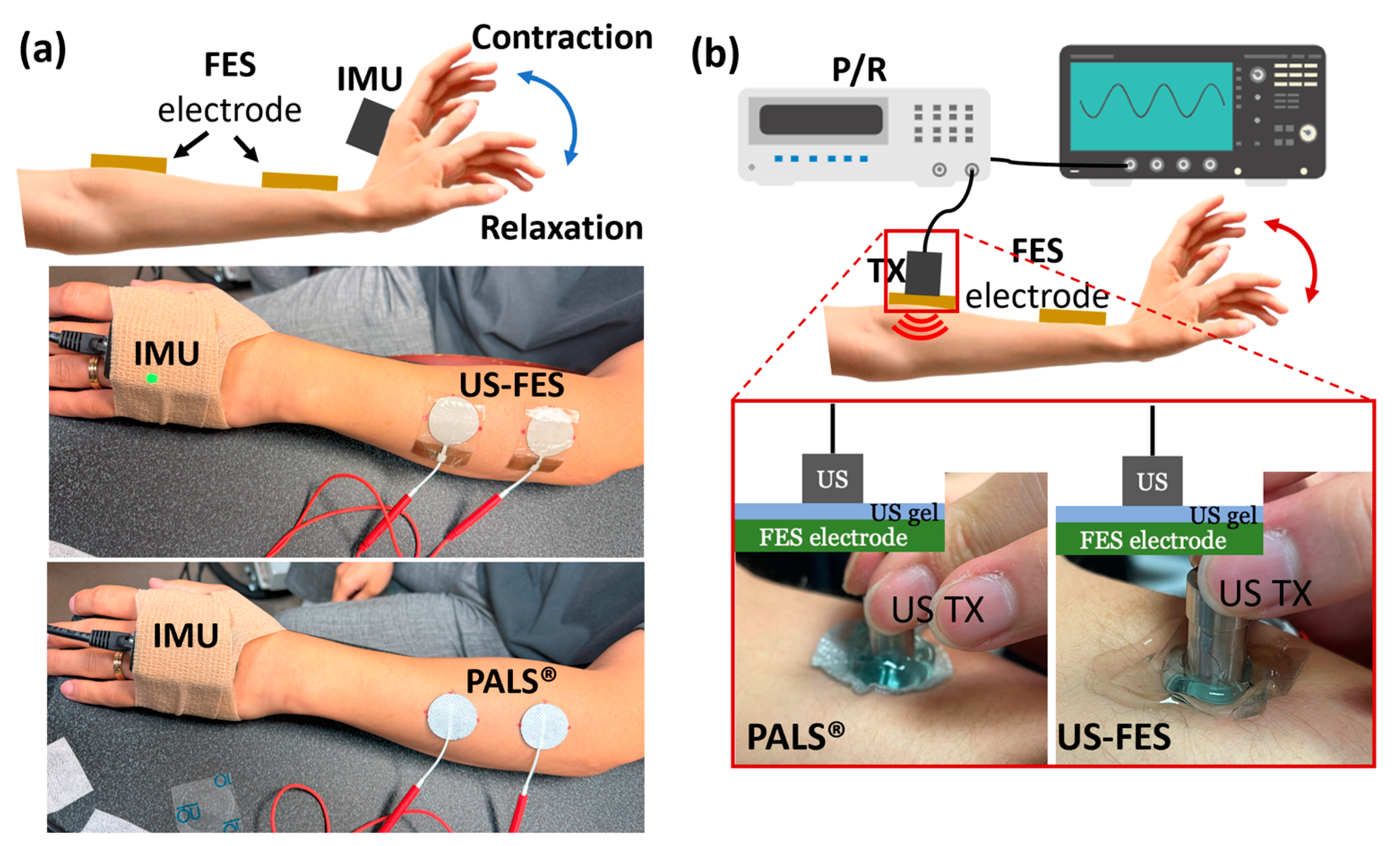
2.2. Evaluation of the Effectiveness of US-FES Electrode during FES Applications
2.3. Ultrasound Compatibility Testing for US-FES Electrode with Single-Element Ultrasound Transducer
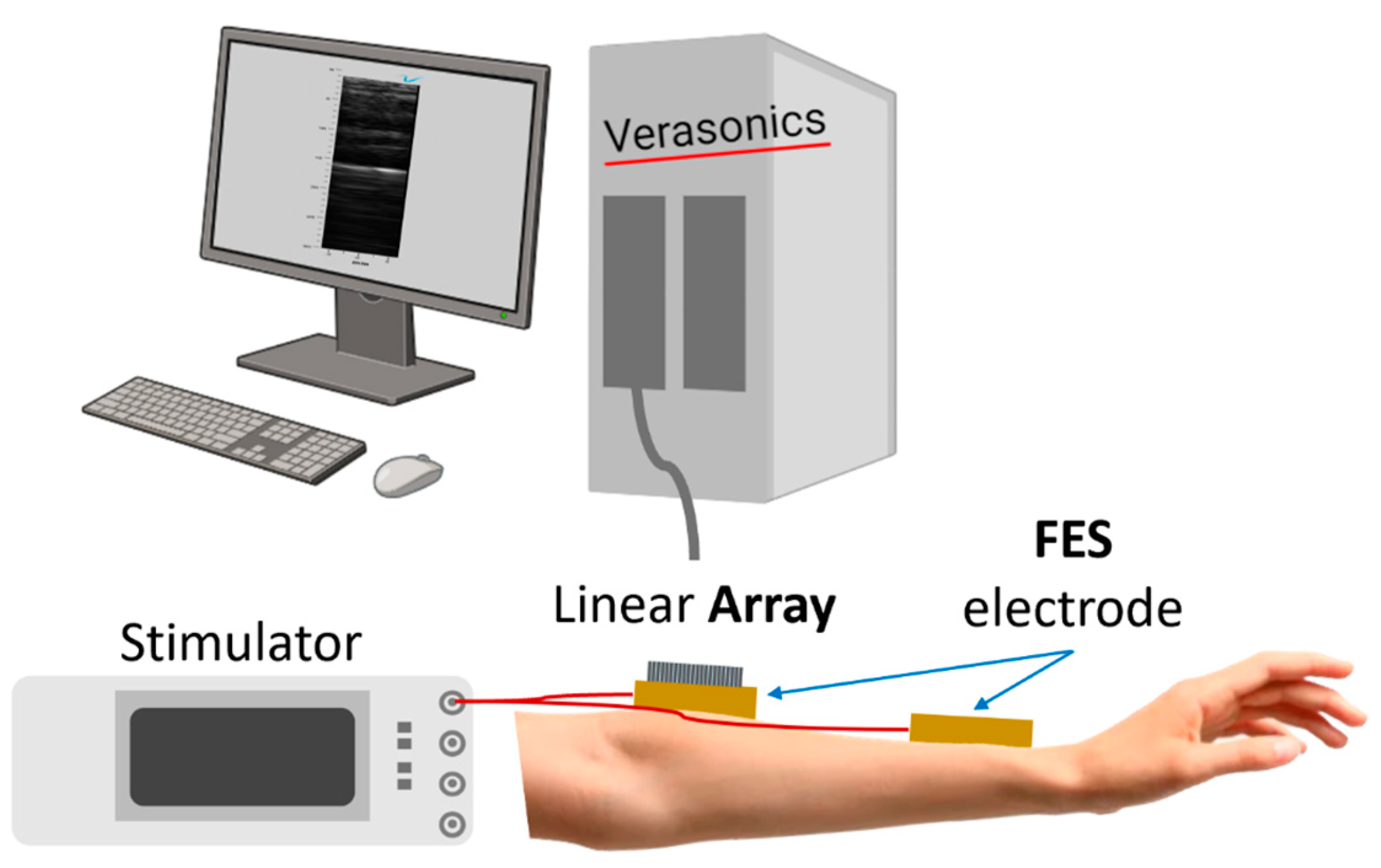
2.4. Ultrasound Compatibility Test of US-FES Electrode with Ultrasound Array
2.5. Phantom Preparation for Resolution Comparison Depending on the Presence of US-FES Electrode during B-Mode Imaging
2.6. Feasibility Assessment of US-FES Electrodes for Tremor Patients
3. Results
3.1. Effectiveness of US-FES Electrode
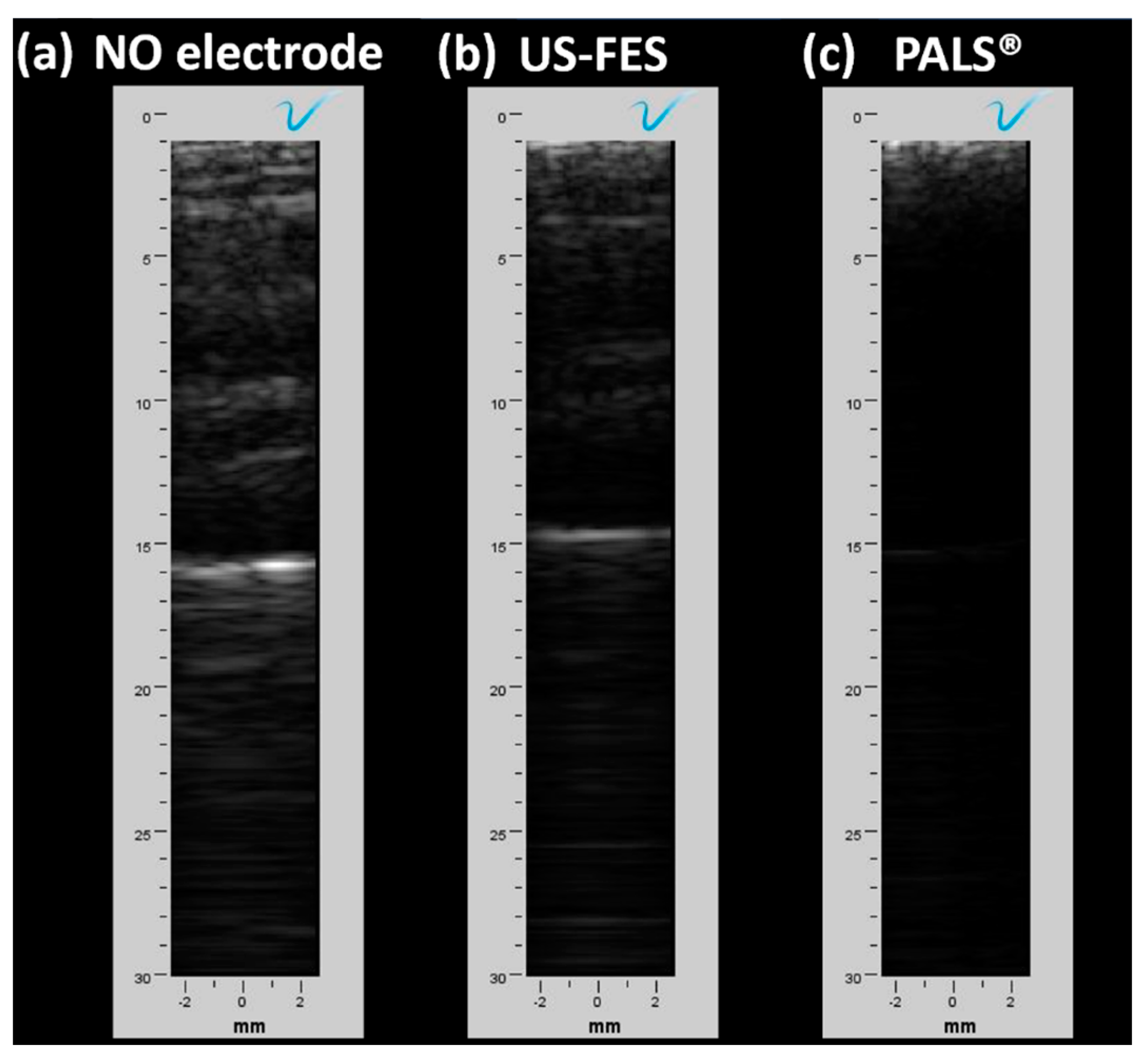
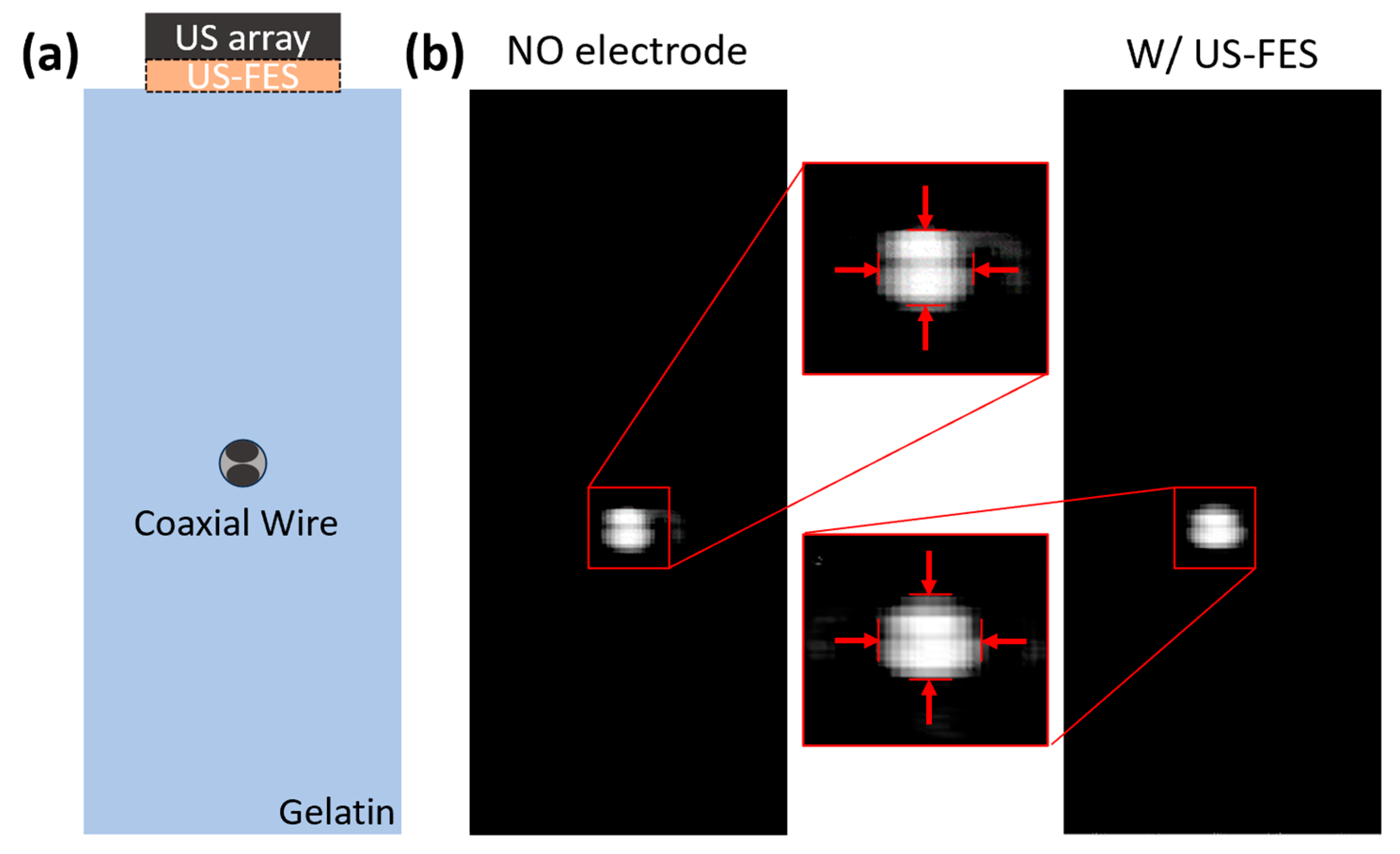
3.2. Feasibility of Ultrasound-Compatible FES Electrode with A-Mode Ultrasound
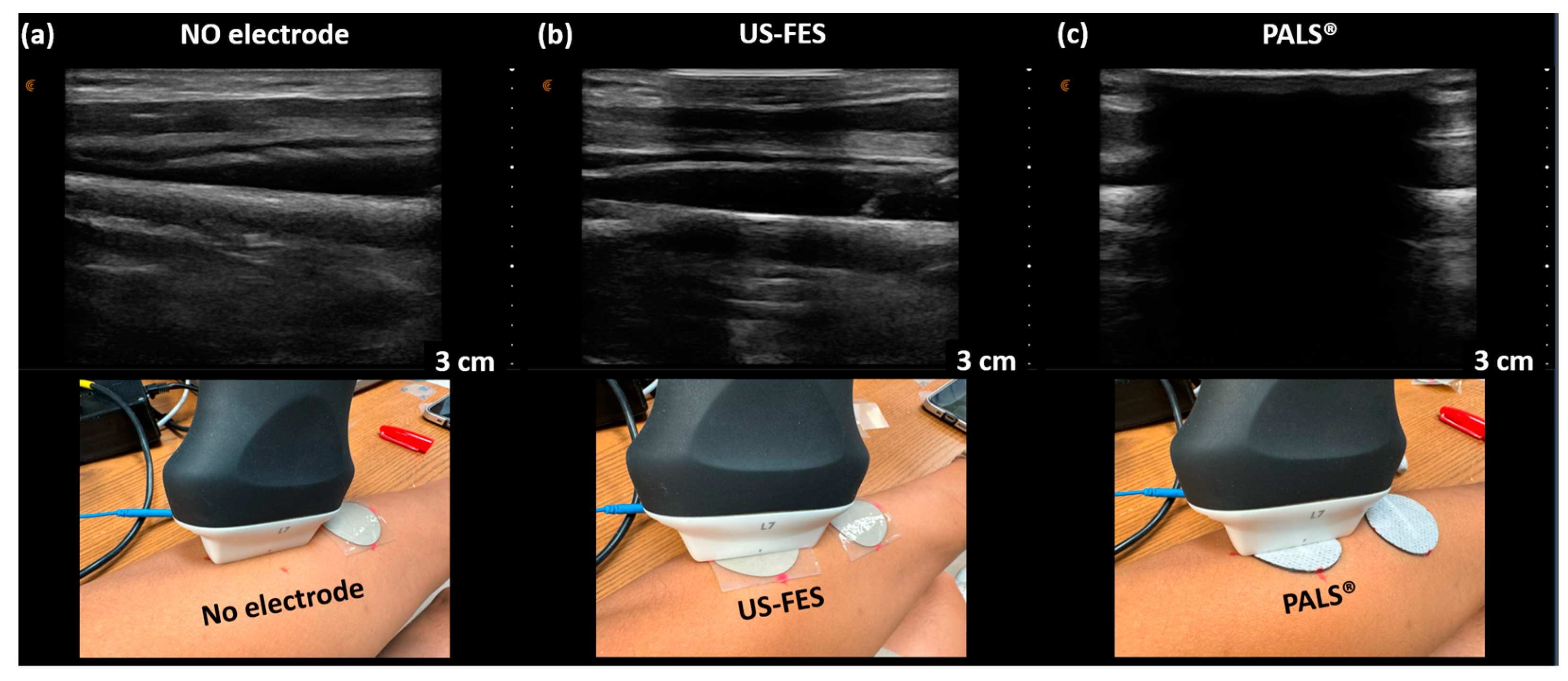
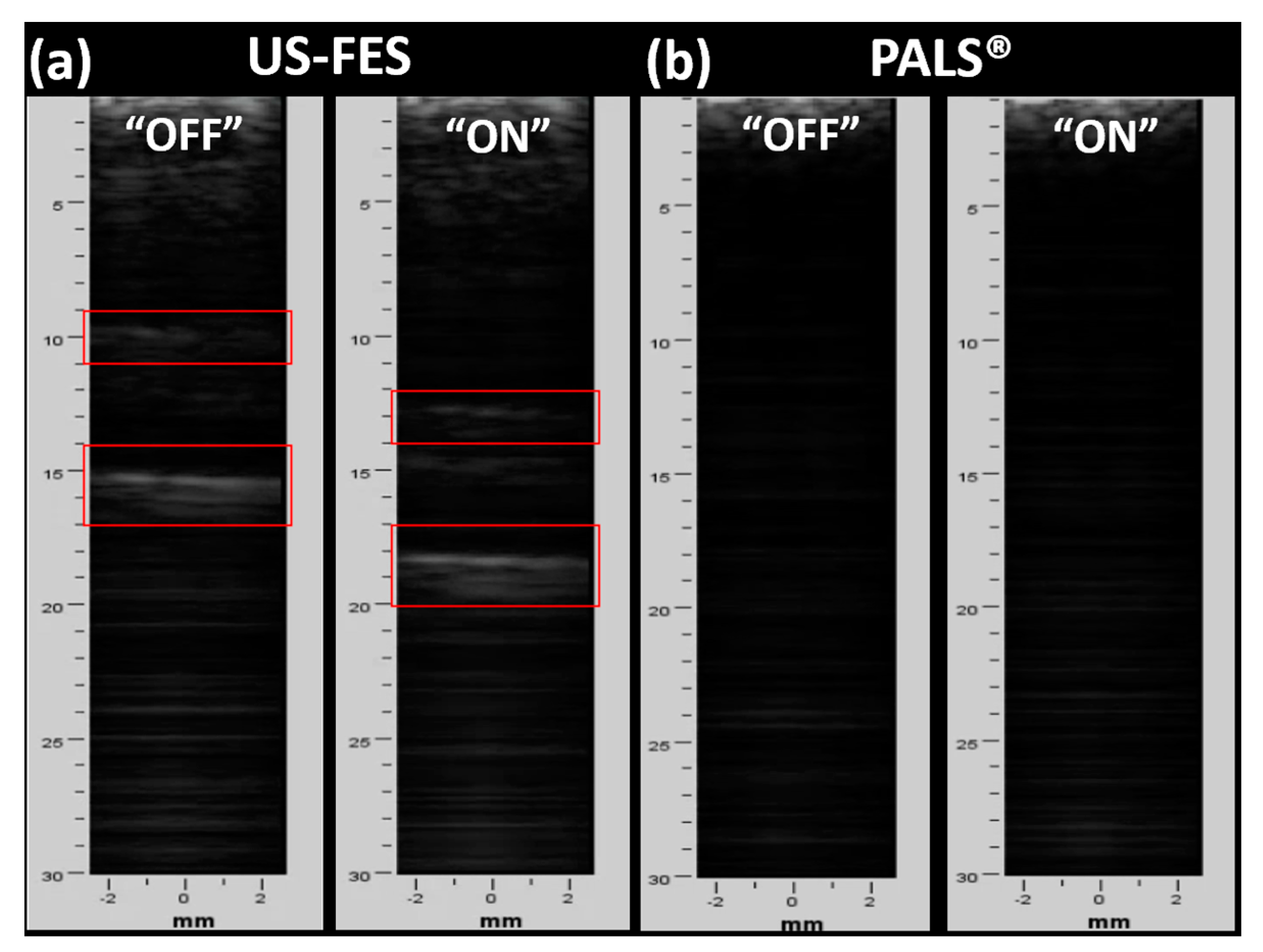
3.3. Feasibility of US-Compatible FES Electrode with B-Mode Imaging
3.4. Tremor Suppression Ratio Calculation and B-Mode Image Evaluation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lasfargues, J.E.; Custis, D.; Morrone, F.; Carswell, J.; Nguyen, T. A model for estimating spinal cord injury prevalence in the United States. Paraplegia 1995, 33, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Jain, N.B.; Ayers, G.D.; Peterson, E.N.; Harris, M.B.; Morse, L.; O’connor, K.C.; Garshick, E. Traumatic spinal cord injury in the United States, 1993–2012. JAMA J. Am. Med. Assoc. 2015, 313, 2236–2243. [Google Scholar] [CrossRef] [PubMed]
- Marquez-Chin, C.; Popovic, M.R. Functional electrical stimulation therapy for restoration of motor function after spinal cord injury and stroke: A review. Biomed. Eng. Online 2020, 19, 34. [Google Scholar] [CrossRef] [PubMed]
- Tysnes, O.B.; Storstein, A. Epidemiology of Parkinson’s disease. J. Neural Transm. 2017, 124, 901–905. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.; Jin, M.; Zhu, X.; Ming, D. Peripherical Electrical Stimulation for Parkinsonian Tremor: A Systematic Review. Front. Aging Neurosci. 2022, 14, 795454. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, K.P.; Bain, P.; Bajaj, N.; Elble, R.J.; Hallett, M.; Louis, E.D.; Raethjen, J.; Stamelou, M.; Testa, C.M.; Deuschl, G.; et al. Consensus Statement on the Classification of Tremors. From the Task Force on Tremor of the International Parkinson and Movement Disorder Society. Mov. Disord. Mov. Disord. 2018, 33, 75–87. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.; Xue, X.; Iyer, A.; Jiang, X.; Roque, D. Towards ultrasound imaging-based closed-loop peripheral nerve stimulation for tremor suppression. Curr. Opin. Biomed. Eng. 2023, 28, 100484. [Google Scholar] [CrossRef]
- Rabelo, M.; de Moura Jucá, R.V.; Lima, L.A.; Resende-Martins, H.; Bó, A.P.; Fattal, C.; Azevedo-Coste, C.; Fachin-Martins, E. Overview of FES-Assisted Cycling Approaches and Their Benefits on Functional Rehabilitation and Muscle Atrophy. Adv. Exp. Med. Biol. 2018, 1088, 561–583. [Google Scholar] [CrossRef] [PubMed]
- Magrinelli, F.; Picelli, A.; Tocco, P.; Federico, A.; Roncari, L.; Smania, N.; Zanette, G.; Tamburin, S. Pathophysiology of Motor Dysfunction in Parkinson’s Disease as the Rationale for Drug Treatment and Rehabilitation. Parkinson’s Dis. 2016, 2016, 9832839. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, N.; Izumi, S.I. Rehabilitation with Poststroke Motor Recovery: A Review with a Focus on Neural Plasticity. Stroke Res. Treat. 2013, 2013, 13. [Google Scholar] [CrossRef]
- Sun, X.; Li, Y.; Liu, H. Muscle fatigue assessment using one-channel single-element ultrasound transducer. In Proceedings of the International IEEE/EMBS Conference on Neural Engineering, NER, Shanghai, China, 25–28 May 2017; pp. 122–125. [Google Scholar] [CrossRef]
- de Oliveira, J.; de Souza, M.A.; Assef, A.A.; Maia, J.M. Multi-Sensing Techniques with Ultrasound for Musculoskeletal Assessment: A Review. Sensors 2022, 22, 9232. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Iyer, A.; Lambeth, K.; Kim, K.; Sharma, N. Ultrasound Echogenicity as an Indicator of Muscle Fatigue during Functional Electrical Stimulation. Sensors 2022, 22, 335. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Chen, J.; Yang, Y. A Method for Suppressing Electrical Stimulation Artifacts from Electromyography. Int. J. Neural Syst. 2019, 29, 1850054. [Google Scholar] [CrossRef] [PubMed]
- Sankowski, A.J.; Łebkowska, U.M.; Ćwikła, J.; Walecka, I.; Walecki, J. The comparison of efficacy of different imaging techniques (conventional radiography, ultrasonography, magnetic resonance) in assessment of wrist joints and metacarpophalangeal joints in patients with psoriatic arthritis. Pol. J. Radiol. 2013, 78, 18. [Google Scholar] [CrossRef]
- Yu, J.Y.; Jeong, J.G.; Lee, B.H. Evaluation of muscle damage using ultrasound imaging. J. Phys. Ther. Sci. 2015, 27, 531. [Google Scholar] [CrossRef]
- Isaka, M.; Sugimoto, K.; Akasaka, H.; Yasunobe, Y.; Takahashi, T.; Xie, K.; Onishi, Y.; Yoshida, S.; Minami, T.; Yamamoto, K.; et al. The Muscle Thickness Assessment Using Ultrasonography is a Useful Alternative to Skeletal Muscle Mass by Bioelectrical Impedance Analysis. Clin. Interv. Aging 2022, 17, 1851–1861. [Google Scholar] [CrossRef] [PubMed]
- Talib, I.; Sundaraj, K.; Lam, C.K.; Hussain, J.; Ali, M.A. A review on crosstalk in myographic signals. Eur. J. Appl. Physiol. 2019, 119, 9–28. [Google Scholar] [CrossRef] [PubMed]
- Correa-de-Araujo, R.; Harris-Love, M.O.; Miljkovic, I.; Fragala, M.S.; Anthony, B.W.; Manini, T.M. The Need for Standardized Assessment of Muscle Quality in Skeletal Muscle Function Deficit and Other Aging-Related Muscle Dysfunctions: A Symposium Report. Front. Physiol. 2017, 8, 241863. [Google Scholar] [CrossRef]
- Huang, Z.H.; Ma, C.Z.H.; Wang, L.K.; Wang, X.Y.; Fu, S.N.; Zheng, Y.P. Real-Time Visual Biofeedback via Wearable Ultrasound Imaging Can Enhance the Muscle Contraction Training Outcome of Young Adults. J. Strength. Cond. Res. 2022, 36, 941–947. [Google Scholar] [CrossRef]
- Xue, X.; Zhang, B.; Moon, S.; Xu, G.-X.; Huang, C.-C.; Sharma, N.; Jiang, X. Development of a Wearable Ultrasound Transducer for Sensing Muscle Activities in Assistive Robotics Applications. Biosensors 2023, 13, 134. [Google Scholar] [CrossRef]
- Sheng, Z.; Iyer, A.; Sun, Z.; Kim, K.; Sharma, N. A Hybrid Knee Exoskeleton Using Real-Time Ultrasound-Based Muscle Fatigue Assessment. IEEE/ASME Trans. Mechatron. 2022, 27, 1854–1862. [Google Scholar] [CrossRef]
- Sikdar, S.; Wei, Q.; Cortes, N. Dynamic Ultrasound Imaging Applications to Quantify Musculoskeletal Function. Exerc. Sport. Sci. Rev. 2014, 42, 126. [Google Scholar] [CrossRef] [PubMed]
- Sarto, F.; Spörri, J.; Fitze, D.P.; Quinlan, J.I.; Narici, M.V.; Franchi, M.V. Implementing Ultrasound Imaging for the Assessment of Muscle and Tendon Properties in Elite Sports: Practical Aspects, Methodological Considerations and Future Directions. Sports Med. 2021, 51, 1151. [Google Scholar] [CrossRef] [PubMed]
- van Hooren, B.; Teratsias, P.; Hodson-Tole, E.F. Ultrasound imaging to assess skeletal muscle architecture during movements: A systematic review of methods, reliability, and challenges. J. Appl. Physiol. 2020, 128, 978–999. [Google Scholar] [CrossRef]
- Moon, S.; Xue, X.; Shukla, D.; Ganesh, V.; Zhu, Y.; Sharma, N.; Jiang, X. Evaluation of a Body-Conforming Electrode for Functional Ultrasound Compatible Electrical Stimulation. In Proceedings of the IEEE International Ultrasonics Symposium, IUS, Montreal, QC, Canada, 3–8 September 2023. [Google Scholar] [CrossRef]
- Kim, T.; Cui, Z.; Chang, W.Y.; Kim, H.; Zhu, Y.; Jiang, X. Flexible 1-3 Composite Ultrasound Transducers with Silver-Nanowire-Based Stretchable Electrodes. IEEE Trans. Ind. Electron. 2020, 67, 6955–6962. [Google Scholar] [CrossRef]
- Moon, S.; Xue, X.; Chen, M.; Shukla, D.; Wu, H.; Zhu, Y.; Feng, W.; Sharma, N.; Jiang, X. A Flexible Ultrasound Transducer with Tunable Focusing for Non-Invasive Brain Stimulation. In Proceedings of the IEEE International Ultrasonics Symposium, IUS, Montreal, QC, Canada, 3–8 September 2023. [Google Scholar] [CrossRef]
- Shukla, D.; Liu, Y.; Zhu, Y. Eco-friendly screen printing of silver nanowires for flexible and stretchable electronics. Nanoscale 2023, 15, 2767–2778. [Google Scholar] [CrossRef] [PubMed]
- Stewart, A.M.; Pretty, C.G.; Chen, X.Q. An Evaluation of the Effect of Stimulation Parameters and Electrode Type on Bicep Muscle Response for a Voltage-controlled Functional Electrical Stimulator. IFAC-PapersOnLine 2017, 50, 15109–15114. [Google Scholar] [CrossRef]
- Chen, W.; Liu, J.; Lei, S.; Yang, Z.; Zhang, Q.; Li, Y.; Huang, J.; Dong, Y.; Zheng, H.; Wu, D.; et al. Flexible Ultrasound Transducer With Embedded Optical Shape Sensing Fiber for Biomedical Imaging Applications. IEEE Trans. Biomed. Eng. 2023, 70, 2841–2851. [Google Scholar] [CrossRef]


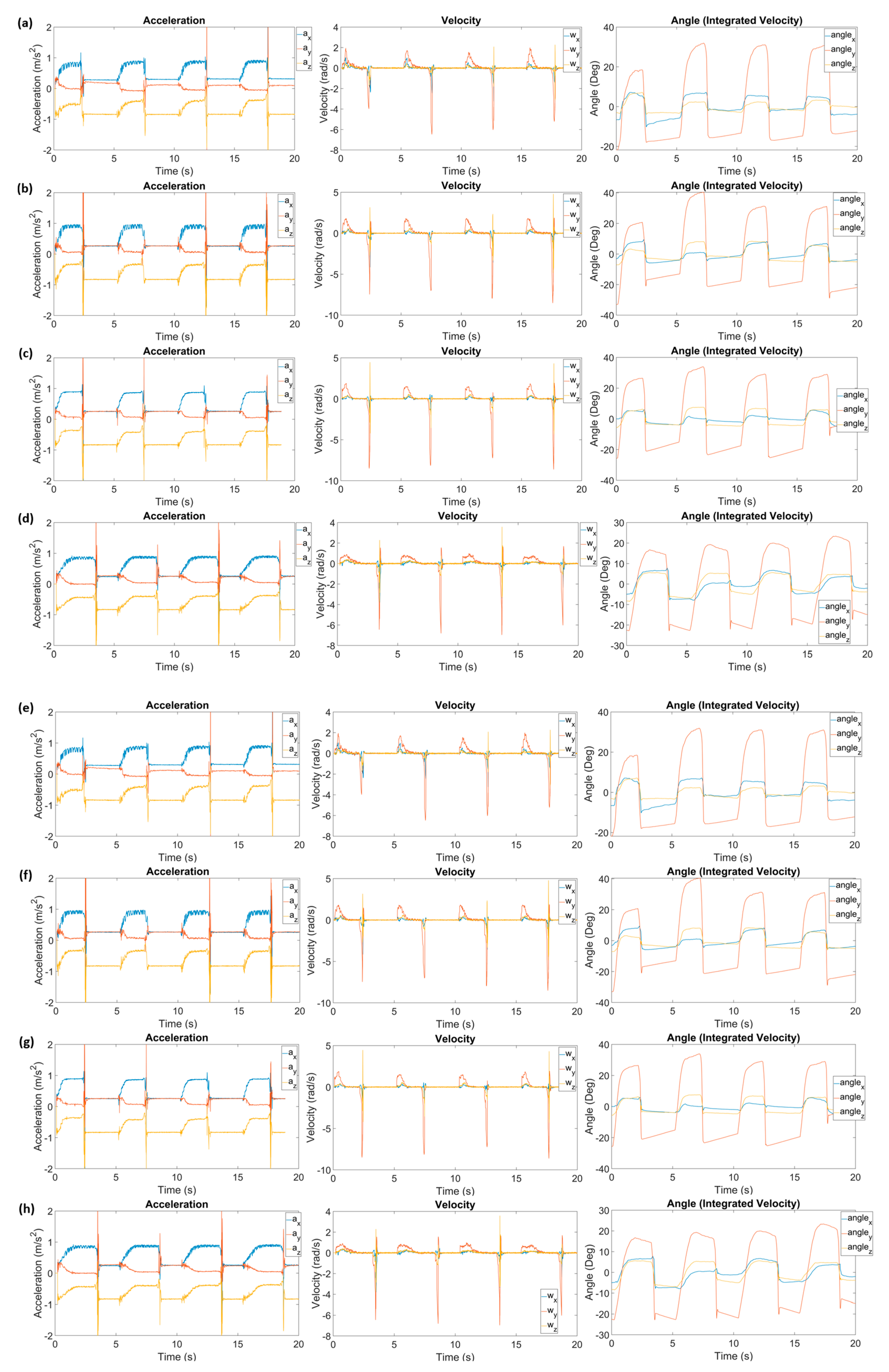




| Parameters | Wrist Angle (PALS®) | Wrist Angle (US-FES) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| #1 | #2 | #3 | #4 | Ave. | #1 | #2 | #3 | #4 | Ave. | ||
| Amplitude | 20 Hz, 5 mA, 40% | ||||||||||
| 20 Hz, 8 mA, 40% | |||||||||||
| 20 Hz, 11 mA, 40% | |||||||||||
| Frequency | 20 Hz, 8 mA, 40% | ||||||||||
| 30 Hz, 8 mA, 40% | |||||||||||
| 40 Hz, 8 mA, 40% | |||||||||||
| Pulse Duration | 20 Hz, 8 mA, 20% | ||||||||||
| 20 Hz, 8 mA, 40% | |||||||||||
| 20 Hz, 8 mA, 60% | |||||||||||
| Parameters | Time Delay (PALS®) | Time Delay (US-FES) | |||||||||
| #1 | #2 | #3 | #4 | Ave. | #1 | #2 | #3 | #4 | Ave. | ||
| Amplitude | 20 Hz, 5 mA, 40% | 0.13 s | 0.30 s | 0.35 s | 0.42 s | 0.30 s | 0.13 s | 0.27 s | 0.35 s | 0.38 s | 0.28 s |
| 20 Hz, 8 mA, 40% | 0.11 s | 0.26 s | 0.31 s | 0.39 s | 0.27 s | 0.11 s | 0.23 s | 0.35 s | 0.45 s | 0.29 s | |
| 20 Hz, 11 mA, 40% | 0.07 s | 0.25 s | 0.37 s | 0.49 s | 0.30 s | 0.11 s | 0.27 s | 0.31 s | 0.39 s | 0.27 s | |
| Frequency | 20 Hz, 8 mA, 40% | 0.11 s | 0.26 s | 0.31 s | 0.39 s | 0.27 s | 0.11 s | 0.23 s | 0.35 s | 0.45 s | 0.29 s |
| 30 Hz, 8 mA, 40% | 0.12 s | 0.26 s | 0.33 s | 0.42 s | 0.28 s | 0.10 s | 0.21 s | 0.28 s | 0.39 s | 0.25 s | |
| 40 Hz, 8 mA, 40% | 0.10 s | 0.29 s | 0.49 s | 0.63 s | 0.38 s | 0.11 s | 0.20 s | 0.32 s | 0.45 s | 0.27 s | |
| Pulse Duration | 20 Hz, 8 mA, 20% | 0.05 s | 0.16 s | 0.26 s | 0.40 s | 0.22 s | 0.13 s | 0.26 s | 0.47 s | 0.63 s | 0.37 s |
| 20 Hz, 8 mA, 40% | 0.11 s | 0.26 s | 0.31 s | 0.39 s | 0.27 s | 0.11 s | 0.23 s | 0.35 s | 0.45 s | 0.29 s | |
| 20 Hz, 8 mA, 60% | 0.14 s | 0.26 s | 0.33 s | 0.39 s | 0.28 s | 0.11 s | 0.25 s | 0.40 s | 0.40 s | 0.29 s | |
| Parameters | Rate of Angle Change (PALS®) | Rate of Angle Change (US-FES) | |||||||||
| #1 | #2 | #3 | #4 | Ave. | #1 | #2 | #3 | #4 | Ave. | ||
| Amplitude | 20 Hz, 5 mA, 40% | 0.09 rad/s | 0.07 rad/s | 0.07 rad/s | 0.09 rad/s | 0.08 rad/s | 0.37 rad/s | 0.22 rad/s | 0.34 rad/s | 0.32 rad/s | 0.31 rad/s |
| 20 Hz, 8 mA, 40% | 1.27 rad/s | 1.53 rad/s | 1.53 rad/s | 1.52 rad/s | 1.46 rad/s | 1.95 rad/s | 1.71 rad/s | 1.56 rad/s | 1.99 rad/s | 1.80 rad/s | |
| 20 Hz, 11 mA, 40% | 1.44 rad/s | 1.66 rad/s | 1.64 rad/s | 1.46 rad/s | 1.55 rad/s | 1.82 rad/s | 1.78 rad/s | 1.80 rad/s | 1.81 rad/s | 1.80 rad/s | |
| Frequency | 20 Hz, 8 mA, 40% | 1.27 rad/s | 1.53 rad/s | 1.53 rad/s | 1.52 rad/s | 1.46 rad/s | 1.95 rad/s | 1.71 rad/s | 1.56 rad/s | 1.99 rad/s | 1.80 rad/s |
| 30 Hz, 8 mA, 40% | 1.57 rad/s | 2.19 rad/s | 2.18 rad/s | 2.23 rad/s | 2.04 rad/s | 1.68 rad/s | 2.57 rad/s | 1.76 rad/s | 1.68 rad/s | 1.92 rad/s | |
| 40 Hz, 8 mA, 40% | 1.85 rad/s | 1.85 rad/s | 1.85 rad/s | 1.72 rad/s | 1.82 rad/s | 1.87 rad/s | 1.53 rad/s | 1.48 rad/s | 1.83 rad/s | 1.68 rad/s | |
| Pulse Duration | 20 Hz, 8 mA, 20% | 0.91 rad/s | 0.99 rad/s | 1.11 rad/s | 1.13 rad/s | 1.04 rad/s | 1.03 rad/s | 1.26 rad/s | 0.97 rad/s | 0.88 rad/s | 1.04 rad/s |
| 20 Hz, 8 mA, 40% | 1.27 rad/s | 1.53 rad/s | 1.53 rad/s | 1.52 rad/s | 1.46 rad/s | 1.95 rad/s | 1.71 rad/s | 1.56 rad/s | 1.99 rad/s | 1.80 rad/s | |
| 20 Hz, 8 mA, 60% | 1.37 rad/s | 1.09 rad/s | 1.16 rad/s | 1.25 rad/s | 1.22 rad/s | 1.91 rad/s | 1.55 rad/s | 1.82 rad/s | 1.77 rad/s | 1.76 rad/s | |
| Trial # | ) | ) | (%) |
|---|---|---|---|
| 1 | 13.3746 | 8.6476 | 35.34 |
| 2 | 10.6083 | 3.5944 | 66.11 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moon, S.; Xue, X.; Ganesh, V.; Shukla, D.; Kreager, B.C.; Cai, Q.; Wu, H.; Zhu, Y.; Sharma, N.; Jiang, X. Ultrasound-Compatible Electrode for Functional Electrical Stimulation. Biomedicines 2024, 12, 1741. https://doi.org/10.3390/biomedicines12081741
Moon S, Xue X, Ganesh V, Shukla D, Kreager BC, Cai Q, Wu H, Zhu Y, Sharma N, Jiang X. Ultrasound-Compatible Electrode for Functional Electrical Stimulation. Biomedicines. 2024; 12(8):1741. https://doi.org/10.3390/biomedicines12081741
Chicago/Turabian StyleMoon, Sunho, Xiangming Xue, Vidisha Ganesh, Darpan Shukla, Benjamin C. Kreager, Qianqian Cai, Huaiyu Wu, Yong Zhu, Nitin Sharma, and Xiaoning Jiang. 2024. "Ultrasound-Compatible Electrode for Functional Electrical Stimulation" Biomedicines 12, no. 8: 1741. https://doi.org/10.3390/biomedicines12081741
APA StyleMoon, S., Xue, X., Ganesh, V., Shukla, D., Kreager, B. C., Cai, Q., Wu, H., Zhu, Y., Sharma, N., & Jiang, X. (2024). Ultrasound-Compatible Electrode for Functional Electrical Stimulation. Biomedicines, 12(8), 1741. https://doi.org/10.3390/biomedicines12081741







