Development of Liver Fibrosis Represented by the Fibrosis-4 Index Is a Specific Risk Factor for Tubular Injury in Individuals with Type 2 Diabetes
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects and Ethics Statement
2.2. Biochemical Analyses
2.3. Classification of Participants Based on Urinary DKD Biomarkers
2.4. Statistical Analyses
3. Results
3.1. Clinical Characteristics of the Subjects
3.2. Associations of Urinary Biomarkers and Risk Factors across DKD Subtypes
4. Discussion
4.1. Liver Fibrosis Is Specifically Associated with Tubular Injury in Individuals with T2D
4.2. Role of Inflammation in Tubular Injury and Liver Fibrosis in T2D
4.3. Role of Free Fatty Acid Metabolism in Tubular Injury and Liver Fibrosis in T2D
4.4. Role of Advanced Glycation End Products (AGEs) in Tubular Injury and Liver Fibrosis in T2D
4.5. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Reidy, K.; Kang, H.M.; Hostetter, T.; Susztak, K. Molecular mechanisms of diabetic kidney disease. J. Clin. Investig. 2014, 124, 2333–2340. [Google Scholar] [CrossRef] [PubMed]
- Sagoo, M.K.; Gnudi, L. Diabetic Nephropathy: An Overview. Methods Mol. Biol. 2020, 2067, 3–7. [Google Scholar] [CrossRef] [PubMed]
- Retnakaran, R.; Cull, C.A.; Thorne, K.I.; Adler, A.I.; Holman, R.R. Risk factors for renal dysfunction in type 2 diabetes: U.K. Prospective Diabetes Study 74. Diabetes 2006, 55, 1832–1839. [Google Scholar] [CrossRef] [PubMed]
- Tervaert, T.W.; Mooyaart, A.L.; Amann, K.; Cohen, A.H.; Cook, H.T.; Drachenberg, C.B.; Ferrario, F.; Fogo, A.B.; Haas, M.; de Heer, E.; et al. Pathologic classification of diabetic nephropathy. J. Am. Soc. Nephrol. 2010, 21, 556–563. [Google Scholar] [CrossRef] [PubMed]
- Scilletta, S.; Di Marco, M.; Miano, N.; Filippello, A.; Di Mauro, S.; Scamporrino, A.; Musmeci, M.; Coppolino, G.; Di Giacomo Barbagallo, F.; Bosco, G.; et al. Update on Diabetic Kidney Disease (DKD): Focus on Non-Albuminuric DKD and Cardiovascular Risk. Biomolecules 2023, 13, 752. [Google Scholar] [CrossRef] [PubMed]
- Kamijo-Ikemori, A.; Sugaya, T.; Ichikawa, D.; Hoshino, S.; Matsui, K.; Yokoyama, T.; Yasuda, T.; Hirata, K.; Kimura, K. Urinary liver type fatty acid binding protein in diabetic nephropathy. Clin. Chim. Acta 2013, 424, 104–108. [Google Scholar] [CrossRef] [PubMed]
- Musso, G.; Cassader, M.; Cohney, S.; Pinach, S.; Saba, F.; Gambino, R. Emerging Liver-Kidney Interactions in Nonalcoholic Fatty Liver Disease. Trends Mol. Med. 2015, 21, 645–662. [Google Scholar] [CrossRef] [PubMed]
- Friedman, S.L.; Neuschwander-Tetri, B.A.; Rinella, M.; Sanyal, A.J. Mechanisms of NAFLD development and therapeutic strategies. Nat. Med. 2018, 24, 908–922. [Google Scholar] [CrossRef] [PubMed]
- Saito, H.; Tanabe, H.; Kudo, A.; Machii, N.; Higa, M.; Yamaguchi, S.; Maimaituxun, G.; Abe, K.; Takahashi, A.; Tanaka, K.; et al. High FIB4 index is an independent risk factor of diabetic kidney disease in type 2 diabetes. Sci. Rep. 2021, 11, 11753. [Google Scholar] [CrossRef]
- Li, X.; Bhattacharya, D.; Yuan, Y.; Wei, C.; Zhong, F.; Ding, F.; D’Agati, V.D.; Lee, K.; Friedman, S.L.; He, J.C. Chronic kidney disease in a murine model of non-alcoholic steatohepatitis (NASH). Kidney Int. 2024, 105, 540–561. [Google Scholar] [CrossRef]
- 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2019. Diabetes Care 2019, 42, S13–S28. [CrossRef]
- Imai, E.; Horio, M.; Nitta, K.; Yamagata, K.; Iseki, K.; Tsukamoto, Y.; Ito, S.; Makino, H.; Hishida, A.; Matsuo, S. Modification of the Modification of Diet in Renal Disease (MDRD) Study equation for Japan. Am. J. Kidney Dis. 2007, 50, 927–937. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.G.; Lydecker, A.; Murray, K.; Tetri, B.N.; Contos, M.J.; Sanyal, A.J. Comparison of noninvasive markers of fibrosis in patients with nonalcoholic fatty liver disease. Clin. Gastroenterol. Hepatol. 2009, 7, 1104–1112. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Kim, D.; Kim, H.J.; Lee, C.H.; Yang, J.I.; Kim, W.; Kim, Y.J.; Yoon, J.H.; Cho, S.H.; Sung, M.W.; et al. Hepatic steatosis index: A simple screening tool reflecting nonalcoholic fatty liver disease. Dig. Liver Dis. 2010, 42, 503–508. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, S.E.; Sugaya, T.; Hovind, P.; Baba, T.; Parving, H.H.; Rossing, P. Urinary liver-type fatty acid-binding protein predicts progression to nephropathy in type 1 diabetic patients. Diabetes Care 2010, 33, 1320–1324. [Google Scholar] [CrossRef] [PubMed]
- Targher, G.; Bertolini, L.; Rodella, S.; Zoppini, G.; Lippi, G.; Day, C.; Muggeo, M. Non-alcoholic fatty liver disease is independently associated with an increased prevalence of chronic kidney disease and proliferative/laser-treated retinopathy in type 2 diabetic patients. Diabetologia 2008, 51, 444–450. [Google Scholar] [CrossRef] [PubMed]
- Faul, F.; Erdfelder, E.; Buchner, A.; Lang, A.G. Statistical power analyses using G*Power 3.1: Tests for correlation and regression analyses. Behav. Res. Methods 2009, 41, 1149–1160. [Google Scholar] [CrossRef] [PubMed]
- Portillo-Sanchez, P.; Bril, F.; Maximos, M.; Lomonaco, R.; Biernacki, D.; Orsak, B.; Subbarayan, S.; Webb, A.; Hecht, J.; Cusi, K. High Prevalence of Nonalcoholic Fatty Liver Disease in Patients With Type 2 Diabetes Mellitus and Normal Plasma Aminotransferase Levels. J. Clin. Endocrinol. Metab. 2015, 100, 2231–2238. [Google Scholar] [CrossRef] [PubMed]
- Jia, G.; Di, F.; Wang, Q.; Shao, J.; Gao, L.; Wang, L.; Li, Q.; Li, N. Non-Alcoholic Fatty Liver Disease Is a Risk Factor for the Development of Diabetic Nephropathy in Patients with Type 2 Diabetes Mellitus. PLoS ONE 2015, 10, e0142808. [Google Scholar] [CrossRef]
- Targher, G.; Chonchol, M.; Bertolini, L.; Rodella, S.; Zenari, L.; Lippi, G.; Franchini, M.; Zoppini, G.; Muggeo, M. Increased risk of CKD among type 2 diabetics with nonalcoholic fatty liver disease. J. Am. Soc. Nephrol. 2008, 19, 1564–1570. [Google Scholar] [CrossRef]
- Truong, E.; Noureddin, M. The Interplay Between Nonalcoholic Fatty Liver Disease and Kidney Disease. Clin. Liver Dis. 2022, 26, 213–227. [Google Scholar] [CrossRef] [PubMed]
- Perdomo, C.M.; Frühbeck, G.; Escalada, J. Impact of Nutritional Changes on Nonalcoholic Fatty Liver Disease. Nutrients 2019, 11, 677. [Google Scholar] [CrossRef]
- Targher, G.; Chonchol, M.; Miele, L.; Zoppini, G.; Pichiri, I.; Muggeo, M. Nonalcoholic fatty liver disease as a contributor to hypercoagulation and thrombophilia in the metabolic syndrome. Semin. Thromb. Hemost. 2009, 35, 277–287. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Ge, X.; Li, X.; He, J.; Wei, X.; Du, J.; Sun, J.; Li, X.; Xun, Z.; Liu, W.; et al. High-fat diet promotes renal injury by inducing oxidative stress and mitochondrial dysfunction. Cell Death Dis. 2020, 11, 914. [Google Scholar] [CrossRef]
- Sikalidis, A.K.; Maykish, A. The Gut Microbiome and Type 2 Diabetes Mellitus: Discussing a Complex Relationship. Biomedicines 2020, 8, 8. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.P.; Zhang, J.X.; Li, X.Q.; Li, L.; Wu, Q.Y.; Liu, L.; Wang, G.H.; Ruan, X.Z.; Ma, K.L. Outer membrane vesicles derived from gut microbiota mediate tubulointerstitial inflammation: A potential new mechanism for diabetic kidney disease. Theranostics 2023, 13, 3988–4003. [Google Scholar] [CrossRef]
- Safari, Z.; Gérard, P. The links between the gut microbiome and non-alcoholic fatty liver disease (NAFLD). Cell Mol. Life Sci. 2019, 76, 1541–1558. [Google Scholar] [CrossRef] [PubMed]
- Selen, E.S.; Choi, J.; Wolfgang, M.J. Discordant hepatic fatty acid oxidation and triglyceride hydrolysis leads to liver disease. JCI Insight 2021, 6, e135626. [Google Scholar] [CrossRef]
- Francque, S.; Verrijken, A.; Caron, S.; Prawitt, J.; Paumelle, R.; Derudas, B.; Lefebvre, P.; Taskinen, M.R.; Van Hul, W.; Mertens, I.; et al. PPARalpha gene expression correlates with severity and histological treatment response in patients with non-alcoholic steatohepatitis. J. Hepatol. 2015, 63, 164–173. [Google Scholar] [CrossRef]
- Chung, K.W.; Lee, E.K.; Lee, M.K.; Oh, G.T.; Yu, B.P.; Chung, H.Y. Impairment of PPARalpha and the Fatty Acid Oxidation Pathway Aggravates Renal Fibrosis during Aging. J. Am. Soc. Nephrol. 2018, 29, 1223–1237. [Google Scholar] [CrossRef]
- Kang, H.M.; Ahn, S.H.; Choi, P.; Ko, Y.A.; Han, S.H.; Chinga, F.; Park, A.S.; Tao, J.; Sharma, K.; Pullman, J.; et al. Defective fatty acid oxidation in renal tubular epithelial cells has a key role in kidney fibrosis development. Nat. Med. 2015, 21, 37–46. [Google Scholar] [CrossRef]
- Fernando, D.H.; Forbes, J.M.; Angus, P.W.; Herath, C.B. Development and Progression of Non-Alcoholic Fatty Liver Disease: The Role of Advanced Glycation End Products. Int. J. Mol. Sci. 2019, 20, 5037. [Google Scholar] [CrossRef] [PubMed]
- Leung, C.; Herath, C.B.; Jia, Z.; Goodwin, M.; Mak, K.Y.; Watt, M.J.; Forbes, J.M.; Angus, P.W. Dietary glycotoxins exacerbate progression of experimental fatty liver disease. J. Hepatol. 2014, 60, 832–838. [Google Scholar] [CrossRef] [PubMed]
- Rabbani, N.; Thornalley, P.J. Advanced glycation end products in the pathogenesis of chronic kidney disease. Kidney Int. 2018, 93, 803–813. [Google Scholar] [CrossRef]
- Semba, R.D.; Nicklett, E.J.; Ferrucci, L. Does accumulation of advanced glycation end products contribute to the aging phenotype? J. Gerontol. A Biol. Sci. Med. Sci. 2010, 65, 963–975. [Google Scholar] [CrossRef] [PubMed]
- Miyata, T.; Ueda, Y.; Horie, K.; Nangaku, M.; Tanaka, S.; van Ypersele de Strihou, C.; Kurokawa, K. Renal catabolism of advanced glycation end products: The fate of pentosidine. Kidney Int. 1998, 53, 416–422. [Google Scholar] [CrossRef]
- Waanders, F.; Greven, W.L.; Baynes, J.W.; Thorpe, S.R.; Kramer, A.B.; Nagai, R.; Sakata, N.; van Goor, H.; Navis, G. Renal accumulation of pentosidine in non-diabetic proteinuria-induced renal damage in rats. Nephrol. Dial. Transplant. 2005, 20, 2060–2070. [Google Scholar] [CrossRef] [PubMed]
- Genuth, S.; Sun, W.; Cleary, P.; Sell, D.R.; Dahms, W.; Malone, J.; Sivitz, W.; Monnier, V.M. Glycation and carboxymethyllysine levels in skin collagen predict the risk of future 10-year progression of diabetic retinopathy and nephropathy in the diabetes control and complications trial and epidemiology of diabetes interventions and complications participants with type 1 diabetes. Diabetes 2005, 54, 3103–3111. [Google Scholar] [CrossRef]
- Yamagishi, S.; Inagaki, Y.; Okamoto, T.; Amano, S.; Koga, K.; Takeuchi, M. Advanced glycation end products inhibit de novo protein synthesis and induce TGF-beta overexpression in proximal tubular cells. Kidney Int. 2003, 63, 464–473. [Google Scholar] [CrossRef]
- Yamagishi, S.; Inagaki, Y.; Okamoto, T.; Amano, S.; Koga, K.; Takeuchi, M.; Makita, Z. Advanced glycation end product-induced apoptosis and overexpression of vascular endothelial growth factor and monocyte chemoattractant protein-1 in human-cultured mesangial cells. J. Biol. Chem. 2002, 277, 20309–20315. [Google Scholar] [CrossRef]
- Yamagami, H.; Hara, T.; Yasui, S.; Hosoki, M.; Hori, T.; Kaneko, Y.; Mitsui, Y.; Kurahashi, K.; Harada, T.; Yoshida, S.; et al. Cross-Sectional and Longitudinal Associations between Skin Autofluorescence and Tubular Injury Defined by Urinary Excretion of Liver-Type Fatty Acid-Binding Protein in People with Type 2 Diabetes. Biomedicines 2023, 11, 3020. [Google Scholar] [CrossRef] [PubMed]
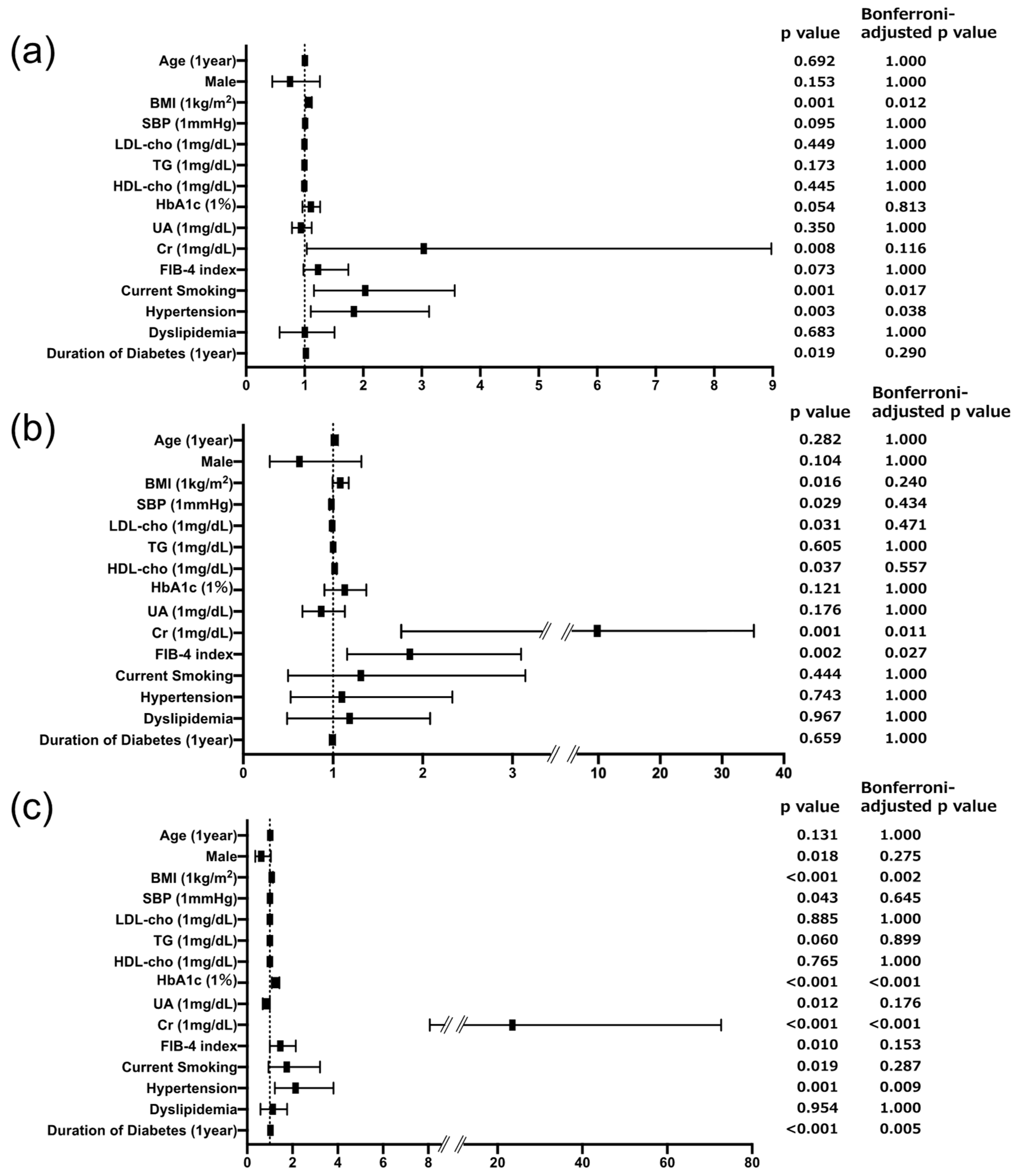
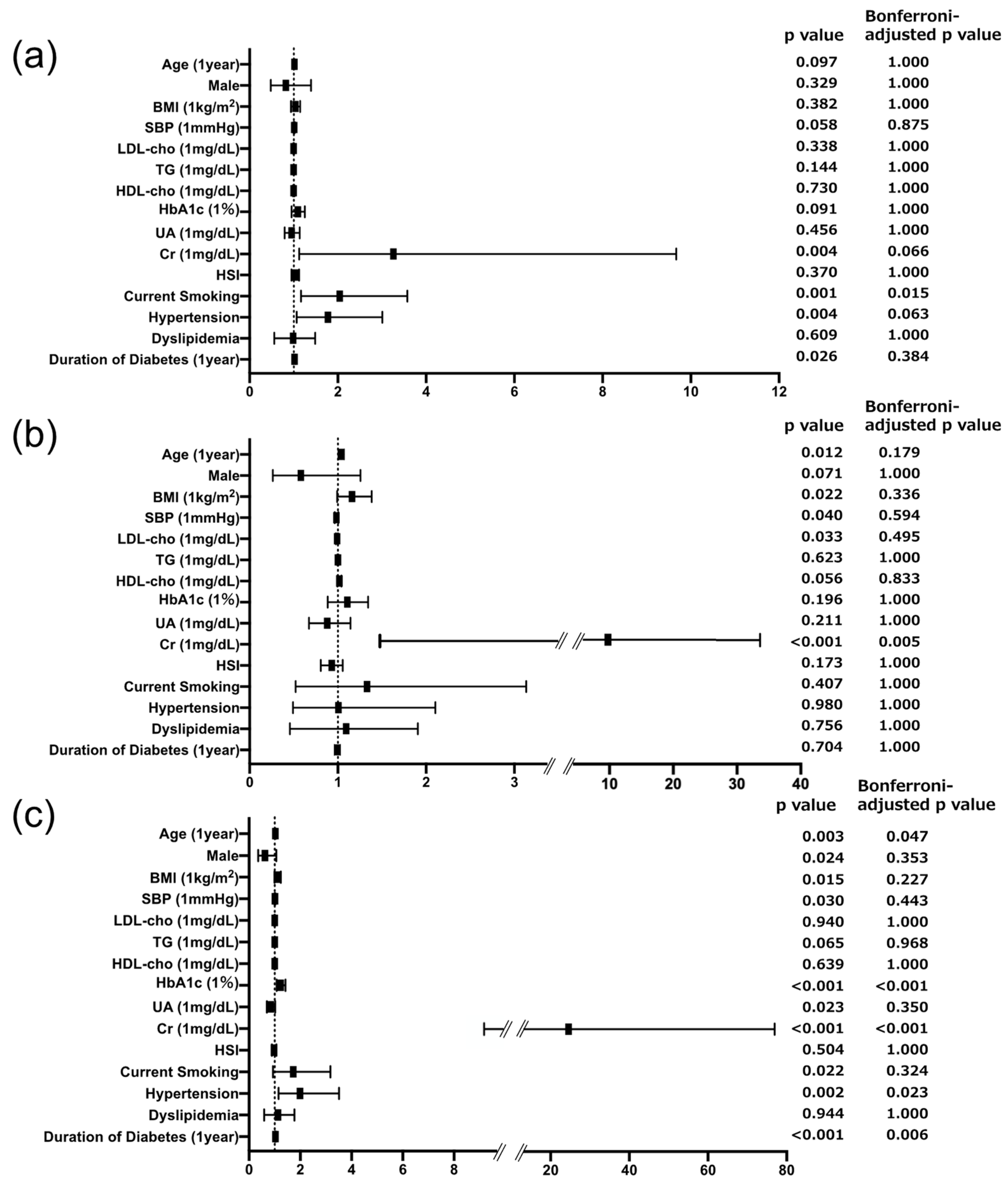
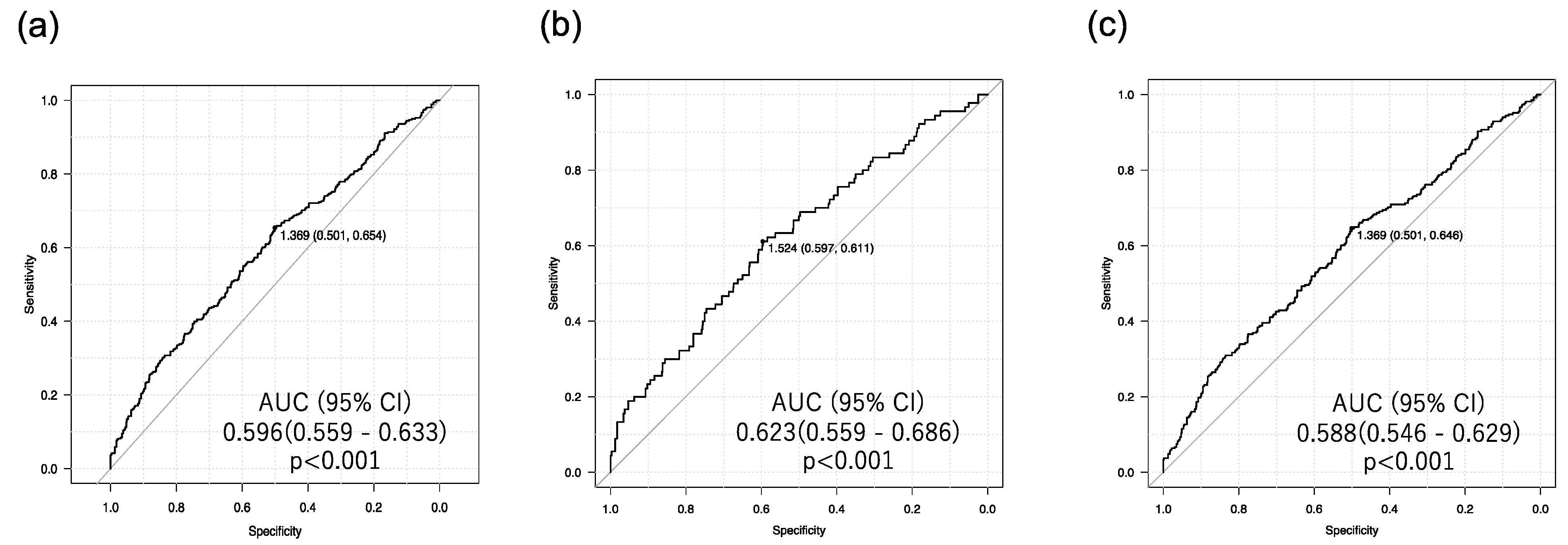
| Group (n) | N (n = 637) | G (n = 248) | T (n = 90) | D (n = 268) | p Value |
|---|---|---|---|---|---|
| Age (years) | 68.0 (58.4, 74.0) | 70.0 (60.0, 76.0) | 72.0 (62.2, 78.0) * | 73.0 (62.0, 78.5) * | <0.001 |
| Male (n, (%)) | 349 (54.8) | 142 (57.3) | 49 (54.4) | 159 (59.3) | 0.614 |
| BMI (kg/m2) | 24.5 (21.9, 27.3) | 25.0 (22.8, 28.0) | 23.7 (22.4, 26.7) | 25.2 (22.4, 28.2) * | 0.005 |
| SBP (mmHg) | 130 ± 16 | 135 ± 18 * | 127 ± 17 | 136 ± 17 *‡ | <0.001 |
| LDL-cho (mg/dL) | 99 (82, 120) | 97 (76, 116) | 88 (74, 106) | 93 (77, 115) | 0.003 |
| TG (mg/dL) | 110 (78, 159) | 122(84, 161) | 110 (81, 157) | 132 (85, 197) * | 0.009 |
| HDL-cho (mg/dL) | 53 (45, 63) | 52 (44, 63) | 55 (47, 65) | 53 (43, 62) | 0.140 |
| Casual PG (mg/dL) | 131 (112, 165) | 137 (118, 175) | 140 (111, 186) | 147 (121, 193) *† | <0.001 |
| HbA1c (%) | 6.8 (6.3, 7.4) | 6.9 (6.5, 7.4) | 6.8 (6.4, 7.3) | 7.1 (6.6, 8.0) *†‡ | <0.001 |
| UA (mg/dL) | 5.0 (4.1, 5.9) | 5.0 (4.2, 5.8) | 4.8 (4.1, 5.8) | 5.2 (4.3, 5.9) | 0.309 |
| Cr (mg/dL) | 0.73 (0.60, 0.87) | 0.75 (0.61, 0.93) | 0.80 (0.59, 1.09) | 0.86 (0.69, 1.15) *† | <0.001 |
| eGFR (ml/min/1.73 m2) | 74.8 ± 18.0 | 72.4 ± 21.5 | 70.1 ± 26.4 | 61.6 ± 22.8 *† | <0.001 |
| Albumin (g/dL) | 4.2 ± 0.4 | 4.2 ± 0.4 | 4.1 ± 0.4 | 4.2 ± 0.5 | 0.069 |
| AST (U/L) | 20 (17, 25) | 21 (18, 28) | 22 (18, 28) | 22 (18, 29) * | 0.009 |
| ALT (U/L) | 21 (15, 29) | 21 (16, 30) | 19 (14, 31) | 20 (14, 33) | 0.568 |
| Platelets (109/L) | 216 (189, 256) | 222 (188, 261) | 208 (179, 260) | 220 (182, 266) | 0.671 |
| uACR (mg/gCr) | 9.8 (6.0, 15.6) | 58.8 (41.3, 123.3) *‡ | 14.7 (8.6, 20.3) | 209.5 (78.0, 550.5) *†‡ | <0.001 |
| uL-FABPCR (μg/gCr) | 2.0 (1.3, 3.0) | 2.6 (1.9, 3.5) * | 6.7 (5.5, 9.0) *† | 13.1 (7.6, 21.7) *† | <0.001 |
| FIB-4 index | 1.4 (1.0, 1.9) | 1.5 (1.1, 2.0) | 1.7 (1.3, 2.3) * | 1.6 (1.1, 2.2) | <0.001 |
| HSI | 35.6 (32.1, 39.3) | 35.8 (32.6, 41.0) | 34.5 (31.1, 38.4) | 36.1 (32.0, 40.9) * | 0.048 |
| Current Smoking (n, (%)) | 101 (15.9) | 54 (21.8) | 14 (15.6) | 51 (19.0) | 0.181 |
| Hypertension (n, (%)) | 383 (60.1) | 194 (78.2) | 59 (65.6) | 225 (84.0) | <0.001 |
| Dyslipidemia (n, (%)) | 459 (72.1) | 184 (74.2) | 63 (70.0) | 215 (80.2) | 0.053 |
| Duration of T2D (years) | 9 (3, 15) | 11 (5, 18) * | 11 (5, 17) | 13 (7, 22) * | <0.001 |
| ARB or ACEi (n, (%)) | 222 (34.9) | 136 (54.8) * | 43 (47.8) | 140 (52.2) * | <0.001 |
| CCB (n, (%)) | 193 (30.3) | 121 (48.8) * | 35 (38.9) | 165 (61.6) *†‡ | <0.001 |
| β blocker (n, (%)) | 46 (7.2) | 16 (6.5) | 6 (6.7) | 24 (9.0) | 0.730 |
| MR antagonist (n, (%)) | 15 (2.4) | 9 (3.6) | 1 (1.1) | 6 (2.2) | 0.620 |
| Statin (n, (%)) | 305 (47.9) | 135 (54.4) | 46 (51.1) | 133 (49.6) | 0.595 |
| Ezetimibe (n, (%)) | 51 (8.0) | 14 (5.6) | 6 (6.7) | 33 (12.3) | 0.050 |
| Other Hypolipidemics (n, (%)) | 16 (2.5) | 13 (5.2) | 4 (4.4) | 15 (5.6) | 0.061 |
| Antiplatelet (n, (%)) | 67 (10.5) | 33 (13.3) * | 8 (8.9) | 48 (17.9) * | 0.017 |
| SU or Glinide (n, (%)) | 88 (13.8) | 49 (19.8) | 22 (24.4) | 77 (28.7) *† | <0.001 |
| Metformin (n, (%)) | 313 (49.1) | 133 (53.6) | 40 (44.4) | 123 (45.9) | 0.273 |
| Imeglimin (n, (%)) | 17 (2.7) | 5 (2.0) | 1 (1.1) | 8 (3.0) | 0.833 |
| DPP-4i (n, (%)) | 331 (52.0) | 137 (55.2) | 58 (64.4) | 132 (49.3) | 0.071 |
| SGLT2i (n, (%)) | 215 (33.8) | 106 (42.7) | 34 (37.8) | 137 (51.1) *†‡ | <0.001 |
| αGI (n, (%)) | 79 (12.4) | 43 (17.3) | 19 (21.1) | 41 (15.3) | 0.065 |
| Pioglitazone (n, (%)) | 17 (2.7) | 9 (3.6) | 3 (3.3) | 17 (6.3) | 0.075 |
| Insulin (n, (%)) | 139 (21.8) | 57 (23.0) | 21 (23.3) | 76 (28.4) | 0.214 |
| GLP-1RA (n, (%)) | 101 (15.9) | 58 (23.4) * | 19 (21.1) | 75 (28.0) * | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hara, T.; Watanabe, T.; Yamagami, H.; Miyataka, K.; Yasui, S.; Asai, T.; Kaneko, Y.; Mitsui, Y.; Masuda, S.; Kurahashi, K.; et al. Development of Liver Fibrosis Represented by the Fibrosis-4 Index Is a Specific Risk Factor for Tubular Injury in Individuals with Type 2 Diabetes. Biomedicines 2024, 12, 1789. https://doi.org/10.3390/biomedicines12081789
Hara T, Watanabe T, Yamagami H, Miyataka K, Yasui S, Asai T, Kaneko Y, Mitsui Y, Masuda S, Kurahashi K, et al. Development of Liver Fibrosis Represented by the Fibrosis-4 Index Is a Specific Risk Factor for Tubular Injury in Individuals with Type 2 Diabetes. Biomedicines. 2024; 12(8):1789. https://doi.org/10.3390/biomedicines12081789
Chicago/Turabian StyleHara, Tomoyo, Takeshi Watanabe, Hiroki Yamagami, Kohsuke Miyataka, Saya Yasui, Takahito Asai, Yousuke Kaneko, Yukari Mitsui, Shiho Masuda, Kiyoe Kurahashi, and et al. 2024. "Development of Liver Fibrosis Represented by the Fibrosis-4 Index Is a Specific Risk Factor for Tubular Injury in Individuals with Type 2 Diabetes" Biomedicines 12, no. 8: 1789. https://doi.org/10.3390/biomedicines12081789
APA StyleHara, T., Watanabe, T., Yamagami, H., Miyataka, K., Yasui, S., Asai, T., Kaneko, Y., Mitsui, Y., Masuda, S., Kurahashi, K., Otoda, T., Yuasa, T., Kuroda, A., Endo, I., Honda, S., Kondo, A., Matsuhisa, M., & Aihara, K.-i. (2024). Development of Liver Fibrosis Represented by the Fibrosis-4 Index Is a Specific Risk Factor for Tubular Injury in Individuals with Type 2 Diabetes. Biomedicines, 12(8), 1789. https://doi.org/10.3390/biomedicines12081789








