Phytotherapy in Alzheimer’s Disease—A Narrative Review
Abstract
1. Introduction
2. Materials and Methods
3. Pathomechanism of Alzheimer’s Disease
4. Herbs
4.1. Curcuma longa L.
4.2. Panax ginseng
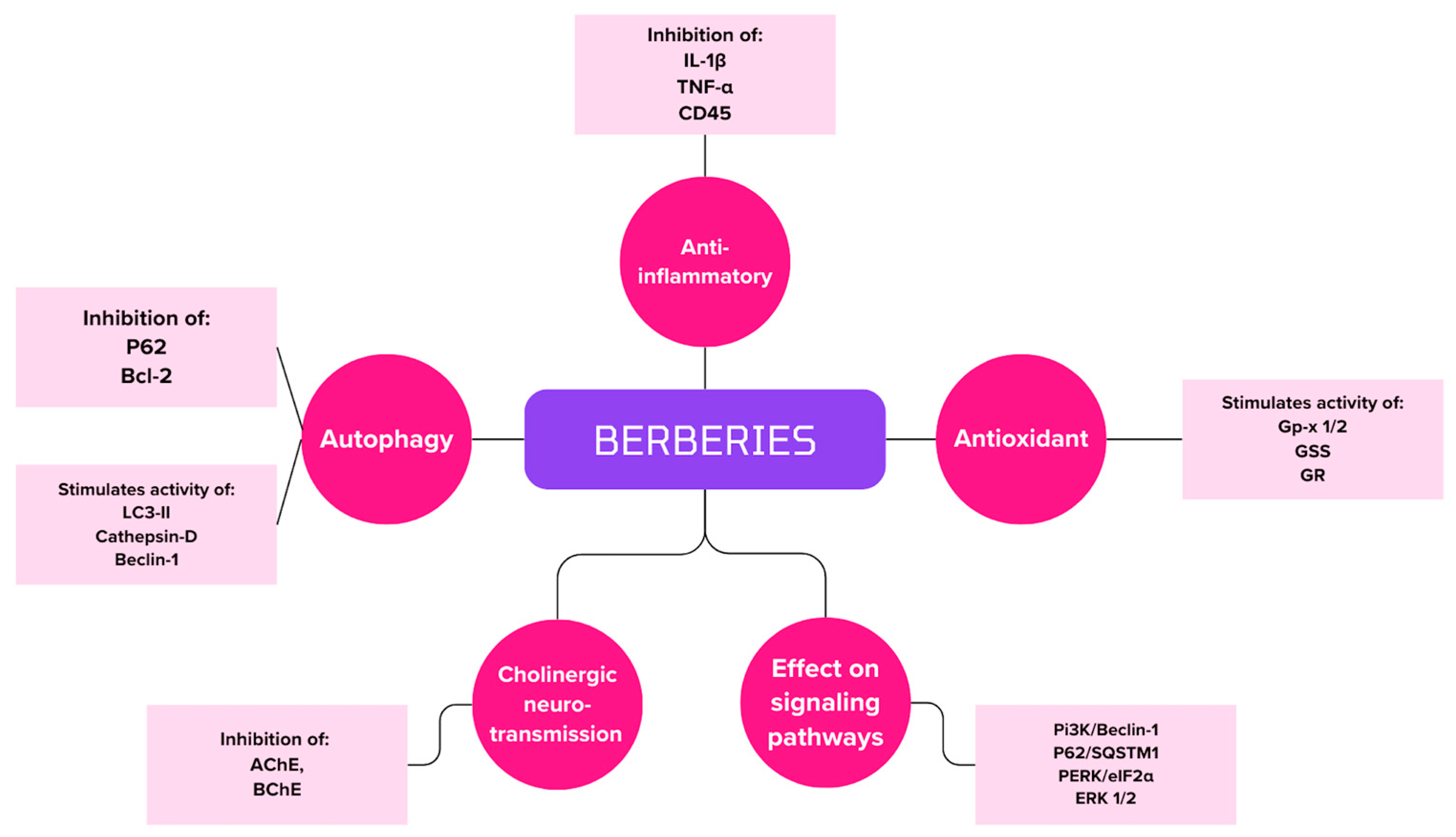
4.3. Berberis L.
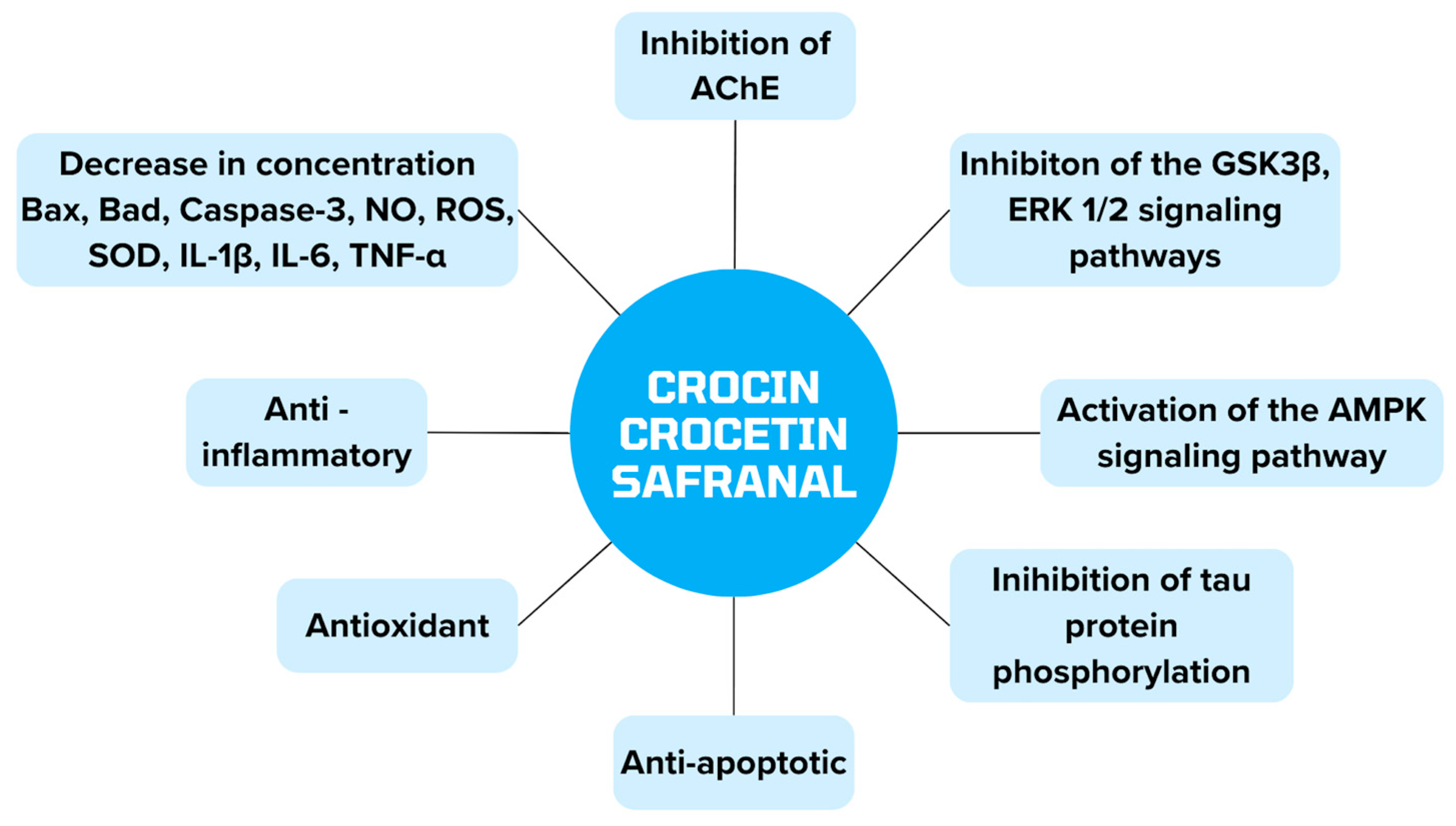
4.4. Crocus sativus
4.5. Herbal Summary
5. Alzheimer’s Disease Therapy
6. Alternative Therapies for Alzheimer’s Disease
6.1. Curcuma longa
6.2. Panax ginseng
6.3. Berberis
6.4. Crocus sativus
7. Summary
8. Discussion
9. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| Abbreviation | Meaning of the Abbreviation |
| AD | Alzheimer’s disease |
| WHO | World Health Organization |
| NMDA | N-methyl-D-aspartate |
| Aβ | Beta-amyloid |
| APP | Amyloid precursor protein |
| PSEN 1 | Presenilin 1 |
| PSEN 2 | Presenilin 2 |
| MHC-II | Major histocompatibility complex II |
| iNOS | Inducible nitric oxide |
| TNF | Tumor necrosis factor |
| NFTs | Neurofibrillary tangles |
| BBB | Blood–brain barriers |
| ROS | Reactive oxygen species |
| mtDNA | Mitochondrial DNA |
| PGC-1α | Peroxisome proliferator-activated receptor-γ coactivator 1 α |
| APOE | Apolipoprotein E |
| LRP 1 | Lipoprotein receptor-related Protein 1 |
| MAPK | Mitogen-activated protein kinase |
| FDA | Food and Drug Administration |
| JECFA | FAO/WHO Joint Expert Committee on Food Additives |
| EFSA | European Food Safety Authority |
| TGS | Ginsenosides |
| PPD | Protopanaxadiol type |
| PPT | Protopanaxatriol type |
| Nrf2 | Nuclear erythroid factor 2 |
| PPAR γ | Peroxisome proliferator-activated receptor γ |
| NO | Nitric oxide |
| PS-1 | Presenilin-1 |
| IGF-1 AChEI | Insulin-like growth factor Acetylcholinesterase inhibitor |
| COX-2 | Cyclooxygenase-2 |
| NGF | Nerve growth factor |
| BDNF | Brain-derived neurotrophic factor |
| PI3K | Phosphatidylinositol 3-kinase |
| Akt | Protein kinase B |
| GSK-3β | Glycogen synthase kinase-3β |
| IAPP | Islet amyloid polypeptide |
| MMSE | Mini-Mental State Examination |
| STZ | Streptozotocin |
| CN | Curcumin nanoparticles |
| AChE | Acetylcholinesterase |
| ACh | Acetylcholine |
| CUR | Curcumin |
| GBE | Ginkgo biloba |
| HMM | Heavy metal |
| SCO + HMM | Model control group |
| MEM | Memantine |
| CMC | Carboxymethylcellulose |
| OMM | outer mitochondrial membrane |
| PINK1-PARKIN | Serine/threonine protein kinase1-E3 ubiquitin ligase |
| GDNF | Glial cell-derived neurotrophic factor |
| BACE-1 | β-amyloid cleaving enzyme 1 |
| ASK1-JNK | Apoptosis signal-regulating kinase 1/c-Jun N-terminal kinases |
| ChAT | Choline acetyltransferase |
| VAChT | Vesicular acetylcholine transporter |
| mTOR | Serine-threonine protein kinase |
| ULK1 | Unc-51-like kinase 1 |
| AβO | β-amyloid oligomer |
| ADAS-cog | Alzheimer’s Disease Assessment Scale—Cognitive Subscale |
| ERK 1/2 | Extracellular signal-regulated kinases 1/2 |
| EGF | Epidermal growth factor |
| PERK | Protein kinase RNA-like endoplasmic reticulum kinase |
| eIF2α | Eukaryotic translation initiation factor-2α |
| BChE | Butyrylcholinesterase |
| MAO | Monoamine oxidase |
| NTPDase | Ectonucleoside triphosphate |
| Asc | Vitamin C |
| %AC | Antioxidant capacity |
| PP2A | Protein phosphatase 2 |
| P62/SQSTM1 | Protein sequestosome 1 |
| GSS | Glutathione synthetase |
| GPx-1/2 | Glutathione peroxidase |
| GR | Glutathione reductase |
| GFAP | Glial fibrillary acidic protein |
| OD | Relative density |
| LC3-II | Microtubule-associated protein light chain 3-II |
| GSH | Glutathione |
| SOD | Superoxide dismutase |
| STK11/LKB1 | Serine/threonine kinase 11 |
| AMPK | AMP-activated protein kinase |
| CDR | Clinical Dementia Rating Scale |
| H&E | Hematoxylin and eosin |
| SCIRS | Severe Cognitive Impairment Rating Scale |
| SD | Mean |
References
- Scheltens, P.; De Strooper, B.; Kivipelto, M.; Holstege, H.; Chételat, G.; Teunissen, C.E.; Cummings, J.; van der Flier, W.M. Alzheimer’s disease. Lancet 2021, 397, 1577–1590. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-X.; Tian, Y.; Wang, Z.-T.; Ma, Y.-H.; Tan, L.; Yu, J.-T. The Epidemiology of Alzheimer’s Disease Modifiable Risk Factors and Prevention. J. Prev. Alzheimer’s Dis. 2021, 8, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Graff-Radford, J.; Yong, K.X.; Apostolova, L.G.; Bouwman, F.H.; Carrillo, M.; Dickerson, B.C.; Rabinovici, G.D.; Schott, J.M.; Jones, D.T.; Murray, M.E. New insights into atypical Alzheimer’s disease in the era of biomarkers. Lancet Neurol. 2021, 20, 222–234. [Google Scholar] [CrossRef] [PubMed]
- Mani, S.; Dubey, R.; Lai, I.-C.; Babu, M.A.; Tyagi, S.; Swargiary, G.; Mody, D.; Singh, M.; Agarwal, S.; Iqbal, D.; et al. Oxidative Stress and Natural Antioxidants: Back and Forth in the Neurological Mechanisms of Alzheimer’s Disease. J. Alzheimer’s Dis. 2023, 96, 877–912. [Google Scholar] [CrossRef] [PubMed]
- Andrade-Guerrero, J.; Santiago-Balmaseda, A.; Jeronimo-Aguilar, P.; Vargas-Rodríguez, I.; Cadena-Suárez, A.R.; Sánchez-Garibay, C.; Pozo-Molina, G.; Méndez-Catalá, C.F.; Cardenas-Aguayo, M.-D.; Diaz-Cintra, S.; et al. Alzheimer’s Disease: An Updated Overview of Its Genetics. Int. J. Mol. Sci. 2023, 24, 3754. [Google Scholar] [CrossRef] [PubMed]
- Alzheimer’s Association. 2023 Alzheimer’s Disease Facts and Figures. Alzheimer’s Dement. J. Alzheimer’s Assoc. 2023, 19, 1598–1695. [Google Scholar] [CrossRef] [PubMed]
- Ossenkoppele, R.; van der Kant, R.; Hansson, O. Tau biomarkers in Alzheimer’s disease: Towards implementation in clinical practice and trials. Lancet Neurol. 2022, 21, 726–734. [Google Scholar] [CrossRef] [PubMed]
- Twarowski, B.; Herbet, M. Inflammatory Processes in Alzheimer’s Disease—Pathomechanism, Diagnosis and Treatment: A Review. Int. J. Mol. Sci. 2023, 24, 6518. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Gupta, V.; Sharma, S. Donepezil. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. Available online: http://www.ncbi.nlm.nih.gov/books/NBK513257/ (accessed on 15 May 2024).
- Kalola, U.K.; Patel, P.; Nguyen, H. Galantamine. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. Available online: http://www.ncbi.nlm.nih.gov/books/NBK574546/ (accessed on 15 May 2024).
- Kuns, B.; Rosani, A.; Patel, P.; Varghese, D. Memantine. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. Available online: http://www.ncbi.nlm.nih.gov/books/NBK500025/ (accessed on 15 May 2024).
- Patel, P.H.; Gupta, V. Rivastigmine. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. Available online: http://www.ncbi.nlm.nih.gov/books/NBK557438/ (accessed on 15 May 2024).
- Passeri, E.; Elkhoury, K.; Morsink, M.; Broersen, K.; Linder, M.; Tamayol, A.; Malaplate, C.; Yen, F.T.; Arab-Tehrany, E. Alzheimer’s Disease: Treatment Strategies and Their Limitations. Int. J. Mol. Sci. 2022, 23, 13954. [Google Scholar] [CrossRef] [PubMed]
- De la Rosa, A.; Olaso-Gonzalez, G.; Arc-Chagnaud, C.; Millan, F.; Salvador-Pascual, A.; García-Lucerga, C.; Blasco-Lafarga, C.; Garcia-Dominguez, E.; Carretero, A.; Correas, Á.G.; et al. Physical exercise in the prevention and treatment of Alzheimer’s disease. J. Sport Health Sci. 2020, 9, 394–404. [Google Scholar] [CrossRef]
- Breijyeh, Z.; Karaman, R. Comprehensive Review on Alzheimer’s Disease: Causes and Treatment. Molecules 2020, 25, 5789. [Google Scholar] [CrossRef] [PubMed]
- Long, J.M.; Holtzman, D.M. Alzheimer Disease: An Update on Pathobiology and Treatment Strategies. Cell 2019, 179, 312–339. [Google Scholar] [CrossRef] [PubMed]
- LaRocca, T.J.; Cavalier, A.N.; Roberts, C.M.; Lemieux, M.R.; Ramesh, P.; Garcia, M.A.; Link, C.D. Amyloid beta acts synergistically as a pro-inflammatory cytokine. Neurobiol. Dis. 2021, 159, 105493. [Google Scholar] [CrossRef] [PubMed]
- Cervellati, C.; Zuliani, G. Frontier on Alzheimer’s Disease. Int. J. Mol. Sci. 2023, 24, 7748. [Google Scholar] [CrossRef] [PubMed]
- Merighi, S.; Nigro, M.; Travagli, A.; Gessi, S. Microglia and Alzheimer’s Disease. Int. J. Mol. Sci. 2022, 23, 12990. [Google Scholar] [CrossRef] [PubMed]
- Thal, D.R.; Tomé, S.O. The Central Role of Tau in Alzheimer’s Disease: From Neurofibrillary Tangle Maturation to the Induction of Cell Death. Brain Res. Bull. 2022, 190, 204–217. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Macyczko, J.R.; Liu, C.-C.; Bu, G. ApoE4 Reduction: An Emerging and Promising Therapeutic Strategy for Alzheimer’s Disease. Neurobiol. Aging 2022, 115, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Ionescu-Tucker, A.; Cotman, C.W. Emerging roles of oxidative stress in brain aging and Alzheimer’s disease. Neurobiol. Aging 2021, 107, 86–95. [Google Scholar] [CrossRef] [PubMed]
- Ton, A.M.M.; Campagnaro, B.P.; Alves, G.A.; Aires, R.; Côco, L.Z.; Arpini, C.M.; Oliveira, T.G.E.; Campos-Toimil, M.; Meyrelles, S.S.; Pereira, T.M.C.; et al. Oxidative Stress and Dementia in Alzheimer’s Patients: Effects of Synbiotic Supplementation. Oxid. Med. Cell. Longev. 2020, 2020, 2638703. [Google Scholar] [CrossRef] [PubMed]
- Veselov, I.M.; Vinogradova, D.V.; Maltsev, A.V.; Shevtsov, P.N.; Spirkova, E.A.; Bachurin, S.O.; Shevtsova, E.F. Mitochondria and Oxidative Stress as a Link between Alzheimer’s Disease and Diabetes Mellitus. Int. J. Mol. Sci. 2023, 24, 14450. [Google Scholar] [CrossRef] [PubMed]
- Pires, M.; Rego, A.C. Apoe4 and Alzheimer’s Disease Pathogenesis—Mitochondrial Deregulation and Targeted Therapeutic Strategies. Int. J. Mol. Sci. 2023, 24, 778. [Google Scholar] [CrossRef] [PubMed]
- Serrano-Pozo, A.; Das, S.; Hyman, B.T. APOE and Alzheimer’s disease: Advances in genetics, pathophysiology, and therapeutic approaches. Lancet Neurol. 2021, 20, 68–80. [Google Scholar] [CrossRef] [PubMed]
- Kotha, R.R.; Luthria, D.L. Curcumin: Biological, Pharmaceutical, Nutraceutical, and Analytical Aspects. Molecules 2019, 24, 2930. [Google Scholar] [CrossRef] [PubMed]
- Berry, A.; Collacchi, B.; Masella, R.; Varì, R.; Cirulli, F. Curcuma Longa, the “Golden Spice” to Counteract Neuroinflammaging and Cognitive Decline—What Have We Learned and What Needs to Be Done. Nutrients 2021, 13, 1519. [Google Scholar] [CrossRef] [PubMed]
- Assi, A.-A.; Farrag, M.M.Y.; Badary, D.M.; Allam, E.A.H.; Nicola, M.A. Protective effects of curcumin and Ginkgo biloba extract combination on a new model of Alzheimer’s disease. Inflammopharmacology 2023, 31, 1449–1464. [Google Scholar] [CrossRef] [PubMed]
- Kushwah, S.; Maurya, N.S.; Kushwaha, S.; Scotti, L.; Chawade, A.; Mani, A. Herbal Therapeutics for Alzheimer’s Disease: Ancient Indian Medicine System from the Modern Viewpoint. Curr. Neuropharmacol. 2023, 21, 764–776. [Google Scholar] [CrossRef]
- Elhawary, E.A.; Moussa, A.Y.; Singab, A.N.B. Genus Curcuma: Chemical and ethnopharmacological role in aging process. BMC Complement. Med. Ther. 2024, 24, 31. [Google Scholar] [CrossRef] [PubMed]
- Ahsan, R.; Arshad, M.; Khushtar, M.; Ahmad, M.A.; Muazzam, M.; Akhter, M.S.; Gupta, G.; Muzahid, M. A Comprehensive Review on Physiological Effects of Curcumin. Drug Res. 2020, 70, 441–447. [Google Scholar] [CrossRef]
- Ataei, M.; Gumpricht, E.; Kesharwani, P.; Jamialahmadi, T.; Sahebkar, A. Recent advances in curcumin-based nanoformulations in diabetes. J. Drug Target. 2023, 31, 671–684. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-Y.; Sun, L.-W.; Zhao, D.-Q. Current Status and Problem-Solving Strategies for Ginseng Industry. Chin. J. Integr. Med. 2019, 25, 883–886. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.-S.; Kang, K.S.; Kim, S.-Y. Panax ginseng Pharmacopuncture: Current Status of the Research and Future Challenges. Biomolecules 2019, 10, 33. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-Y.; Liu, Q.-P.; An, P.; Jia, M.; Luan, X.; Tang, J.-Y.; Zhang, H. Ginsenoside Rd: A promising natural neuroprotective agent. Phytomedicine 2022, 95, 153883. [Google Scholar] [CrossRef] [PubMed]
- Piao, X.; Zhang, H.; Kang, J.P.; Yang, D.U.; Li, Y.; Pang, S.; Jin, Y.; Yang, D.C.; Wang, Y. Advances in Saponin Diversity of Panax ginseng. Molecules 2020, 25, 3452. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Zeng, J.; Wong, A.S.T. Chemical Structures and Pharmacological Profiles of Ginseng Saponins. Molecules 2019, 24, 2443. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Abid, S.; Ahn, J.C.; Mathiyalagan, R.; Kim, Y.-J.; Yang, D.-C.; Wang, Y. Characteristics of Panax ginseng Cultivars in Korea and China. Molecules 2020, 25, 2635. [Google Scholar] [CrossRef] [PubMed]
- Shan, M.; Bai, Y.; Fang, X.; Lan, X.; Zhang, Y.; Cao, Y.; Zhu, D.; Luo, H. American Ginseng for the Treatment of Alzheimer’s Disease: A Review. Molecules 2023, 28, 5716. [Google Scholar] [CrossRef] [PubMed]
- Cambria, C.; Sabir, S.; Shorter, I.C. Ginseng. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. Available online: http://www.ncbi.nlm.nih.gov/books/NBK538198/ (accessed on 17 April 2024).
- Mohi-Ud-Din, R.; Mir, R.H.; Mir, P.A.; Farooq, S.; Raza, S.N.; Raja, W.Y.; Masoodi, M.H.; Singh, I.P.; Bhat, Z.A. Ethnomedicinal uses, Phytochemistry and Pharmacological Aspects of the Genus Berberis Linn: A Comprehensive Review. Comb. Chem. High Throughput Screen. 2021, 24, 624–644. [Google Scholar] [CrossRef] [PubMed]
- Redha, A.A.; Siddiqui, S.A.; Ibrahim, S.A. Advanced extraction techniques for Berberis species phytochemicals: A review. Int. J. Food Sci. Technol. 2021, 56, 5485–5496. [Google Scholar] [CrossRef]
- Salehi, B.; Selamoglu, Z.; Sener, B.; Kilic, M.; Jugran, A.K.; de Tommasi, N.; Sinisgalli, C.; Milella, L.; Rajkovic, J.; Morais-Braga, M.F.B.; et al. Berberis Plants—Drifting from Farm to Food Applications, Phytotherapy, and Phytopharmacology. Foods 2019, 8, 522. [Google Scholar] [CrossRef] [PubMed]
- Kalmarzi, R.N.; Naleini, S.N.; Ashtary-Larky, D.; Peluso, I.; Jouybari, L.; Rafi, A.; Ghorat, F.; Heidari, N.; Sharifian, F.; Mardaneh, J.; et al. Anti-Inflammatory and Immunomodulatory Effects of Barberry (Berberis vulgaris) and Its Main Compounds. Oxidative Med. Cell. Longev. 2019, 2019, e6183965. [Google Scholar] [CrossRef] [PubMed]
- Khoshandam, A.; Imenshahidi, M.; Hosseinzadeh, H. Pharmacokinetic of berberine, the main constituent of Berberis vulgaris L.: A comprehensive review. Phytother. Res. 2022, 36, 4063–4079. [Google Scholar] [CrossRef] [PubMed]
- Shayganfard, M. Berberine: Is it a Promising Agent for Mental Disorders Treatment? Curr. Mol. Pharmacol. 2023, 16, 307–320. [Google Scholar] [CrossRef]
- El Midaoui, A.; Ghzaiel, I.; Vervandier-Fasseur, D.; Ksila, M.; Zarrouk, A.; Nury, T.; Khallouki, F.; El Hessni, A.; Ibrahimi, S.O.; Latruffe, N.; et al. Saffron (Crocus sativus L.): A Source of Nutrients for Health and for the Treatment of Neuropsychiatric and Age-Related Diseases. Nutrients 2022, 14, 597. [Google Scholar] [CrossRef] [PubMed]
- Pitsikas, N. Crocus sativus L. Extracts and Its Constituents Crocins and Safranal; Potential Candidates for Schizophrenia Treatment? Molecules 2021, 26, 1237. [Google Scholar] [CrossRef] [PubMed]
- Butnariu, M.; Quispe, C.; Herrera-Bravo, J.; Sharifi-Rad, J.; Singh, L.; Aborehab, N.M.; Bouyahya, A.; Venditti, A.; Sen, S.; Acharya, K.; et al. The Pharmacological Activities of Crocus sativus L.: A Review Based on the Mechanisms and Therapeutic Opportunities of its Phytoconstituents. Oxidative Med. Cell. Longev. 2022, 2022, e8214821. [Google Scholar] [CrossRef] [PubMed]
- Azami, S.; Shahriari, Z.; Asgharzade, S.; Farkhondeh, T.; Sadeghi, M.; Ahmadi, F.; Vahedi, M.M.; Forouzanfar, F. Therapeutic Potential of Saffron (Crocus sativus L.) in Ischemia Stroke. Evid.-Based Complement. Altern. Med. 2021, 2021, e6643950. [Google Scholar] [CrossRef] [PubMed]
- Munirah, M.P.; Norhayati, M.N.; Noraini, M. Crocus Sativus for Insomnia: A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2022, 19, 11658. [Google Scholar] [CrossRef] [PubMed]
- Pourbagher-Shahri, A.M.; Forouzanfar, F. Saffron (Crocus sativus) and its constituents for pain management: A review of current evidence. Phytother. Res. 2023, 37, 5041–5057. [Google Scholar] [CrossRef] [PubMed]
- Soria-Lopez, J.A.; González, H.M.; Léger, G.C. Chapter 13—Alzheimer’s disease. In Handbook of Clinical Neurology; Dekosky, S.T., Asthana, S., Eds.; Geriatric Neurology; Elsevier: Amsterdam, The Netherlands, 2019; Volume 167, pp. 231–255. [Google Scholar] [CrossRef]
- Chvojkova, M.; Kolar, D.; Kovacova, K.; Cejkova, L.; Misiachna, A.; Hakenova, K.; Vales, K. Pro-cognitive effects of dual tacrine derivatives acting as cholinesterase inhibitors and NMDA receptor antagonists. Biomed. Pharmacother. 2024, 176, 116821. [Google Scholar] [CrossRef] [PubMed]
- Marucci, G.; Buccioni, M.; Ben, D.D.; Lambertucci, C.; Volpini, R.; Amenta, F. Efficacy of acetylcholinesterase inhibitors in Alzheimer’s disease. Neuropharmacology 2021, 190, 108352. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Peng, Y.; Zhuo, L.; Wang, Y.; Zeng, G.; Wang, S.; Long, L.; Li, X.; Wang, Z. Recent advance on pleiotropic cholinesterase inhibitors bearing amyloid modulation efficacy. Eur. J. Med. Chem. 2022, 242, 114695. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Wang, Z.; Liu, R.; Huang, Y.; Zhang, N.; Zhang, R. Memantine, Donepezil, or Combination Therapy—What is the best therapy for Alzheimer’s Disease? A Network Meta-Analysis. Brain Behav. 2020, 10, e01831. [Google Scholar] [CrossRef] [PubMed]
- Marotta, G.; Basagni, F.; Rosini, M.; Minarini, A. Memantine Derivatives as Multitarget Agents in Alzheimer’s Disease. Molecules 2020, 25, 4005. [Google Scholar] [CrossRef] [PubMed]
- Dhillon, S. Aducanumab: First Approval. Drugs 2021, 81, 1437–1443. [Google Scholar] [CrossRef] [PubMed]
- Haeberlein, S.B.; Aisen, P.; Barkhof, F.; Chalkias, S.; Chen, T.; Cohen, S.; Dent, G.; Hansson, O.; Harrison, K.; von Hehn, C.; et al. Two Randomized Phase 3 Studies of Aducanumab in Early Alzheimer’s Disease. J. Prev. Alzheimer’s Dis. 2022, 9, 197–210. [Google Scholar] [CrossRef] [PubMed]
- Van Dyck, C.H.; Swanson, C.J.; Aisen, P.; Bateman, R.J.; Chen, C.; Gee, M.; Kanekiyo, M.; Li, D.; Reyderman, L.; Cohen, S.; et al. Lecanemab in Early Alzheimer’s Disease. N. Engl. J. Med. 2023, 388, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Office of the Commissioner. FDA Approves First Drug to Treat Agitation Symptoms Associated with Dementia Due to Alzheimer’s Disease, FDA. Available online: https://www.fda.gov/news-events/press-announcements/fda-approves-first-drug-treat-agitation-symptoms-associated-dementia-due-alzheimers-disease (accessed on 18 May 2024).
- Office of the Commissioner. FDA Converts Novel Alzheimer’s Disease Treatment to Traditional Approval, FDA. Available online: https://www.fda.gov/news-events/press-announcements/fda-converts-novel-alzheimers-disease-treatment-traditional-approval (accessed on 18 May 2024).
- Koul, B.; Farooq, U.; Yadav, D.; Song, M. Phytochemicals: A Promising Alternative for the Prevention of Alzheimer’s Disease. Life 2023, 13, 999. [Google Scholar] [CrossRef] [PubMed]
- Ege, D. Action Mechanisms of Curcumin in Alzheimer’s Disease and Its Brain Targeted Delivery. Materials 2021, 14, 3332. [Google Scholar] [CrossRef] [PubMed]
- Chainoglou, E.; Hadjipavlou-Litina, D. Curcumin in Health and Diseases: Alzheimer’s Disease and Curcumin Analogues, Derivatives, and Hybrids. Int. J. Mol. Sci. 2020, 21, 1975. [Google Scholar] [CrossRef] [PubMed]
- Nebrisi, E.E. Neuroprotective Activities of Curcumin in Parkinson’s Disease: A Review of the Literature. Int. J. Mol. Sci. 2021, 22, 11248. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Zeng, F.; Luo, Y.; Zheng, C.; Ran, C.; Yang, J. Curcumin Scaffold as a Multifunctional Tool for Alzheimer’s Disease Research. Molecules 2022, 27, 3879. [Google Scholar] [CrossRef] [PubMed]
- Bhat, A.; Mahalakshmi, A.M.; Ray, B.; Tuladhar, S.; Hediyal, T.A.; Manthiannem, E.; Padamati, J.; Chandra, R.; Chidambaram, S.B.; Sakharkar, M.K. Benefits of curcumin in brain disorders. BioFactors 2019, 45, 666–689. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Venigalla, M.; Raju, R.; Münch, G. Pharmacological considerations for treating neuroinflammation with curcumin in Alzheimer’s disease. J. Neural Transm. 2022, 129, 755–771. [Google Scholar] [CrossRef] [PubMed]
- Su, I.-J.; Chang, H.-Y.; Wang, H.-C.; Tsai, K.-J. A Curcumin Analog Exhibits Multiple Biologic Effects on the Pathogenesis of Alzheimer’s Disease and Improves Behavior, Inflammation, and β-Amyloid Accumulation in a Mouse Model. Int. J. Mol. Sci. 2020, 21, 5459. [Google Scholar] [CrossRef] [PubMed]
- Calabrò, M.; Rinaldi, C.; Santoro, G.; Crisafulli, C. The biological pathways of Alzheimer disease: A review. AIMS Neurosci. 2021, 8, 86–132. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Huang, N.-Q.; Yan, F.; Jin, H.; Zhou, S.-Y.; Shi, J.-S.; Jin, F. Diabetes mellitus and Alzheimer’s disease: GSK-3β as a potential link. Behav. Brain Res. 2018, 339, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Thota, R.N.; Rosato, J.I.; Dias, C.B.; Burrows, T.L.; Martins, R.N.; Garg, M.L. Dietary Supplementation with Curcumin Reduce Circulating Levels of Glycogen Synthase Kinase-3β and Islet Amyloid Polypeptide in Adults with High Risk of Type 2 Diabetes and Alzheimer’s Disease. Nutrients 2020, 12, 1032. [Google Scholar] [CrossRef] [PubMed]
- Farkhondeh, T.; Samarghandian, S.; Pourbagher-Shahri, A.M.; Sedaghat, M. The impact of curcumin and its modified formulations on Alzheimer’s disease. J. Cell. Physiol. 2019, 234, 16953–16965. [Google Scholar] [CrossRef] [PubMed]
- Noor, N.A.; Hosny, E.N.; Khadrawy, Y.A.; Mourad, I.M.; Othman, A.I.; Ezz, H.S.A.; Mohammed, H.S. Effect of curcumin nanoparticles on streptozotocin-induced male Wistar rat model of Alzheimer’s disease. Metab. Brain Dis. 2022, 37, 343–357. [Google Scholar] [CrossRef]
- Kim, M.; Mok, H.; Yeo, W.-S.; Ahn, J.-H.; Choi, Y.K. Role of ginseng in the neurovascular unit of neuroinflammatory diseases focused on the blood-brain barrier. J. Ginseng Res. 2021, 45, 599–609. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Yang, J.; Chen, R.; Liu, Y.; Liu, S.; Pan, Y.; Lei, Q.; Wang, Y.; He, L.; Song, Y.; et al. Ginsenoside Rg1 ameliorates Alzheimer’s disease pathology via restoring mitophagy. J. Ginseng Res. 2023, 47, 448–457. [Google Scholar] [CrossRef] [PubMed]
- Quinn, P.M.J.; Moreira, P.I.; Ambrósio, A.F.; Alves, C.H. PINK1/PARKIN signalling in neurodegeneration and neuroinflammation. Acta Neuropathol. Commun. 2020, 8, 189. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yang, D.; Li, Z.; Zhao, M.; Wang, D.; Sun, Z.; Wen, P.; Dai, Y.; Gou, F.; Ji, Y.; et al. PINK1/Parkin-mediated mitophagy in neurodegenerative diseases. Ageing Res. Rev. 2023, 84, 101817. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Rubin, L.; Silva, M.; Li, S.; Yang, C.; Lazarovici, P.; Zheng, W. Current Progress on Neuroprotection Induced by Artemisia, Ginseng, Astragalus, and Ginkgo Traditional Chinese Medicines for the Therapy of Alzheimer’s Disease. Oxidative Med. Cell. Longev. 2022, 2022, 3777021. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Li, N.; Pu, Y.; Zhang, T.; Wang, B. Neuroprotective Effects of Ginseng Phytochemicals: Recent Perspectives. Molecules 2019, 24, 2939. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Huang, Q.; Chen, J.; Qi, H.; Liu, J.; Chen, Z.; Zhao, D.; Wang, Z.; Li, X. Neuroprotective Potentials of Panax ginseng Against Alzheimer’s Disease: A Review of Preclinical and Clinical Evidences. Front. Pharmacol. 2021, 12, 688490. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Yan, Y.; Fang, Q.; Zhang, N.; Kumar, G.; Zhang, J.; Song, L.-J.; Yu, J.; Zhao, L.; Zhang, H.-T.; et al. The Rho kinase inhibitor fasudil attenuates Aβ1–42-induced apoptosis via the ASK1/JNK signal pathway in primary cultures of hippocampal neurons. Metab. Brain Dis. 2019, 34, 1787–1801. [Google Scholar] [CrossRef]
- Razani, E.; Pourbagheri-Sigaroodi, A.; Safaroghli-Azar, A.; Zoghi, A.; Shanaki-Bavarsad, M.; Bashash, D. The PI3K/Akt signaling axis in Alzheimer’s disease: A valuable target to stimulate or suppress? Cell Stress Chaperones 2021, 26, 871–887. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Li, S.; Huang, H.; Lv, J.; Chen, S.; Dias, A.C.P.; Li, Y.; Liu, X.; Wang, Q. Comparison of the Protective Effects of Ginsenosides Rb1 and Rg1 on Improving Cognitive Deficits in SAMP8 Mice Based on Anti-Neuroinflammation Mechanism. Front. Pharmacol. 2020, 11, 834. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Li, T.; Tian, X.; Zhao, L. Ginsenoside Rd Attenuates Tau Phosphorylation in Olfactory Bulb, Spinal Cord, and Telencephalon by Regulating Glycogen Synthase Kinase 3β and Cyclin-Dependent Kinase 5. Evid.-Based Complement. Altern. Med. 2021, 2021, 4485957. [Google Scholar] [CrossRef] [PubMed]
- Davoody, S.; Taei, A.A.; Khodabakhsh, P.; Dargahi, L. mTOR signaling and Alzheimer’s disease: What we know and where we are? CNS Neurosci. Ther. 2024, 30, e14463. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Jia, J. Magnolol improves Alzheimer’s disease-like pathologies and cognitive decline by promoting autophagy through activation of the AMPK/mTOR/ULK1 pathway. Biomed. Pharmacother. 2023, 161, 114473. [Google Scholar] [CrossRef] [PubMed]
- Razgonova, M.P.; Veselov, V.V.; Zakharenko, A.M.; Golokhvast, K.S.; Nosyrev, A.E.; Cravotto, G.; Tsatsakis, A.; Spandidos, D.A. Panax ginseng components and the pathogenesis of Alzheimer’s disease (Review). Mol. Med. Rep. 2019, 19, 2975–2998. [Google Scholar] [CrossRef] [PubMed]
- Yuan, N.-N.; Cai, C.-Z.; Wu, M.-Y.; Su, H.-X.; Li, M.; Lu, J.-H. Neuroprotective effects of berberine in animal models of Alzheimer’s disease: A systematic review of pre-clinical studies. BMC Complement. Altern. Med. 2019, 19, 109. [Google Scholar] [CrossRef] [PubMed]
- Akbar, M.; Shabbir, A.; Rehman, K.; Akash, M.S.H.; Shah, M.A. Neuroprotective potential of berberine in modulating Alzheimer’s disease via multiple signaling pathways. J. Food Biochem. 2021, 45, e13936. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.K.; Singh, S.K.; Nandi, M.K.; Mishra, G.; Maurya, A.; Rai, A.; Rai, G.K.; Awasthi, R.; Sharma, B.; Kulkarni, G.T. Berberine: A Plant-derived Alkaloid with Therapeutic Potential to Combat Alzheimer’s disease. Central Nerv. Syst. Agents Med. Chem. 2019, 19, 154–170. [Google Scholar] [CrossRef] [PubMed]
- Fang, Z.; Tang, Y.; Ying, J.; Tang, C.; Wang, Q. Traditional Chinese medicine for anti-Alzheimer’s disease: Berberine and evodiamine from Evodia rutaecarpa. Chin. Med. 2020, 15, 82. [Google Scholar] [CrossRef]
- Sahu, R.; Upadhayay, S.; Mehan, S. Inhibition of extracellular regulated kinase (ERK)-1/2 signaling pathway in the prevention of ALS: Target inhibitors and influences on neurological dysfunctions. Eur. J. Cell Biol. 2021, 100, 151179. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Ye, C.; Chen, Y.; Chen, Y.; Diao, S.; Huang, M. Berberine Improves Behavioral and Cognitive Deficits in a Mouse Model of Alzheimer’s Disease via Regulation of β-Amyloid Production and Endoplasmic Reticulum Stress. ACS Chem. Neurosci. 2021, 12, 1894–1904. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Kang, C.; Che, S.; Su, J.; Sun, Q.; Ge, T.; Guo, Y.; Lv, J.; Sun, Z.; Yang, W.; et al. Berberine: A Promising Treatment for Neurodegenerative Diseases. Front. Pharmacol. 2022, 13, 845591. [Google Scholar] [CrossRef] [PubMed]
- Raju, M.; Kunde, S.S.; Auti, S.T.; Kulkarni, Y.A.; Wairkar, S. Nanostrukturalny nośnik lipidowy obciążony berberyną dla choroby Alzheimera: Projektowanie, optymalizacja statystyczna i ulepszona wydajność in vivo. Life Sci. 2021, 285, 119990. [Google Scholar] [CrossRef] [PubMed]
- Rajasekhar, K.; Samanta, S.; Bagoband, V.; Murugan, N.A.; Govindaraju, T. Antioxidant Berberine-Derivative Inhibits Multifaceted Amyloid Toxicity. iScience 2020, 23, 101005. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Chen, Y.; Liang, Y.; Chen, H.; Ji, X.; Huang, M. Berberine mitigates cognitive decline in an Alzheimer’s Disease Mouse Model by targeting both tau hyperphosphorylation and autophagic clearance. Biomed. Pharmacother. 2020, 121, 109670. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Wang, C.; Chen, Y.; He, Y.; Cai, Z. Berberine attenuates cognitive impairment and ameliorates tau hyperphosphorylation by limiting the self-perpetuating pathogenic cycle between NF-κB signaling, oxidative stress and neuroinflammation. Pharmacol. Rep. 2017, 69, 1341–1348. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Jiang, X.; Liang, Y.; Liu, Q.; Chen, S.; Guo, Y. Berberine improves cognitive impairment by promoting autophagic clearance and inhibiting production of β-amyloid in APP/tau/PS1 mouse model of Alzheimer’s disease. Exp. Gerontol. 2017, 91, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Hseu, Y.-C.; Gowrisankar, Y.V.; Wang, L.-W.; Zhang, Y.-Z.; Chen, X.-Z.; Huang, P.-J.; Yen, H.-R.; Yang, H.-L. The in vitro and in vivo depigmenting activity of pterostilbene through induction of autophagy in melanocytes and inhibition of UVA-irradiated α-MSH in keratinocytes via Nrf2-mediated antioxidant pathways. Redox Biol. 2021, 44, 102007. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.; Chen, Y.; Cheng, D.; He, Z.; Shi, X.; Du, B.; Xi, X.; Gao, Y.; Guo, Y. YAP inhibits autophagy and promotes progression of colorectal cancer via upregulating Bcl-2 expression. Cell Death Dis. 2021, 12, 457. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yang, K.-B.; Chen, W.; Mai, J.; Wu, X.-Q.; Sun, T.; Wu, R.-Y.; Jiao, L.; Li, D.-D.; Ji, J.; et al. CUL3 (cullin 3)-mediated ubiquitination and degradation of BECN1 (beclin 1) inhibit autophagy and promote tumor progression. Autophagy 2021, 17, 4323–4340. [Google Scholar] [CrossRef]
- Hossain, R.; Quispe, C.; Herrera-Bravo, J.; Beltrán, J.F.; Islam, M.T.; Shaheen, S.; Cruz-Martins, N.; Martorell, M.; Kumar, M.; Sharifi-Rad, J.; et al. Neurobiological Promises of the Bitter Diterpene Lactone Andrographolide. Oxidative Med. Cell. Longev. 2022, 2022, 3079577. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Yao, J.-X.; Zhang, T.-T.; Wen, J.-Y.; Zhang, Z.; Luo, Y.-M.; Cao, Y.; Li, H. Network pharmacology reveals that Berberine may function against Alzheimer’s disease via the AKT signaling pathway. Front. Neurosci. 2023, 17, 1059496. [Google Scholar] [CrossRef] [PubMed]
- D’onofrio, G.; Nabavi, S.M.; Sancarlo, D.; Greco, A.; Pieretti, S. Crocus sativus L. (Saffron) in Alzheimer’s Disease Treatment: Bioactive Effects on Cognitive Impairment. Curr. Neuropharmacol. 2021, 19, 1606–1616. [Google Scholar] [CrossRef]
- Sanaie, S.; Nikanfar, S.; Kalekhane, Z.Y.; Azizi-Zeinalhajlou, A.; Sadigh-Eteghad, S.; Araj-Khodaei, M.; Ayati, M.H.; Andalib, S. Saffron as a promising therapy for diabetes and Alzheimer’s disease: Mechanistic insights. Metab. Brain Dis. 2022, 38, 137–162. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Cai, X.; Hu, W.; Li, Z.; Kong, F.; Chen, X.; Wang, D. Investigation of the neuroprotective effects of crocin via antioxidant activities in HT22 cells and in mice with Alzheimer’s disease. Int. J. Mol. Med. 2018, 43, 956–966. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S.; Ambegaokar, S.S.; Jackson, G.R.; Mudher, A. Insulin-Mediated Changes in Tau Hyperphosphorylation and Autophagy in a Drosophila Model of Tauopathy and Neuroblastoma Cells. Front. Neurosci. 2019, 13, 801. [Google Scholar] [CrossRef] [PubMed]
- Saeedi, M.; Rashidy-Pour, A. Association between chronic stress and Alzheimer’s disease: Therapeutic effects of Saffron. Biomed. Pharmacother. 2020, 133, 110995. [Google Scholar] [CrossRef] [PubMed]
- Seibel, R.; Schneider, R.H.; Gottlieb, M.G. Effects of Spices (Saffron, Rosemary, Cinnamon, Turmeric and Ginger) in Alzheimer’s Disease. Curr. Alzheimer Res. 2021, 18, 347–357. [Google Scholar] [CrossRef] [PubMed]
- Chalatsa, I.; Arvanitis, D.A.; Koulakiotis, N.S.; Giagini, A.; Skaltsounis, A.L.; Papadopoulou-Daifoti, Z.; Tsarbopoulos, A.; Sanoudou, D. The Crocus sativus Compounds trans-Crocin 4 and trans-Crocetin Modulate the Amyloidogenic Pathway and Tau Misprocessing in Alzheimer Disease Neuronal Cell Culture Models. Front. Neurosci. 2019, 13, 249. [Google Scholar] [CrossRef] [PubMed]
- Wani, A.; Al Rihani, S.B.; Sharma, A.; Weadick, B.; Govindarajan, R.; Khan, S.U.; Sharma, P.R.; Dogra, A.; Nandi, U.; Reddy, C.N.; et al. Crocetin promotes clearance of amyloid-β by inducing autophagy via the STK11/LKB1-mediated AMPK pathway. Autophagy 2021, 17, 3813–3832. [Google Scholar] [CrossRef]
- Asadi, F.; Jamshidi, A.H.; Khodagholi, F.; Yans, A.; Azimi, L.; Faizi, M.; Vali, L.; Abdollahi, M.; Ghahremani, M.H.; Sharifzadeh, M. Reversal effects of crocin on amyloid β-induced memory deficit: Modification of autophagy or apoptosis markers. Pharmacol. Biochem. Behav. 2015, 139, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Gregory, J.; Vengalasetti, Y.V.; Bredesen, D.E.; Rao, R.V. Neuroprotective Herbs for the Management of Alzheimer’s Disease. Biomolecules 2021, 11, 543. [Google Scholar] [CrossRef]
- Su, W.; Wang, Y.; Shao, S.; Ye, X. Crocin ameliorates neuroinflammation and cognitive impairment in mice with Alzheimer’s disease by activating PI3K/AKT pathway. Brain Behav. 2024, 14, e3503. [Google Scholar] [CrossRef] [PubMed]
- Baluchnejadmojarad, T.; Mohamadi-Zarch, S.-M.; Roghani, M. Safranal, an active ingredient of saffron, attenuates cognitive deficits in amyloid β-induced rat model of Alzheimer’s disease: Underlying mechanisms. Metab. Brain Dis. 2019, 34, 1747–1759. [Google Scholar] [CrossRef] [PubMed]
- Farokhnia, M.; Sabet, M.S.; Iranpour, N.; Gougol, A.; Yekehtaz, H.; Alimardani, R.; Farsad, F.; Kamalipour, M.; Akhondzadeh, S. Comparing the efficacy and safety of Crocus sativus L. with memantine in patients with moderate to severe Alzheimer’s disease: A double-blind randomized clinical trial. Hum. Psychopharmacol. Clin. Exp. 2014, 29, 351–359. [Google Scholar] [CrossRef] [PubMed]
- Lopes, C.M.C.; Lima, S.M.R.R.; Veiga, E.C.d.A.; Soares-Jr, J.M.; Baracat, E.C. Phytotherapeutic medicines: Reality or myth? Front. Public Health 2019, 65, 292–294. [Google Scholar] [CrossRef] [PubMed]
- Williams, G.; Hahn, D.; Stephens, J.H.; Craig, J.C.; Hodson, E.M. Cranberries for preventing urinary tract infections. Cochrane Database Syst. Rev. 2023, 2023, CD001321. [Google Scholar] [CrossRef]
- Venkateswaran, M.R.; Vadivel, T.E.; Jayabal, S.; Murugesan, S.; Rajasekaran, S.; Periyasamy, S. A review on network pharmacology based phytotherapy in treating diabetes—An environmental perspective. Environ. Res. 2021, 202, 111656. [Google Scholar] [CrossRef] [PubMed]
- CFR Part 582—Substances Generally Recognized as Safe. Available online: https://www.ecfr.gov/current/title-21/part-582 (accessed on 22 May 2024).
- Howes, M.-J.R.; Houghton, P.J. Ethnobotanical treatment strategies against Alzheimer’s disease. Curr. Alzheimer Res. 2012, 9, 67–85. [Google Scholar] [CrossRef] [PubMed]





| Activity μmol SH/min/g | Control | Model AD | Model AD |
|---|---|---|---|
| Cortex | 1.1 | 2.7 | 1.9 |
| Hippocampus | 1.7 | 3.6 | 1.5 |
| CUR Concentration in: | Plasma (ng/mL) | Hippocampus (ng/g) |
|---|---|---|
| CUR after 30 min | 57.31 ± 4.134 | 49.46 ± 3.763 |
| CUR + GBE after 30 min | 80.58 ± 3.297 | 175.9 ± 8.346 |
| CUR after 60 min | 60.38 ± 2.747 | 119.7 ± 3.069 |
| CUR + GBE after 60 min | 100.3 ± 5.463 | 409.5 ± 6.766 |
| Relative Intensity Tau Proteins | Brain | Spinal Cord | Olfactory Bulb |
|---|---|---|---|
| Baseline | 5–7 | 4–7 | 3–7 |
| Rd 10 mg/kg post-application value | 1–3 | 1–2 | 1–7 |
| Relative Protein Expression | p-GSK3β/GSK3β | p-Akt/Akt | p-PP2A/PP2A |
|---|---|---|---|
| Control group | 1.0 | 1.0 | 1.0 |
| Research group | 1.3 | 1.35 | 0.8 |
| Relative OD (% of β-actin) | Control Group | Berberine 50 mg/kg/d | Berberine 100 mg/kg/d |
|---|---|---|---|
| GPx-1/2 | 0.55 | 1.55 | 1.7 |
| GSS | 0.55 | 1.6 | 1.65 |
| GR | 0.65 | 1.8 | 1.7 |
| CD45 | 0.8 | 0.25 | 0.3 |
| GFAP | 1.25 | 0.5 | 0.45 |
| IL-1β | 1.4 | 0.6 | 0.65 |
| TNF-α | 1.3 | 0.6 | 0.5 |
| Expression Level | LC3-II | P62 | Bcl-2 | Cathepsin-D | Beclin-1 |
|---|---|---|---|---|---|
| Control | 0.9 | 1.25 | 1.2 | 0.8 | 0.75 |
| Berberine 50 mg/kg/d | 1.1 | 0.9 | 0.9 | 1.0 | 1.1 |
| Berberine 100 mg/kg/d | 1.4 | 0.65 | 0.75 | 1.05 | 1.3 |
| Arbitrary Unit | Caspase-3 | Beclin-1 | Bax/Bcl-2 | LC3-II/LC3-I |
|---|---|---|---|---|
| Control | 1.0 | 1.0 | 1.0 | 1.0 |
| Aβ | 2.8 | 1.4 | 1.3 | 1.3 |
| Aβ + Crocin 150 nmol/side | 0.8 | 1.5 | 0.8 | 1.4 |
| Aβ + Crocin 300 nmol/side | 0.6 | 1.7 | 0.7 | 1.5 |
| Aβ + Crocin 600 nmol/side | 0.55 | 1.8 | 0.6 | 1.6 |
| Activity/Concentration Level | Sham | Sham + Safranal 0.2 mL/kg | Aβ | Aβ + Safranal 0.025 mL/kg | Aβ + Safranal 0.1 mL/kg | Aβ + Safranal 0.2 mL/kg |
|---|---|---|---|---|---|---|
| ROS (AFU) | 80 | 75 | 140 | 135 | 100 | 90 |
| Catalase (Unit/mg) | 2 | 2.2 | 1 | 1.2 | 1.4 | 1.6 |
| SOD (Unit/mg) | 4 | 4.2 | 2 | 2.4 | 3 | 3.2 |
| GSH (nmol/mg) | 4 | 3.9 | 2.6 | 2.8 | 3.4 | 3.6 |
| IL-1β (pg/mg) | 25 | 28 | 45 | 40 | 38 | 28 |
| IL-6 (pg/mg) | 22 | 24 | 42 | 40 | 30 | 28 |
| TNF α (pg/mg) | 28 | 26 | 44 | 42 | 34 | 32 |
| Caspase-3 (OD) | 0.6 | 0.7 | 1.2 | 1.1 | 0.9 | 0.8 |
| AChE (nmol/min/mg) | 28 | 30 | 48 | 42 | 36 | 38 |
| Year | Plant | Substance | Dose | Population | Result | Citation |
|---|---|---|---|---|---|---|
| 2024 | Crocus sativus | Crocin | 40 mg/kg/d | 30 ICR mice | Decrease in expression of IL-1β, IL-6, TNF- α Increase in PI3K and Akt activity | [115] |
| 2023 | Curcuma longa | Curcumin | 160 ppm | Transgenic mice | Decrease in IL-1β expression | [29] |
| 2023 | Curcuma longa | Curcumin | 100 mg/kg | 48 rats | Decrease in tau and Aβ protein levels | [28] |
| 2023 | Panax ginseng | Rg1 | 10 mg/kg/d 1 uM | 5XFAD mice | Inhibition of mTOR and ULK1 Increased PINK-Parkin pathway and autophagy | [74] |
| 2023 | Berberis | Berberine | 50 mg/kg/d | Human SH-SY5Y cells | Inhibition of neuronal damage Improving memory | [104] |
| 2022 | Curcuma longa | Curcumin | 50 mg/kg | 20 mice 3xTg AD 10 wild type C57 mice | Decrease in AChE activity and tau protein levels | [72] |
| 2021 | Panax ginseng | Rd1 | 10 mg/kg | 30 rats | Decrease in the concentration of tau protein | [83] |
| 2021 | Crocus sativus | Crocin | 30 mg/d | APP transgenic mice | Improving cognitive functions | [114] |
| 2020 | Curcuma longa | Curcumin | 500 mg | 29 people | Decrease in GSK3β activation and IAPP levels | [70] |
| 2020 | Panax ginseng | Rb1 Rg1 | 30/60 µmol/kg 30/60 µmol/kg | 66 SAMP8 mice 12 SAMR1 mice | Decrease in the concentration of Aβ protein | [82] |
| 2020 | Berberis | Berberine | 100 mg/kg/d | 24 mice 3xTg AD | Increase in PP2A and Akt activity Increase in autophagy Decrease in GSK3β activity | [97] |
| 2019 | Panax ginseng | Ginsenosides | 1 g/d 3 g/d 4.5 g/d | 40 people | Improved cognitive function in tests: MMSE, ADAS | [86] |
| 2019 | Crocus sativus | Safranal | 0.025 mL/kg 0.1 mL/kg 0.2 mL/kg | 66 Wistar rats | Increase in concentration/activity of GSH, catalase, GSH, SOD Decrease in concentration/activity of ROS, IL-1β, IL-6, TNF, Caspase-3 and AChE | [116] |
| 2017 | Berberis | Berberine | 50 mg/kg 100 mg/kg | 30 APP/PS1 mice | Increased activity of antioxidant enzymes: GPx-1/2, GSS, GR Decrease in inflammatory markers | [98] |
| 2017 | Berberis | Berberine | 50 mg/kg/d 100 mg/kg/d | 36 mice 3xTg AD | Increase in activity of LC3-II, Cathepsin-D, Beclin-1 Decrease in the activity of P62 and Bcl-2 | [99] |
| 2016 | Crocus sativus | Crocin | 150 nmol/side 300 nmol/side 600 nmol/side 30 mg/kg | Mice | Decrease in Caspase-3 activity Increase in Beclin-1 and LC3-II activity | [113] |
| 2014 | Crocus sativus | Saffron extract | 30 mg/d | 68 people | No significant difference in improvement of MMSE and SCIRS scores compared with memantine treated group | [117] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piekarz, J.; Picheta, N.; Burdan, O.; Kurek, M.; Chrościńska-Krawczyk, M. Phytotherapy in Alzheimer’s Disease—A Narrative Review. Biomedicines 2024, 12, 1812. https://doi.org/10.3390/biomedicines12081812
Piekarz J, Picheta N, Burdan O, Kurek M, Chrościńska-Krawczyk M. Phytotherapy in Alzheimer’s Disease—A Narrative Review. Biomedicines. 2024; 12(8):1812. https://doi.org/10.3390/biomedicines12081812
Chicago/Turabian StylePiekarz, Julia, Natalia Picheta, Oliwia Burdan, Marcelina Kurek, and Magdalena Chrościńska-Krawczyk. 2024. "Phytotherapy in Alzheimer’s Disease—A Narrative Review" Biomedicines 12, no. 8: 1812. https://doi.org/10.3390/biomedicines12081812
APA StylePiekarz, J., Picheta, N., Burdan, O., Kurek, M., & Chrościńska-Krawczyk, M. (2024). Phytotherapy in Alzheimer’s Disease—A Narrative Review. Biomedicines, 12(8), 1812. https://doi.org/10.3390/biomedicines12081812






