Establishment and Evaluation of a Mouse Model of Experimental Ulcerative Colitis Induced by the Gavage Administration of Dextran Sulfate Sodium
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Animals
2.3. Design and Establishment of UC Mouse Model
2.4. Evaluation of Acute UC Mouse Model
2.4.1. Alterations in the Activity and Body Weight of Mice
2.4.2. Visual Alterations in the Colon
2.4.3. Histopathological and Biochemical Analysis
2.4.4. Analysis of MPO
2.4.5. Analysis of NO
2.4.6. Analysis of ROS
2.4.7. Analysis of GSH
2.5. Statistical Analysis
3. Results
3.1. Mouse Weight and Survival Rate
3.2. Length of Mouse Colon
3.3. Colon Tissue Section
3.4. Inflammatory Indicators of UC
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jadhav, A.; Jagtap, S.; Vyavahare, S.; Sharbidre, A.; Kunchiraman, B. Reviewing the potential of probiotics, prebiotics and synbiotics: Advancements in treatment of ulcerative colitis. Front. Cell Infect. Microbiol. 2023, 13, 1268041. [Google Scholar] [CrossRef] [PubMed]
- Gros, B.; Kaplan, G.G. Ulcerative Colitis in Adults: A Review. JAMA 2023, 330, 951–965. [Google Scholar] [CrossRef]
- Ng, S.C.; Shi, H.Y.; Hamidi, N.; Underwood, F.E.; Tang, W.; Benchimol, E.I.; Panaccione, R.; Ghosh, S.; Wu, J.C.Y.; Chan, F.K.L.; et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: A systematic review of population-based studies. Lancet 2017, 390, 2769–2778. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Tsujinaka, S.; Miura, T.; Kitamura, Y.; Suzuki, H.; Shibata, C. Inflammatory Bowel Disease and Colorectal Cancer: Epidemiology, Etiology, Surveillance, and Management. Cancers 2023, 15, 4154. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Shang, S.; Yu, S.; Cui, L.; Li, S.; He, N. Identification and exploration of pharmacological pyroptosis-related biomarkers of ulcerative colitis. Front. Immunol. 2022, 13, 998470. [Google Scholar] [CrossRef]
- Bilsborough, J.; Fiorino, M.F.; Henkle, B.W. Select animal models of colitis and their value in predicting clinical efficacy of biological therapies in ulcerative colitis. Expert. Opin. Drug Discov. 2021, 16, 567–577. [Google Scholar] [CrossRef] [PubMed]
- Low, D.; Nguyen, D.D.; Mizoguchi, E. Animal models of ulcerative colitis and their application in drug research. Drug Des. Dev. Ther. 2013, 7, 1341–1357. [Google Scholar]
- Ren, D.-D.; Chen, K.-C.; Li, S.-S.; Zhang, Y.-T.; Li, Z.-M.; Liu, S.; Sun, Y.-S. Panax quinquefolius polysaccharides ameliorate ulcerative colitis in mice induced by dextran sulfate sodium. Front. Immunol. 2023, 14, 1161625. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Geng, W.; Chen, S.; Wang, L.; Rong, X.; Wang, S.; Wang, T.; Xiong, L.; Huang, J.; Pang, X.; et al. Ginger Alleviates DSS-Induced Ulcerative Colitis Severity by Improving the Diversity and Function of Gut Microbiota. Front. Pharmacol. 2021, 12, 632569. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.-y.; Fan, Y.-m.; Ga, Y.; Zhang, Y.-n.; Han, J.-c.; Hao, Z.-h. Shaoyao decoction attenuates DSS-induced ulcerative colitis, macrophage and NLRP3 inflammasome activation through the MKP1/NF-κB pathway. Phytomedicine 2021, 92, 153743. [Google Scholar] [CrossRef] [PubMed]
- Heng, Y.; Li, X.; Sun, T.; Zhang, Y.; Yang, P. Comparison of Ulcerative Colitis Models Respectively Induced by Free Drinking and Intragastric Administration of Dextran Sodium Sulfate in Mice. China Pharm. 2017, 12, 603–606. [Google Scholar]
- Yang, Y.; Gong, P.; Long, X.; Jiang, Y.; Ye, M.; Tao, S.; Su, Y.; Yang, F.; Tian, L. Microcystin-LR Induces and Aggravates Colitis through NLRP3 Inflammasome-Mediated Pyroptosis in Mice. Toxins 2023, 15, 447. [Google Scholar] [CrossRef] [PubMed]
- Mo, J.; Ni, J.; Zhang, M.; Xu, Y.; Li, Y.; Karim, N.; Chen, W. Mulberry Anthocyanins Ameliorate DSS-Induced Ulcerative Colitis by Improving Intestinal Barrier Function and Modulating Gut Microbiota. Antioxidants 2022, 11, 1674. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Zhao, D.; Nian, Y.; Li, C. Casein-fed mice showed faster recovery from DSS-induced colitis than chicken-protein-fed mice. Food Funct. 2021, 12, 5806–5820. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.-J.; Huang, X.-J.; Shi, X.-D.; Chen, H.-H.; Cui, S.W.; Nie, S.-P. Protective effect of three glucomannans from different plants against DSS induced colitis in female BALB/c mice. Food Funct. 2019, 10, 1928–1939. [Google Scholar] [CrossRef] [PubMed]
- Cooper, H.S.; Murthy, S.; Shah, R.; Sedergran, D. Clinicopathologic study of dextran sulfate sodium experimental murine colitis. Lab. Investig. A J. Tech. Methods Pathol. 1993, 69, 238–249. [Google Scholar]
- Wu, Y.; Ran, L.; Yang, Y.; Gao, X.; Peng, M.; Liu, S.; Sun, L.; Wan, J.; Wang, Y.; Yang, K.; et al. Deferasirox alleviates DSS-induced ulcerative colitis in mice by inhibiting ferroptosis and improving intestinal microbiota. Life Sci. 2023, 314, 121312. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; He, W.; Tian, H.; Zhan, P.; Liu, J. Thyme (Thymus vulgaris L.) polyphenols ameliorate DSS-induced ulcerative colitis of mice by mitigating intestinal barrier damage, regulating gut microbiota, and suppressing TLR4/NF-κB-NLRP3 inflammasome pathways. Food Funct. 2023, 14, 1113–1132. [Google Scholar] [CrossRef] [PubMed]
- Xue, L.; Zhao, Y.; Wang, H.; Li, Z.; Wu, T.; Liu, R.; Sui, W.; Zhang, M. The effects of live and pasteurized Akkermansia muciniphila on DSS-induced ulcerative colitis, gut microbiota, and metabolomics in mice. Food Funct. 2023, 14, 4632–4646. [Google Scholar] [CrossRef] [PubMed]
- Naini, B.V.; Cortina, G. A histopathologic scoring system as a tool for standardized reporting of chronic (ileo)colitis and independent risk assessment for inflammatory bowel disease. Hum. Pathol. 2012, 43, 2187–2196. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Guo, X.; Qu, L.; Tu, J.; Li, S.; Cao, G.; Liu, Y. Preventive effect of Atractylodis Rhizoma extract on DSS-induced acute ulcerative colitis through the regulation of the MAPK/NF-κB signals in vivo and in vitro. J. Ethnopharmacol. 2022, 292, 115211. [Google Scholar] [CrossRef] [PubMed]
- Sangaraju, R.; Nalban, N.; Alavala, S.; Rajendran, V.; Jerald, M.K.; Sistla, R. Protective effect of galangin against dextran sulfate sodium (DSS)-induced ulcerative colitis in Balb/c mice. Inflamm. Res. 2019, 68, 691–704. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Ding, L.; Yu, Z.; Zhang, T.; Ma, S.; Liu, J. Intracellular ROS scavenging and antioxidant enzyme regulating capacities of corn gluten meal-derived antioxidant peptides in HepG2 cells. Food Res. Int. 2016, 90, 33–41. [Google Scholar] [CrossRef]
- Maurer, L.H.; Cazarin, C.B.B.; Quatrin, A.; Nichelle, S.M.; Minuzzi, N.M.; Teixeira, C.F.; Manica da Cruz, I.B.; Maróstica Júnior, M.R.; Emanuelli, T. Dietary fiber and fiber-bound polyphenols of grape peel powder promote GSH recycling and prevent apoptosis in the colon of rats with TNBS-induced colitis. J. Funct. Foods 2020, 64, 103644. [Google Scholar] [CrossRef]
- Wang, D.; Sun, M.; Zhang, Y.; Chen, Z.; Zang, S.; Li, G.; Li, G.; Clark, A.R.; Huang, J.; Si, L. Enhanced therapeutic efficacy of a novel colon-specific nanosystem loading emodin on DSS-induced experimental colitis. Phytomedicine 2020, 78, 153293. [Google Scholar] [CrossRef] [PubMed]
- Wirtz, S.; Neurath, M.F. Mouse models of inflammatory bowel disease. Adv. Drug Deliv. Rev. 2007, 59, 1073–1083. [Google Scholar] [CrossRef] [PubMed]
- Stremmel, W.; Staffer, S.; Schneider, M.J.; Gan-Schreier, H.; Wannhoff, A.; Stuhrmann, N.; Gauss, A.; Wolburg, H.; Mahringer, A.; Swidsinski, A.; et al. Genetic Mouse Models with Intestinal-Specific Tight Junction Deletion Resemble an Ulcerative Colitis Phenotype. J. Crohn’s Colitis 2017, 11, 1247–1257. [Google Scholar] [CrossRef]
- Steinhoff, U.; Visekruna, A. 15—Mucosal Immunity and Inflammation. In Methods in Microbiology; Kabelitz, D., Kaufmann, S.H.E., Eds.; Academic Press: Cambridge, MA, USA, 2010; Volume 37, pp. 353–367. [Google Scholar]
- Zhang, H.; Wang, Y.; Su, Y.; Fang, X.; Guo, W. The alleviating effect and mechanism of Bilobalide on ulcerative colitis. Food Funct. 2021, 12, 6226–6239. [Google Scholar] [CrossRef] [PubMed]
- Almutary, A.G.; Alnuqaydan, A.M.; Almatroodi, S.A.; Tambuwala, M.M. Comparative Analysis of the Effect of Different Concentrations of Dextran Sodium Sulfate on the Severity and Extent of Inflammation in Experimental Ulcerative Colitis. Appl. Sci. 2023, 13, 3233. [Google Scholar] [CrossRef]
- Yu, T.-Y.; Feng, Y.-M.; Kong, W.-S.; Li, S.-N.; Sun, X.-J.; Zhou, G.; Xie, R.-F.; Zhou, X. Gallic acid ameliorates dextran sulfate sodium-induced ulcerative colitis in mice via inhibiting NLRP3 inflammasome. Front. Pharmacol. 2023, 14, 1095721. [Google Scholar] [CrossRef] [PubMed]
- Chassaing, B.; Aitken, J.D.; Malleshappa, M.; Vijay-Kumar, M. Dextran Sulfate Sodium (DSS)-Induced Colitis in Mice. Curr. Protoc. Immunol. 2014, 104, 15.25.11–15.25.14. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, T.; Suzuki, M.; Fujimura, J.; Hisada, K.; Yoshikazu, O.; Obinata, K.; Yamashiro, Y. The Relationship Between the Concentration of Dextran Sodium Sulfate and the Degree of Induced Experimental Colitis in Weanling Rats. J. Pediatr. Gastroenterol. Nutr. 2003, 37, 481–486. [Google Scholar] [PubMed]
- Tong, L.; Hao, H.; Zhang, Z.; Lv, Y.; Liang, X.; Liu, Q.; Liu, T.; Gong, P.; Zhang, L.; Cao, F. Milk-derived extracellular vesicles alleviate ulcerative colitis by regulating the gut immunity and reshaping the gut microbiota. Theranostics 2021, 11, 8570. [Google Scholar] [CrossRef] [PubMed]
- Gustafsson, J.K.; Johansson, M.E.V. The role of goblet cells and mucus in intestinal homeostasis. Nat. Rev. Gastroenterol. Hepatol. 2022, 19, 785–803. [Google Scholar] [CrossRef] [PubMed]
- Kennelly, J.P.; Carlin, S.; Ju, T.; van der Veen, J.N.; Nelson, R.C.; Buteau, J.; Thiesen, A.; Richard, C.; Willing, B.P.; Jacobs, R.L. Intestinal Phospholipid Disequilibrium Initiates an ER Stress Response That Drives Goblet Cell Necroptosis and Spontaneous Colitis in Mice. Cell. Mol. Gastroenterol. Hepatol. 2021, 11, 999–1021. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, R.d.P.d.; Machado, A.P.d.F.; Galvez, J.; Cazarin, C.B.B.; Maróstica Junior, M.R. Ulcerative colitis: Gut microbiota, immunopathogenesis and application of natural products in animal models. Life Sci. 2020, 258, 118129. [Google Scholar] [CrossRef] [PubMed]
- Jeon, Y.-D.; Lee, J.-H.; Lee, Y.-M.; Kim, D.-K. Puerarin inhibits inflammation and oxidative stress in dextran sulfate sodium-induced colitis mice model. Biomed. Pharmacother. 2020, 124, 109847. [Google Scholar] [CrossRef] [PubMed]
- Wallace, J.L. Nitric oxide in the gastrointestinal tract: Opportunities for drug development. Br. J. Pharmacol. 2019, 176, 147–154. [Google Scholar] [CrossRef] [PubMed]
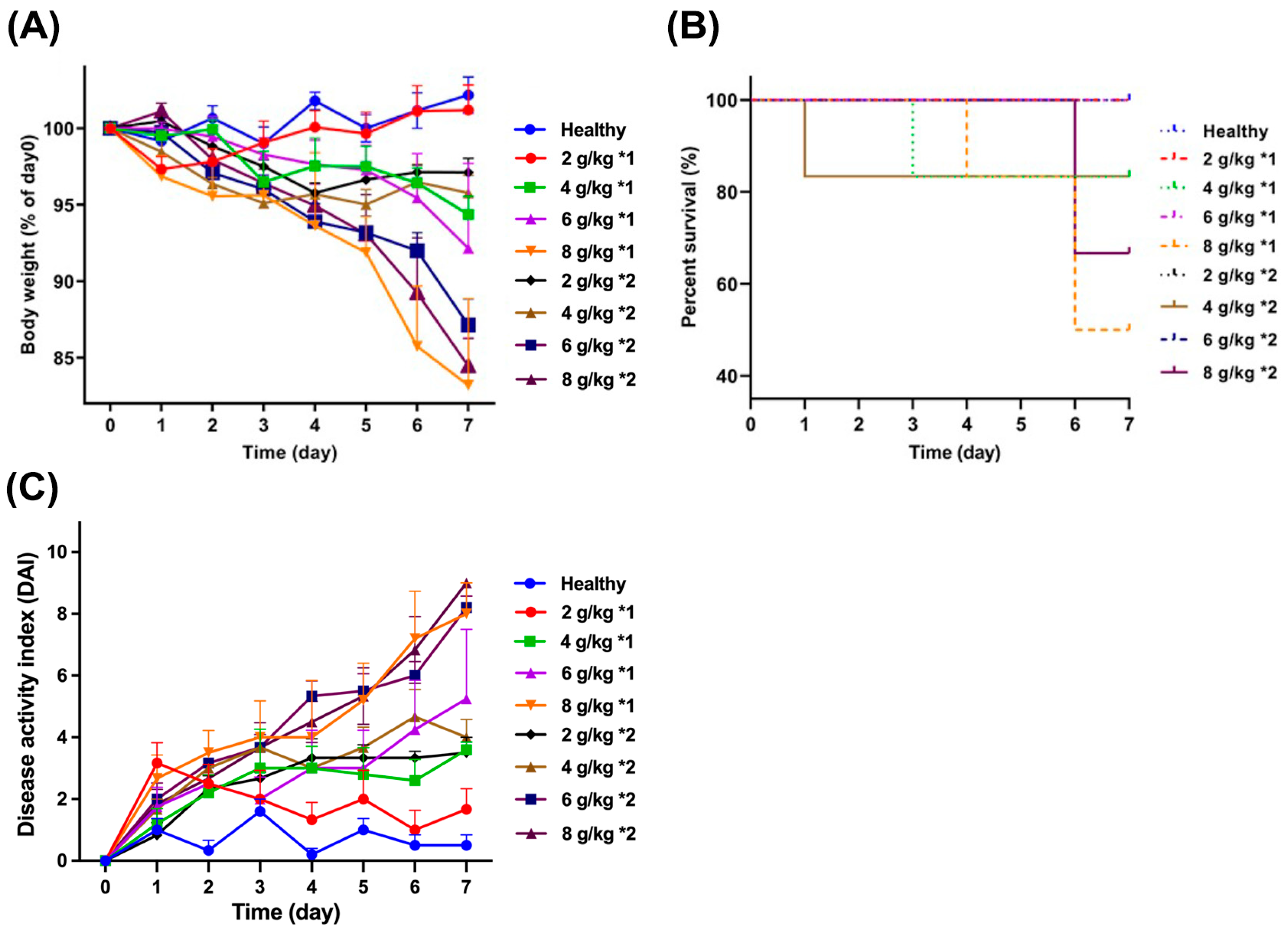



| Dosage (g/kg) | Frequency | Body Weight Percentage | Survival Rate | DAI | Colon Length (cm) |
|---|---|---|---|---|---|
| 2 | 1 | 101.2 ± 1.65 | 100 | 1.67 ± 0.67 | 9.13 ± 0.16 *** |
| 2 | 97.1 ± 0.93 | 100 | 3.50 ± 0.50 | 9.66 ± 0.09 * | |
| 4 | 1 | 94.4 ± 1.21 ** | 83.3 | 3.60 ± 0.24 | 8.40 ± 0.23 *** |
| 2 | 95.8 ± 1.14 | 83.3 | 4.00 ± 0.58 | 9.22 ± 0.29 *** | |
| 6 | 1 | 92.2 ± 5.55 *** | 100 | 5.25 ± 2.25 | 8.67 ± 0.49 *** |
| 2 | 87.1 ± 1.69 *** | 83.3 | 8.20 ± 0.37 ** | 7.35 ± 0.36 *** | |
| 8 | 1 | 83.2 ± 5.66 *** | 33.3 | 8.00 ± 1.00 ** | 7.67 ± 0.15 *** |
| 2 | 84.5 ± 1.18 *** | 50.0 | 9.00 ± 0.00 ** | 7.03 ± 0.23 *** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, D.; Chen, W.; Cao, J.; Si, L.; Chen, Z. Establishment and Evaluation of a Mouse Model of Experimental Ulcerative Colitis Induced by the Gavage Administration of Dextran Sulfate Sodium. Biomedicines 2024, 12, 1764. https://doi.org/10.3390/biomedicines12081764
Wang D, Chen W, Cao J, Si L, Chen Z. Establishment and Evaluation of a Mouse Model of Experimental Ulcerative Colitis Induced by the Gavage Administration of Dextran Sulfate Sodium. Biomedicines. 2024; 12(8):1764. https://doi.org/10.3390/biomedicines12081764
Chicago/Turabian StyleWang, Dan, Wei Chen, Jie Cao, Luqin Si, and Zehong Chen. 2024. "Establishment and Evaluation of a Mouse Model of Experimental Ulcerative Colitis Induced by the Gavage Administration of Dextran Sulfate Sodium" Biomedicines 12, no. 8: 1764. https://doi.org/10.3390/biomedicines12081764




