Sodium Butyrate (NaB) and Sodium Propionate (NaP) Reduce Cyclin A2 Expression, Inducing Cell Cycle Arrest and Proliferation Inhibition of Different Breast Cancer Subtypes, Leading to Apoptosis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Culture of MDA-MB-231 Cells
2.2. Cell Proliferation Assay
2.3. Cell Cycle Analysis
2.4. Assessment of Apoptosis
2.5. Real-Time PCR
2.6. Statistical Analyses
3. Results
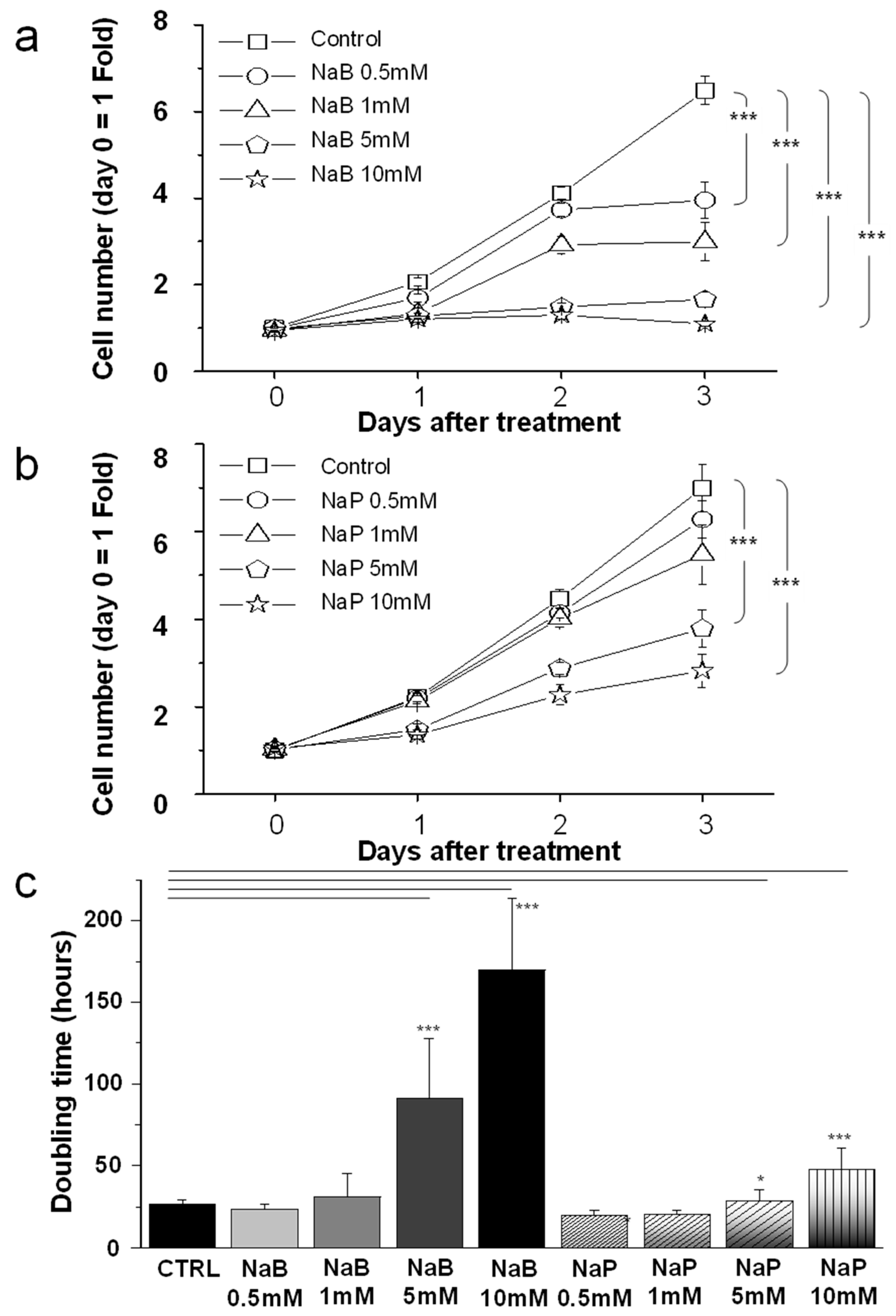
3.1. MDA-MB-231 Cell Proliferation Was Reduced by NaB and NaP Treatment in a Dose- and Time-Dependent Manner
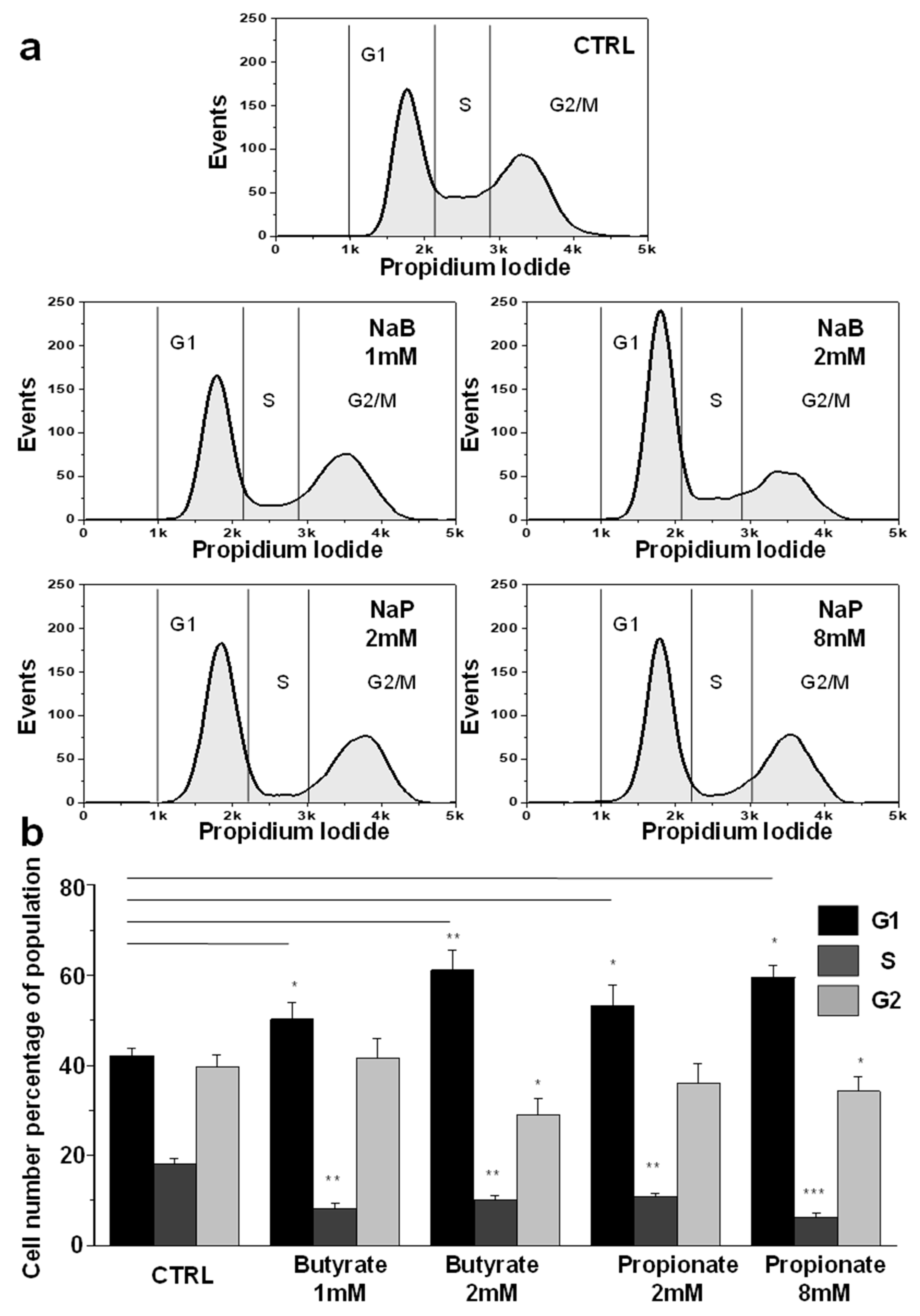
3.2. NaB and NaP Treatment Induced Cell Cycle Arrest and Blockage of MDA-MB-231 Cells in G1 Phase
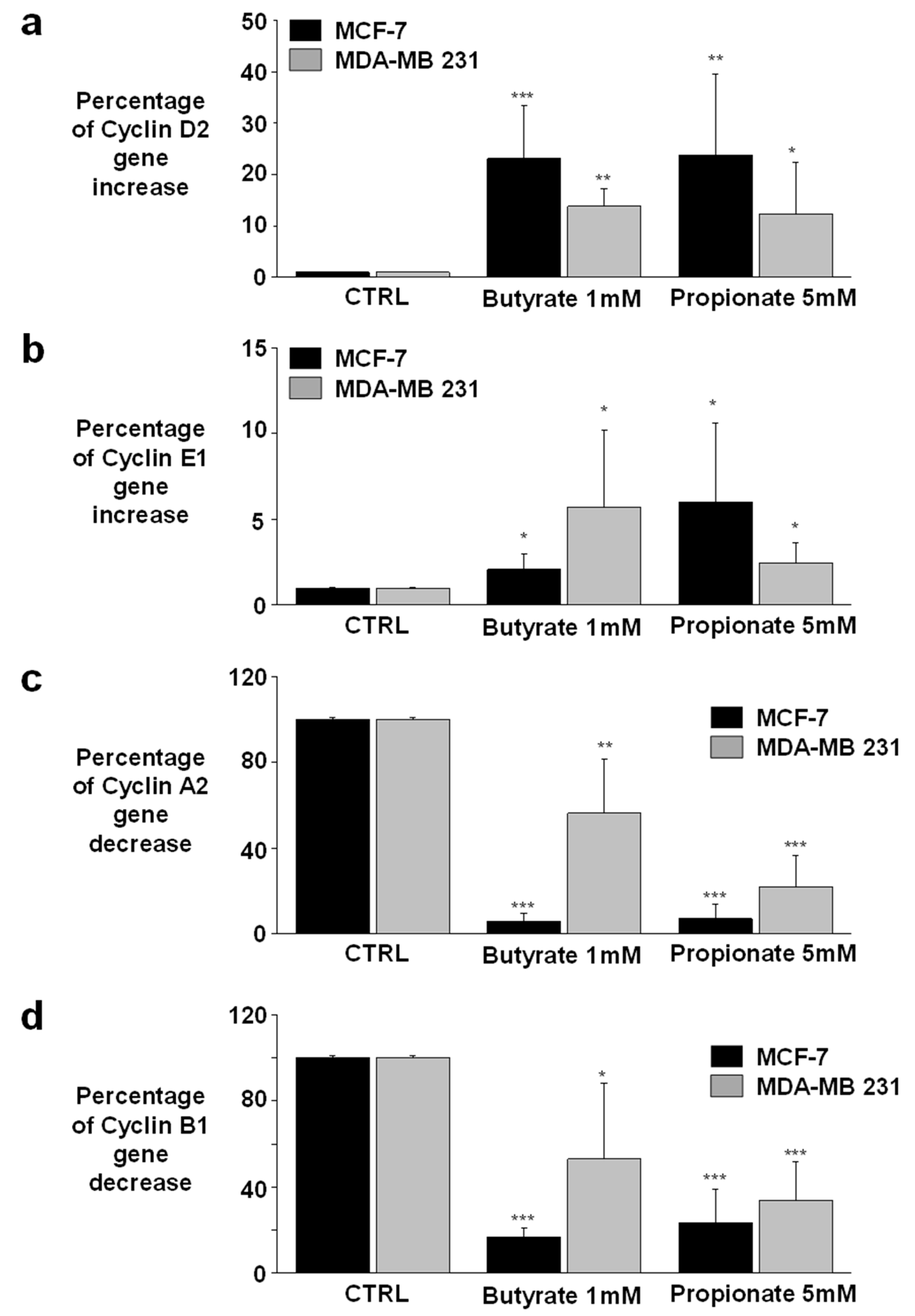
3.3. NaB and NaP Treatment Induced Cell Cycle Arrest in MCF-7 and MDA-MB-231 by Reducing Cyclin A2 Gene Expression
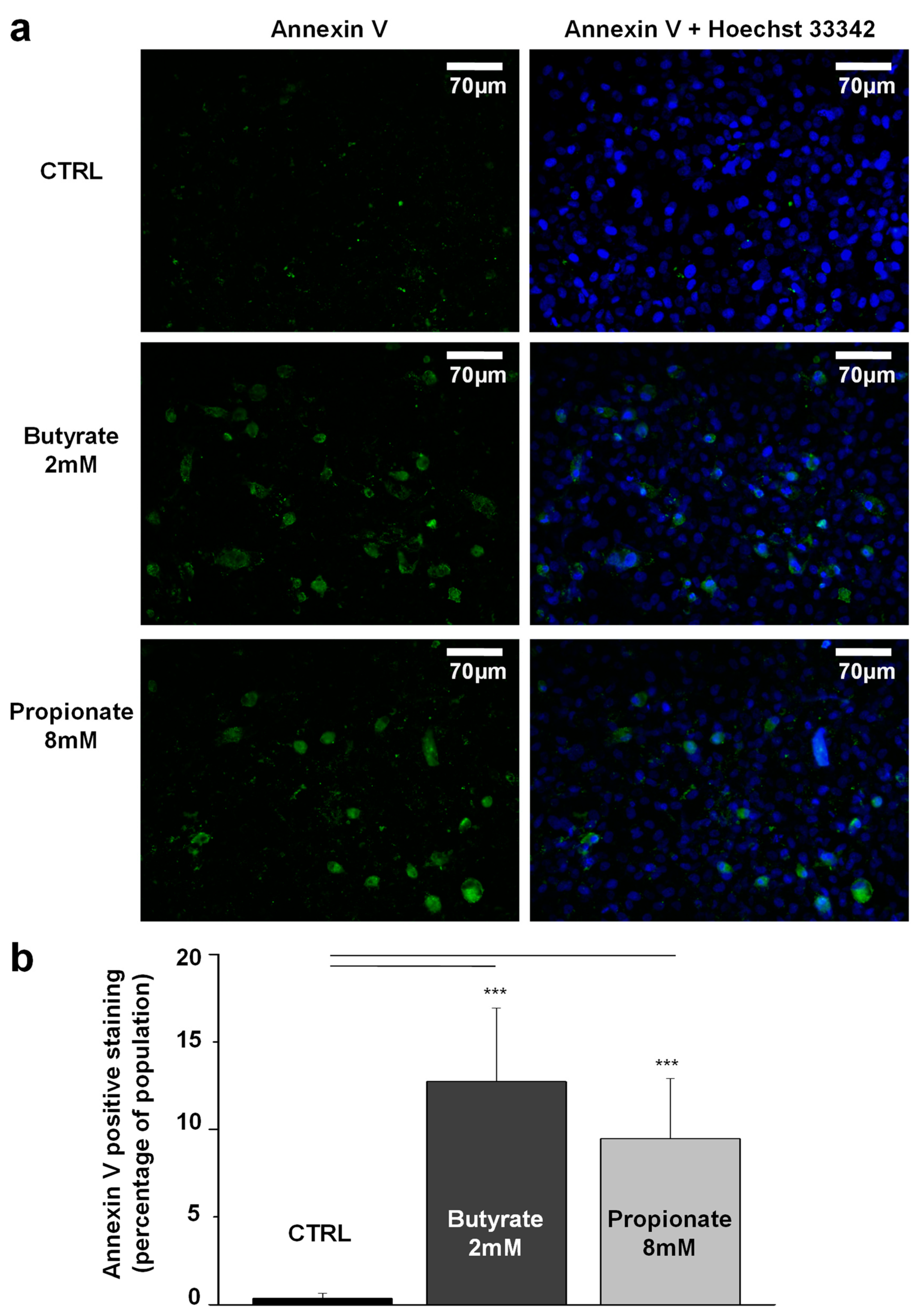
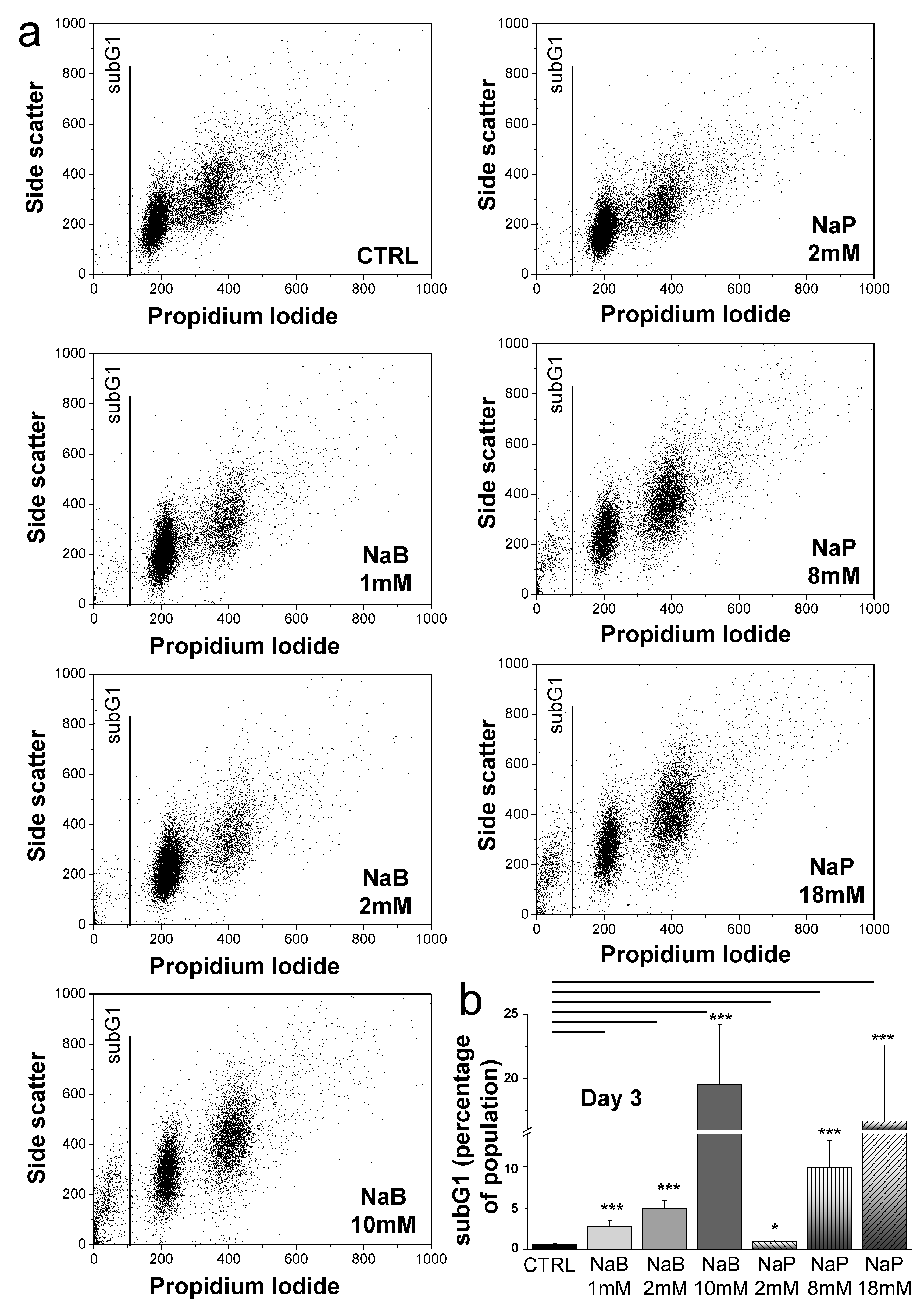
3.4. NaB and NaP Treatment Induced Cell Apoptosis in MDA-MB-231 Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Arnold, M.; Morgan, E.; Rumgay, H.; Mafra, A.; Singh, D.; Laversanne, M.; Vignat, J.; Gralow, J.R.; Cardoso, F.; Siesling, S.; et al. Current and future burden of breast cancer: Global statistics for 2020 and 2040. Breast 2022, 66, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Cheng, H.; Bai, Z.; Li, J. Breast Cancer Cell Line Classification and Its Relevance with Breast Tumor Subtyping. J. Cancer 2017, 8, 3131–3141. [Google Scholar] [CrossRef] [PubMed]
- Prat, A.; Pineda, E.; Adamo, B.; Galvan, P.; Fernandez, A.; Gaba, L.; Diez, M.; Viladot, M.; Arance, A.; Munoz, M. Clinical implications of the intrinsic molecular subtypes of breast cancer. Breast 2015, 24 (Suppl. 2), S26–S35. [Google Scholar] [CrossRef] [PubMed]
- van der Hee, B.; Wells, J.M. Microbial Regulation of Host Physiology by Short-chain Fatty Acids. Trends Microbiol. 2021, 29, 700–712. [Google Scholar] [CrossRef] [PubMed]
- Correa-Oliveira, R.; Fachi, J.L.; Vieira, A.; Sato, F.T.; Vinolo, M.A. Regulation of immune cell function by short-chain fatty acids. Clin. Transl. Immunol. 2016, 5, e73. [Google Scholar] [CrossRef] [PubMed]
- Machado, M.G.; Sencio, V.; Trottein, F. Short-Chain Fatty Acids as a Potential Treatment for Infections: A Closer Look at the Lungs. Infect. Immun. 2021, 89, e0018821. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Ren, C.; Huang, G.; Liu, J.; Liu, W.; Wang, L.; Zhu, B.; Feng, X.; Shi, J.; Li, J.; et al. Inhibition of store-operated Ca(2+) entry counteracts the apoptosis of nasopharyngeal carcinoma cells induced by sodium butyrate. Oncol. Lett. 2017, 13, 921–929. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Park, H.; Im, J.Y.; Choi, W.S.; Kim, H.S. Sodium butyrate regulates androgen receptor expression and cell cycle arrest in human prostate cancer cells. Anticancer Res. 2007, 27, 3285–3292. [Google Scholar]
- Ma, J.; Zhang, Y.; Wang, J.; Zhao, T.; Ji, P.; Song, J.; Zhang, H.; Luo, W. Proliferative effects of gamma-amino butyric acid on oral squamous cell carcinoma cells are associated with mitogen-activated protein kinase signaling pathways. Int. J. Mol. Med. 2016, 38, 305–311. [Google Scholar] [CrossRef]
- Pant, K.; Yadav, A.K.; Gupta, P.; Islam, R.; Saraya, A.; Venugopal, S.K. Butyrate induces ROS-mediated apoptosis by modulating miR-22/SIRT-1 pathway in hepatic cancer cells. Redox Biol. 2017, 12, 340–349. [Google Scholar] [CrossRef]
- Hinnebusch, B.F.; Meng, S.; Wu, J.T.; Archer, S.Y.; Hodin, R.A. The effects of short-chain fatty acids on human colon cancer cell phenotype are associated with histone hyperacetylation. J. Nutr. 2002, 132, 1012–1017. [Google Scholar] [CrossRef] [PubMed]
- Mahalakshmi, R.; Husayn Ahmed, P.; Mahadevan, V. HDAC inhibitors show differential epigenetic regulation and cell survival strategies on p53 mutant colon cancer cells. J. Biomol. Struct. Dyn. 2018, 36, 938–955. [Google Scholar] [CrossRef]
- Siavoshian, S.; Blottiere, H.M.; Cherbut, C.; Galmiche, J.P. Butyrate stimulates cyclin D and p21 and inhibits cyclin-dependent kinase 2 expression in HT-29 colonic epithelial cells. Biochem. Biophys. Res. Commun. 1997, 232, 169–172. [Google Scholar] [CrossRef] [PubMed]
- Semaan, J.; El-Hakim, S.; Ibrahim, J.N.; Safi, R.; Elnar, A.A.; El Boustany, C. Comparative effect of sodium butyrate and sodium propionate on proliferation, cell cycle and apoptosis in human breast cancer cells MCF-7. Breast Cancer 2020, 27, 696–705. [Google Scholar] [CrossRef] [PubMed]
- Habibi, A.; Sadat Shandiz, S.A.; Salehzadeh, A.; Moradi-Shoeili, Z. Novel pyridinecarboxaldehyde thiosemicarbazone conjugated magnetite nanoparticulates (MNPs) promote apoptosis in human lung cancer A549 cells. J. Biol. Inorg. Chem. 2020, 25, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Moshfegh, A.; Salehzadeh, A.; Sadat Shandiz, S.A.; Shafaghi, M.; Naeemi, A.S.; Salehi, S. Phytochemical Analysis, Antioxidant, Anticancer and Antibacterial Properties of the Caspian Sea Red Macroalgae, Laurencia caspica. Iran. J. Sci. Technol. Trans. A Sci. 2019, 43, 49–56. [Google Scholar] [CrossRef]
- Vandesompele, J.; De Preter, K.; Pattyn, F.; Poppe, B.; Van Roy, N.; De Paepe, A.; Speleman, F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002, 3, RESEARCH0034. [Google Scholar] [CrossRef] [PubMed]
- Salimi, V.; Shahsavari, Z.; Safizadeh, B.; Hosseini, A.; Khademian, N.; Tavakoli-Yaraki, M. Sodium butyrate promotes apoptosis in breast cancer cells through reactive oxygen species (ROS) formation and mitochondrial impairment. Lipids Health Dis. 2017, 16, 208. [Google Scholar] [CrossRef] [PubMed]
- Pattayil, L.; Balakrishnan-Saraswathi, H.T. In Vitro Evaluation of Apoptotic Induction of Butyric Acid Derivatives in Colorectal Carcinoma Cells. Anticancer Res. 2019, 39, 3795–3801. [Google Scholar] [CrossRef]
- Abe, M.; Kufe, D.W. Effect of sodium butyrate on human breast carcinoma (MCF-7) cellular proliferation, morphology, and CEA production. Breast Cancer Res. Treat. 1984, 4, 269–274. [Google Scholar] [CrossRef]
- Fernandes, M.R.; Aggarwal, P.; Costa, R.G.F.; Cole, A.M.; Trinchieri, G. Targeting the gut microbiota for cancer therapy. Nat. Rev. Cancer 2022, 22, 703–722. [Google Scholar] [CrossRef] [PubMed]
- Mirzaei, R.; Afaghi, A.; Babakhani, S.; Sohrabi, M.R.; Hosseini-Fard, S.R.; Babolhavaeji, K.; Khani Ali Akbari, S.; Yousefimashouf, R.; Karampoor, S. Role of microbiota-derived short-chain fatty acids in cancer development and prevention. Biomed. Pharmacother. 2021, 139, 111619. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Dong, H.; Xu, Q.; Zhang, Y. Histone deacetylase (HDAC) inhibitors in cancer: A patent review (2017–present). Expert. Opin. Ther. Pat. 2020, 30, 263–274. [Google Scholar] [CrossRef] [PubMed]
- West, A.C.; Johnstone, R.W. New and emerging HDAC inhibitors for cancer treatment. J. Clin. Investig. 2014, 124, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Peng, L.; Seto, E. Histone Deacetylase 10 Regulates the Cell Cycle G2/M Phase Transition via a Novel Let-7-HMGA2-Cyclin A2 Pathway. Mol. Cell. Biol. 2015, 35, 3547–3565. [Google Scholar] [CrossRef] [PubMed]
- Davie, J.R. Inhibition of histone deacetylase activity by butyrate. J. Nutr. 2003, 133, 2485S–2493S. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Hu, P.C.; Ma, Y.B.; Fan, R.; Gao, F.F.; Zhang, J.W.; Wei, L. Sodium butyrate-induced apoptosis and ultrastructural changes in MCF-7 breast cancer cells. Ultrastruct. Pathol. 2016, 40, 200–204. [Google Scholar] [CrossRef] [PubMed]
- Sealy, L.; Chalkley, R. The effect of sodium butyrate on histone modification. Cell 1978, 14, 115–121. [Google Scholar] [CrossRef]
- Kazmierczak-Siedlecka, K.; Marano, L.; Merola, E.; Roviello, F.; Polom, K. Sodium butyrate in both prevention and supportive treatment of colorectal cancer. Front. Cell Infect. Microbiol. 2022, 12, 1023806. [Google Scholar] [CrossRef]
- Goncalves, P.; Martel, F. Butyrate and colorectal cancer: The role of butyrate transport. Curr. Drug Metab. 2013, 14, 994–1008. [Google Scholar] [CrossRef]
- Li, Z.; Wang, Y.; Yuan, C.; Zhu, Y.; Qiu, J.; Zhang, W.; Qi, B.; Wu, H.; Ye, J.; Jiang, H.; et al. Oncogenic roles of Bmi1 and its therapeutic inhibition by histone deacetylase inhibitor in tongue cancer. Lab. Investig. 2014, 94, 1431–1445. [Google Scholar] [CrossRef] [PubMed]
- Chopin, V.; Toillon, R.A.; Jouy, N.; Le Bourhis, X. Sodium butyrate induces P53-independent, Fas-mediated apoptosis in MCF-7 human breast cancer cells. Br. J. Pharmacol. 2002, 135, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Kwon, O.; Ryu, T.Y.; Jung, C.R.; Kim, J.; Min, J.K.; Kim, D.S.; Son, M.Y.; Cho, H.S. Propionate of a microbiota metabolite induces cell apoptosis and cell cycle arrest in lung cancer. Mol. Med. Rep. 2019, 20, 1569–1574. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, M.F.; Carvalho, E.; Pezzuto, P.; Rumjanek, F.D.; Amoedo, N.D. Reciprocal modulation of histone deacetylase inhibitors sodium butyrate and trichostatin A on the energy metabolism of breast cancer cells. J. Cell Biochem. 2015, 116, 797–808. [Google Scholar] [CrossRef]
- Schafer, K.A. The cell cycle: A review. Vet. Pathol. 1998, 35, 461–478. [Google Scholar] [CrossRef] [PubMed]
- Vermeulen, K.; Van Bockstaele, D.R.; Berneman, Z.N. The cell cycle: A review of regulation, deregulation and therapeutic targets in cancer. Cell Prolif. 2003, 36, 131–149. [Google Scholar] [CrossRef]
- Zhang, Z.; Lei, A.; Xu, L.; Chen, L.; Chen, Y.; Zhang, X.; Gao, Y.; Yang, X.; Zhang, M.; Cao, Y. Similarity in gene-regulatory networks suggests that cancer cells share characteristics of embryonic neural cells. J. Biol. Chem. 2017, 292, 12842–12859. [Google Scholar] [CrossRef] [PubMed]
- Cao, M.; Zhang, Z.; Han, S.; Lu, X. Butyrate inhibits the proliferation and induces the apoptosis of colorectal cancer HCT116 cells via the deactivation of mTOR/S6K1 signaling mediated partly by SIRT1 downregulation. Mol. Med. Rep. 2019, 19, 3941–3947. [Google Scholar] [CrossRef]
- Jia, X.; Zheng, Y.; Guo, Y.; Chen, K. Sodium butyrate and panobinostat induce apoptosis of chronic myeloid leukemia cells via multiple pathways. Mol. Genet. Genom. Med. 2019, 7, e613. [Google Scholar] [CrossRef]
- Taylor, M.A.; Khathayer, F.; Ray, S.K. Quercetin and Sodium Butyrate Synergistically Increase Apoptosis in Rat C6 and Human T98G Glioblastoma Cells Through Inhibition of Autophagy. Neurochem. Res. 2019, 44, 1715–1725. [Google Scholar] [CrossRef]
- Ryu, T.Y.; Kim, K.; Son, M.Y.; Min, J.K.; Kim, J.; Han, T.S.; Kim, D.S.; Cho, H.S. Downregulation of PRMT1, a histone arginine methyltransferase, by sodium propionate induces cell apoptosis in colon cancer. Oncol. Rep. 2019, 41, 1691–1699. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.A.; Ahn, Y.-H. Application of sodium propionate to the suspension culture of Chinese hamster ovary cells for enhanced production of follicle-stimulating hormone. Biotechnol. Bioprocess. Eng. 2007, 12, 497–501. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ibrahim, J.-N.; El-Hakim, S.; Semaan, J.; Ghosn, S.; El Ayoubi, H.; Elnar, A.A.; Tohme, N.; El Boustany, C. Sodium Butyrate (NaB) and Sodium Propionate (NaP) Reduce Cyclin A2 Expression, Inducing Cell Cycle Arrest and Proliferation Inhibition of Different Breast Cancer Subtypes, Leading to Apoptosis. Biomedicines 2024, 12, 1779. https://doi.org/10.3390/biomedicines12081779
Ibrahim J-N, El-Hakim S, Semaan J, Ghosn S, El Ayoubi H, Elnar AA, Tohme N, El Boustany C. Sodium Butyrate (NaB) and Sodium Propionate (NaP) Reduce Cyclin A2 Expression, Inducing Cell Cycle Arrest and Proliferation Inhibition of Different Breast Cancer Subtypes, Leading to Apoptosis. Biomedicines. 2024; 12(8):1779. https://doi.org/10.3390/biomedicines12081779
Chicago/Turabian StyleIbrahim, José-Noel, Sandy El-Hakim, Josiane Semaan, Stéphanie Ghosn, Hiba El Ayoubi, Arpiné Ardzivian Elnar, Najat Tohme, and Charbel El Boustany. 2024. "Sodium Butyrate (NaB) and Sodium Propionate (NaP) Reduce Cyclin A2 Expression, Inducing Cell Cycle Arrest and Proliferation Inhibition of Different Breast Cancer Subtypes, Leading to Apoptosis" Biomedicines 12, no. 8: 1779. https://doi.org/10.3390/biomedicines12081779
APA StyleIbrahim, J.-N., El-Hakim, S., Semaan, J., Ghosn, S., El Ayoubi, H., Elnar, A. A., Tohme, N., & El Boustany, C. (2024). Sodium Butyrate (NaB) and Sodium Propionate (NaP) Reduce Cyclin A2 Expression, Inducing Cell Cycle Arrest and Proliferation Inhibition of Different Breast Cancer Subtypes, Leading to Apoptosis. Biomedicines, 12(8), 1779. https://doi.org/10.3390/biomedicines12081779




