The Impact of Environmental Factors on the Development of Autoimmune Thyroiditis—Review
Abstract
1. Introduction
- symptoms of hypothyroidism combined with elevated Thyroid Stimulating Hormone (TSH) levels, with or without low thyroid hormone levels;
- enlargement of the thyroid gland (goiter);
- elevated thyroid antibody levels.
2. Materials and Methods
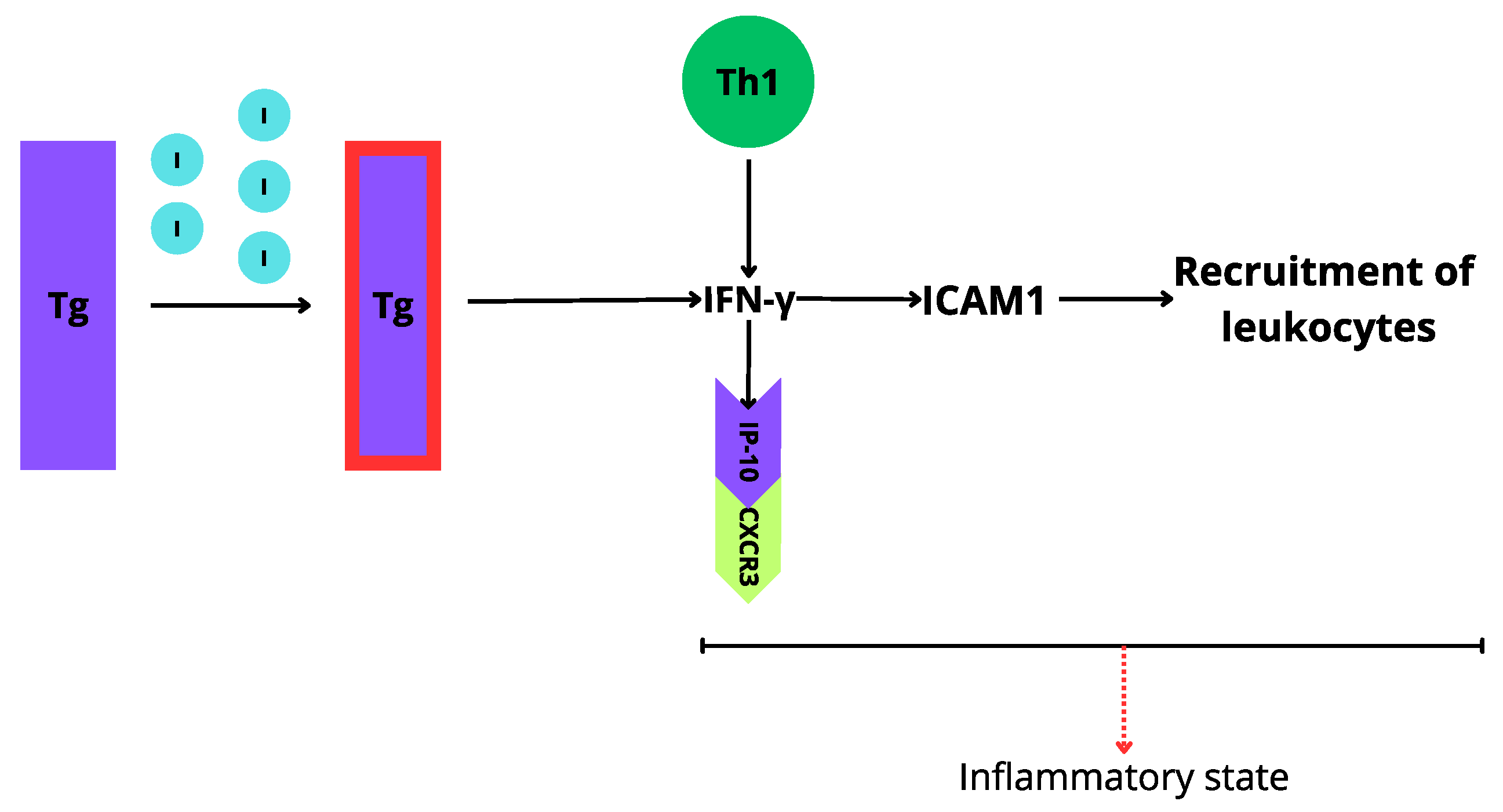
3. Environmental Factors in Autoimmune Thyroids Disorders
3.1. Iodine Excess
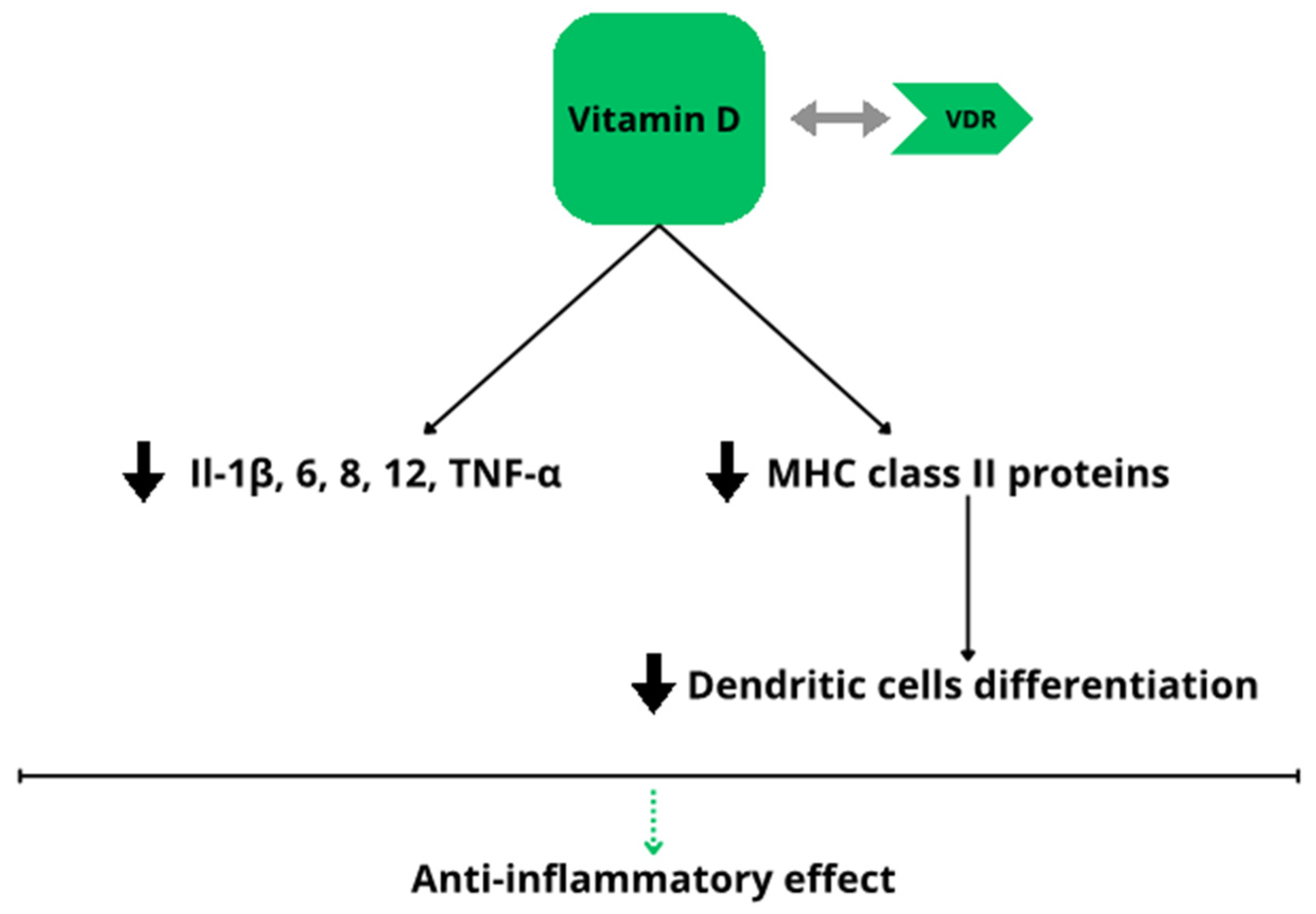
3.2. Vitamin D Deficiency
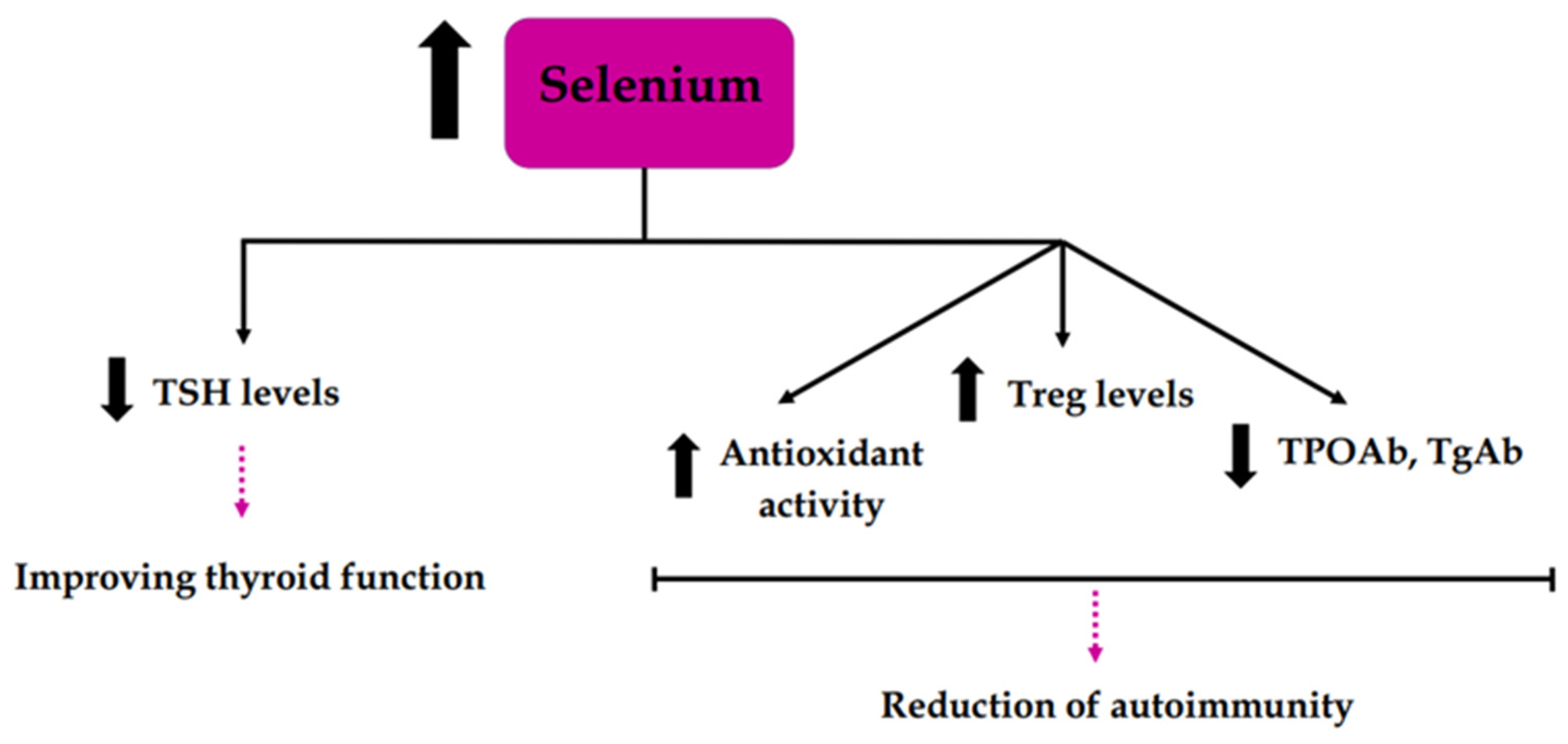
3.3. Selenium Deficiency
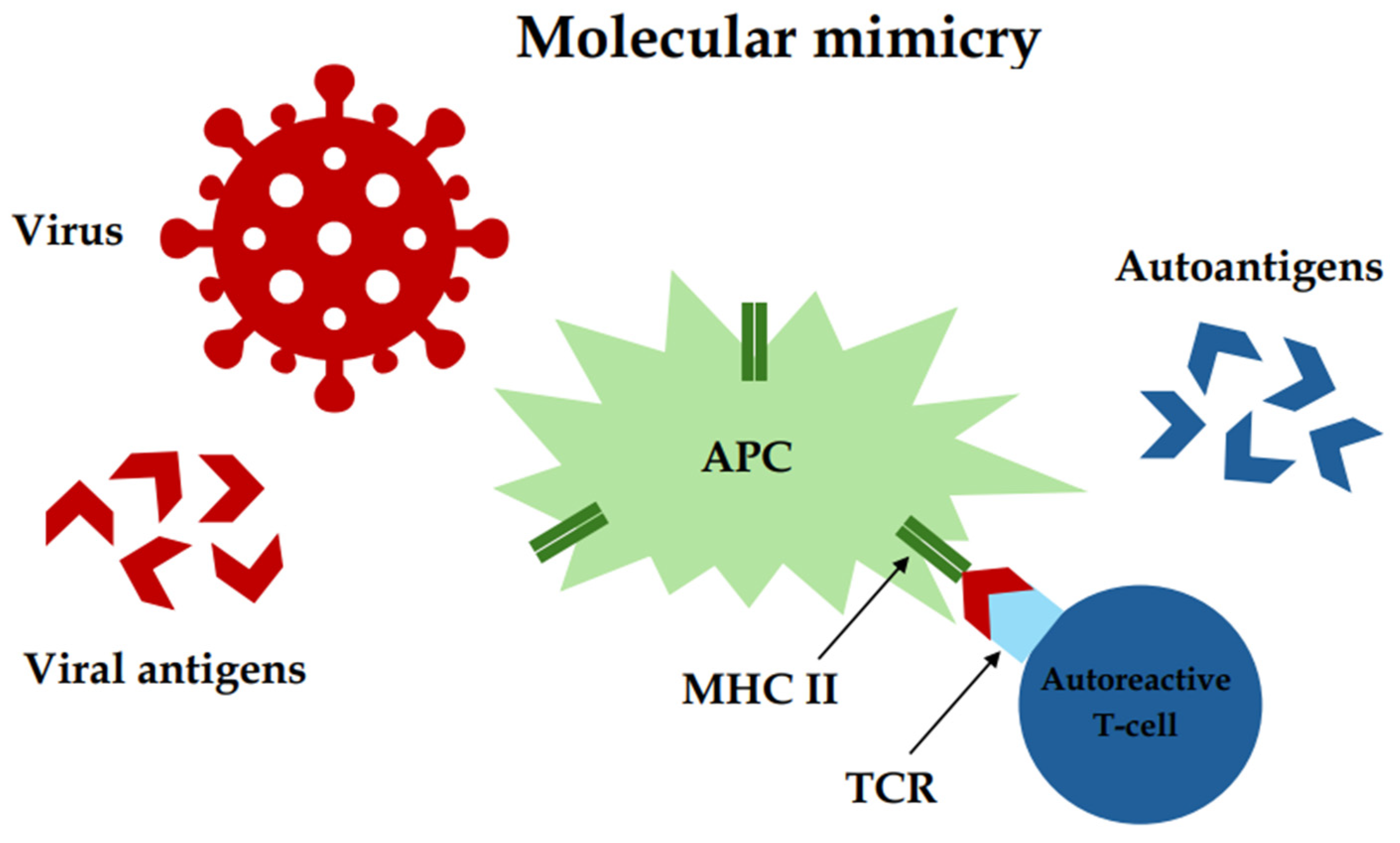
3.4. Viral Infections
3.5. Bacterial Infections
3.6. Microbiome Disruption
3.7. Medications
3.7.1. Interferon-Alpha
3.7.2. Amiodarone
3.7.3. Lithium
3.7.4. Tyrosine Kinase Inhibitors
3.8. Stress
3.9. Smoking
3.10. Climate
3.11. The Influence of Environmental and Genetic Factors on Immunological Processes in AIT
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hashimoto, H. Zur Kenntnis der lymphomatösen Veränderung der Schilddrüse (Struma lymphomatosa). Arch. Klin. Chir. 1912, 97, 219–248. [Google Scholar]
- Pyzik, A.; Grywalska, E.; Matyjaszek-Matuszek, B.; Roliński, J. Immune disorders in Hashimoto’s thyroiditis: What do we know so far? J. Immunol. Res. 2015, 2015, 979167. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.H.; Fu, D.G. Autoimmune thyroid disease: Mechanism, genetics and current knowledge. Eur. Rev. Med. Pharmacol. Sci. 2014, 18, 3611–3618. [Google Scholar] [PubMed]
- Zeber-Lubecka, N.; Hennig, E.E. Genetic Susceptibility to Joint Occurrence of Polycystic Ovary Syndrome and Hashimoto’s Thyroiditis: How Far Is Our Understanding? Front. Immunol. 2021, 12, 606620. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Chen, Y.; Shen, Y.; Zhou, S.; Fei, W.; Yang, Y.; Que, H. Correlation between Hashimoto’s thyroiditis and polycystic ovary syndrome: A systematic review and meta-analysis. Front. Endocrinol. 2022, 13, 1025267. [Google Scholar] [CrossRef] [PubMed]
- Kyritsi, E.M.; Kanaka-Gantenbein, C. Autoimmune Thyroid Disease in Specific Genetic Syndromes in Childhood and Adolescence. Front. Endocrinol. 2020, 11, 543. [Google Scholar] [CrossRef] [PubMed]
- American Thyroid Association. Available online: https://www.thyroid.org/hashimotos-thyroiditis/ (accessed on 10 February 2024).
- Vargas-Uricoechea, H.; Nogueira, J.P.; Pinzón-Fernández, M.V.; Schwarzstein, D. The Usefulness of Thyroid Antibodies in the Diagnostic Approach to Autoimmune Thyroid Disease. Antibodies 2023, 12, 48. [Google Scholar] [CrossRef]
- Almahari, S.A.; Maki, R.; Al Teraifi, N.; Alshaikh, S.; Chandran, N.; Taha, H. Hashimoto Thyroiditis beyond Cytology: A Correlation between Cytological, Hormonal, Serological, and Radiological Findings. J. Thyroid Res. 2023, 2023, 5707120. [Google Scholar] [CrossRef]
- Klubo-Gwiezdzinska, J.; Wartofsky, L. Hashimoto thyroiditis: An evidence-based guide to etiology, diagnosis and treatment. Pol. Arch. Intern. Med. 2022, 132, 16222. [Google Scholar] [CrossRef]
- Fröhlich, E.; Wahl, R. Thyroid Autoimmunity: Role of Anti-thyroid Antibodies in Thyroid and Extra-Thyroidal Diseases. Front. Immunol. 2017, 8, 521. [Google Scholar] [CrossRef]
- Caturegli, P.; De Remigis, A.; Rose, N.R. Hashimoto thyroiditis: Clinical and diagnostic criteria. Autoimmun. Rev. 2014, 13, 391–397. [Google Scholar] [CrossRef]
- Wu, G.; Zou, D.; Cai, H.; Liu, Y. Ultrasonography in the diagnosis of Hashimoto’s thyroiditis. Front. Biosci. 2016, 21, 1006–1012. [Google Scholar] [CrossRef]
- Omidan, N.; Zahir, S.T.; Fateh, A. Cytological and Pathological Evaluation of Hashimoto’s Thyroiditis. Maedica 2019, 14, 98–103. [Google Scholar] [CrossRef] [PubMed]
- Siegmann, E.M.; Müller, H.H.O.; Luecke, C.; Philipsen, A.; Kornhuber, J.; Grömer, T.W. Association of Depression and Anxiety Disorders With Autoimmune Thyroiditis: A Systematic Review and Meta-analysis. JAMA Psychiatry 2018, 75, 577–584. [Google Scholar] [CrossRef]
- Bates, J.N.; Kohn, T.P.; Pastuszak, A.W. Effect of Thyroid Hormone Derangements on Sexual Function in Men and Women. Sex. Med. Rev. 2020, 8, 217–230. [Google Scholar] [CrossRef] [PubMed]
- Quintino-Moro, A.; Zantut-Wittmann, D.E.; Tambascia, M.; Machado Hda, C.; Fernandes, A. High Prevalence of Infertility among Women with Graves’ Disease and Hashimoto’s Thyroiditis. Int. J. Endocrinol. 2014, 2014, 982705. [Google Scholar] [CrossRef] [PubMed]
- Udovcic, M.; Pena, R.H.; Patham, B.; Tabatabai, L.; Kansara, A. Hypothyroidism and the Heart. Methodist Debakey Cardiovasc. J. 2017, 13, 55–59. [Google Scholar] [CrossRef]
- Hu, X.; Wang, X.; Liang, Y.; Chen, X.; Zhou, S.; Fei, W.; Yang, Y.; Que, H. Cancer Risk in Hashimoto’s Thyroiditis: A Systematic Review and Meta-Analysis. Front. Endocrinol. 2022, 13, 937871. [Google Scholar] [CrossRef]
- Lee, Y.J.; Cho, S.W.; Lim, Y.H.; Kim, B.N.; Kim, J.I.; Hong, Y.C.; Park, Y.J.; Shin, C.H.; Lee, Y.A. Relationship of iodine excess with thyroid function in 6-year-old children living in an iodine-replete area. Front. Endocrinol. 2023, 14, 1099824. [Google Scholar] [CrossRef]
- Lisco, G.; De Tullio, A.; Triggiani, D.; Zupo, R.; Giagulli, V.A.; De Pergola, G.; Piazzolla, G.; Guastamacchia, E.; Sabbà, C.; Triggiani, V. Iodine Deficiency and Iodine Prophylaxis: An Overview and Update. Nutrients 2023, 15, 1004. [Google Scholar] [CrossRef]
- Yu, Z.; Yu, Y.; Wan, Y.; Fan, J.; Meng, H.; Li, S.; Wang, Y.; Wang, T.; Ling, R. Iodine intake level and incidence of thyroid disease in adults in Shaanxi province: A cross-sectional study. Ann. Transl. Med. 2021, 9, 1567. [Google Scholar] [CrossRef]
- Duntas, L.H. The Role of Iodine and Selenium in Autoimmune Thyroiditis. Horm. Metab. Res. 2015, 47, 721–726. [Google Scholar] [CrossRef] [PubMed]
- Kasiyan, O.; Tkachenko, H.; Kurhaluk, N.; Yurchenko, S.; Manenko, A. Relationship Between Thyroid Hormonal Status in Patients with a Hypothyroid Form of Hashimoto’s Thyroiditis and Iodine Concentrations in Drinking Water. Biol. Trace Elem. Res. 2022, 200, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Novak, N.; Biček, A.; Zaletel, K.; Gaberšček, S. Amelioration of iodine supply is notably associated with thyroid function in healthy subjects and in patients with euthyroid Hashimoto’s thyroiditis. Slov. Med. J. 2016, 85, 531–540. [Google Scholar] [CrossRef]
- Li, H.; Ma, J.; Wang, C.; Liu, J.; Chen, Y.; Liu, C.; Hou, Z. Correlation Analysis Between Thyroid Function and Autoantibodies in Hashimoto Thyroiditis Patients with Different Iodine Nutritional Status. Am. J. Biomed. Life Sci. 2021, 9, 10–19. [Google Scholar] [CrossRef]
- Palaniappan, S.; Shanmughavelu, L.; Prasad, H.K.; Subramaniam, S.; Krishnamoorthy, N.; Lakkappa, L. Improving iodine nutritional status and increasing prevalence of autoimmune thyroiditis in children. Indian J. Endocrinol. Metab. 2017, 21, 85–89. [Google Scholar] [CrossRef] [PubMed]
- Yokomichi, H.; Mochizuki, M.; Kojima, R.; Horiuchi, S.; Ooka, T.; Akiyama, Y.; Miyake, K.; Kushima, M.; Otawa, S.; Shinohara, R.; et al. Mother’s iodine exposure and infants’ hypothyroidism: The Japan Environment and Children’s Study (JECS). Endocr. J. 2022, 69, 9–21. [Google Scholar] [CrossRef]
- Kaličanin, D.; Cvek, M.; Barić, A.; Škrabić, V.; Punda, A.; Boraska Perica, V. Associations between vitamin D levels and dietary patterns in patients with Hashimoto’s thyroiditis. Front. Nutr. 2023, 10, 1188612. [Google Scholar] [CrossRef] [PubMed]
- Czarnywojtek, A.; Florek, E.; Pietrończyk, K.; Sawicka-Gutaj, N.; Ruchała, M.; Ronen, O.; Nixon, I.J.; Shaha, A.R.; Rodrigo, J.P.; Tufano, R.P.; et al. The Role of Vitamin D in Autoimmune Thyroid Diseases: A Narrative Review. J. Clin. Med. 2023, 12, 1452. [Google Scholar] [CrossRef]
- Siddiq, A.; Naveed, A.K.; Ghaffar, N.; Aamir, M.; Ahmed, N. Association of Pro-Inflammatory Cytokines with Vitamin D in Hashimoto’s Thyroid Autoimmune Disease. Medicina 2023, 59, 853. [Google Scholar] [CrossRef]
- Maciejewski, A.; Kowalczyk, M.J.; Herman, W.; Czyżyk, A.; Kowalska, M.; Żaba, R.; Łącka, K. Vitamin D Receptor Gene Polymorphisms and Autoimmune Thyroiditis: Are They Associated with Disease Occurrence and Its Features? Biomed. Res. Int. 2019, 2019, 8197580. [Google Scholar] [CrossRef] [PubMed]
- Chao, G.; Zhu, Y.; Fang, L. Correlation Between Hashimoto’s Thyroiditis-Related Thyroid Hormone Levels and 25-Hydroxyvitamin D. Front. Endocrinol. 2020, 11, 4. [Google Scholar] [CrossRef]
- Maciejewski, A.; Wójcicka, M.; Roszak, M.; Losy, J.; Łącka, K. Assessment of Vitamin D Level in Autoimmune Thyroiditis Patients and a Control Group in the Polish Population. Adv. Clin. Exp. Med. 2015, 24, 801–806. [Google Scholar] [CrossRef]
- Gasic, S.; Smiljic, S.; Milanovic, Z.; Gasic, M.; Ilic, S.; Bogosavlijevic, I.; Dejanovic, M.; Nestrovic, V.; Matic, T. Relationship between low vitamin D levels with Hashimoto thyroiditis. Srp. Arh. Za Celok. Lek. 2023, 151, 296–301. [Google Scholar] [CrossRef]
- Xu, J.; Zhu, X.Y.; Sun, H.; Xu, X.Q.; Xu, S.A.; Suo, Y.; Cao, L.J.; Zhou, Q.; Yu, H.J.; Cao, W.Z. Low vitamin D levels are associated with cognitive impairment in patients with Hashimoto thyroiditis. BMC Endocr. Disord. 2018, 18, 87. [Google Scholar] [CrossRef] [PubMed]
- Kim, D. Low vitamin D status is associated with hypothyroid Hashimoto’s thyroiditis. Hormones 2016, 15, 385–393. [Google Scholar] [CrossRef] [PubMed]
- Gierach, M.; Junik, R. The role of vitamin D in women with Hashimoto’s thyroiditis. Endokrynol. Pol. 2023, 74, 176–180. [Google Scholar] [CrossRef]
- Sönmezgöz, E.; Ozer, S.; Yilmaz, R.; Önder, Y.; Bütün, I.; Bilge, S. Hypovitaminosis D in children with Hashimoto’s thyroiditis. Rev. Méd. Chile 2016, 144, 611–616. [Google Scholar] [CrossRef]
- Evliyaoğlu, O.; Acar, M.; Özcabı, B.; Erginöz, E.; Bucak, F.; Ercan, O.; Kucur, M. Vitamin D deficiency and Hashimoto’s thyroiditis in children and adolescents: A critical vitamin D level for this association? J. Clin. Res. Pediatr. Endocrinol. 2015, 7, 128–133. [Google Scholar] [CrossRef]
- Rola, R.; Trusewicz, E.; Bieńkowski, T.; Studzińska, S. Application of dried blood spots and serum samples for the determination of vitamin D metabolites in the group of healthy women and with Hashimoto’s thyroiditis. Chromatographia 2021, 84, 695–701. [Google Scholar] [CrossRef]
- Anaraki, P.V.; Aminorroaya, A.; Amini, M.; Momeni, F.; Feizi, A.; Iraj, B.; Tabatabaei, A. Effect of vitamin D deficiency treatment on thyroid function and autoimmunity markers in Hashimoto’s thyroiditis: A double-blind randomized placebo-controlled clinical trial. J. Res. Med. Sci. 2017, 22, 103. [Google Scholar] [CrossRef]
- Yavuzer, H.; Işık, S.; Cengiz, M.; Bolayırlı, I.M.; Döventaş, A.; Erdinçler, D.S. The relationship between vitamin D levels and receptor activator of nuclear factor ligand in Hashimoto’s thyroiditis. Med. Bull. Haseki 2017, 55, 261–268. [Google Scholar] [CrossRef]
- Cvek, M.; Kaličanin, D.; Barić, A.; Vuletić, M.; Gunjača, I.; Lovrić, V.T.; Škrabić, V.; Punda, A.; Perica, V.B. Vitamin D and Hashimoto’s thyroiditis: Observations from CROHT Biobank. Nutrients 2021, 13, 2793. [Google Scholar] [CrossRef] [PubMed]
- Botelho, I.M.B.; Neto, A.M.; Silva, C.A.; Tambascia, M.A.; Alegre, S.M.; Zantut-Wittmann, D.E. Vitamin D in Hashimoto’s thyroiditis and its relationship with thyroid function and inflammatory status. Endocr. J. 2018, 65, 1029–1037. [Google Scholar] [CrossRef] [PubMed]
- Filipova, L.; Lazurova, Z.; Fulop, P.; Lazurova, I. Vitamin D insufficiency is not associated with thyroid autoimmunity in Slovak women with Hashimoto’s disease. Bratisl. Med. J. 2023, 124, 182–186. [Google Scholar] [CrossRef] [PubMed]
- Ke, W.; Sun, T.; Zhang, Y.; He, L.; Wu, Q.; Liu, J.; Zha, B. 25-Hydroxyvitamin D serum level in Hashimoto’s thyroiditis, but not Graves’ disease is relatively deficient. Endocr. J. 2017, 64, 581–587. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Wu, D.; Li, C.; Fan, C.; Chao, N.; Liu, J.; Li, Y.; Wang, R.; Miao, W.; Guan, H.; et al. Lower serum 25-hydroxyvitamin D level is associated with 3 types of autoimmune thyroid diseases. Medicine 2015, 94, e1639. [Google Scholar] [CrossRef] [PubMed]
- Lacka, K.; Szeliga, A. Significance of selenium in thyroid physiology and pathology. Pol. Merkur. Lek. 2015, 38, 348–353. [Google Scholar] [PubMed]
- Szeliga, A.; Czyżyk, A.; Niedzielski, P.; Mleczek, M.; Maciejewski, A.; Dorszewska, J.; Łącka, K. Assessment of serum selenium concentration in patients with autoimmune thyroiditis in Poznan district. Pol. Merkur. Lek. 2018, 45, 150–153. [Google Scholar]
- Wu, Q.; Wang, Y.; Chen, P.; Wei, J.; Lv, H.; Wang, S.; Wu, Y.; Zhao, X.; Peng, X.; Rijntjes, E.; et al. Increased incidence of Hashimoto thyroiditis in selenium deficiency: A prospective 6-year cohort study. J. Clin. Endocrinol. Metab. 2022, 107, e3603–e3611. [Google Scholar] [CrossRef]
- Cinemre, D.A.; Cinemre, G.C.; Serinkan, F.B.; Degirmencioglu, S.; Bahtiyar, N.; Aydemir, B. The role of selenium, selenoproteins and oxidative DNA damage in etiopathogenesis of Hashimoto thyroiditis. J. Elem. 2022, 27, 755–764. [Google Scholar] [CrossRef]
- Rostami, R.; Nourooz-Zadeh, S.; Mohammadi, A.; Khalkhali, H.R.; Ferns, G.; Nourooz-Zadeh, J. Serum selenium status and its interrelationship with serum biomarkers of thyroid function and antioxidant defense in Hashimoto’s thyroiditis. Antioxidants 2020, 9, 1070. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Feng, W.; Chen, H.; Shi, H.; Jiang, L.; Zheng, X.; Liu, X.; Zhang, W.; Ge, Y.; Liu, Y.; et al. Effect of selenium on thyroid autoimmunity and regulatory T cells in patients with Hashimoto’s thyroiditis: A prospective randomized-controlled trial. Clin. Transl. Sci. 2021, 14, 1390–1402. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.F.; Sun, R.X.; Li, C.F.; Wang, X.H. The effects of selenium supplementation on antibody titres in patients with Hashimoto’s thyroiditis. Endokrynol. Pol. 2021, 72, 666–667. [Google Scholar] [CrossRef] [PubMed]
- Pirola, I.; Gandossi, E.; Agosti, B.; Delbarba, A.; Cappelli, C. Selenium supplementation could restore euthyroidism in subclinical hypothyroid patients with autoimmune thyroiditis. Endokrynol. Pol. 2016, 67, 567–571. [Google Scholar] [CrossRef] [PubMed]
- Manevska, N.; Stojanoski, S.; Makazlieva, T. Selenium treatment effect in auto-immune Hashimoto thyroiditis in Macedonian population. J. Endocrinol. Metab. 2019, 9, 22–28. [Google Scholar] [CrossRef]
- Hogestyn, J.M.; Mock, D.J.; Mayer-Proschel, M. Contributions of neurotropic human herpesviruses herpes simplex virus 1 and human herpesvirus 6 to neurodegenerative disease pathology. Neural Regen. Res. 2018, 13, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Tesch, F.; Ehm, F.; Vivirito, A.; Wende, D.; Batram, M.; Loser, F.; Menzer, S.; Jacob, J.; Roessler, M.; Seifert, M.; et al. Incident autoimmune diseases in association with SARS-CoV-2 infection: A matched cohort study. Clin. Rheumatol. 2023, 42, 2905–2914. [Google Scholar] [CrossRef] [PubMed]
- Al-Rammahi, A.A.H.A.; Al-Khilkhali, H.J.B. Detection of Epstein-Barr virus and Hashimoto’s autoimmune in patients with a thyroid disorder. Department of Biology, Faculty of Science, University of Kufa, Iraq. BIO Web Conf. 2023, 65, 05046. [Google Scholar] [CrossRef]
- Assaad, S.N.; Meheissen, M.A.; Elsayed, E.T.; Alnakhal, S.N.; Salem, T.M. Study of Epstein–Barr virus serological profile in Egyptian patients with Hashimoto’s thyroiditis: A case-control study. J. Clin. Transl. Endocrinol. 2020, 20, 100222. [Google Scholar] [CrossRef]
- Heidari, Z.; Jami, M. Parvovirus B19 Infection Is Associated with Autoimmune Thyroid Disease in Adults. Int. J. Endocrinol. Metab. 2021, 19, e115592. [Google Scholar] [CrossRef] [PubMed]
- Seyyedi, N.; Dehbidi, G.R.; Karimi, M.; Asgari, A.; Esmaeili, B.; Zare, F.; Farhadi, A.; Dabbaghmanesh, M.H.; Saki, F.; Behzad-Behbahani, A. Human herpesvirus 6A active infection in patients with autoimmune Hashimoto’s thyroiditis. Braz. J. Infect. Dis. 2019, 23, 435–440. [Google Scholar] [CrossRef] [PubMed]
- Readhead, B.; Haure-Mirande, J.V.; Funk, C.C.; Richards, M.; Shannon, P.; Haroutunian, V.; Sano, M.; Liang, W.; Beckmann, N.; Price, N.; et al. Multiscale Analysis of Independent Alzheimer’s Cohorts Finds Disruption of Molecular, Genetic, and Clinical Networks by Human Herpesvirus. Neuron 2018, 99, 64–82.e7. [Google Scholar] [CrossRef]
- Marci, R.; Gentili, V.; Bortolotti, D.; Monte, G.; Caselli, E.; Bolzani, S.; Rotola, A.; Di Luca, D.; Rizzoet, R. Presence of HHV-6A in Endometrial Epithelial Cells from Women with Primary Unexplained Infertility. PLoS ONE 2016, 11, e0158304. [Google Scholar] [CrossRef]
- Coulam, C.B.; Bilal, M.; Salazar Garcia, M.D.; Katukurundage, D.; Elazzamy, H.; Fernandez, E.; Kwak-Kim, J.; Beaman, K.; Dambaeva, S. Prevalence of HHV-6 in endometrium from women with recurrent implantation failure. Am. J. Reprod. Immunol. 2018, 80, e12862. [Google Scholar] [CrossRef] [PubMed]
- Bortolotti, D.; Gentili, V.; Rotola, A.; Cultrera, R.; Marci, R.; Di Luca, D.; Rizzo, R. HHV-6A infection of endometrial epithelial cells affects immune profile and trophoblast invasion. Am. J. Reprod. Immunol. 2019, 82, e13174. [Google Scholar] [CrossRef]
- Weider, T.; Genoni, A.; Broccolo, F.; Paulsen, T.H.; Dahl-Jørgensen, K.; Toniolo, A.; Hammerstad, S.S. High Prevalence of Common Human Viruses in Thyroid Tissue. Front. Endocrinol. 2022, 13, 938633. [Google Scholar] [CrossRef] [PubMed]
- Desailloud, R.; Hober, D. Viruses and thyroiditis: An update. Virol. J. 2009, 6, 5. [Google Scholar] [CrossRef]
- Abdullah, Y.J.; Essa, R.H.; Jumaa, M.G. Incidence of Helicobacter pylori Infection among Hashimoto’s Thyroiditis Patients in Amara City, Iraq. Jordan J. Biol. Sci. 2022, 15, 537–541. [Google Scholar] [CrossRef]
- Figura, N.; Di Cairano, G.; Moretti, E.; Iacoponi, F.; Santucci, A.; Bernardini, G.; Gonnelli, S.; Giordano, N.; Ponzetto, A. Helicobacter pylori Infection and Autoimmune Thyroid Diseases: The Role of Virulent Strains. Antibiotics 2019, 9, 12. [Google Scholar] [CrossRef]
- Dore, M.P.; Fanciulli, G.; Manca, A.; Pes, G.M. Association of Helicobacter pylori Infection with Autoimmune Thyroid Disease in the Female Sex. J. Clin. Med. 2023, 12, 5150. [Google Scholar] [CrossRef] [PubMed]
- Shmuely, H.; Shimon, I.; Azulay Gitter, L. Helicobacter pylori infection in women with Hashimoto thyroiditis: A case-control study. Medicine 2016, 95, e4074. [Google Scholar] [CrossRef]
- Quigley, E.M. Gut bacteria in health and disease. Gastroenterol. Hepatol. 2013, 9, 560–569. [Google Scholar]
- Shen, S.; Wong, C.H. Bugging inflammation: Role of the gut microbiota. Clin. Transl. Immunol. 2016, 5, e72. [Google Scholar] [CrossRef]
- Cayres, L.C.; de Salis, L.V.; Rodrigues, G.S.P.; Lengert, A.H.; Biondi, A.P.C.; Sargentini, L.D.B.; Brisotti, J.L.; Gomes, E.; de Oliveira, G.L.V. Detection of Alterations in the Gut Microbiota and Intestinal Permeability in Patients With Hashimoto Thyroiditis. Front. Immunol. 2021, 12, 579140. [Google Scholar] [CrossRef]
- Liu, J.; Qin, X.; Lin, B.; Cui, J.; Liao, J.; Zhang, F.; Lin, Q. Analysis of gut microbiota diversity in Hashimoto’s thyroiditis patients. BMC Microbiol. 2022, 22, 318. [Google Scholar] [CrossRef]
- Sawicka-Gutaj, N.; Gruszczyński, D.; Zawalna, N.; Nijakowski, K.; Muller, I.; Karpiński, T.; Salvi, M.; Ruchała, M. Microbiota Alterations in Patients with Autoimmune Thyroid Diseases: A Systematic Review. Int. J. Mol. Sci. 2022, 23, 13450. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; An, Y.; Cao, B.; Fliers, E.; Nieuwdorp, M. The Composition of Gut Microbiota in Patients Bearing Hashimoto’s Thyroiditis with Euthyroidism and Hypothyroidism. Int. J. Endocrinol. 2020, 2020, 5036959. [Google Scholar] [CrossRef] [PubMed]
- Fenneman, A.C.; Rampanelli, E.; van der Spek, A.H.; Fliers, E.; Nieuwdorp, M. Protocol for a double-blinded randomised controlled trial to assess the effect of faecal microbiota transplantations on thyroid reserve in patients with subclinical autoimmune hypothyroidism in the Netherlands: The IMITHOT trial. BMJ Open 2023, 13, e073971. [Google Scholar] [CrossRef]
- Faustino, L.C.; Lombardi, A.; Madrigal-Matute, J.; Owen, R.; Libutti, S.; Tomer, Y. Interferon-α Triggers Autoimmune Thyroid Diseases via Lysosomal-Dependent Degradation of Thyroglobulin. J. Clin. Endocrinol. Metab. 2018, 103, 3678–3687. [Google Scholar] [CrossRef]
- Chou, S.M.; Yeh, H.J.; Lin, T.M.; Chang, Y.S.; Hsu, H.C.; Shen, Y.C.; Kuo, T.T.; Chen, J.H.; Chen, S.H.; Chang, C.C. Association of interferon-based therapy with risk of autoimmune diseases in patients with chronic hepatitis C virus infection: A population-based Taiwanese cohort study. Front. Immunol. 2022, 13, 992819. [Google Scholar] [CrossRef] [PubMed]
- Luo, W.; Wu, S.; Chen, H.; Wu, Y.; Peng, J. Thyroid dysfunction is associated with the loss of hepatitis B surface antigen in patients with chronic hepatitis B undergoing treatment with a-interferon. J. Int. Med. Res. 2021, 49, 3000605211025139. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.K.; Tseng, Y.T.; Chen, K.H.; Chen, K.T. Long-term outcomes and risk factors of thyroid dysfunction during pegylated interferon and ribavirin treatment in patients with chronic hepatitis C infection in Taiwan. BMC Endocr. Disord. 2019, 19, 36. [Google Scholar] [CrossRef] [PubMed]
- Rodia, R.; Meloni, P.E.; Mascia, C.; Balestrieri, C.; Ruggiero, V.; Serra, G.; Conti, M.; Loi, M.; Pes, F.; Onaliet, S.; et al. Direct-acting antivirals used in HCV-related liver disease do not affect thyroid function and autoimmunity. J. Endocrinol. Investig. 2023, 46, 359–366. [Google Scholar] [CrossRef] [PubMed]
- Medić, F.; Bakula, M.; Alfirević, M.; Bakula, M.; Mucić, K.; Marić, N. Amiodarone and thyroid dysfunction. Acta Clin. Croat. 2022, 61, 327–341. [Google Scholar] [CrossRef] [PubMed]
- Weetman, A.P.; Bhandal, S.K.; Burrin, J.M.; Robinson, K.; McKenna, W. Amiodarone and thyroid autoimmunity in the United Kingdom. BMJ 1988, 297, 33. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Safran, M.; Martino, E.; Aghini-Lombardi, F.; Bartalena, L.; Balzano, S.; Pinchera, A.; Braverman, L. Effect of amiodarone on circulating antithyroid antibodies. BMJ 1988, 297, 456–457. [Google Scholar] [CrossRef] [PubMed]
- Permoda-Osip, A.; Abramowicz, M.; Kraszewska, A.; Suwalska, A.; Chlopocka-Wozniak, M.; Rybakowski, J.K. Kidney, thyroid and other organ functions after 40 years or more of lithium therapy: A case series of five patients. Ther. Adv. Psychopharmacol. 2016, 6, 277–282. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Xue, S.; Zhang, L.; Chen, G. Trace elements and the thyroid. Front. Endocrinol. 2022, 13, 904889. [Google Scholar] [CrossRef]
- Kuman Tunçel, Ö.; Akdeniz, F.; Özbek, S.S.; Kavukçu, G.; Ünal Kocabaş, G. Thyroid function and ultrasonography abnormalities in lithium-treated bipolar patients: A cross-sectional study with healthy controls. Arch. Neuropsychiatry 2017, 54, 108–115. [Google Scholar] [CrossRef]
- Snijders, G.J.L.; de Witte, L.D.; van den Berk, D.; van der Laan, C.; Regeer, E.; Begemann, M.J.H.; Berdenis van Berlekom, A.; Litjens, M.; Boks, M.P.; Ophoff, R.A.; et al. No association between anti-thyroidperoxidase antibodies and bipolar disorder: A study in the Dutch Bipolar Cohort and a meta-analysis. Psychoneuroendocrinology 2020, 112, 104518. [Google Scholar] [CrossRef] [PubMed]
- Rodia, R.; Pani, F.; Caocci, G.; La Nasa, G.; Simula, M.P.; Mulas, O.; Velluzzi, F.; Loviselli, A.; Mariotti, S.; Boi, F. Thyroid autoimmunity and hypothyroidism are associated with deep molecular response in patients with chronic myeloid leukemia on tyrosine kinase inhibitors. J. Endocrinol. Investig. 2022, 45, 291–300. [Google Scholar] [CrossRef] [PubMed]
- Vaivode, I.; Zake, T.; Strele, I.; Upmale-Engela, S.; Gogins, D.; Gersone, G.; Skesters, A.; Dambrova, M.; Konrade, I. Stress-Related Immune Response and Selenium Status in Autoimmune Thyroid Disease Patients. Int. J. Mol. Sci. 2023, 24, 2440. [Google Scholar] [CrossRef] [PubMed]
- Hong, H.; Lee, J. Thyroid-Stimulating Hormone as a Biomarker for Stress After Thyroid Surgery: A Prospective Cohort Study. Med. Sci. Monit. 2022, 28, e937957. [Google Scholar] [CrossRef] [PubMed]
- Wiersinga, W.M. Smoking and thyroid. Clin. Endocrinol. 2013, 79, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Strieder, T.G.; Prummel, M.F.; Tijssen, J.G.; Endert, E.; Wiersinga, W.M. Risk factors for and prevalence of thyroid disorders in a cross-sectional study among healthy female relatives of patients with autoimmune thyroid disease. Clin. Endocrinol. 2003, 59, 396–401. [Google Scholar] [CrossRef] [PubMed]
- Belin, R.M.; Astor, B.C.; Powe, N.R.; Ladenson, P. Smoke exposure is associated with a lower prevalence of serum thyroid autoantibodies and thyrotropin concentration elevation and a higher prevalence of mild thyrotropin concentration suppression in the third National Health and Nutrition Examination Survey (NHANES III). J. Clin. Endocrinol. Metab. 2004, 89, 6077–6086. [Google Scholar]
- Mehran, L.; Amouzgar, A.; Delshad, H.; Azizi, F. The association of cigarette smoking with serum TSH concentration and thyroperoxidase antibody. Exp. Clin. Endocrinol. Diabetes 2012, 120, 80–83. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, I.B.; Laurberg, P.; Knudsen, N.; Jørgensen, T.; Perrild, H.; Ovesen, L.; Rasmussen, L. Smoking is negatively associated with the presence of thyroglobulin autoantibody and to a lesser degree with thyroid peroxidase autoantibody in serum: A population study. Eur. J. Endocrinol. 2008, 158, 367–373. [Google Scholar] [CrossRef]
- Wang, D.W.; Zhou, R.B.; Yao, Y.M.; Zhu, X.; Yin, Y.; Zhao, G.; Dong, N.; Sheng, Z. Stimulation of α7 nicotinic acetylcholine receptor by nicotine increases suppressive capacity of naturally occurring CD4+CD25+ regulatory T cells in mice in vitro. J. Pharmacol. Exp. 2010, 335, 553–561. [Google Scholar] [CrossRef]
- Caturegli, P.; De Remigis, A.; Ferlito, M.; Landek-Salgado, M.; Iwama, S.; Tzou, S.; Ladensonet, P. Anatabine ameliorates experimental autoimmune thyroiditis. Endocrinology 2012, 153, 4580–4587. [Google Scholar] [CrossRef]
- Krassas, G.E.; Segni, M.; Wiersinga, W.M. Childhood Graves’ ophthalmopathy: Results of a European questionnaire study. Eur. J. Endocrinol. 2005, 153, 515–521. [Google Scholar] [CrossRef]
- Ajjan, R.A.; Weetman, A.P. The Pathogenesis of Hashimoto’s Thyroiditis: Further Developments in our Understanding. Horm. Metab. Res. 2015, 47, 702–710. [Google Scholar] [CrossRef]
- Cepon, T.J.; Snodgrass, J.J.; Leonard, W.R.; Tarskaia, L.A.; Klimova, T.M.; Fedorova, V.I.; Baltakhinova, M.E.; Krivoshapkin, V.G. Circumpolar adaptation, social change, and the development of autoimmune thyroid disorders among the Yakut (Sakha) of Siberia. Am. J. Hum. Biol. 2011, 23, 703–709. [Google Scholar] [CrossRef]
- Mikosch, P.; Aistleitner, A.; Oehrlein, M.; Trifina-Mikosch, E. Hashimoto’s thyroiditis and coexisting disorders in correlation with HLA status-an overview. Wien. Med. Wochenschr. 2023, 173, 41–53. [Google Scholar] [CrossRef]
- Zaletel, K.; Gaberscek, S. Hashimoto’s thyroiditis: From genes to the disease. Curr. Genom. 2011, 12, 576–588. [Google Scholar] [CrossRef]
- Mikoś, H.; Mikoś, M.; Obara-Moszyńska, M.; Niedziela, M. The role of the immune system and cytokines involved in the pathogenesis of autoimmune thyroid disease (AITD). Endokrynol. Pol. 2014, 65, 150–155. [Google Scholar] [CrossRef]
- Lacka, K.; Paradowska-Gorycka, A.; Maciejewski, A.; Kramer, L.; Herman, W.A.; Lacki, J.K. Interleukin 1 beta (IL1beta) gene polymorphisms (SNP-511 and SNP+3953) in Hashimoto’s thyroiditis among the Polish population. Exp. Clin. Endocrinol. Diabetes 2014, 122, 544–547. [Google Scholar] [CrossRef]
- Wang, S.H.; Baker, J.R. The role of apoptosis in thyroid autoimmunity. Thyroid 2007, 17, 975–979. [Google Scholar] [CrossRef] [PubMed]
- Berger, A. Th1 and Th2 responses: What are they? BMJ 2000, 321, 424. [Google Scholar] [CrossRef] [PubMed]
- de Brito, B.B.; da Silva, F.A.F.; Soares, A.S.; Pereira, V.A.; Santos, M.L.C.; Sampaio, M.M.; Neves, P.H.M.; de Melo, F.F. Pathogenesis and clinical management of Helicobacter pylori gastric infection. World J. Gastroenterol. 2019, 25, 5578–5589. [Google Scholar] [CrossRef]
- Zhou, A.; Hyppönen, E. Vitamin D deficiency and C-reactive protein: A bidirectional Mendelian randomization study. Int. J. Epidemiol. 2023, 52, 260–271. [Google Scholar] [CrossRef]
- Gombart, A.F.; Pierre, A.; Maggini, S. A Review of Micronutrients and the Immune System-Working in Harmony to Reduce the Risk of Infection. Nutrients 2020, 12, 236. [Google Scholar] [CrossRef]
- Qiu, F.; Liang, C.L.; Liu, H.; Zeng, Y.; Hou, S.; Huang, S.; Lai, X.; Dai, Z. Impacts of cigarette smoking on immune responsiveness: Up and down or upside down? Oncotarget 2017, 8, 268–284. [Google Scholar] [CrossRef]
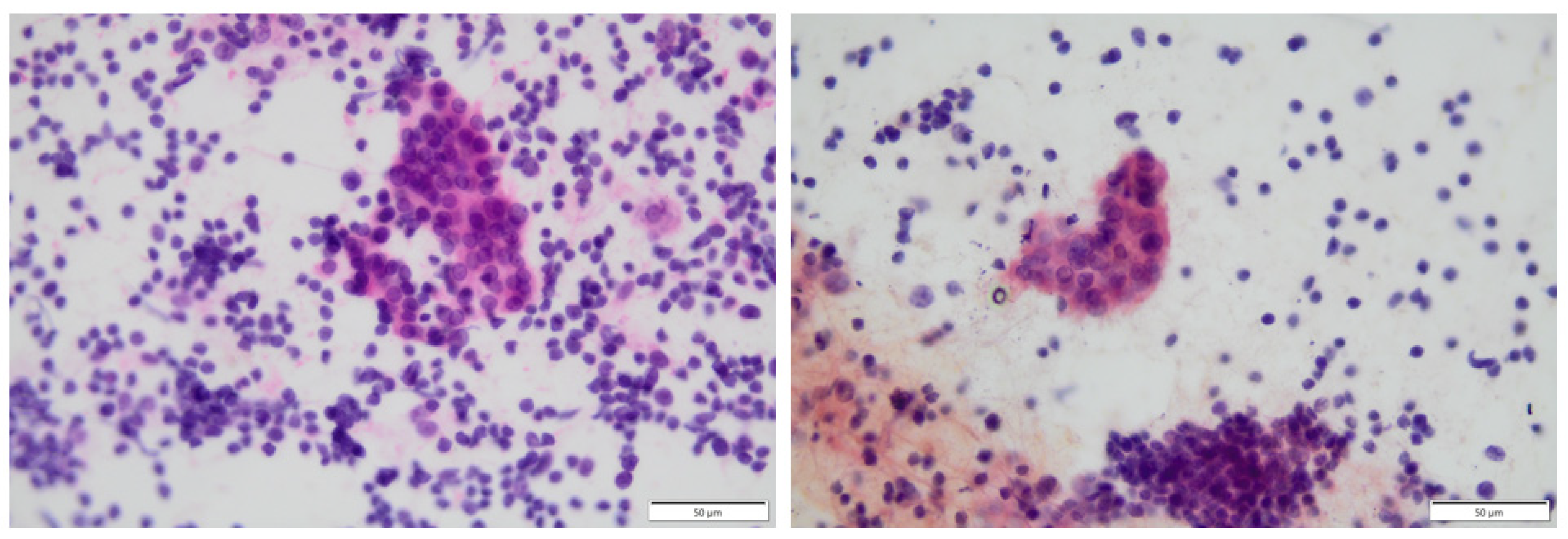
| First Author | Patient Population (Nationality) | Total Patients | Control Group | Iodine Level | p | Iodine Excess Induces AIT |
|---|---|---|---|---|---|---|
| Lee et al. [20] | South Korean | 439 | No control group | UIC: 606.2 µg/L | 0.021 | -(hypothyroidism) |
| Yokomichi et al. [28] | Japanese | 100,286 | No control group | - | - | -(hypothyroidism) |
| Kasiyan et al. [24] | Ukrainian | 168 | 68 | - | - | + |
| Yu et al. [22] | Chinese | 1159 | 182 | MUI: 233.20 µg/L | <0.05 | + |
| Hongyan et al. [26] | Chinese | 160 | 60 | MUI: iodine excess group: 212.69 µg/L iodine over-dose group: 302.51 µg/L | - | + |
| Palaniappan et al. [27] | Indian | 86 | 43 | UIE: 329.53 µg/L | <0.001 | + |
| Novak et al. [25] | Slovenian | 2889 | 1399 | - | - | -(hypothyroidism) |
| First Author | Patient Population (Nationality) | Total Patients | Control Group | Mean Vitamin D Level in AIT Patients | p | Vitamin D Deficiency Induces AIT |
|---|---|---|---|---|---|---|
| Maciejewski et al. [34] | Polish | 94 | 32 | 20.09 nmol/L | 0.014 | + |
| Siddiq et al. [31] | Pakistani | 144 | 72 | 21.89 nmol/L | 0.001 | + |
| Chao et al. [33] | Chinese | 5230 | 4889 | 15.81 ng/mL | 0.014 | + |
| Gašić et al. [35] | Serbian | 156 | 48 | 20.23 ng/mL | <0.001 | + |
| Ke et al. [47] | Chinese | 175 | 63 | 45.77 nmol/L | <0.001 | + |
| Rola et al. [41] | Polish | 98 | 42 | 18.3 ng/mL | - | - |
| Anaraki et al. [42] | Iranian | 65 | 32 | - | - | - |
| Sönmezgöz et al. [39] | Turkish | 136 | 68 | 16.85 ng/mL | <0.001 | + |
| Xu et al. [36] | Chinese | 394 | 200 | 40.4 nmol/L | <0.001 | + |
| Kim et al. [37] | South Korean | 776 | 407 | 92.1 nmol/L | >0.05 | + |
| Ma et al. [48] | Chinese | 210 | 70 | 31.00 nmol/L | <0.001 | + |
| Yavuzer et al. [43] | Turkish | 83 | 34 | 19.5 ng/mL | 0.27 | - |
| Gierach et al. [38] | Polish | 370 | 125 | 27.01 ng/mL | <0.001 | + |
| Cvek et al. [44] | Croatian | 637 | 176 | 17.1 ng/mL | 0.277 | - |
| Evliyaoğlu et al. [40] | Turkish | 169 | 79 | 16.67 ng/mL | 0.001 | + |
| Botelho et al. [45] | Brazilian | 159 | 71 | 26.4 ng/mL | 0.1917 | - |
| Filipova et al. [46] | Slovakian | 98 | 41 | 73 nmol/L a,b 55.7 nmol/L a,c | 0.8 0.9 | - |
| First Author | Patient Population (Nationality) | Total Patients | Control Group | Mean Se Level in AIT Patients | p | Selenium Deficiency Induces AIT |
|---|---|---|---|---|---|---|
| Wu et al. [51] | Chinese | 1254 | Unknown number d | <80 μg/L | - | + |
| Cinemre et al. [52] | Turkish | 82 | 42 | 148.90 ± 32.30 μg/L | 0.002 | + |
| Rostami et al. [53] | Iranian | 99 | 50 | 0.87 ± 0.29 µmol/L | <0.001 | + |
| Hu et al. [54] | Chinese | 90 | 47 | 73.3 μg/L | 0.493 | - f |
| Wang et al. [55] | Chinese | 89 | No control group | 72.93 ± 43.242 ng/mL | <0.05 e | - f |
| Pirola et al. [56] | Italian | 192 | 96 | - | - | - f |
| Manevska et al. [57] | North Macedonian | 500 | No control group | - | - | - f |
| Szeliga et al. [50] | Polish | 53 | 36 | 56.67 μg/L | p > 0.05 | - |
| First Author | Patient Population (Nationality) | Total Patients | Control Group | Viral Infections Induce AIT | Type of Virus |
|---|---|---|---|---|---|
| Al-Rammahi & Al-Khilkhali [60] | Iraqi | 120 | 60 | + | EBV |
| Assaad et al. [61] | Egyptian | 120 | 60 | + | EBV |
| Heidari & Jami [62] | Iranian | 1132 | 480 | + | PVB19 |
| Seyyedi et al. [63] | Iranian | 242 | 32 | + | HHV-6A |
| Tesch et al. [59] | German | 2,201,764 | 1,560,357 | + | SARS-CoV-2 |
| Weider et al. [68] | Norwegian | 53 | 18 | + | HHV-6A, Enteroviruses BPV19 |
| Environmental Factors Associated with Autoimmune Thyroiditis | |||
|---|---|---|---|
| Iodine excess | |||
| Vitamin D deficiency | |||
| Selenium deficiency | |||
| Viral infections | |||
| EBV 13 | PVB19 14 | HHV-6A 15 | SARS-CoV-2 16 |
| Bacterial infections | |||
| Helicobacter pylori | |||
| Microbiome disruption | |||
| Medications | |||
| Interferon-alpha | Amiodarone | Lithium | Tyrosine kinase inhibitors |
| Stress | |||
| Smoking | |||
| Climate | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cyna, W.; Wojciechowska, A.; Szybiak-Skora, W.; Lacka, K. The Impact of Environmental Factors on the Development of Autoimmune Thyroiditis—Review. Biomedicines 2024, 12, 1788. https://doi.org/10.3390/biomedicines12081788
Cyna W, Wojciechowska A, Szybiak-Skora W, Lacka K. The Impact of Environmental Factors on the Development of Autoimmune Thyroiditis—Review. Biomedicines. 2024; 12(8):1788. https://doi.org/10.3390/biomedicines12081788
Chicago/Turabian StyleCyna, Wojciech, Aleksandra Wojciechowska, Weronika Szybiak-Skora, and Katarzyna Lacka. 2024. "The Impact of Environmental Factors on the Development of Autoimmune Thyroiditis—Review" Biomedicines 12, no. 8: 1788. https://doi.org/10.3390/biomedicines12081788
APA StyleCyna, W., Wojciechowska, A., Szybiak-Skora, W., & Lacka, K. (2024). The Impact of Environmental Factors on the Development of Autoimmune Thyroiditis—Review. Biomedicines, 12(8), 1788. https://doi.org/10.3390/biomedicines12081788







