P-tau217 as a Reliable Blood-Based Marker of Alzheimer’s Disease
Abstract
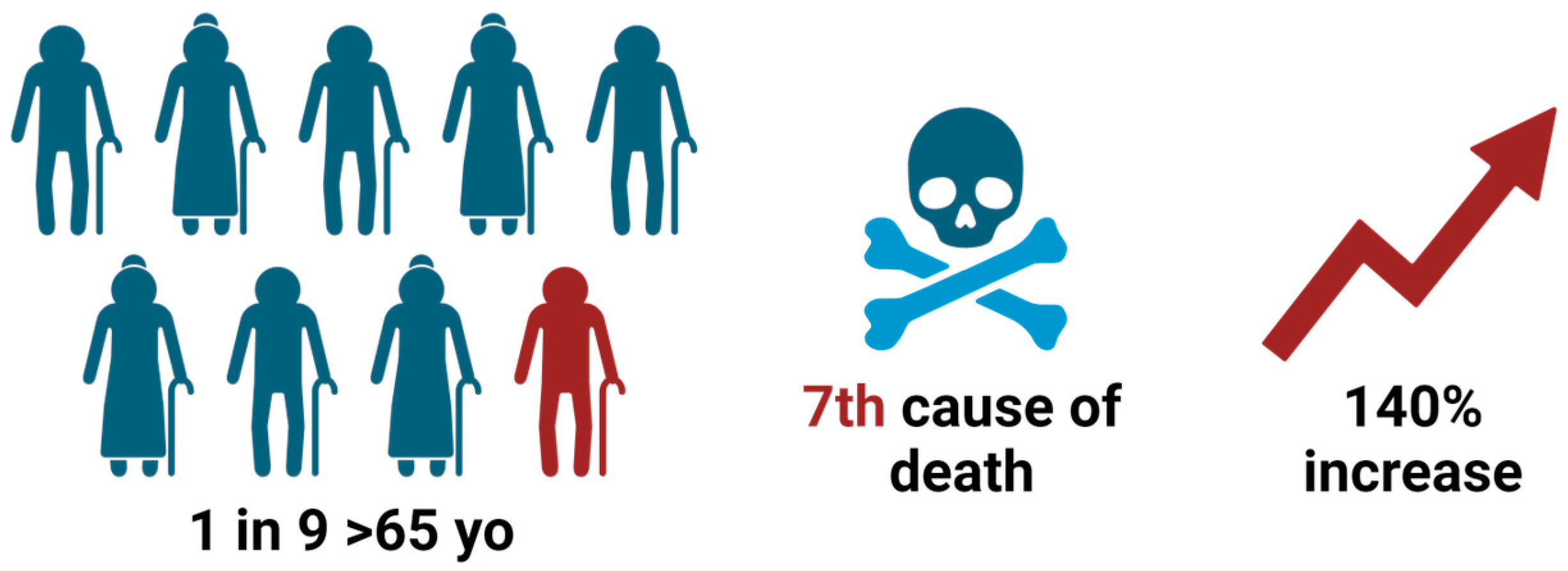
1. Introduction
2. AD Pathology and Biomarkers
3. Tau as a Biomarker
Tau in Distinct Brain Regions
4. P-tau217 in CSF and Plasma
4.1. CSF P-tau217
4.2. Plasma P-tau217
5. P-tau217 as an AD Biomarker
5.1. Clinical AD
5.2. Preclinical AD
5.3. Role of P-tau217 in AD Prognosis
5.4. Role of P-tau217 in Differentiating AD from Non-AD Dementia
6. Screening Assays for P-tau Analytes
| ELISA | Mass Spectrometry | CLEIA | |
|---|---|---|---|
| p-tau181 (plasma) | Sen: 86.6% [89] | Sen: No data | Sen: No data |
| Spe: 80.0% [89] | Spe: No data | Spe: No data | |
| AUC: 0.889 [89] | AUC: 0.98 [47] | AUC: 0.910 [90] | |
| p-tau217 (plasma) | Sen: 95% [91] | Sen: No data | Sen: No data |
| Spe: 94% [91] | Spe: No data | Spe: No data | |
| AUC: 0.98 [91] | AUC: 0.98 [47] | AUC: 0.952 [87] | |
| p-tau231 (plasma) | Sen: 81.2% [5] | Sen: No data | Sen: No data |
| Spe: 93.3% [5] | Spe: No data | Spe: No data | |
| AUC: 0.94 [5] | AUC: 0.834 [59] | AUC: No data | |
| p-tau181 (CSF) | Sen: 91.8% [89] | Sen: No data | Sen: 90% [86] |
| Spe: 90.5% [89] | Spe: No data | Spe: 90% [86] | |
| AUC: 0.954 [89] | AUC: 0.95 [47] | AUC: 0.98 [86] | |
| p-tau217 (CSF) | Sen: No data | Sen: No data | Sen: No data |
| Spe: No data | Spe: No data | Spe: No data | |
| AUC: 0.95 [41] | AUC: 1.00 [47] | AUC: No data | |
| p-tau231 (CSF) | Sen: No data | Sen: No data | Sen: No data |
| Spe: No data | Spe: No data | Spe: No data | |
| AUC: 0.88 [41] | AUC: 0.9873 [92] | AUC: No data |
7. Role of P-tau217 in Clinical Trials
8. Challenges and Limitations
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
| Aβ | amyloid-beta |
| Aβ42/Aβ40 | ratio of Aβ42 to Aβ40 |
| AD | Alzheimer’s disease |
| APP | amyloid precursor protein |
| AUC | area under the curve |
| BMI | body mass index |
| CERAD | Consortium to Establish a Registry for Alzheimer’s Disease |
| CKD | chronic kidney disease |
| CLEIA | chemiluminescent enzyme immunoassay |
| CSF | cerebrospinal fluid |
| CU | cognitively unimpaired |
| ELISA | enzyme-linked immunosorbent assay |
| EV | extracellular vesicle |
| FTD | frontotemporal dementia |
| GVB | granulovacuolar body |
| lvPPA | logopenic variant primary progressive aphasia |
| MCI-AD | mild cognitive impairment due to AD |
| MI | myocardial infarction |
| nbM | nucleus basalis of Meynert |
| NfL | neurofilament light chain |
| PCA | posterior cortical atrophy |
| PET | positron emission tomography |
| p-tau | phosphorylated tau |
| p-tau217 | phosphorylated tau at threonine 217 |
| p/t-tau | p-tau to t-tau ratio |
| pT217/T217 | ratio of phosphorylated to unphosphorylated tau |
| t-tau | total tau |
References
- Bloom, G.S. Amyloid-β and Tau: The Trigger and Bullet in Alzheimer Disease Pathogenesis. JAMA Neurol. 2014, 71, 505. [Google Scholar] [CrossRef] [PubMed]
- 2024 Alzheimer’s Disease Facts and Figures. Alzheimer’s Dement. 2024, 20, 3708–3821. [CrossRef]
- Nelson, P.T.; Alafuzoff, I.; Bigio, E.H.; Bouras, C.; Braak, H.; Cairns, N.J.; Castellani, R.J.; Crain, B.J.; Davies, P.; Tredici, K.D.; et al. Correlation of Alzheimer Disease Neuropathologic Changes With Cognitive Status: A Review of the Literature. J. Neuropathol. Exp. Neurol. 2012, 71, 362–381. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Ortiz, F.; Kac, P.R.; Brum, W.S.; Zetterberg, H.; Blennow, K.; Karikari, T.K. Plasma Phospho-Tau in Alzheimer’s Disease: Towards Diagnostic and Therapeutic Trial Applications. Mol. Neurodegener. 2023, 18, 18. [Google Scholar] [CrossRef] [PubMed]
- Pais, M.V.; Forlenza, O.V.; Diniz, B.S. Plasma Biomarkers of Alzheimer’s Disease: A Review of Available Assays, Recent Developments, and Implications for Clinical Practice. J. Alzheimer’s Dis. Rep. 2023, 7, 355–380. [Google Scholar] [CrossRef] [PubMed]
- Janelidze, S.; Stomrud, E.; Smith, R.; Palmqvist, S.; Mattsson, N.; Airey, D.C.; Proctor, N.K.; Chai, X.; Shcherbinin, S.; Sims, J.R.; et al. Cerebrospinal Fluid P-Tau217 Performs Better than p-Tau181 as a Biomarker of Alzheimer’s Disease. Nat. Commun. 2020, 11, 1683. [Google Scholar] [CrossRef] [PubMed]
- Wildsmith, K.R.; Barthélemy, N.R.; Bohorquez, S.S.; Manser, P.T.; Teng, E.; Ward, M.; Keeley, M.; Bateman, R.J.; Weimer, R. O3-14-02: Tau Burden Measured Using [18F]GTP1 Correlates with CSF TAU Phosphorylation at Sites T217 and T205 More Closely than T181. Alzheimer’s Dement. 2018, 14, P1059–P1060. [Google Scholar] [CrossRef]
- Hampel, H.; Hardy, J.; Blennow, K.; Chen, C.; Perry, G.; Kim, S.H.; Villemagne, V.L.; Aisen, P.; Vendruscolo, M.; Iwatsubo, T.; et al. The Amyloid-β Pathway in Alzheimer’s Disease. Mol. Psychiatry 2021, 26, 5481–5503. [Google Scholar] [CrossRef]
- Lichtenthaler, S.F. Alpha-Secretase in Alzheimer’s Disease: Molecular Identity, Regulation and Therapeutic Potential: Alpha-Secretase in Alzheimer’s Disease. J. Neurochem. 2011, 116, 10–21. [Google Scholar] [CrossRef]
- MacLeod, R.; Hillert, E.-K.; Cameron, R.T.; Baillie, G.S. The Role and Therapeutic Targeting of α-, β- and γ-Secretase in Alzheimer’s Disease. Future Sci. OA 2015, 1, FSO11. [Google Scholar] [CrossRef]
- Chow, V.W.; Mattson, M.P.; Wong, P.C.; Gleichmann, M. An Overview of APP Processing Enzymes and Products. Neuromol. Med. 2010, 12, 1–12. [Google Scholar] [CrossRef]
- Karisetty, B.C.; Bhatnagar, A.; Armour, E.M.; Beaver, M.; Zhang, H.; Elefant, F. Amyloid-β Peptide Impact on Synaptic Function and Neuroepigenetic Gene Control Reveal New Therapeutic Strategies for Alzheimer’s Disease. Front. Mol. Neurosci. 2020, 13, 577622. [Google Scholar] [CrossRef]
- Kinney, J.W.; Bemiller, S.M.; Murtishaw, A.S.; Leisgang, A.M.; Salazar, A.M.; Lamb, B.T. Inflammation as a Central Mechanism in Alzheimer’s Disease. AD Transl. Res. Clin. Interv. 2018, 4, 575–590. [Google Scholar] [CrossRef] [PubMed]
- Metaxas, A.; Kempf, S. Neurofibrillary Tangles in Alzheimer′s Disease: Elucidation of the Molecular Mechanism by Immunohistochemistry and Tau Protein Phospho-Proteomics. Neural Regen. Res. 2016, 11, 1579. [Google Scholar] [CrossRef] [PubMed]
- Beharry, C.; Cohen, L.S.; Di, J.; Ibrahim, K.; Briffa-Mirabella, S.; Alonso, A.D.C. Tau-Induced Neurodegeneration: Mechanisms and Targets. Neurosci. Bull. 2014, 30, 346–358. [Google Scholar] [CrossRef] [PubMed]
- Götz, J.; Chen, F.; Van Dorpe, J.; Nitsch, R.M. Formation of Neurofibrillary Tangles in P301L Tau Transgenic Mice Induced by Aβ42 Fibrils. Science 2001, 293, 1491–1495. [Google Scholar] [CrossRef] [PubMed]
- Lewis, J.; Dickson, D.W.; Lin, W.-L.; Chisholm, L.; Corral, A.; Jones, G.; Yen, S.-H.; Sahara, N.; Skipper, L.; Yager, D.; et al. Enhanced Neurofibrillary Degeneration in Transgenic Mice Expressing Mutant Tau and APP. Science 2001, 293, 1487–1491. [Google Scholar] [CrossRef] [PubMed]
- Leroy, K.; Ando, K.; Laporte, V.; Dedecker, R.; Suain, V.; Authelet, M.; Héraud, C.; Pierrot, N.; Yilmaz, Z.; Octave, J.-N.; et al. Lack of Tau Proteins Rescues Neuronal Cell Death and Decreases Amyloidogenic Processing of APP in APP/PS1 Mice. Am. J. Pathol. 2012, 181, 1928–1940. [Google Scholar] [CrossRef]
- Nussbaum, J.M.; Seward, M.E.; Bloom, G.S. Alzheimer Disease: A Tale of Two Prions. Prion 2013, 7, 14–19. [Google Scholar] [CrossRef]
- McKhann, G.; Drachman, D.; Folstein, M.; Katzman, R.; Price, D.; Stadlan, E.M. Clinical Diagnosis of Alzheimer’s Disease: Report of the NINCDS-ADRDA Work Group under the Auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology 1984, 34, 939–944. [Google Scholar] [CrossRef]
- Kovacs, G.G.; Milenkovic, I.; Wöhrer, A.; Höftberger, R.; Gelpi, E.; Haberler, C.; Hönigschnabl, S.; Reiner-Concin, A.; Heinzl, H.; Jungwirth, S.; et al. Non-Alzheimer Neurodegenerative Pathologies and Their Combinations Are More Frequent than Commonly Believed in the Elderly Brain: A Community-Based Autopsy Series. Acta Neuropathol. 2013, 126, 365–384. [Google Scholar] [CrossRef] [PubMed]
- Jack, C.R.; Bennett, D.A.; Blennow, K.; Carrillo, M.C.; Feldman, H.H.; Frisoni, G.B.; Hampel, H.; Jagust, W.J.; Johnson, K.A.; Knopman, D.S.; et al. A/T/N: An Unbiased Descriptive Classification Scheme for Alzheimer Disease Biomarkers. Neurology 2016, 87, 539–547. [Google Scholar] [CrossRef] [PubMed]
- Hampel, H.; Cummings, J.; Blennow, K.; Gao, P.; Jack, C.R.; Vergallo, A. Developing the ATX(N) Classification for Use across the Alzheimer Disease Continuum. Nat. Rev. Neurol. 2021, 17, 580–589. [Google Scholar] [CrossRef] [PubMed]
- Therriault, J.; Pascoal, T.A.; Lussier, F.Z.; Tissot, C.; Chamoun, M.; Bezgin, G.; Servaes, S.; Benedet, A.L.; Ashton, N.J.; Karikari, T.K.; et al. Biomarker Modeling of Alzheimer’s Disease Using PET-Based Braak Staging. Nat. Aging 2022, 2, 526–535. [Google Scholar] [CrossRef] [PubMed]
- The Dominantly Inherited Alzheimer Network; Barthélemy, N.R.; Li, Y.; Joseph-Mathurin, N.; Gordon, B.A.; Hassenstab, J.; Benzinger, T.L.S.; Buckles, V.; Fagan, A.M.; Perrin, R.J.; et al. A Soluble Phosphorylated Tau Signature Links Tau, Amyloid and the Evolution of Stages of Dominantly Inherited Alzheimer’s Disease. Nat. Med. 2020, 26, 398–407. [Google Scholar] [CrossRef] [PubMed]
- Palmqvist, S.; Janelidze, S.; Quiroz, Y.T.; Zetterberg, H.; Lopera, F.; Stomrud, E.; Su, Y.; Chen, Y.; Serrano, G.E.; Leuzy, A.; et al. Discriminative Accuracy of Plasma Phospho-Tau217 for Alzheimer Disease vs Other Neurodegenerative Disorders. JAMA 2020, 324, 772–781. [Google Scholar] [CrossRef] [PubMed]
- Suárez-Calvet, M.; Karikari, T.K.; Ashton, N.J.; Lantero Rodríguez, J.; Milà-Alomà, M.; Gispert, J.D.; Salvadó, G.; Minguillon, C.; Fauria, K.; Shekari, M.; et al. Novel Tau Biomarkers Phosphorylated at T181, T217 or T231 Rise in the Initial Stages of the Preclinical Alzheimer’s Continuum When Only Subtle Changes in Aβ Pathology Are Detected. EMBO Mol. Med. 2020, 12, e12921. [Google Scholar] [CrossRef] [PubMed]
- Hirota, Y.; Sakakibara, Y.; Ibaraki, K.; Takei, K.; Iijima, K.M.; Sekiya, M. Distinct Brain Pathologies Associated with Alzheimer’s Disease Biomarker-Related Phospho-Tau 181 and Phospho-Tau 217 in App Knock-in Mouse Models of Amyloid-β Amyloidosis. Brain Commun. 2022, 4, fcac286. [Google Scholar] [CrossRef] [PubMed]
- Hansson, O. Biomarkers for Neurodegenerative Diseases. Nat. Med. 2021, 27, 954–963. [Google Scholar] [CrossRef]
- Wennström, M.; Janelidze, S.; Nilsson, K.P.R.; The Netherlands Brain Bank; Serrano, G.E.; Beach, T.G.; Dage, J.L.; Hansson, O. Cellular Localization of P-Tau217 in Brain and Its Association with p-Tau217 Plasma Levels. Acta Neuropathol. Commun. 2022, 10, 3. [Google Scholar] [CrossRef]
- Murray, M.E.; Moloney, C.M.; Kouri, N.; Syrjanen, J.A.; Matchett, B.J.; Rothberg, D.M.; Tranovich, J.F.; Sirmans, T.N.H.; Wiste, H.J.; Boon, B.D.C.; et al. Global Neuropathologic Severity of Alzheimer’s Disease and Locus Coeruleus Vulnerability Influences Plasma Phosphorylated Tau Levels. Mol. Neurodegener. 2022, 17, 85. [Google Scholar] [CrossRef] [PubMed]
- Prinzi, C.; Kostenko, A.; De Leo, G.; Gulino, R.; Leanza, G.; Caccamo, A. Selective Noradrenaline Depletion in the Neocortex and Hippocampus Induces Working Memory Deficits and Regional Occurrence of Pathological Proteins. Biology 2023, 12, 1264. [Google Scholar] [CrossRef]
- Murphy, G.M.; Greenberg, B.D.; Ellis, W.G.; Forno, L.S.; Salamat, S.M.; Gonzalez-DeWhitt, P.A.; Lowery, D.E.; Tinklenberg, J.R.; Eng, L.F. Alzheimer’s Disease. Beta-Amyloid Precursor Protein Expression in the Nucleus Basalis of Meynert. Am. J. Pathol. 1992, 141, 357–361. [Google Scholar]
- Thal, D.R.; Del Tredici, K.; Ludolph, A.C.; Hoozemans, J.J.M.; Rozemuller, A.J.; Braak, H.; Knippschild, U. Stages of Granulovacuolar Degeneration: Their Relation to Alzheimer’s Disease and Chronic Stress Response. Acta Neuropathol. 2011, 122, 577–589. [Google Scholar] [CrossRef]
- Koper, M.J.; Van Schoor, E.; Ospitalieri, S.; Vandenberghe, R.; Vandenbulcke, M.; Von Arnim, C.A.F.; Tousseyn, T.; Balusu, S.; De Strooper, B.; Thal, D.R. Necrosome Complex Detected in Granulovacuolar Degeneration Is Associated with Neuronal Loss in Alzheimer’s Disease. Acta Neuropathol. 2020, 139, 463–484. [Google Scholar] [CrossRef]
- Leuzy, A.; Janelidze, S.; Mattsson-Carlgren, N.; Palmqvist, S.; Jacobs, D.; Cicognola, C.; Stomrud, E.; Vanmechelen, E.; Dage, J.L.; Hansson, O. Comparing the Clinical Utility and Diagnostic Performance of CSF P-Tau181, P-Tau217, and P-Tau231 Assays. Neurology 2021, 97, e1681–e1694. [Google Scholar] [CrossRef] [PubMed]
- Karikari, T.K.; Emeršič, A.; Vrillon, A.; Lantero-Rodriguez, J.; Ashton, N.J.; Kramberger, M.G.; Dumurgier, J.; Hourregue, C.; Čučnik, S.; Brinkmalm, G.; et al. Head-to-head Comparison of Clinical Performance of CSF Phospho-tau T181 and T217 Biomarkers for Alzheimer’s Disease Diagnosis. Alzheimer’s Dement. 2021, 17, 755–767. [Google Scholar] [CrossRef] [PubMed]
- Barthélemy, N.R.; Toth, B.; Manser, P.T.; Sanabria-Bohórquez, S.; Teng, E.; Keeley, M.; Bateman, R.J.; Weimer, R.M.; Wildsmith, K.R. Site-Specific Cerebrospinal Fluid Tau Hyperphosphorylation in Response to Alzheimer’s Disease Brain Pathology: Not All Tau Phospho-Sites Are Hyperphosphorylated. J. Alzheimer’s Dis. 2022, 85, 415–429. [Google Scholar] [CrossRef]
- Meeker, K.L.; Luckett, P.H.; Barthélemy, N.R.; Hobbs, D.A.; Chen, C.; Bollinger, J.; Ovod, V.; Flores, S.; Keefe, S.; Henson, R.L.; et al. Comparison of Cerebrospinal Fluid, Plasma and Neuroimaging Biomarker Utility in Alzheimer’s Disease. Brain Commun. 2024, 6, fcae081. [Google Scholar] [CrossRef]
- Zhang, D.; Zhang, W.; Ming, C.; Gao, X.; Yuan, H.; Lin, X.; Mao, X.; Wang, C.; Guo, X.; Du, Y.; et al. P-Tau217 Correlates with Neurodegeneration in Alzheimer’s Disease, and Targeting p-Tau217 with Immunotherapy Ameliorates Murine Tauopathy. Neuron 2024, 112, 1676–1693.e12. [Google Scholar] [CrossRef]
- Mendes, A.J.; Ribaldi, F.; Lathuiliere, A.; Ashton, N.J.; Janelidze, S.; Zetterberg, H.; Scheffler, M.; Assal, F.; Garibotto, V.; Blennow, K.; et al. Head-to-Head Study of Diagnostic Accuracy of Plasma and Cerebrospinal Fluid p-Tau217 versus p-Tau181 and p-Tau231 in a Memory Clinic Cohort. J. Neurol. 2024, 271, 2053–2066. [Google Scholar] [CrossRef] [PubMed]
- Brickman, A.M.; Manly, J.J.; Honig, L.S.; Sanchez, D.; Reyes-Dumeyer, D.; Lantigua, R.A.; Lao, P.J.; Stern, Y.; Vonsattel, J.P.; Teich, A.F.; et al. Plasma P-tau181, P-tau217, and Other Blood-based Alzheimer’s Disease Biomarkers in a Multi-ethnic, Community Study. Alzheimer’s Dement. 2021, 17, 1353–1364. [Google Scholar] [CrossRef] [PubMed]
- Arranz, J.; Zhu, N.; Rubio-Guerra, S.; Rodríguez-Baz, Í.; Ferrer, R.; Carmona-Iragui, M.; Barroeta, I.; Illán-Gala, I.; Santos-Santos, M.; Fortea, J.; et al. Diagnostic Performance of Plasma pTau 217, pTau 181, Aβ 1-42 and Aβ 1-40 in the LUMIPULSE Automated Platform for the Detection of Alzheimer Disease. Res. Sq. 2023, preprint. [Google Scholar] [CrossRef]
- Milà-Alomà, M.; Ashton, N.J.; Shekari, M.; Salvadó, G.; Ortiz-Romero, P.; Montoliu-Gaya, L.; Benedet, A.L.; Karikari, T.K.; Lantero-Rodriguez, J.; Vanmechelen, E.; et al. Plasma P-Tau231 and p-Tau217 as State Markers of Amyloid-β Pathology in Preclinical Alzheimer’s Disease. Nat. Med. 2022, 28, 1797–1801. [Google Scholar] [CrossRef] [PubMed]
- Therriault, J.; Vermeiren, M.; Servaes, S.; Tissot, C.; Ashton, N.J.; Benedet, A.L.; Karikari, T.K.; Lantero-Rodriguez, J.; Brum, W.S.; Lussier, F.Z.; et al. Association of Phosphorylated Tau Biomarkers With Amyloid Positron Emission Tomography vs Tau Positron Emission Tomography. JAMA Neurol. 2023, 80, 188–199. [Google Scholar] [CrossRef] [PubMed]
- Thijssen, E.H.; La Joie, R.; Strom, A.; Fonseca, C.; Iaccarino, L.; Wolf, A.; Spina, S.; Allen, I.E.; Cobigo, Y.; Heuer, H.; et al. Plasma Phosphorylated Tau 217 and Phosphorylated Tau 181 as Biomarkers in Alzheimer’s Disease and Frontotemporal Lobar Degeneration: A Retrospective Diagnostic Performance Study. Lancet Neurol. 2021, 20, 739–752. [Google Scholar] [CrossRef] [PubMed]
- Barthélemy, N.R.; Horie, K.; Sato, C.; Bateman, R.J. Blood Plasma Phosphorylated-Tau Isoforms Track CNS Change in Alzheimer’s Disease. J. Exp. Med. 2020, 217, e20200861. [Google Scholar] [CrossRef]
- Mohs, R.C.; Beauregard, D.; Dwyer, J.; Gaudioso, J.; Bork, J.; MaGee-Rodgers, T.; Key, M.N.; Kerwin, D.R.; Hughes, L.; Cordell, C.B.; et al. The Bio-Hermes Study: Biomarker Database Developed to Investigate Blood-based and Digital Biomarkers in Community-based, Diverse Populations Clinically Screened for Alzheimer’s Disease. Alzheimer’s Dement. 2024, 20, 2752–2765. [Google Scholar] [CrossRef]
- Pontecorvo, M.J.; Lu, M.; Burnham, S.C.; Schade, A.E.; Dage, J.L.; Shcherbinin, S.; Collins, E.C.; Sims, J.R.; Mintun, M.A. Association of Donanemab Treatment With Exploratory Plasma Biomarkers in Early Symptomatic Alzheimer Disease: A Secondary Analysis of the TRAILBLAZER-ALZ Randomized Clinical Trial. JAMA Neurol. 2022, 79, 1250–1259. [Google Scholar] [CrossRef]
- Pichet Binette, A.; Janelidze, S.; Cullen, N.; Dage, J.L.; Bateman, R.J.; Zetterberg, H.; Blennow, K.; Stomrud, E.; Mattsson-Carlgren, N.; Hansson, O. Confounding Factors of Alzheimer’s Disease Plasma Biomarkers and Their Impact on Clinical Performance. Alzheimer’s Dement. 2023, 19, 1403–1414. [Google Scholar] [CrossRef]
- Therriault, J.; Servaes, S.; Tissot, C.; Rahmouni, N.; Ashton, N.J.; Benedet, A.L.; Karikari, T.K.; Macedo, A.C.; Lussier, F.Z.; Stevenson, J.; et al. Equivalence of Plasma P-tau217 with Cerebrospinal Fluid in the Diagnosis of Alzheimer’s Disease. Alzheimer’s Dement. 2023, 19, 4967–4977. [Google Scholar] [CrossRef] [PubMed]
- Ashton, N.J.; Brum, W.S.; Di Molfetta, G.; Benedet, A.L.; Arslan, B.; Jonaitis, E.; Langhough, R.E.; Cody, K.; Wilson, R.; Carlsson, C.M.; et al. Diagnostic Accuracy of a Plasma Phosphorylated Tau 217 Immunoassay for Alzheimer Disease Pathology. JAMA Neurol. 2024, 81, 255. [Google Scholar] [CrossRef] [PubMed]
- Ossenkoppele, R.; Van Der Kant, R.; Hansson, O. Tau Biomarkers in Alzheimer’s Disease: Towards Implementation in Clinical Practice and Trials. Lancet Neurol. 2022, 21, 726–734. [Google Scholar] [CrossRef]
- Barthélemy, N.R.; Salvadó, G.; Schindler, S.E.; He, Y.; Janelidze, S.; Collij, L.E.; Saef, B.; Henson, R.L.; Chen, C.D.; Gordon, B.A.; et al. Highly Accurate Blood Test for Alzheimer’s Disease Is Similar or Superior to Clinical Cerebrospinal Fluid Tests. Nat. Med. 2024, 30, 1085–1095. [Google Scholar] [CrossRef]
- Woo, M.S.; Tissot, C.; Lantero-Rodriguez, J.; Snellman, A.; Therriault, J.; Rahmouni, N.; Macedo, A.C.; Servaes, S.; Wang, Y.; Arias, J.F.; et al. Plasma pTau-217 and N-terminal Tau (NTA) Enhance Sensitivity to Identify Tau PET Positivity in Amyloid-β Positive Individuals. Alzheimer’s Dement. 2023, 20, 1166–1174. [Google Scholar] [CrossRef] [PubMed]
- Jack, C.R.; Wiste, H.J.; Algeciras-Schimnich, A.; Figdore, D.J.; Schwarz, C.G.; Lowe, V.J.; Ramanan, V.K.; Vemuri, P.; Mielke, M.M.; Knopman, D.S.; et al. Predicting Amyloid PET and Tau PET Stages with Plasma Biomarkers. Brain 2023, 146, 2029–2044. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, P.C.L.; Therriault, J.; Tissot, C.; Ferrari-Souza, J.P.; Benedet, A.L.; Povala, G.; Bellaver, B.; Leffa, D.T.; Brum, W.S.; Lussier, F.Z.; et al. Plasma P-tau231 and P-tau217 Inform on Tau Tangles Aggregation in Cognitively Impaired Individuals. Alzheimer’s Dement. 2023, 19, 4463–4474. [Google Scholar] [CrossRef] [PubMed]
- Janelidze, S.; Palmqvist, S.; Leuzy, A.; Stomrud, E.; Verberk, I.M.W.; Zetterberg, H.; Ashton, N.J.; Pesini, P.; Sarasa, L.; Allué, J.A.; et al. Detecting Amyloid Positivity in Early Alzheimer’s Disease Using Combinations of Plasma Aβ42/Aβ40 and P-tau. Alzheimer’s Dement. 2022, 18, 283–293. [Google Scholar] [CrossRef]
- Montoliu-Gaya, L.; Alosco, M.L.; Yhang, E.; Tripodis, Y.; Sconzo, D.; Ally, M.; Grötschel, L.; Ashton, N.J.; Lantero-Rodriguez, J.; Sauer, M.; et al. Optimal Blood Tau Species for the Detection of Alzheimer’s Disease Neuropathology: An Immunoprecipitation Mass Spectrometry and Autopsy Study. Acta Neuropathol. 2024, 147, 5. [Google Scholar] [CrossRef]
- Salvadó, G.; Ossenkoppele, R.; Ashton, N.J.; Beach, T.G.; Serrano, G.E.; Reiman, E.M.; Zetterberg, H.; Mattsson-Carlgren, N.; Janelidze, S.; Blennow, K.; et al. Specific Associations between Plasma Biomarkers and Postmortem Amyloid Plaque and Tau Tangle Loads. EMBO Mol. Med. 2023, 15, e17123. [Google Scholar] [CrossRef]
- Zabala-Findlay, A.; Penny, L.K.; Lofthouse, R.A.; Porter, A.J.; Palliyil, S.; Harrington, C.R.; Wischik, C.M.; Arastoo, M. Utility of Blood-Based Tau Biomarkers for Mild Cognitive Impairment and Alzheimer’s Disease: Systematic Review and Meta-Analysis. Cells 2023, 12, 1184. [Google Scholar] [CrossRef] [PubMed]
- Yamoah, A.; Tripathi, P.; Sechi, A.; Köhler, C.; Guo, H.; Chandrasekar, A.; Nolte, K.W.; Wruck, C.J.; Katona, I.; Anink, J.; et al. Aggregates of RNA Binding Proteins and ER Chaperones Linked to Exosomes in Granulovacuolar Degeneration of the Alzheimer’s Disease Brain. J. Alzheimer’s Dis. 2020, 75, 139–156. [Google Scholar] [CrossRef] [PubMed]
- Jonaitis, E.M.; Janelidze, S.; Cody, K.A.; Langhough, R.; Du, L.; Chin, N.A.; Mattsson-Carlgren, N.; Hogan, K.J.; Christian, B.T.; Betthauser, T.J.; et al. Plasma Phosphorylated Tau 217 in Preclinical Alzheimer’s Disease. Brain Commun. 2023, 5, fcad057. [Google Scholar] [CrossRef] [PubMed]
- Ashton, N.J.; Janelidze, S.; Mattsson-Carlgren, N.; Binette, A.P.; Strandberg, O.; Brum, W.S.; Karikari, T.K.; González-Ortiz, F.; Di Molfetta, G.; Meda, F.J.; et al. Differential Roles of Aβ42/40, p-Tau231 and p-Tau217 for Alzheimer’s Trial Selection and Disease Monitoring. Nat. Med. 2022, 28, 2555–2562. [Google Scholar] [CrossRef] [PubMed]
- Mielke, M.M.; Aakre, J.A.; Algeciras-Schimnich, A.; Proctor, N.K.; Machulda, M.M.; Eichenlaub, U.; Knopman, D.S.; Vemuri, P.; Graff-Radford, J.; Jack, C.R.; et al. Comparison of CSF Phosphorylated Tau 181 and 217 for Cognitive Decline. Alzheimer’s Dement. 2022, 18, 602–611. [Google Scholar] [CrossRef] [PubMed]
- Mattsson-Carlgren, N.; Janelidze, S.; Palmqvist, S.; Cullen, N.; Svenningsson, A.L.; Strandberg, O.; Mengel, D.; Walsh, D.M.; Stomrud, E.; Dage, J.L.; et al. Longitudinal Plasma P-Tau217 Is Increased in Early Stages of Alzheimer’s Disease. Brain 2020, 143, 3234–3241. [Google Scholar] [CrossRef] [PubMed]
- Hansson, O.; Blennow, K.; Zetterberg, H.; Dage, J. Blood Biomarkers for Alzheimer’s Disease in Clinical Practice and Trials. Nat. Aging 2023, 3, 506–519. [Google Scholar] [CrossRef] [PubMed]
- Palmqvist, S.; Tideman, P.; Cullen, N.; Zetterberg, H.; Blennow, K.; the Alzheimer’s Disease Neuroimaging Initiative; Dage, J.L.; Stomrud, E.; Janelidze, S.; Mattsson-Carlgren, N.; et al. Prediction of Future Alzheimer’s Disease Dementia Using Plasma Phospho-Tau Combined with Other Accessible Measures. Nat. Med. 2021, 27, 1034–1042. [Google Scholar] [CrossRef] [PubMed]
- Mattsson-Carlgren, N.; Salvadó, G.; Ashton, N.J.; Tideman, P.; Stomrud, E.; Zetterberg, H.; Ossenkoppele, R.; Betthauser, T.J.; Cody, K.A.; Jonaitis, E.M.; et al. Prediction of Longitudinal Cognitive Decline in Preclinical Alzheimer Disease Using Plasma Biomarkers. JAMA Neurol. 2023, 80, 360–369. [Google Scholar] [CrossRef]
- Gicas, K.M.; Honer, W.G.; Petyuk, V.A.; Wilson, R.S.; Boyle, P.A.; Leurgans, S.E.; Schneider, J.A.; De Jager, P.L.; Bennett, D.A. Primacy and Recency Effects in Verbal Memory Are Differentially Associated with Post-Mortem Frontal Cortex p-Tau 217 and 202 Levels in a Mixed Sample of Community-Dwelling Older Adults. J. Clin. Exp. Neuropsychol. 2023, 45, 770–785. [Google Scholar] [CrossRef]
- Campese, N.; Palermo, G.; Del Gamba, C.; Beatino, M.F.; Galgani, A.; Belli, E.; Del Prete, E.; Della Vecchia, A.; Vergallo, A.; Siciliano, G.; et al. Progress Regarding the Context-of-Use of Tau as Biomarker of Alzheimer’s Disease and Other Neurodegenerative Diseases. Expert Rev. Proteom. 2021, 18, 27–48. [Google Scholar] [CrossRef] [PubMed]
- Arai, H.; Morikawa, Y.; Higuchi, M.; Matsui, T.; Clark, C.M.; Miura, M.; Machida, N.; Lee, V.M.-Y.; Trojanowski, J.Q.; Sasaki, H. Cerebrospinal Fluid Tau Levels in Neurodegenerative Diseases with Distinct Tau-Related Pathology. Biochem. Biophys. Res. Commun. 1997, 236, 262–264. [Google Scholar] [CrossRef]
- Schoonenboom, N.S.M.; Reesink, F.E.; Verwey, N.A.; Kester, M.I.; Teunissen, C.E.; Van De Ven, P.M.; Pijnenburg, Y.A.L.; Blankenstein, M.A.; Rozemuller, A.J.; Scheltens, P.; et al. Cerebrospinal Fluid Markers for Differential Dementia Diagnosis in a Large Memory Clinic Cohort. Neurology 2012, 78, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Mukaetova-Ladinska, E.B.; Monteith, R.; Perry, E.K. Cerebrospinal Fluid Biomarkers for Dementia with Lewy Bodies. Int. J. Alzheimer’s Dis. 2010, 2010, 536538. [Google Scholar] [CrossRef] [PubMed]
- Abu-Rumeileh, S.; Mometto, N.; Bartoletti-Stella, A.; Polischi, B.; Oppi, F.; Poda, R.; Stanzani-Maserati, M.; Cortelli, P.; Liguori, R.; Capellari, S.; et al. Cerebrospinal Fluid Biomarkers in Patients with Frontotemporal Dementia Spectrum: A Single-Center Study. J. Alzheimer’s Dis. 2018, 66, 551–563. [Google Scholar] [CrossRef] [PubMed]
- Meeter, L.H.H.; Vijverberg, E.G.; Del Campo, M.; Rozemuller, A.J.M.; Donker Kaat, L.; De Jong, F.J.; Van Der Flier, W.M.; Teunissen, C.E.; Van Swieten, J.C.; Pijnenburg, Y.A.L. Clinical Value of Neurofilament and Phospho-Tau/Tau Ratio in the Frontotemporal Dementia Spectrum. Neurology 2018, 90, e1231–e1239. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Tian, C.; Shi, Y.; Song, X.-R.; Yin, W.; Tao, Q.-Q.; Liu, J.; Peng, G.-P.; Wu, Z.-Y.; Wang, Y.-J.; et al. Blood-Based CNS Regionally and Neuronally Enriched Extracellular Vesicles Carrying pTau217 for Alzheimer’s Disease Diagnosis and Differential Diagnosis. Acta Neuropathol. Commun. 2024, 12, 38. [Google Scholar] [CrossRef] [PubMed]
- Hanes, J.; Kovac, A.; Kvartsberg, H.; Kontsekova, E.; Fialova, L.; Katina, S.; Kovacech, B.; Stevens, E.; Hort, J.; Vyhnalek, M.; et al. Evaluation of a Novel Immunoassay to Detect P-Tau Thr217 in the CSF to Distinguish Alzheimer Disease from Other Dementias. Neurology 2020, 95, e3026–e3035. [Google Scholar] [CrossRef] [PubMed]
- Ashton, N.J.; Janelidze, S.; Al Khleifat, A.; Leuzy, A.; Van Der Ende, E.L.; Karikari, T.K.; Benedet, A.L.; Pascoal, T.A.; Lleó, A.; Parnetti, L.; et al. A Multicentre Validation Study of the Diagnostic Value of Plasma Neurofilament Light. Nat. Commun. 2021, 12, 3400. [Google Scholar] [CrossRef]
- Korecka, M.; Shaw, L.M. Mass Spectrometry-based Methods for Robust Measurement of Alzheimer’s Disease Biomarkers in Biological Fluids. J. Neurochem. 2021, 159, 211–233. [Google Scholar] [CrossRef]
- Buerger, K.; Zinkowski, R.; Teipel, S.J.; Tapiola, T.; Arai, H.; Blennow, K.; Andreasen, N.; Hofmann-Kiefer, K.; DeBernardis, J.; Kerkman, D.; et al. Differential Diagnosis of Alzheimer Disease With Cerebrospinal Fluid Levels of Tau Protein Phosphorylated at Threonine 231. Arch. Neurol. 2002, 59, 1267–1272. [Google Scholar] [CrossRef] [PubMed]
- Barthélemy, N.R.; Mallipeddi, N.; Moiseyev, P.; Sato, C.; Bateman, R.J. Tau Phosphorylation Rates Measured by Mass Spectrometry Differ in the Intracellular Brain vs. Extracellular Cerebrospinal Fluid Compartments and Are Differentially Affected by Alzheimer’s Disease. Front. Aging Neurosci. 2019, 11, 121. [Google Scholar] [CrossRef] [PubMed]
- Janelidze, S.; Bali, D.; Ashton, N.J.; Barthélemy, N.R.; Vanbrabant, J.; Stoops, E.; Vanmechelen, E.; He, Y.; Dolado, A.O.; Triana-Baltzer, G.; et al. Head-to-Head Comparison of 10 Plasma Phospho-Tau Assays in Prodromal Alzheimer’s Disease. Brain 2023, 146, 1592–1601. [Google Scholar] [CrossRef] [PubMed]
- Therriault, J.; Woo, M.S.; Salvadó, G.; Gobom, J.; Karikari, T.K.; Janelidze, S.; Servaes, S.; Rahmouni, N.; Tissot, C.; Ashton, N.J.; et al. Comparison of Immunoassay- with Mass Spectrometry-Derived p-Tau Quantification for the Detection of Alzheimer’s Disease Pathology. Mol. Neurodegener. 2024, 19, 2. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.; Shah, S.H.; Salman, M.; Abdullah, M.; Hayat, F.; Akbar, S. Enzyme-Linked Immunosorbent Assay versus Chemiluminescent Immunoassay: A General Overview. Glob. J. Med. Pharm. Biomed. Update 2023, 18, 1. [Google Scholar] [CrossRef]
- Arcaro, M.; Fenoglio, C.; Serpente, M.; Arighi, A.; Fumagalli, G.G.; Sacchi, L.; Floro, S.; D’Anca, M.; Sorrentino, F.; Visconte, C.; et al. A Novel Automated Chemiluminescence Method for Detecting Cerebrospinal Fluid Amyloid-Beta 1-42 and 1-40, Total Tau and Phosphorylated-Tau: Implications for Improving Diagnostic Performance in Alzheimer’s Disease. Biomedicines 2022, 10, 2667. [Google Scholar] [CrossRef]
- Pilotto, A.; Quaresima, V.; Trasciatti, C.; Tolassi, C.; Bertoli, D.; Mordenti, C.; Galli, A.; Rizzardi, A.; Caratozzolo, S.; Zancanaro, A.; et al. Plasma P-Tau217 in Alzheimer’s Disease: Lumipulse and ALZpath SIMOA Head-to-Head Comparison. medRxiv 2024. [Google Scholar] [CrossRef]
- Therriault, J.; Zimmer, E.R.; Benedet, A.L.; Pascoal, T.A.; Gauthier, S.; Rosa-Neto, P. Staging of Alzheimer’s Disease: Past, Present, and Future Perspectives. Trends Mol. Med. 2022, 28, 726–741. [Google Scholar] [CrossRef] [PubMed]
- Baiardi, S.; Quadalti, C.; Mammana, A.; Dellavalle, S.; Zenesini, C.; Sambati, L.; Pantieri, R.; Polischi, B.; Romano, L.; Suffritti, M.; et al. Diagnostic Value of Plasma P-Tau181, NfL, and GFAP in a Clinical Setting Cohort of Prevalent Neurodegenerative Dementias. Alzheimer’s Res. Ther. 2022, 14, 153. [Google Scholar] [CrossRef]
- Giacomucci, G.; Mazzeo, S.; Crucitti, C.; Ingannato, A.; Bagnoli, S.; Padiglioni, S.; Galdo, G.; Emiliani, F.; Frigerio, D.; Moschini, V.; et al. Plasma P-Tau181 as a Promising Non-Invasive Biomarker of Alzheimer’s Disease Pathology in Subjective Cognitive Decline and Mild Cognitive Impairment. J. Neurol. Sci. 2023, 453, 120805. [Google Scholar] [CrossRef]
- Kivisäkk, P.; Fatima, H.A.; Cahoon, D.S.; Otieno, B.; Chacko, L.; Minooei, F.; Demos, C.; Stengelin, M.; Sigal, G.; Wohlstadter, J.; et al. Clinical Evaluation of a Novel Plasma pTau217 Electrochemiluminescence Immunoassay in Alzheimer’s Disease. Sci. Rep. 2024, 14, 629. [Google Scholar] [CrossRef]
- Gobom, J.; Benedet, A.L.; Mattsson-Carlgren, N.; Montoliu-Gaya, L.; Schultz, N.; Ashton, N.J.; Janelidze, S.; Servaes, S.; Sauer, M.; Pascoal, T.A.; et al. Antibody-Free Measurement of Cerebrospinal Fluid Tau Phosphorylation across the Alzheimer’s Disease Continuum. Mol. Neurodegener. 2022, 17, 81. [Google Scholar] [CrossRef] [PubMed]
- Tariot, P.N.; Reiman, E.M.; Alexander, R.C.; Langbaum, J.B.; Holdridge, K.; Ferguson, M.B.; Yaari, R.; Sims, J.R. TRAILBLAZER-ALZ 3 Trial Design and Rationale. J. Prev. Alzheimer’s Dis. 2021, 8 (Suppl. 1), S3. [Google Scholar]
- Shcherbinin, S.; Andersen, S.W.; Evans, C.D.; Lo, A.C.; Lu, M.; Navitsky, M.; Collins, E.C.; Sims, J.R.; Brooks, D.A.; Mintun, M.A. TRAILBLAZER-ALZ Study: Dynamics of Amyloid Reduction after Donanemab Treatment. Alzheimer’s Dement. 2021, 17, e057492. [Google Scholar] [CrossRef]
- Sims, J.R.; Lu, M.; Schade, A.E.; Brooks, D.A.; Mintun, M.A. TRAILBLAZER-ALZ Studies: Plasma P-Tau Assays and the Initial Performance in Clinical Trials. J. Prev. Alzheimer’s Dis. 2021, 8 (Suppl. 1), S2. [Google Scholar]
- Brum, W.S.; Ashton, N.J.; Simrén, J.; Di Molfetta, G.; Karikari, T.K.; Benedet, A.L.; Zimmer, E.R.; Lantero-Rodriguez, J.; Montoliu-Gaya, L.; Jeromin, A.; et al. Biological Variation Estimates of Alzheimer’s Disease Plasma Biomarkers in Healthy Individuals. Alzheimer’s Dement. 2024, 20, 1284–1297. [Google Scholar] [CrossRef]
- Mielke, M.M.; Dage, J.L.; Frank, R.D.; Algeciras-Schimnich, A.; Knopman, D.S.; Lowe, V.J.; Bu, G.; Vemuri, P.; Graff-Radford, J.; Jack, C.R.; et al. Performance of Plasma Phosphorylated Tau 181 and 217 in the Community. Nat. Med. 2022, 28, 1398–1405. [Google Scholar] [CrossRef]
- Vila-Castelar, C.; Chen, Y.; Langella, S.; Lopera, F.; Zetterberg, H.; Hansson, O.; Dage, J.L.; Janelidzde, S.; Su, Y.; Chen, K.; et al. Sex Differences in Blood Biomarkers and Cognitive Performance in Individuals with Autosomal Dominant Alzheimer’s Disease. Alzheimer’s Dement. 2023, 19, 4127–4138. [Google Scholar] [CrossRef] [PubMed]
- Kelleher, R.J.; Shen, J. Presenilin-1 Mutations and Alzheimer’s Disease. Proc. Natl. Acad. Sci. USA 2017, 114, 629–631. [Google Scholar] [CrossRef]
- Janelidze, S.; Barthélemy, N.R.; He, Y.; Bateman, R.J.; Hansson, O. Mitigating the Associations of Kidney Dysfunction With Blood Biomarkers of Alzheimer Disease by Using Phosphorylated Tau to Total Tau Ratios. JAMA Neurol. 2023, 80, 516–522. [Google Scholar] [CrossRef]
- Rates. Center for Alzheimer’s Disease Research. Available online: https://alz.carney.brown.edu/facilities-resources/biomarkers-facility/rates (accessed on 1 July 2024).
- Schindler, S.E.; Li, Y.; Li, M.; Despotis, A.; Park, E.; Vittert, L.; Hamilton, B.H.; Womack, K.B.; Saef, B.; Holtzman, D.M.; et al. Using Alzheimer’s Disease Blood Tests to Accelerate Clinical Trial Enrollment. Alzheimer’s Dement. 2023, 19, 1175–1183. [Google Scholar] [CrossRef] [PubMed]


| Procedure | Cost |
|---|---|
| Simoa® pTau217 for HD-X | USD 194 [101] |
| Simoa® pTau181 for HD-X | USD 172 [101] |
| Simoa® pTau231 for HD-X | USD 141 [101] |
| Simoa® GFAP for HD-X | USD 145 [101] |
| Simoa® Neurofilament Light (NfL) for HD-X | USD 207 [101] |
| Simoa® Neurology 4-Plex E for HD-X (AB40, AB42, GFAP, NF-light) | USD 401 [101] |
| Simoa® Neurology 3-Plex A for HD-X (AB40, AB42, Tau) | USD 338 [101] |
| SMC™ Amyloid Beta 40 | USD 137 [101] |
| SMC™ Amyloid Beta 42 | USD 137 [101] |
| Amyloid-PET scan | USD 6487 [102] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lai, R.; Li, B.; Bishnoi, R. P-tau217 as a Reliable Blood-Based Marker of Alzheimer’s Disease. Biomedicines 2024, 12, 1836. https://doi.org/10.3390/biomedicines12081836
Lai R, Li B, Bishnoi R. P-tau217 as a Reliable Blood-Based Marker of Alzheimer’s Disease. Biomedicines. 2024; 12(8):1836. https://doi.org/10.3390/biomedicines12081836
Chicago/Turabian StyleLai, Roy, Brenden Li, and Ram Bishnoi. 2024. "P-tau217 as a Reliable Blood-Based Marker of Alzheimer’s Disease" Biomedicines 12, no. 8: 1836. https://doi.org/10.3390/biomedicines12081836
APA StyleLai, R., Li, B., & Bishnoi, R. (2024). P-tau217 as a Reliable Blood-Based Marker of Alzheimer’s Disease. Biomedicines, 12(8), 1836. https://doi.org/10.3390/biomedicines12081836







