Abstract
The purpose of the study is to elucidate whether irisin is a promising predictive biomarker for kidney-related events in patients with T2DM and concomitant asymptomatic HF. We prospectively enrolled 146 T2DM patients who had either evidence of structural cardiac abnormality or elevated levels of N-terminal brain natriuretic pro-peptide (NT-proBNP) > 125 pmol/mL and followed them for 52 weeks. Structural cardiac abnormalities were used as the minimum from the following criteria: abnormal left ventricular (LV) global longitudinal strain (GLS) < −16%, LV hypertrophy, left atrial volume index > 34 mL/m2, abnormal ratio of early transmitral diastolic filling velocity/early mitral annular velocity ≥ 13 units. All the patients underwent echocardiographic and Doppler examinations by two blinded, highly experienced echocardiographers. NT-proBNP, irisin, TNF-alpha, and hs-CRP were quantified in the serum at baseline, at 26 weeks, and at the end of the study. The kidney-related outcomes consisted of an eGFR reduction by 40% from baseline, or end-stage kidney disease, or kidney replacement therapy. We found that levels of irisin at baseline < 4.15 ng/mL and/or its decrease > 20% from baseline in T2DM patients predicted kidney-related events better than baseline levels/dynamic NT-proBNP and the use of SGLT2 inhibitors. In conclusion, we established that a low baseline level of irisin and its 20% decrease correlated with newly kidney-related events in T2DM patients with asymptomatic HFpEF/HFmrEF.
1. Introduction
In recent decades, the global prevalence of type 2 diabetes mellitus (T2DM) has demonstrated a steady trend to increase due to the aging of the population, an elevated prevalence of metabolic risk factors, and genetic/epigenetic causes widely penetrating in different ethnicities and genders [1,2]. Individuals with T2DM are at a higher risk of developing a range of complications including diabetic cardiomyopathy, ischemia- and non-ischemia-induced heart failure (HF), chronic kidney disease (CKD), and coronary artery disease (CAD), which intervene in terms of the prognosis and quality of life [3]. Potential molecular mechanisms contributing to functional, morphological, and structural impairments, known as diabetes-induced cardiomyopathy, affect altered cardiac metabolism, and are the result of the following: glucose and lipid toxicity, mitochondrial DNA damage, insulin resistance and impaired cardiac insulin metabolic signaling, cardiac myocytes endoplasmic reticulum stress, mitochondrial stress and dysfunction due to oxidative stress, inflammation, neurohumoral activation and adipose tissue dysfunction, impaired vascular integrity, and endothelial dysfunction [4,5]. These pathophysiological factors link CV and metabolic conditions with myocardial cell death pathways, the over-expression of pro-inflammatory genes, the accumulation of extracellular matrix and fibrotic changes, and cardiac autonomic neuropathy, which are considered the main causes leading to adverse cardiac remodeling and consequently the development and progression of HF with a preserved (HFpEF) and reduced/mildly reduced (HFrEF/HFmrEF) ejection fraction [6,7].
CKD is a common comorbidity in T2DM patients that is increasing in prevalence and affects about 50% of T2DM patients globally [8]. It has been found that HF along with the duration of T2DM are the most important risk factors related to a reduction in the glomerular function rate (GFR) and contribute to kidney-related events [9,10]. On the contrary, worsening GFR is frequently associated with the development of HFpEF, which in non-dialysis CKD individuals is the second cause of death after CAD [11,12]. Meanwhile, besides the serial assessment of creatinine levels, which are biomarkers of kidney damage, no other approach to the detection of CKD has been suggested, while numerous promising biomarkers are widely considered [13]. There is limited evidence of circulating biomarkers regarding the prediction of kidney-related outcomes in patients with asymptomatic HFpEF [14]. Conventionally used natriuretic peptides (NPs) exhibited their inadequate discriminative potency on clinical outcomes in individuals with metabolic risk factors including those who were overweight and obese as well as those with T2DM [15]. Moreover, a relative deficiency of brain NP (BNP) was found to be associated with any phenotype of HF including those who had asymptomatic diastolic dysfunction [16]. In this connection, the discovery of novel biomarkers appears to be intriguing and promising.
Irisin is a multifunctional myokine, which is derived from skeletal muscles and cardiomyocytes due to the cleavage of fibronectin type III domain-containing 5 and contributes to mitochondrial homeostasis, fat browning, and glucose and fatty acid oxidation [17]. Therefore, irisin exerts several cardio and renal protective capabilities through regulating vascular tone, blood pressure, and mitochondrial thermogenesis and integrity as well as enhancing the over-expression of angiopoietin-Tie2, interleukin (IL)-8, IL-13, transforming growth factor (TGF)-beta, and thrombopoietin [18,19]. Finally, it protects ischemic and reperfusion injury, necrosis, apoptosis, and ferroptosis, potentiates tissue reparation, reduces the inflammatory response and improves injured cell survival, and ameliorates kidney and skeletal muscle injury, osteopenia, and myopathy through the SIRT-1/Nrf2 pathway and AMP kinase / Drp-1 and ERK1/2 signaling [20,21]. Yet, irisin in an animal model decreased serum creatinine, urea, and phosphorous levels as well as prevented vascular calcification in CKD [22]. In clinical settings, irisin demonstrated its ability to be a predictor for T2DM-related complications and symptomatic HF [23,24,25]. However, the discriminative value of irisin for kidney-related outcomes in T2DM individuals with asymptomatic HFpEF/HFmrEF remains uncertain. The purpose of this study is to elucidate whether irisin is a promising predictive biomarker for kidney-related events in patients with T2DM and concomitant asymptomatic HF.
2. Materials and Methods
2.1. Patient Population and Study Design
A total of 738 patients with T2DM were identified through a local database of the “Vita Center” (Zaporozhye, Ukraine). The inclusion criteria were both genders with an age of ≥18 years, established T2DM, asymptomatic HFpEF/HFmrEF (left ventricular ejection fraction [LVEF] > 40%), glycosylated hemoglobin [HbAc1] < 6.9%, and informed consent to participate in the study. We prospectively enrolled 146 T2DM patients who had either evidence of structural cardiac abnormality or elevated levels of N-terminal brain natriuretic pro-peptide (NT-proBNP) > 125 pmol/mL and followed them for 52 weeks (Figure 1). Structural cardiac abnormalities were used as the minimum from the following criteria: abnormal left ventricular (LV) global longitudinal strain (GLS) < −16%, increased LV mass index (LVMI) ≥ 95 g/m2 in women or ≥115 g/m2 in men, left atrial volume index (LAVI) > 34 mL/m2, abnormal ratio of early transmitral diastolic filling velocity/early mitral annular velocity (E/e′) ≥ 13 units. These criteria corresponded to the diagnostic algorithm according to the 2021 ESC HF guidelines [26].
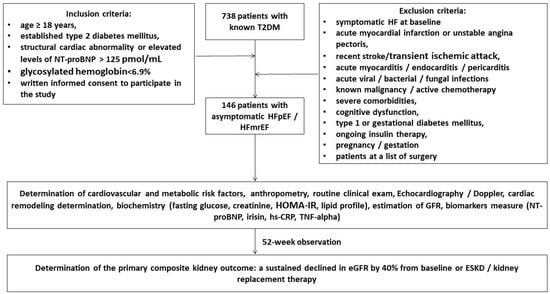
Figure 1.
Flow chart of study design. Abbreviations: T2DM, type 2 diabetes mellitus; HFpEF, heart failure with preserved ejection fraction; HFmrEF, heart failure mildly reduced ejection fraction; NT-proBNP, N-terminal brain natriuretic pro-peptide; hs-CRP, high-sensitive C-reactive peptide; GFR, glomerular filtration rate; TNF, tumor necrosis factor.
The exclusion criteria were symptomatic acute or chronic HF, acute coronary syndrome/myocardial infarction or unstable angina pectoris, recent stroke/transient ischemic attack, acute myocarditis/endocarditis/pericarditis, known malignancy and/or chemotherapy, acute viral/bacterial/fungal infections, severe comorbidities (anemia, chronic obstructive pulmonary disease, bronchial asthma, liver cirrhosis, known inherited and acquired valvular heart defect, symptomatic severe hypoglycemia, morbid obesity, systemic connective tissue diseases, end-stage of renal disease, autoimmune disease, cognitive dysfunction, and thyroid disorders), pregnancy/gestation, type 1 or gestational diabetes mellitus, or current therapy with insulin.
During the 52-week study period, we collected patient data from a variety of sources, including medical records, databases, discharge summaries, autopsy reports, and direct calls to patients and/or their relatives.
2.2. Collection of Relevant Medical Data and Background Information
Demographics and anthropomorphic data, basic clinical characteristics, and comorbidities were collected at the baseline and at the study end.
2.3. Echocardiography Examination
All the patients underwent echocardiographic and Doppler examinations by two blinded, highly experienced echocardiographers according to the guidelines of the American Society of Echocardiography [27]. Records were taken in the standard apical 2- and 4-chamber views at baseline and at the 52-week interval of observation using a GE HealthCare Vivid E95 scanner (General Electric Company, Horton, Norway). The conventional hemodynamic parameters included LVEF by Simpson’s method, left ventricular end-diastolic (LVEDV) and end-systolic (LVESV) volumes, LAVI, early diastolic blood filling (E), and the mean longitudinal strain ratio (e′). The estimated E/e′ ratio was expressed as the ratio of the E wave velocity to the averaged medial and lateral e’ velocity. LV GLS was received by 2D Speckle Tracking Image after obtaining high-quality echocardiographic records during at least three cardiac cycles. The data were stored using the DICOM format for further evaluation.
2.4. Determination of Composite Kidney Outcomes
We determined the kidney-related outcome as a composite of the estimated GFR (eGFR) reduction by 40% from baseline, ESKD, or kidney replacement therapy.
2.5. Blood Sampling
Venous blood samples (3–5 mL) were collected from fasting patients in vacutainer tubes at three time points: baseline, 26 weeks, and the end of the study. Pooled samples were centrifuged (3000 r/min, 30 min). Sera were collected and immediately frozen and stored at −70 °C until analysis before utilization. All the routine biochemical tests were performed using standard biochemical techniques on a Roche P800 analyzer (Basel, Switzerland).
2.6. Biomarker Analysis
N-terminal brain natriuretic pro-peptide (NT-proBNP), irisin, tumor necrosis factor (TNF)-alpha, and high-sensitive C-reactive protein (hs-CRP) were quantified in the serum using commercial enzyme-linked immunosorbent assay (ELISA) kits manufactured by Elabscience (Houston, TX, USA). The intra- and inter-assay coefficients of variation for all the kits were <10%.
2.7. Glomerular Filtration Rate and Insulin Resistance Determination
The conventional CKD-EPI formula was used to estimate the glomerular filtration rate (eGFR) [28]. The Homeostatic Assessment Model of Insulin Resistance (HOMA-IR) was used to assess insulin resistance [29].
2.8. Statistics
The Kolmogorov–Smirnov test was used to assess normality, and the Levene test was used to assess homogeneity. The continuous variables were presented as the mean (M) and standard deviation (SD) or median (Me) and 25–75% interquartile range (IQR), depending on their distribution. The categorical variables were presented as proportions and percentages of the total. Chi-square, Mann–Whitney U, and Kruskal–Wallis tests were used to compare variance according to the distribution. Spearman’s correlation coefficient was calculated to determine the correlation between the variables. Plausible predictors of the composite renal outcome were identified using univariate logistic regression and backward stepwise multivariate logistic regression. An odds ratio (OR) and 95% confidence interval (CI) were calculated for each predictor. The reliability of the predictive models was determined by receiver operating curve (ROC) analysis with further calculation of the area under the curve (AUC), its CI, sensitivity (Se), specificity (Sp), and likelihood ratio (LR) for each predictor. The Youden test was used to estimate the cut-off points for irisin and its trajectory. We compared the incremental prognostic capacity of the models on a binary prediction methodology based on the estimation of integrated discrimination indices (IDI) and net reclassification improvement (NRI). A two-sided p < 0.05 was considered significant. The variables were tested using SPSS v. 23 (IBM, Armonk, New York, NY, USA) and GraphPad Prism v. 9 (GraphPad Software, San Diego, CA, USA).
3. Results
3.1. General Characteristics of the Patients
The study participants had a mean age of 56 years and 51.7% were male (Table 1). They had the following comorbidity and CV risk profile that included dyslipidemia (78.8%), hypertension (74.0%), stable coronary artery disease (31.5%), atrial fibrillation (17.1%), abdominal obesity (37.0%), left ventricular hypertrophy (90.4%), CKD 1–3 grades (26.0%), and smoking (41.7%). All the patients had stable hemodynamics at baseline, with a mean LVEF of 47% (ranging from 41% to 51%), an LV GLS of −14.6 (−12.6; −15.9), an E/e′ of 16 ± 5 units, and moderate LV and left atrial cavity dilation. The mean LV myocardial mass index and LAVI were 138 ± 11 g/m2 and 41 (35–50) mL/m2, respectively. The mean eGFR was 78 ± 18 mL/min/1.73 m2, and the mean HOMA-IR was 7.10 ± 2.5 units. The mean level of NT-proBNP was 615 (168–1145) pmol/mL. All the individuals received optimal therapy depending on their clinical state, phenotype of HF, fasting glucose, lipid profile and comorbidities, which included antihypertensive agents (angiotensin-converting enzyme inhibitors [ACEi], angiotensin-II receptor blockers [ARBs], calcium channel blockers, and thiazide-like diuretics), beta-blocker and ivabradine when needed, antiplatelet agents, and statins. Patients with atrial fibrillation were treated with anticoagulants. The therapy of HFpEF/HFmrEF included beta-blockers, ACEi or ARBs, sodium-glucose cotransporter-2 (SGLT2), and thiazide-like diuretics. The therapy of T2DM included diet, metformin, SGLT2 inhibitors, DPP-4 inhibitors, and glucagon-like peptide-1 receptor agonists (GLP-1-RAs).

Table 1.
General characteristics of eligible patients at admission.
We found that 38 patients met the criteria for kidney-related outcomes, whereas 108 individuals did not. According to these findings, we divided the entire group into two groups. The patients from both groups did not demonstrate significant differences in gender, demographic parameters (body mass index, waist circumference, waist-to-hip ratio), CV and comorbidity profile, systolic and diastolic blood pressure, hemodynamics, E/e′, LVMMI, LAVI, HOMA-IR, fasting glucose, glycated hemoglobin, lipids, hs-CRP, TNF-alpha, and NT-proBNP. However, the patients from the kidney-related event group were older, frequently had CAD, exhibited a higher serum creatinine and urinary albumin/creatinine ratio, and lower GLS, eGFR, and irisin levels, as well as being frequently treated with beta-blockers and rarely SGLT-2 inhibitors compared with those from another group.
3.2. Dynamic Changes of Irisin in the Patients Included in the Study
The changes in the biomarker levels during the observation period are reported in Figure 2. We found that the levels of NT-proBNP were not significantly changed in the group with the kidney-related events (Δ% = 3.5%; from 690 pmol/mL [25–75% IQR = 240–1270 pmol/mL] to 715 pmol/mL [25–75% IQR = 296–1112 pmol/mL], p = 0.64), whereas in the patients without kidney-related events, there was a borderline decrease in the serum levels of peptide (Δ% =−19.7%; from 589 pmol/mL [25–75% IQR = 190–994 pmol/mL] to 473 pmol/mL [25–75% IQR = 216–789 pmol/mL], p = 0.046).
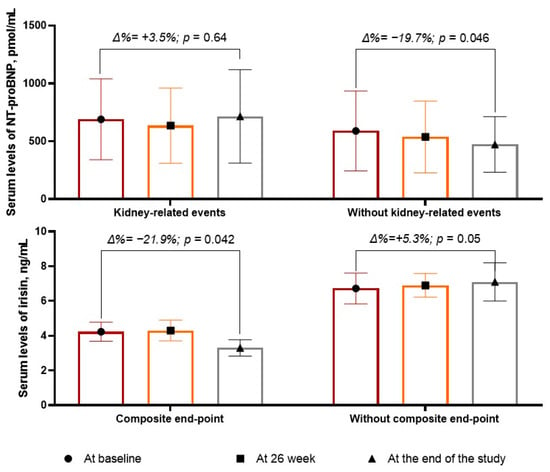
Figure 2.
The dynamic of serum levels of NT-proBNP and irisin.
The levels of irisin significantly (p = 0.05) increased in the patients without kidney-related events (Δ% = +5.3%, from 6.72 ng/mL [25–75% IQR = 5.17–8.10 ng/mL] to 7.10 ng/mL [25–75% IQR = 5.90–8.50 ng/mL], p = 0.05), whereas in those with kidney-related events, the levels of the biomarker decreased up to 21.9% (from 4.23 ng/mL; 25–75% IQR = 3.22–5.98 ng/mL to 3.30 ng/mL [25–75% IQR = 2.10–4.56 ng/mL]) with statistical significance (p = 0.042).
3.3. Spearman’s Correlation between the Levels of Biomarkers at Baseline and Other Parameters
The NT-proBNP levels were positively associated with E/e′ (r = 0.34, p = 0.001), LAVI (r = 0.31, p = 0.001), LV hypertrophy (r = 0.27, p = 0.044), and age (r = 0.22, p = 0.048) and were inversely associated with GLS (r = −0.35, p = 0.001) and eGFR (r = −0.32, p = 0.001). The irisin levels correlated positively with GLS (r = 0.38, p = 0.001) and negatively with LAVI (r = −0.32, p = 0.001), fasting plasma glucose (r = −0.30, p = 0.001), HOMA-IR (r = −0.27, p = 0.042), and HbA1c (r = −0.23, p = 0.046). There were no significant associations between the levels of circulating biomarkers, such as NT-proBNP, irisin, TNF-alpha, and hs-CRP with UACR.
3.4. Predictive Values of Serum Irisin at Baseline and the Trajectory of Irisin: The Results of the ROC Curve Analysis
We found that serum levels of irisin < 4.15 ng/mL (area under the curve [AUC] = 0.81; 95% confidence interval [CI] = 0.74–0.88; sensitivity [Se] = 78.7%, specificity [Sp] = 78.3%; likelihood ratio [LR] = 3.5; p = 0.0001) predicted kidney-related outcomes (Figure 3). Yet, a trend to decrease the levels of irisin (>20% from the baseline) exerted higher discriminative potency (AUC = 0.88; 95% CI = 0.83–0.93; Se = 82.9%, Sp = 79.3%; LR = 4.3; p < 0.0001) than its baseline level.
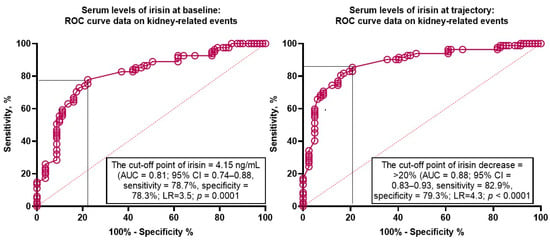
Figure 3.
Receiver operator curve analysis for kidney-related events: The optimal cut-off points of irisin levels at baseline and the trajectory to decrease. Abbreviations: AUC, area under the curve; CI, confidence interval; LR, likelihood ratio.
3.5. Predictors for Kidney-Related Events in T2DM Patients with Asymptomatic HFpEF: Univariate and Multivariate Logistic Regression Analysis
We used the median value for age and NT-proBNP at baseline for this analysis (Table 2). The univariate logistic regression unveiled that kidney-related events were not predicted by an age > 61 years (odds ratio [OR] = 1.03; p = 0.464) and serum levels of NT-proBNP ≥ 690 pmol/mL (OR = 1.06; p = 0.834), whereas irisin at a baseline of ≤ 4.15 ng/mL (OR = 1.16; p = 0.001), a decrease in irisin of >20% (OR = 1.24; p = 0.001), UACR (OR = 1.07; p = 0.012), and the use of SGLT2i (OR = 0.91; p = 0.044) did predict kidney-related events.

Table 2.
Predictors for kidney-related events: The results of the logistic regression.
The multivariate logistic regression revealed that irisin at baseline ≤ 4.15 ng/mL (OR = 1.14; p = 0.016), a decrease in irisin > 20% (OR = 1.27; p = 0.001), and the use of SGLT2i (OP = 0.94; p = 0.041) were independently associated with kidney-related events. The UACR exerted borderline significance for this dependent variable (OP = 1.04; p = 0.050).
3.6. Comparison of the Predictive Models
We compared predictive models for kidney-related outcomes and found that Model 1 (the discriminative value of irisin < 4.15 ng/mL) was not superior to Model 3 (administration of SGLT2i), whereas Model 2 (decrease in irisin levels >20%) was significantly better than the reference value (Table 3). Model 1 + Model 2 demonstrated better discriminative potency in comparison with Model 1, whereas Model 3 did not add sufficient predictive information to Model 2.

Table 3.
The comparisons of predictive models for kidney-related outcomes.
3.7. Reproducibility of Biomarker Trajectory
The evaluation of the reproducibility of the irisin trajectory was performed in comparison with NT-proBNP. The intra-class correlation coefficient for the inter-observer reproducibility of NT-proBNP was 0.94 (95% CI = 0.89–0.97), whereas the intra-class correlation coefficient for the intra-observer reproducibility of irisin was 0.92 (95% CI = 0.88–0.95).
4. Discussion
The results of the study revealed that levels of irisin ≤ 4.15 ng/mL and its decrease > 20% from the baseline level showed independent discriminative potency for kidney-related events in T2DM patients with asymptomatic HF. Recently, we established that irisin was a better predictor for the renoprotective effect of the SGLT2 inhibitor dapagliflosin in diabetics with chronic symptomatic HF, regardless of its phenotypes [30]. However, the predictive value of irisin at baseline and its trajectory for long-term observation among T2DM with concomitant asymptomatic HFpEF/HFmrEF was found first.
Although irisin has been previously considered a multifunctional myokine, which is activated by peroxisome proliferator-activated receptor γ coactivator-1α (PGC-1α) during physical exercise in skeletal muscle tissues and thereby links metabolic homeostasis with cardioprotection, its renoprotective capability remains uncertain [31,32]. However, low levels of irisin were found in several metabolic conditions (obesity, metabolic syndrome, metabolic fatty liver syndrome, peri-/post-menopausal osteoporosis) and cardiovascular diseases including heart failure, acute myocardial infarction, and stable coronary artery disease [33,34,35,36]. Nevertheless, lower irisin concentrations were found to be a powerful predictor for poor clinical outcomes, including premature death, in acute myocardial infarction and any phenotype of HF [37,38,39]. At the same time, an increase in irisin plasma levels in HFpEF/HFrEF individuals was associated with an improved clinical status, functional and structural cardiac performances, anti-oxidative, anti-fibrotic and anti-inflammatory effects, which was translated into a reduction in adverse outcomes [30,40,41].
It has been suggested that irisin is able to inhibit angiotensin-II-induced fibroblast trans-differentiation, collagen production, inflammation, and oxidative stress through the suppression of LOXL2 and TGF-β1 as well as via the phosphorylation of Smad2/3 and SIRT-related molecular mechanisms [41,42]. Indeed, several studies of HF, including HF-induced cachexia, have shown a negative correlation between the irisin concentration and hs-CRP, TNF-alpha, and interleukin-6 [43,44,45]. We did not find a sufficient association between irisin concentrations and inflammatory cytokines, such as hs-CRP and TNA-alpha, in patients without clinical presentation of cardiac dysfunction, while the levels of irisin were found to be decreased. Yet, among patients with asymptomatic HFpEF/HFmrEF, there were no serious differences in hemodynamics besides LV GLS depending on the presence of kidney-related events, whereas irisin concentrations were significantly lower in the group with kidney-related events compared with those who did not have a kidney-related event. These findings seem to show that the regulation of irisin and underlying molecular mechanisms, which correspond to the remodeling of remote organs, could distinguish patients with acute HF, severe HFrEF, or cardiac cachexia.
Cumulatively, we suggested that kidney protective mechanisms in T2DM with a conventional comorbidity profile (overweight, mild-to-moderate obesity, dyslipidemia, mild fasting hyperglycemia) are likely to be mediated by irisin, which is not only produced by skeletal muscles but also white adipose tissue and perivascular/pericardial adipose tissues and also acts through both local and central nervous system-related mechanisms, such as enhancing organ perfusion, inducing protective autophagy, alleviating apoptosis and fibrosis, improving kidney function, regulating incretin metabolism, and adipose tissue browning [44,45]. Consequently, irisin is involved in both cardiac and kidney protection by interacting with crucial signaling pathways in regulating glucose metabolism, lipid homeostasis, fibrosis, and inflammation [46]. As a result, a decreasing concentration of irisin is considered a key factor in the inadequate regulation of the endogenous repair system. Indeed, in animal studies, reduced skeletal muscle FNDC5 expression in ischemic cardiomyopathy was likely modulated by inflammatory cytokines and/or angiotensin-II via the down-regulated PGC-1α, which corresponded to low irisin production [47,48].
The lack of a significant predictive value of NT-proBNP in T2DM patients with asymptomatic cardiac dysfunction is another issue that requires a detailed explanation. It appeared that in this group of patients, the peptide did not demonstrate a diagnostically significant trend to predict renal events. Overall, we established that either low levels of irisin or a decrease in its concentration were more powerful predictors for the worsening of kidney function and related outcomes than NPs. Although the inclusion criteria were designed to avoid the overrepresentation of patients with low peptide values, these data confirm the limited ability of NPs to predict renal events in patients with HF in the absence of clinical manifestations and volume overload. Indeed, recent clinical trials for SGLT2i have shown that the benefits of these agents may be extended to patients regardless of T2DM on clinically meaningful HF [49,50]. At the same time, it remains unclear whether measures of NT-proBNP appear to be effective in predicting HF and non-HF outcomes in SGLT2i-treated individuals. The results of the study suggest that low levels of irisin deserve to be serially measured as a plausible predictive biomarker of a kidney-related clinical outcome. Of note, irisin also possesses anti-inflammatory, anti-oxidative, and anti-apoptotic properties, which make it associated with a transition from acute kidney injury to CKD as well as the development of CKD [51].
Although the patients who were included in the study had other reasons for reduced irisin levels besides T2DM, such as CAD, being obese, or being overweight, this does not explain why the lowest myokine concentration was found specifically in the group of patients who had kidney-related events. The comorbidities found are common in both diabetic patients and those with asymptomatic HF. Interestingly, SGLT2i treatment prevented, to some extent, the decrease in circulating irisin levels, which probably explains the obtained correlation between the administration of these drugs and the reduced risk of renal events. The positive effects of SGLT2i on renal structure and function as well as clinically relevant renal events are considered established in many randomized clinical trials [52,53]. However, the underlying mechanism of action by which this class of drugs can improve patient survival and prevent progressive deterioration of renal function remains a matter of scientific debate. The results of our study open prospects for understanding the pathophysiological role of irisin in the implementation of renoprotection and may have an impact on improving approaches to predict adverse renal events in the absence of other potential factors. At the same time, the mechanism by which the renoprotective effect of irisin is translated in T2DM patients with asymptomatic HFpEF/HFmrEF remains unclear and requires further research.
Study Limitations
The study has several limitations. First, we did not evaluate the metabolic and nutritional status of the patients; however, we recommended enhancing a convenient nutrition plan for them during the period of observation. Second, all the T2DM individuals were well treated with a combination of diet and contemporary agents to reach optimal glycaemia control. In fact, the results of the study seem to be extrapolated to a similar population. Third, we did not investigate in the study the role of SGLT2i, which was used as an antidiabetic agent. In addition, in our analyses, we did not evaluate the level of physical activity. Finally, we believe that these limitations will not intervene in the interpretation of the findings.
5. Conclusions
We established that a low baseline level of irisin (≤4.15 ng/mL) and its 20% decrease seem to be independently associated with newly kidney-related events in T2DM patients with asymptomatic cardiac dysfunction. The findings are likely to gain the perspectives of the personifying treatments of asymptomatic HFpEF/HFmrEF in T2DM to prevent kidney-related outcomes.
Author Contributions
Conceptualization, A.E.B.; methodology, M.L. and A.E.B.; software, A.E.B. and O.O.B.; validation, T.A.B., A.E.B., M.L. and U.C.H.; formal analysis, O.O.B., T.A.B. and A.E.B.; investigation, O.O.B. and T.A.B.; resources, T.A.B. and O.O.B.; data curation, A.E.B. and T.A.B.; writing—original draft preparation: T.A.B., O.O.B., U.C.H., M.L. and A.E.B.; writing—review and editing, T.A.B., O.O.B., U.C.H., M.L. and A.E.B.; visualization, O.O.B. and T.A.B.; supervision, A.E.B.; project administration, T.A.B. and O.O.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Institutional Ethics Committee of Zaporozhye Medical Academy of Post-graduate Education (protocol number: 8; date of approval: 10 October 2020).
Informed Consent Statement
Informed consent was received from all the individuals involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author due to privacy restrictions.
Acknowledgments
We thank all the patients who gave their consent to participate in the study and all the administrative staff and doctors of the Private Hospital “Vita-Center” for their study assistance.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Khan, M.A.B.; Hashim, M.J.; King, J.K.; Govender, R.D.; Mustafa, H.; Al Kaabi, J. Epidemiology of Type 2 Diabetes—Global Burden of Disease and Forecasted Trends. J. Epidemiol. Glob. Health 2020, 10, 107–111. [Google Scholar] [CrossRef] [PubMed]
- Tinajero, M.G.; Malik, V.S. An Update on the Epidemiology of Type 2 Diabetes: A Global Perspective. Endocrinol. Metab. Clin. N. Am. 2021, 50, 337–355. [Google Scholar] [CrossRef] [PubMed]
- Einarson, T.R.; Acs, A.; Ludwig, C.; Panton, U.H. Prevalence of cardiovascular disease in type 2 diabetes: A systematic literature review of scientific evidence from across the world in 2007–2017. Cardiovasc. Diabetol. 2018, 17, 83. [Google Scholar] [CrossRef]
- Ritchie, R.H.; Abel, E.D. Basic Mechanisms of Diabetic Heart Disease. Circ. Res. 2020, 126, 1501–1525. [Google Scholar] [CrossRef] [PubMed]
- Peng, M.L.; Fu, Y.; Wu, C.W.; Zhang, Y.; Ren, H.; Zhou, S.S. Signaling Pathways Related to Oxidative Stress in Diabetic Cardiomyopathy. Front. Endocrinol. 2022, 13, 907757. [Google Scholar] [CrossRef] [PubMed]
- Jia, G.; Hill, M.A.; Sowers, J.R. Diabetic Cardiomyopathy: An Update of Mechanisms Contributing to This Clinical Entity. Circ. Res. 2018, 122, 624–638. [Google Scholar] [CrossRef] [PubMed]
- Kato, M.; Natarajan, R. Epigenetics and epigenomics in diabetic kidney disease and metabolic memory. Nat. Rev. Nephrol. 2019, 15, 327–345. [Google Scholar] [CrossRef] [PubMed]
- Rottura, M.; Drago, S.F.A.; Gianguzzo, V.M.; Molonia, A.; Pallio, G.; Scoglio, R.; Marino, S.; Alibrandi, A.; Imbalzano, E.; Squadrito, F.; et al. Chronic kidney disease progression in diabetic patients: Real world data in general practice. Heliyon 2024, 10, e30787. [Google Scholar] [CrossRef] [PubMed]
- Jepson, C.; Hsu, J.Y.; Fischer, M.J.; Kusek, J.W.; Lash, J.P.; Ricardo, A.C.; Schelling, J.R.; Feldman, H.I.; Chronic Renal Insufficiency Cohort (CRIC) Study Investigators. Incident Type 2 Diabetes Among Individuals with CKD: Findings From the Chronic Renal Insufficiency Cohort (CRIC) Study. Am. J. Kidney Dis. 2019, 73, 72–81. [Google Scholar] [CrossRef] [PubMed]
- Vijay, K.; Neuen, B.L.; Lerma, E.V. Heart Failure in Patients with Diabetes and Chronic Kidney Disease: Challenges and Opportunities. Cardiorenal Med. 2022, 12, 1–10. [Google Scholar] [CrossRef] [PubMed]
- House, A.A.; Wanner, C.; Sarnak, M.J.; Piña, I.L.; McIntyre, C.W.; Komenda, P.; Kasiske, B.L.; Deswal, A.; deFilippi, C.R.; Cleland, J.G.F.; et al. Heart failure in chronic kidney disease: Conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) Controversies Conference. Kidney Int. 2019, 95, 1304–1317. [Google Scholar] [CrossRef] [PubMed]
- Löfman, I.; Szummer, K.; Dahlström, U.; Jernberg, T.; Lund, L.H. Associations with and prognostic impact of chronic kidney disease in heart failure with preserved, mid-range, and reduced ejection fraction. Eur. J. Heart Fail. 2017, 19, 1606–1614. [Google Scholar] [CrossRef] [PubMed]
- Ostermann, M.; Zarbock, A.; Goldstein, S.; Kashani, K.; Macedo, E.; Murugan, R.; Bell, M.; Forni, L.; Guzzi, L.; Joannidis, M.; et al. Recommendations on Acute Kidney Injury Biomarkers From the Acute Disease Quality Initiative Consensus Conference: A Consensus Statement. JAMA Netw. Open 2020, 3, e2019209, Erratum in JAMA Netw. Open 2020, 3, e2029182. [Google Scholar] [CrossRef] [PubMed]
- Folkerts, K.; Kelly, A.M.B.; Petruski-Ivleva, N.; Fried, L.; Blankenburg, M.; Gay, A.; Velentgas, P.; Kovesdy, C.P. Cardiovascular and Renal Outcomes in Patients with Type-2 Diabetes and Chronic Kidney Disease Identified in a United States Administrative Claims Database: A Population Cohort Study. Nephron 2021, 145, 342–352. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, C.; Tsukamoto, O.; Hasegawa, T.; Matsuoka, K.; Amaki, M.; Kanzaki, H.; Izumi, C.; Takashima, S.; Ito, S.; Kitakaze, M. Relative B-Type Natriuretic Peptide Deficiency May Exist in Diastolic Dysfunction in Subclinical Population. Circ. Rep. 2024, 6, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Bachmann, K.N.; Gupta, D.K.; Xu, M.; Brittain, E.; Farber-Eger, E.; Arora, P.; Collins, S.; Wells, Q.S.; Wang, T.J. Unexpectedly Low Natriuretic Peptide Levels in Patients with Heart Failure. JACC Heart Fail. 2021, 9, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Ho, M.Y.; Wang, C.Y. Role of Irisin in Myocardial Infarction, Heart Failure, and Cardiac Hypertrophy. Cells 2021, 10, 2103. [Google Scholar] [CrossRef] [PubMed]
- Colaianni, G.; Cinti, S.; Colucci, S.; Grano, M. Irisin and musculoskeletal health. Ann. N. Y Acad. Sci. 2017, 1402, 5–9. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Xie, S.; Guo, L.; Jiang, J.; Chen, H. Irisin: Linking metabolism with heart failure. Am. J. Transl. Res. 2020, 12, 6003–6014. [Google Scholar] [PubMed]
- Guo, M.; Yao, J.; Li, J.; Zhang, J.; Wang, D.; Zuo, H.; Zhang, Y.; Xu, B.; Zhong, Y.; Shen, F.; et al. Irisin ameliorates age-associated sarcopenia and metabolic dysfunction. J. Cachexia Sarcopenia Muscle 2023, 14, 391–405. [Google Scholar] [CrossRef] [PubMed]
- Qiongyue, Z.; Xin, Y.; Meng, P.; Sulin, M.; Yanlin, W.; Xinyi, L.; Xuemin, S. Post-treatment with Irisin Attenuates Acute Kidney Injury in Sepsis Mice Through Anti-Ferroptosis via the SIRT1/Nrf2 Pathway. Front. Pharmacol. 2022, 13, 857067. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, P.W.; Pang, Q.; Zhou, T.; Song, X.Y.; Pan, Y.J.; Jia, L.P.; Zhang, A.H. Irisin alleviates vascular calcification by inhibiting VSMC osteoblastic transformation and mitochondria dysfunction via AMPK/Drp1 signaling pathway in chronic kidney disease. Atherosclerosis 2022, 346, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Pinho-Jr, J.D.S.; Camacho, F.A.; Cavararo, C.D.S.; Baião, P.F.; Medeiros, R.F.; Barroso, S.G.; de Matos, A.C. Irisin and Cardiometabolic Disorders in Obesity: A Systematic Review. Int. J. Inflamm. 2023, 2023, 5810157. [Google Scholar] [CrossRef] [PubMed]
- Berezin, A.A.; Lichtenauer, M.; Boxhammer, E.; Fushtey, I.M.; Berezin, A.E. Serum Levels of Irisin Predict Cumulative Clinical Outcomes in Heart Failure Patients with Type 2 Diabetes Mellitus. Front. Physiol. 2022, 13, 922775. [Google Scholar] [CrossRef] [PubMed]
- Berezin, A.A.; Lichtenauer, M.; Boxhammer, E.; Stöhr, E.; Berezin, A.E. Discriminative Value of Serum Irisin in Prediction of Heart Failure with Different Phenotypes among Patients with Type 2 Diabetes Mellitus. Cells 2022, 11, 2794. [Google Scholar] [CrossRef] [PubMed]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, C.; Rahko, P.S.; Blauwet, L.A.; Canaday, B.; Finstuen, J.A.; Foster, M.C.; Horton, K.; Ogunyankin, K.O.; Palma, R.A.; Velazquez, E.J. Guidelines for Performing a Comprehensive Transthoracic Echocardiographic Examination in Adults: Recommendations from the American Society of Echocardiography. J. Am. Soc. Echocardiogr. 2019, 32, 1–64. [Google Scholar] [CrossRef] [PubMed]
- Levey, A.S.; Stevens, L.A.; Schmid, C.H.; Zhang, Y.L.; Castro, A.F., 3rd; Feldman, H.I.; Kusek, J.W.; Eggers, P.; Van Lente, F.; Greene, T.; et al. A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 2009, 150, 604–612. [Google Scholar] [CrossRef] [PubMed]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Berezina, T.A.; Fushtey, I.M.; Berezin, A.A.; Pavlov, S.V.; Berezin, A.E. Predictors of Kidney Function Outcomes and Their Relation to SGLT2 Inhibitor Dapagliflozin in Patients with Type 2 Diabetes Mellitus Who Had Chronic Heart Failure. Adv. Ther. 2024, 41, 292–314. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Cui, F.; Ning, K.; Wang, Z.; Fu, P.; Wang, D.; Xu, H. Role of irisin in physiology and pathology. Front. Endocrinol. 2022, 13, 962968. [Google Scholar] [CrossRef] [PubMed]
- Chhor, M.; Law, W.; Pavlovic, M.; Aksentijevic, D.; McGrath, K.; McClements, L. Diagnostic and prognostic biomarkers reflective of cardiac remodelling in diabetes mellitus: A scoping review. Diabet. Med. 2023, 40, e15064. [Google Scholar] [CrossRef] [PubMed]
- Maak, S.; Norheim, F.; Drevon, C.A.; Erickson, H.P. Progress and Challenges in the Biology of FNDC5 and Irisin. Endocr. Rev. 2021, 42, 436–456. [Google Scholar] [CrossRef] [PubMed]
- Vaduganathan, M.; Sattar, N.; Xu, J.; Butler, J.; Mahaffey, K.W.; Neal, B.; Shaw, W.; Rosenthal, N.; Pfeifer, M.; Hansen, M.K.; et al. Stress Cardiac Biomarkers, Cardiovascular and Renal Outcomes, and Response to Canagliflozin. J. Am. Coll. Cardiol. 2022, 79, 432–444. [Google Scholar] [CrossRef] [PubMed]
- Shen, S.; Gao, R.; Bei, Y.; Li, J.; Zhang, H.; Zhou, Y.; Yao, W.; Xu, D.; Zhou, F.; Jin, M.; et al. Serum Irisin Predicts Mortality Risk in Acute Heart Failure Patients. Cell Physiol. Biochem. 2017, 42, 615–622. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Mottaleb, N.A.; Galal, H.M.; El Maghraby, K.M.; Gadallah, A.I. Serum irisin level in myocardial infarction patients with or without heart failure. Can. J. Physiol. Pharmacol. 2019, 97, 932–938. [Google Scholar] [CrossRef] [PubMed]
- Castiglione, V.; Aimo, A.; Vergaro, G.; Saccaro, L.; Passino, C.; Emdin, M. Biomarkers for the diagnosis and management of heart failure. Heart Fail. Rev. 2022, 27, 625–643. [Google Scholar] [CrossRef] [PubMed]
- Silvestrini, A.; Bruno, C.; Vergani, E.; Venuti, A.; Favuzzi, A.M.R.; Guidi, F.; Nicolotti, N.; Meucci, E.; Mordente, A.; Mancini, A. Circulating irisin levels in heart failure with preserved or reduced ejection fraction: A pilot study. PLoS ONE 2019, 14, e0210320. [Google Scholar] [CrossRef] [PubMed]
- Berezin, A.A.; Obradovic, A.B.; Fushtey, I.M.; Berezina, T.A.; Lichtenauer, M.; Berezin, A.E. Low Plasma Levels of Irisin Predict Acutely Decompensated Heart Failure in Type 2 Diabetes Mellitus Patients with Chronic Heart Failure. J. Cardiovasc. Dev. Dis. 2023, 10, 136. [Google Scholar] [CrossRef] [PubMed]
- Huerta-Delgado, A.S.; Roffe-Vazquez, D.N.; Luna-Ceron, E.; Gonzalez-Gil, A.M.; Casillas-Fikentscher, A.; Villarreal-Calderon, J.R.; Enriquez, C.; de la Peña-Almaguer, E.; Castillo, E.C.; Silva-Platas, C.; et al. Association of irisin levels with cardiac magnetic resonance, inflammatory, and biochemical parameters in patients with chronic heart failure versus controls. Magn. Reson. Imaging 2022, 93, 62–72. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Luo, J.; Song, X.; Gu, W.; Wang, S.; Hao, S.; Dong, Z.; Ning, Z. Irisin attenuates angiotensin II-induced atrial fibrillation and atrial fibrosis via LOXL2 and TGFβ1/Smad2/3 signaling pathways. Iran. J. Basic. Med. Sci. 2023, 26, 717–724. [Google Scholar] [CrossRef] [PubMed]
- Flori, L.; Benedetti, G.; Calderone, V.; Testai, L. Hydrogen Sulfide and Irisin, Potential Allies in Ensuring Cardiovascular Health. Antioxidants 2024, 13, 543. [Google Scholar] [CrossRef] [PubMed]
- Sobieszek, G.; Powrózek, T.; Mazurek, M.; Skwarek-Dziekanowska, A.; Małecka-Massalska, T. Electrical and Hormonal Biomarkers in Cachectic Elderly Women with Chronic Heart Failure. J. Clin. Med. 2020, 9, 1021. [Google Scholar] [CrossRef] [PubMed]
- Li, R.L.; Wu, S.S.; Wu, Y.; Wang, X.X.; Chen, H.Y.; Xin, J.J.; Li, H.; Lan, J.; Xue, K.Y.; Li, X.; et al. Irisin alleviates pressure overload-induced cardiac hypertrophy by inducing protective autophagy via mTOR-independent activation of the AMPK-ULK1 pathway. J. Mol. Cell Cardiol. 2018, 121, 242–255. [Google Scholar] [CrossRef] [PubMed]
- Peng, Q.; Wang, X.; Wu, K.; Liu, K.; Wang, S.; Chen, X. Irisin attenuates H2O2-induced apoptosis in cardiomyocytes via microRNA-19b/AKT/mTOR signaling pathway. Int. J. Clin. Exp. Pathol. 2017, 10, 7707–7717. [Google Scholar] [PubMed] [PubMed Central]
- Berezin, A.A.; Obradovic, Z.; Novikov, E.V.; Boxhammer, E.; Lichtenauer, M.; Berezin, A.E. Interplay between Myokine Profile and Glycemic Control in Type 2 Diabetes Mellitus Patients with Heart Failure. Diagnostics 2022, 12, 2940. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Matsuo, Y.; Gleitsmann, K.; Mangner, N.; Werner, S.; Fischer, T.; Bowen, T.S.; Kricke, A.; Matsumoto, Y.; Kurabayashi, M.; Schuler, G.; et al. Fibronectin type III domain containing 5 expression in skeletal muscle in chronic heart failure-relevance of inflammatory cytokines. J. Cachexia Sarcopenia Muscle 2015, 6, 62–72. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Hu, C.; Wu, H.M.; Ma, Z.G.; Tang, Q.Z. Fibronectin type III domain-containing 5 in cardiovascular and metabolic diseases: A promising biomarker and therapeutic target. Acta Pharmacol. Sin. 2021, 42, 1390–1400. [Google Scholar] [CrossRef]
- Nassif, M.E.; Windsor, S.L.; Tang, F.; Khariton, Y.; Husain, M.; Inzucchi, S.E.; McGuire, D.K.; Pitt, B.; Scirica, B.M.; Austin, B.; et al. Dapagliflozin Effects on Biomarkers, Symptoms, and Functional Status in Patients with Heart Failure with Reduced Ejection Fraction: The DEFINE-HF Trial. Circulation 2019, 140, 1463–1476. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, H.; Soga, F.; Tatsumi, K.; Mochizuki, Y.; Sano, H.; Toki, H.; Matsumoto, K.; Shite, J.; Takaoka, H.; Doi, T.; et al. Positive effect of dapagliflozin on left ventricular longitudinal function for type 2 diabetic mellitus patients with chronic heart failure. Cardiovasc. Diabetol. 2020, 19, 6. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Lindholm, B. The role of irisin in kidney diseases. Clin. Chim. Acta 2024, 554, 117756. [Google Scholar] [CrossRef] [PubMed]
- Rossing, P.; Anker, S.D.; Filippatos, G.; Pitt, B.; Ruilope, L.M.; Birkenfeld, A.L.; McGill, J.B.; Rosas, S.E.; Joseph, A.; Gebel, M.; et al. Finerenone in Patients with Chronic Kidney Disease and Type 2 Diabetes by Sodium-Glucose Cotransporter 2 Inhibitor Treatment: The FIDELITY Analysis. Diabetes Care 2022, 45, 2991–2998. [Google Scholar] [CrossRef] [PubMed]
- Mark, P.B.; Sarafidis, P.; Ekart, R.; Ferro, C.J.; Balafa, O.; Fernandez-Fernandez, B.; Herrington, W.G.; Rossignol, P.; Del Vecchio, L.; Valdivielso, J.M.; et al. SGLT2i for evidence-based cardiorenal protection in diabetic and non-diabetic chronic kidney disease: A comprehensive review by EURECA-m and ERBP working groups of ERA. Nephrol. Dial. Transpl. 2023, 38, 2444–2455. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).