Extraintestinal Manifestations in Inflammatory Bowel Disease: From Pathophysiology to Treatment
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Pathophysiology of EIMs
3.1.1. EIMs as Extension of Immune Responses from the Intestine
3.1.2. EIMs as Independent Inflammatory Events
3.1.3. Genetic Basis of EIMs
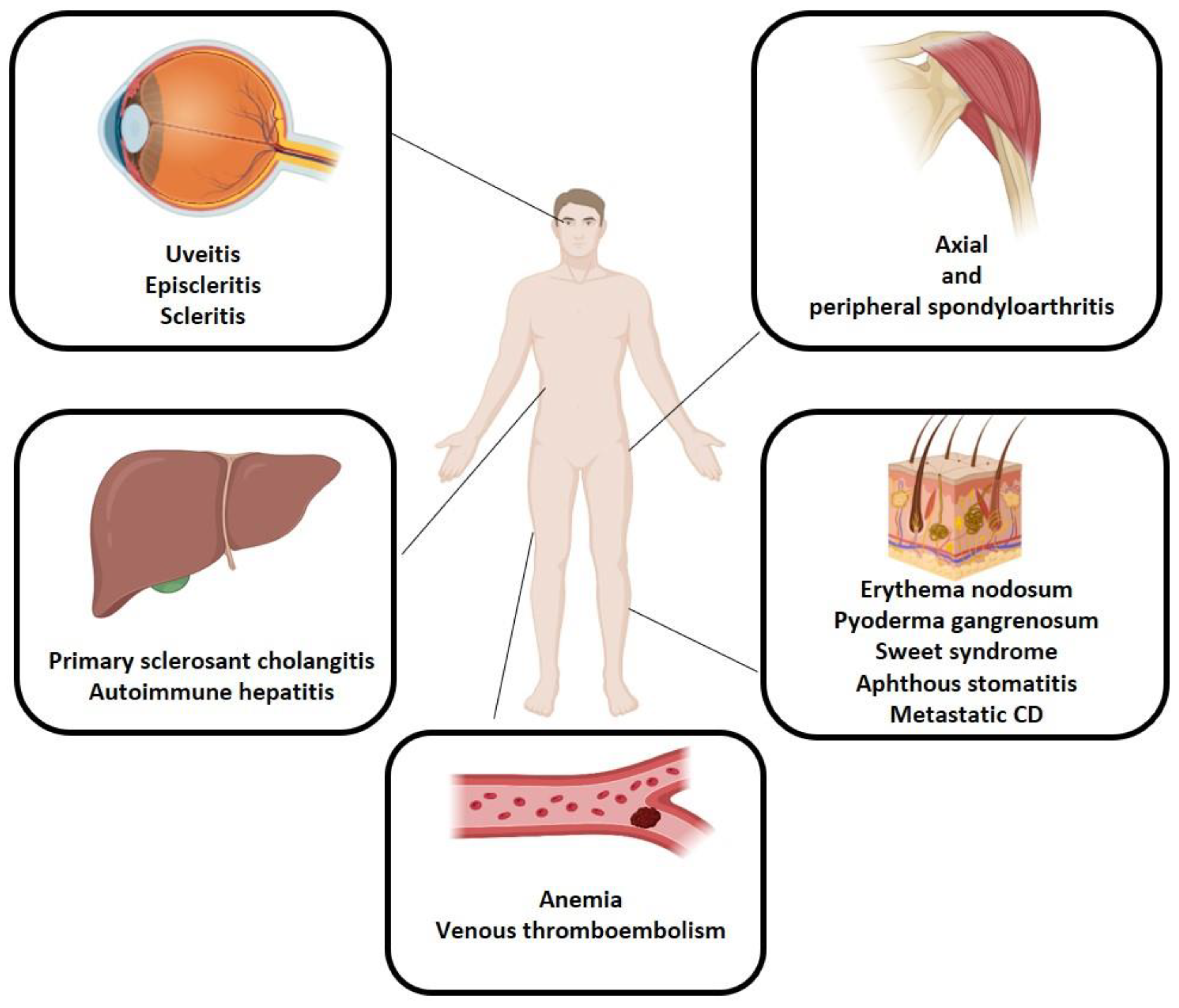
3.2. Musculoskeletal EIMs
3.3. Skin EIMs
3.4. Ocular EIMs
3.5. Hepatobiliary EIMs
3.6. Other EIMs
3.7. Treatments for EIMs: An Overview
3.7.1. Treatment of Musculoskeletal EIMs
3.7.2. Treatment of Cutaneous EIMs
3.7.3. Treatment of Ocular EIMs
3.7.4. Treatment of Hepatobiliary EIMs
3.7.5. Paradoxical Effects of Advanced Therapies
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rogler, G.; Singh, A.; Kavanaugh, A.; Rubin, D.T. Extraintestinal Manifestations of Inflammatory Bowel Disease: Current Concepts, Treatment, and Implications for Disease Management. Gastroenterology 2021, 161, 1118–1132. [Google Scholar] [CrossRef] [PubMed]
- Gordon, H.; Burisch, J.; Ellul, P.; Karmiris, K.; Katsanos, K.; Allocca, M.; Bamias, G.; Barreiro-de Acosta, M.; Braithwaite, T.; Greuter, T.; et al. ECCO Guidelines on Extraintestinal Manifestations in Inflammatory Bowel Disease. J. Crohn’s Colitis 2024, 18, 1–37. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.M.; Cheon, J.H. Pathogenesis and clinical perspectives of extraintestinal manifestations in inflammatory bowel diseases. Intest. Res. 2020, 18, 249–264. [Google Scholar] [CrossRef] [PubMed]
- Bhat, S.; Click, B.; Regueiro, M. Safety and Monitoring of Inflammatory Bowel Disease Advanced Therapies. Inflamm. Bowel Dis. 2024, 30, 829–843. [Google Scholar] [CrossRef] [PubMed]
- Berlin, C.; Berg, E.L.; Briskin, M.J.; Andrew, D.P.; Kilshaw, P.J.; Holzmann, B.; Weissman, I.L.; Hamann, A.; Butcher, E.C. Alpha 4 beta 7 integrin mediates lymphocyte binding to the mucosal vascular addressin MAdCAM-1. Cell 1993, 74, 185–195. [Google Scholar] [CrossRef] [PubMed]
- Grant, A.J.; Lalor, P.F.; Salmi, M.; Jalkanen, S.; Adams, D.H. Homing of mucosal lymphocytes to the liver in the pathogenesis of hepatic complications of inflammatory bowel disease. Lancet 2002, 359, 150–157. [Google Scholar] [CrossRef] [PubMed]
- Chapman, R.W. Aetiology and natural history of primary sclerosing cholangitis—A decade of progress? Gut 1991, 32, 1433–1435. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hedin, C.R.H.; Vavricka, S.R.; Stagg, A.J.; Schoepfer, A.; Raine, T.; Puig, L.; Pleyer, U.; Navarini, A.; van der Meulen-de Jong, A.E.; Maul, J.; et al. The Pathogenesis of Extraintestinal Manifestations: Implications for IBD Research, Diagnosis, and Therapy. J. Crohn’s Colitis 2019, 13, 541–554. [Google Scholar] [CrossRef] [PubMed]
- Kummen, M.; Holm, K.; Anmarkrud, J.A.; Nygård, S.; Vesterhus, M.; Høivik, M.L.; Trøseid, M.; Marschall, H.-U.; Schrumpf, E.; Moum, B.; et al. The gut microbial profile in patients with primary sclerosing cholangitis is distinct from patients with ulcerative colitis without biliary disease and healthy controls. Gut 2017, 66, 611–619. [Google Scholar] [CrossRef]
- Scher, J.U.; Ubeda, C.; Artacho, A.; Attur, M.; Isaac, S.; Reddy, S.M.; Marmon, S.; Neimann, A.; Brusca, S.; Patel, T.; et al. Decreased bacterial diversity characterizes the altered gut microbiota in patients with psoriatic arthritis, resembling dysbiosis in inflammatory bowel disease. Arthritis Rheumatol. 2015, 67, 128–139. [Google Scholar] [CrossRef]
- Tito, R.Y.; Cypers, H.; Joossens, M.; Varkas, G.; Van Praet, L.; Glorieus, E.; Van den Bosch, F.; De Vos, M.; Raes, J.; Elewaut, D. Brief Report: Dialister as a Microbial Marker of Disease Activity in Spondyloarthritis. Arthritis Rheumatol. 2017, 69, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Muñiz Pedrogo, D.A.; Chen, J.; Hillmann, B.; Jeraldo, P.; Al-Ghalith, G.; Taneja, V.; Davis, J.M.; Knights, D.; Nelson, H.; Faubion, W.A.; et al. An Increased Abundance of Clostridiaceae Characterizes Arthritis in Inflammatory Bowel Disease and Rheumatoid Arthritis: A Cross-sectional Study. Inflamm. Bowel Dis. 2019, 25, 902–913. [Google Scholar] [CrossRef] [PubMed]
- Peeters, H.; Vander Cruyssen, B.; Laukens, D.; Coucke, P.; Marichal, D.; Van Den Berghe, M.; Cuvelier, C.; Remaut, E.; Mielants, H.; De Keyser, F.; et al. Radiological sacroiliitis, a hallmark of spondylitis, is linked with CARD15 gene polymorphisms in patients with Crohn’s disease. Ann. Rheum. Dis. 2004, 63, 1131–1134. [Google Scholar] [CrossRef] [PubMed]
- Martin, T.M.; Smith, J.R.; Rosenbaum, J.T. Anterior uveitis: Current concepts of pathogenesis and interactions with the spondyloarthropathies. Curr. Opin. Rheumatol. 2002, 14, 337–341. [Google Scholar] [CrossRef] [PubMed]
- Sheth, T.; Pitchumoni, C.S.; Das, K.M. Musculoskeletal manifestations in inflammatory bowel disease: A revisit in search of immunopathophysiological mechanisms. J. Clin. Gastroenterol. 2014, 48, 308–317. [Google Scholar] [CrossRef] [PubMed]
- Weizman, A.; Huang, B.; Berel, D.; Targan, S.R.; Dubinsky, M.; Fleshner, P.; Ippoliti, A.; Kaur, M.; Panikkath, D.; Brant, S.; et al. Clinical, serologic, and genetic factors associated with pyoderma gangrenosum and erythema nodosum in inflammatory bowel disease patients. Inflamm. Bowel Dis. 2014, 20, 525–533. [Google Scholar] [CrossRef] [PubMed]
- Schett, G.; McInnes, I.B.; Neurath, M.F. Reframing Immune-Mediated Inflammatory Diseases through Signature Cytokine Hubs. N. Engl. J. Med. 2021, 385, 628–639. [Google Scholar] [CrossRef] [PubMed]
- Karreman, M.C.; Luime, J.J.; Hazes, J.M.W.; Weel, A.E.A.M. The Prevalence and Incidence of Axial and Peripheral Spondyloarthritis in Inflammatory Bowel Disease: A Systematic Review and Meta-analysis. J. Crohn’s Colitis 2017, 11, 631–642. [Google Scholar] [CrossRef]
- Felice, C.; Pugliese, D.; Papparella, L.G.; Pizzolante, F.; Onori, E.; Gasbarrini, A.; Rapaccini, G.L.; Guidi, L.; Armuzzi, A. Clinical management of rheumatologic conditions co-occurring with inflammatory bowel diseases. Expert Rev. Clin. Immunol. 2018, 14, 751–759. [Google Scholar] [CrossRef]
- Dougados, M.; van der Linden, S.; Juhlin, R.; Huitfeldt, B.; Amor, B.; Calin, A.; Cats, A.; Dijkmans, B.; Olivieri, I.; Pasero, G. The European Spondylarthropathy Study Group preliminary criteria for the classification of spondylarthropathy. Arthritis Rheum. 1991, 34, 1218–1227. [Google Scholar] [CrossRef]
- Wang, C.-R.; Tsai, H.-W. Seronegative spondyloarthropathy-associated inflammatory bowel disease. World J. Gastroenterol. 2023, 29, 450–468. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Reyna, T.S.; Martínez-Reyes, C.; Yamamoto-Furusho, J.K. Rheumatic manifestations of inflammatory bowel disease. World J. Gastroenterol. 2009, 15, 5517–5524. [Google Scholar] [CrossRef] [PubMed]
- van der Heijde, D.; Ramiro, S.; Landewé, R.; Baraliakos, X.; Van den Bosch, F.; Sepriano, A.; Regel, A.; Ciurea, A.; Dagfinrud, H.; Dougados, M.; et al. 2016 update of the ASAS-EULAR management recommendations for axial spondyloarthritis. Ann. Rheum. Dis. 2017, 76, 978–991. [Google Scholar] [CrossRef] [PubMed]
- Orchard, T.R.; Wordsworth, B.P.; Jewell, D.P. Peripheral arthropathies in inflammatory bowel disease: Their articular distribution and natural history. Gut 1998, 42, 387–391. [Google Scholar] [CrossRef]
- Schett, G.; Lories, R.J.; D’Agostino, M.-A.; Elewaut, D.; Kirkham, B.; Soriano, E.R.; McGonagle, D. Enthesitis: From pathophysiology to treatment. Nat. Rev. Rheumatol. 2017, 13, 731–741. [Google Scholar] [CrossRef] [PubMed]
- Greuter, T.; Navarini, A.; Vavricka, S.R. Skin Manifestations of Inflammatory Bowel Disease. Clin. Rev. Allergy Immunol. 2017, 53, 413–427. [Google Scholar] [CrossRef]
- Antonelli, E.; Bassotti, G.; Tramontana, M.; Hansel, K.; Stingeni, L.; Ardizzone, S.; Genovese, G.; Marzano, A.V.; Maconi, G. Dermatological Manifestations in Inflammatory Bowel Diseases. J. Clin. Med. 2021, 10, 364. [Google Scholar] [CrossRef] [PubMed]
- States, V.; O’Brien, S.; Rai, J.P.; Roberts, H.L.; Paas, M.; Feagins, K.; Pierce, E.J.; Baumgartner, R.N.; Galandiuk, S. Pyoderma Gangrenosum in Inflammatory Bowel Disease: A Systematic Review and Meta-Analysis. Dig. Dis. Sci. 2020, 65, 2675–2685. [Google Scholar] [CrossRef]
- Xu, A.; Balgobind, A.; Strunk, A.; Garg, A.; Alloo, A. Prevalence estimates for pyoderma gangrenosum in the United States: An age- and sex-adjusted population analysis. J. Am. Acad. Dermatol. 2020, 83, 425–429. [Google Scholar] [CrossRef]
- Hung, Y.-T.; Le, P.-H.; Kuo, C.-J.; Tang, Y.-C.; Chiou, M.-J.; Chiu, C.-T.; Kuo, C.-F.; Huang, Y.-H. The Temporal Relationships and Associations between Cutaneous Manifestations and Inflammatory Bowel Disease: A Nationwide Population-Based Cohort Study. J. Clin. Med. 2021, 10, 1311. [Google Scholar] [CrossRef]
- Wang, E.A.; Steel, A.; Luxardi, G.; Mitra, A.; Patel, F.; Cheng, M.Y.; Wilken, R.; Kao, J.; de Ga, K.; Sultani, H.; et al. Classic Ulcerative Pyoderma Gangrenosum Is a T Cell-Mediated Disease Targeting Follicular Adnexal Structures: A Hypothesis Based on Molecular and Clinicopathologic Studies. Front. Immunol. 2017, 8, 1980. [Google Scholar] [CrossRef] [PubMed]
- Giraudo, F.; Miraglia, E.; Garbi, M.L.; Yantorno, M.; Maradeo, M.R.; Correa, G.J.; Tufare, F. Prevalence of pyoderma gangrenosum and Sweet’s syndrome in inflammatory bowel disease at a tertiary healthcare center. Rev. Esp. Enferm. Dig. 2021, 113, 691–697. [Google Scholar] [CrossRef] [PubMed]
- Sleiman, J.; Hitawala, A.A.; Cohen, B.; Falloon, K.; Simonson, M.; Click, B.; Khanna, U.; Fernandez, A.P.; Rieder, F. Systematic Review: Sweet Syndrome Associated with Inflammatory Bowel Disease. J. Crohn’s Colitis 2021, 15, 1864–1876. [Google Scholar] [CrossRef] [PubMed]
- Zunt, S.L. Recurrent aphthous stomatitis. Dermatol. Clin. 2003, 21, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Vavricka, S.R.; Gubler, M.; Gantenbein, C.; Spoerri, M.; Froehlich, F.; Seibold, F.; Protic, M.; Michetti, P.; Straumann, A.; Fournier, N.; et al. Anti-TNF Treatment for Extraintestinal Manifestations of Inflammatory Bowel Disease in the Swiss IBD Cohort Study. Inflamm. Bowel Dis. 2017, 23, 1174–1181. [Google Scholar] [CrossRef] [PubMed]
- Turkcapar, N.; Toruner, M.; Soykan, I.; Aydintug, O.T.; Cetinkaya, H.; Duzgun, N.; Ozden, A.; Duman, M. The prevalence of extraintestinal manifestations and HLA association in patients with inflammatory bowel disease. Rheumatol. Int. 2006, 26, 663–668. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.V.; Jones, D.M.; Hill, J.C.; MacDermott, R.P. Development of metastatic Crohn’s disease of the skin while on anti-TNF biologics. Inflamm. Bowel Dis. 2012, 18, 1188–1190. [Google Scholar] [CrossRef] [PubMed]
- Shah, J.; Shah, A.; Hassman, L.; Gutierrez, A. Ocular Manifestations of Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2021, 27, 1832–1838. [Google Scholar] [CrossRef] [PubMed]
- Biedermann, L.; Renz, L.; Fournier, N.; Rossel, J.-B.; Butter, M.; Bluemel, S.; Vavricka, S.R.; Rogler, G.; Scharl, M. Uveitis manifestations in patients of the Swiss Inflammatory Bowel Disease Cohort Study. Ther. Adv. Gastroenterol. 2019, 12, 1756284819865142. [Google Scholar] [CrossRef]
- Yang, B.R.; Choi, N.-K.; Kim, M.-S.; Chun, J.; Joo, S.H.; Kim, H.; Lee, J. Prevalence of extraintestinal manifestations in Korean inflammatory bowel disease patients. PLoS ONE 2018, 13, e0200363. [Google Scholar] [CrossRef]
- Braithwaite, T.; Adderley, N.J.; Subramanian, A.; Galloway, J.; Kempen, J.H.; Gokhale, K.; Cope, A.P.; Dick, A.D.; Nirantharakumar, K.; Denniston, A.K. Epidemiology of Scleritis in the United Kingdom From 1997 to 2018: Population-Based Analysis of 11 Million Patients and Association between Scleritis and Infectious and Immune-Mediated Inflammatory Disease. Arthritis Rheumatol. 2021, 73, 1267–1276. [Google Scholar] [CrossRef] [PubMed]
- Murthy, S.I.; Sabhapandit, S.; Balamurugan, S.; Subramaniam, P.; Sainz-de-la-Maza, M.; Agarwal, M.; Parvesio, C. Scleritis: Differentiating infectious from non-infectious entities. Indian J. Ophthalmol. 2020, 68, 1818–1828. [Google Scholar] [CrossRef] [PubMed]
- Sainz de la Maza, M.; Molina, N.; Gonzalez-Gonzalez, L.A.; Doctor, P.P.; Tauber, J.; Foster, C.S. Clinical characteristics of a large cohort of patients with scleritis and episcleritis. Ophthalmology 2012, 119, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Saich, R.; Chapman, R. Primary sclerosing cholangitis, autoimmune hepatitis and overlap syndromes in inflammatory bowel disease. World J. Gastroenterol. 2008, 14, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Chazouilleres, O.; Beuers, U.; Bergquist, A.; Karlsen, T.H.; Levy, C.; Samyn, M.; Schramm, C.; Trauner, M. EASL Clinical Practice Guidelines on sclerosing cholangitis. J. Hepatol. 2022, 77, 761–806. [Google Scholar] [CrossRef] [PubMed]
- Barberio, B.; Massimi, D.; Cazzagon, N.; Zingone, F.; Ford, A.C.; Savarino, E.V. Prevalence of Primary Sclerosing Cholangitis in Patients with Inflammatory Bowel Disease: A Systematic Review and Meta-analysis. Gastroenterology 2021, 161, 1865–1877. [Google Scholar] [CrossRef] [PubMed]
- Fraga, M.; Fournier, N.; Safroneeva, E.; Pittet, V.; Godat, S.; Straumann, A.; Nydegger, A.; Vavricka, S.R.; Moradpour, D.; Schoepfer, A.M.; et al. Primary sclerosing cholangitis in the Swiss Inflammatory Bowel Disease Cohort Study: Prevalence, risk factors, and long-term follow-up. Eur. J. Gastroenterol. Hepatol. 2017, 29, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Venkatesh, S.K.; Welle, C.L.; Miller, F.H.; Jhaveri, K.; Ringe, K.I.; Eaton, J.E.; Bungay, H.; Arrivé, L.; Ba-Ssalamah, A.; Grigoriadis, A.; et al. Reporting standards for primary sclerosing cholangitis using MRI and MR cholangiopancreatography: Guidelines from MR Working Group of the International Primary Sclerosing Cholangitis Study Group. Eur. Radiol. 2022, 32, 923–937. [Google Scholar] [CrossRef] [PubMed]
- Gordon, H.; Biancone, L.; Fiorino, G.; Katsanos, K.H.; Kopylov, U.; Al Sulais, E.; Axelrad, J.E.; Balendran, K.; Burisch, J.; de Ridder, L.; et al. ECCO Guidelines on Inflammatory Bowel Disease and Malignancies. J. Crohn’s Colitis 2023, 17, 827–854. [Google Scholar] [CrossRef]
- Ghersin, I.; Khateeb, N.; Katz, L.H.; Daher, S.; Shamir, R.; Assa, A. Comorbidities in adolescents with inflammatory bowel disease: Findings from a population-based cohort study. Pediatr. Res. 2020, 87, 1256–1262. [Google Scholar] [CrossRef]
- Lohse, A.W.; Sebode, M.; Bhathal, P.S.; Clouston, A.D.; Dienes, H.P.; Jain, D.; Gouw, A.S.H.; Guindi, M.; Kakar, S.; Kleiner, D.E.; et al. Consensus recommendations for histological criteria of autoimmune hepatitis from the International AIH Pathology Group: Results of a workshop on AIH histology hosted by the European Reference Network on Hepatological Diseases and the European Society of Pathology: Results of a workshop on AIH histology hosted by the European Reference Network on Hepatological Diseases and the European Society of Pathology. Liver Int. 2022, 42, 1058–1069. [Google Scholar] [CrossRef] [PubMed]
- European Association for the Study of the Liver EASL Clinical Practice Guidelines: Autoimmune hepatitis. J. Hepatol. 2015, 63, 971–1004. [CrossRef] [PubMed]
- Murthy, S.K.; Robertson McCurdy, A.B.; Carrier, M.; McCurdy, J.D. Venous thromboembolic events in inflammatory bowel diseases: A review of current evidence and guidance on risk in the post-hospitalization setting. Thromb. Res. 2020, 194, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Dhaliwal, G.; Patrone, M.V.; Bickston, S.J. Venous Thromboembolism in Patients with Inflammatory Bowel Disease. J. Clin. Med. 2023, 13, 251. [Google Scholar] [CrossRef] [PubMed]
- Ytterberg, S.R.; Bhatt, D.L.; Mikuls, T.R.; Koch, G.G.; Fleischmann, R.; Rivas, J.L.; Germino, R.; Menon, S.; Sun, Y.; Wang, C.; et al. Cardiovascular and Cancer Risk with Tofacitinib in Rheumatoid Arthritis. N. Engl. J. Med. 2022, 386, 316–326. [Google Scholar] [CrossRef] [PubMed]
- Yates, M.; Mootoo, A.; Adas, M.; Bechman, K.; Rampes, S.; Patel, V.; Qureshi, S.; Cope, A.P.; Norton, S.; Galloway, J.B. Venous Thromboembolism Risk with JAK Inhibitors: A Meta-Analysis. Arthritis Rheumatol. 2021, 73, 779–788. [Google Scholar] [CrossRef]
- Olivera, P.A.; Lasa, J.S.; Bonovas, S.; Danese, S.; Peyrin-Biroulet, L. Safety of Janus Kinase Inhibitors in Patients with Inflammatory Bowel Diseases or Other Immune-mediated Diseases: A Systematic Review and Meta-Analysis. Gastroenterology 2020, 158, 1554–1573.e12. [Google Scholar] [CrossRef] [PubMed]
- Olivera, P.A.; Zuily, S.; Kotze, P.G.; Regnault, V.; Al Awadhi, S.; Bossuyt, P.; Gearry, R.B.; Ghosh, S.; Kobayashi, T.; Lacolley, P.; et al. International consensus on the prevention of venous and arterial thrombotic events in patients with inflammatory bowel disease. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 857–873. [Google Scholar] [CrossRef] [PubMed]
- Sandborn, W.J.; Rebuck, R.; Wang, Y.; Zou, B.; Adedokun, O.J.; Gasink, C.; Sands, B.E.; Hanauer, S.B.; Targan, S.; Ghosh, S.; et al. Five-Year Efficacy and Safety of Ustekinumab Treatment in Crohn’s Disease: The IM-UNITI Trial. Clin. Gastroenterol. Hepatol. 2022, 20, 578–590.e4. [Google Scholar] [CrossRef]
- Colombel, J.-F.; Sands, B.E.; Rutgeerts, P.; Sandborn, W.; Danese, S.; D’Haens, G.; Panaccione, R.; Loftus, E.V.; Sankoh, S.; Fox, I.; et al. The safety of vedolizumab for ulcerative colitis and Crohn’s disease. Gut 2017, 66, 839–851. [Google Scholar] [CrossRef]
- Filmann, N.; Rey, J.; Schneeweiss, S.; Ardizzone, S.; Bager, P.; Bergamaschi, G.; Koutroubakis, I.; Lindgren, S.; Morena, F.d.l.; Moum, B.; et al. Prevalence of anemia in inflammatory bowel diseases in european countries: A systematic review and individual patient data meta-analysis. Inflamm. Bowel Dis. 2014, 20, 936–945. [Google Scholar] [CrossRef] [PubMed]
- Dignass, A.U.; Gasche, C.; Bettenworth, D.; Birgegård, G.; Danese, S.; Gisbert, J.P.; Gomollon, F.; Iqbal, T.; Katsanos, K.; Koutroubakis, I.; et al. European consensus on the diagnosis and management of iron deficiency and anaemia in inflammatory bowel diseases. J. Crohn’s Colitis 2015, 9, 211–222. [Google Scholar] [CrossRef] [PubMed]
- Gordon, M.; Sinopoulou, V.; Iheozor-Ejiofor, Z.; Iqbal, T.; Allen, P.; Hoque, S.; Engineer, J.; Akobeng, A.K. Interventions for treating iron deficiency anaemia in inflammatory bowel disease. Cochrane Database Syst. Rev. 2021, 1, CD013529. [Google Scholar] [CrossRef] [PubMed]
- Schreiber, S.; Howaldt, S.; Schnoor, M.; Nikolaus, S.; Bauditz, J.; Gasché, C.; Lochs, H.; Raedler, A. Recombinant erythropoietin for the treatment of anemia in inflammatory bowel disease. N. Engl. J. Med. 1996, 334, 619–623. [Google Scholar] [CrossRef] [PubMed]
- Devalia, V.; Hamilton, M.S.; Molloy, A.M. British Committee for Standards in Haematology Guidelines for the diagnosis and treatment of cobalamin and folate disorders. Br. J. Haematol. 2014, 166, 496–513. [Google Scholar] [CrossRef] [PubMed]
- Weiss, G.; Gasche, C. Pathogenesis and treatment of anemia in inflammatory bowel disease. Haematologica 2010, 95, 175–178. [Google Scholar] [CrossRef] [PubMed]
- Brooklyn, T.N.; Dunnill, M.G.S.; Shetty, A.; Bowden, J.J.; Williams, J.D.L.; Griffiths, C.E.M.; Forbes, A.; Greenwood, R.; Probert, C.S. Infliximab for the treatment of pyoderma gangrenosum: A randomised, double blind, placebo controlled trial. Gut 2006, 55, 505–509. [Google Scholar] [CrossRef]
- Löfberg, R.; Louis, E.V.; Reinisch, W.; Robinson, A.M.; Kron, M.; Camez, A.; Pollack, P.F. Adalimumab produces clinical remission and reduces extraintestinal manifestations in Crohn’s disease: Results from CARE. Inflamm. Bowel Dis. 2012, 18, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Smolen, J.; Landewé, R.B.; Mease, P.; Brzezicki, J.; Mason, D.; Luijtens, K.; van Vollenhoven, R.F.; Kavanaugh, A.; Schiff, M.; Burmester, G.R.; et al. Efficacy and safety of certolizumab pegol plus methotrexate in active rheumatoid arthritis: The RAPID 2 study. A randomised controlled trial. Ann. Rheum. Dis. 2009, 68, 797–804. [Google Scholar] [CrossRef]
- Mease, P.J.; Fleischmann, R.; Deodhar, A.A.; Wollenhaupt, J.; Khraishi, M.; Kielar, D.; Woltering, F.; Stach, C.; Hoepken, B.; Arledge, T.; et al. Effect of certolizumab pegol on signs and symptoms in patients with psoriatic arthritis: 24-week results of a Phase 3 double-blind randomised placebo-controlled study (RAPID-PsA). Ann. Rheum. Dis. 2014, 73, 48–55. [Google Scholar] [CrossRef]
- Landewé, R.; Braun, J.; Deodhar, A.; Dougados, M.; Maksymowych, W.P.; Mease, P.J.; Reveille, J.D.; Rudwaleit, M.; van der Heijde, D.; Stach, C.; et al. Efficacy of certolizumab pegol on signs and symptoms of axial spondyloarthritis including ankylosing spondylitis: 24-week results of a double-blind randomised placebo-controlled Phase 3 study. Ann. Rheum. Dis. 2014, 73, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Rudwaleit, M.; Rosenbaum, J.T.; Landewé, R.; Marzo-Ortega, H.; Sieper, J.; van der Heijde, D.; Davies, O.; Bartz, H.; Hoepken, B.; Nurminen, T.; et al. Observed Incidence of Uveitis Following Certolizumab Pegol Treatment in Patients with Axial Spondyloarthritis. Arthritis Care Res. 2016, 68, 838–844. [Google Scholar] [CrossRef] [PubMed]
- Sieper, J.; van der Heijde, D.; Dougados, M.; Maksymowych, W.P.; Scott, B.B.; Boice, J.A.; Berd, Y.; Bergman, G.; Curtis, S.; Tzontcheva, A.; et al. A randomized, double-blind, placebo-controlled, sixteen-week study of subcutaneous golimumab in patients with active nonradiographic axial spondyloarthritis. Arthritis Rheumatol. 2015, 67, 2702–2712. [Google Scholar] [CrossRef] [PubMed]
- Kavanaugh, A.; McInnes, I.; Mease, P.; Krueger, G.G.; Gladman, D.; Gomez-Reino, J.; Papp, K.; Zrubek, J.; Mudivarthy, S.; Mack, M.; et al. Golimumab, a new human tumor necrosis factor alpha antibody, administered every four weeks as a subcutaneous injection in psoriatic arthritis: Twenty-four-week efficacy and safety results of a randomized, placebo-controlled study. Arthritis Rheum. 2009, 60, 976–986. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y. A review of upadacitinib in rheumatoid arthritis. Mod. Rheumatol. 2020, 30, 779–787. [Google Scholar] [CrossRef] [PubMed]
- van Bentum, R.E.; Heslinga, S.C.; Nurmohamed, M.T.; Gerards, A.H.; Griep, E.N.; Koehorst, C.B.J.M.; Kok, M.R.; Schilder, A.M.; Verhoef, M.; van der Horst-Bruinsma, I.E. Reduced Occurrence Rate of Acute Anterior Uveitis in Ankylosing Spondylitis Treated with Golimumab—The GO-EASY Study. J. Rheumatol. 2019, 46, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Narula, N.; Aruljothy, A.; Wong, E.C.L.; Homenauth, R.; Alshahrani, A.-A.; Marshall, J.K.; Reinisch, W. The impact of ustekinumab on extraintestinal manifestations of Crohn’s disease: A post hoc analysis of the UNITI studies. United Eur. Gastroenterol. J. 2021, 9, 581–589. [Google Scholar] [CrossRef] [PubMed]
- Guillo, L.; D’Amico, F.; Danese, S.; Peyrin-Biroulet, L. Ustekinumab for Extra-intestinal Manifestations of Inflammatory Bowel Disease: A Systematic Literature Review. J. Crohn’s Colitis 2021, 15, 1236–1243. [Google Scholar] [CrossRef]
- Pugliese, D.; Daperno, M.; Fiorino, G.; Savarino, E.; Mosso, E.; Biancone, L.; Testa, A.; Sarpi, L.; Cappello, M.; Bodini, G.; et al. Real-life effectiveness of ustekinumab in inflammatory bowel disease patients with concomitant psoriasis or psoriatic arthritis: An IG-IBD study. Dig. Liver Dis. 2019, 51, 972–977. [Google Scholar] [CrossRef]
- Chateau, T.; Bonovas, S.; Le Berre, C.; Mathieu, N.; Danese, S.; Peyrin-Biroulet, L. Vedolizumab Treatment in Extra-Intestinal Manifestations in Inflammatory Bowel Disease: A Systematic Review. J. Crohn’s Colitis 2019, 13, 1569–1577. [Google Scholar] [CrossRef]
- Feagan, B.G.; Sandborn, W.J.; Colombel, J.-F.; Byrne, S.O.; Khalid, J.M.; Kempf, C.; Geransar, P.; Bhayat, F.; Rubin, D.T. Incidence of Arthritis/Arthralgia in Inflammatory Bowel Disease with Long-term Vedolizumab Treatment: Post Hoc Analyses of the GEMINI Trials. J. Crohn’s Colitis 2019, 13, 50–57. [Google Scholar] [CrossRef] [PubMed]
- De Galan, C.; Truyens, M.; Peeters, H.; Mesonero Gismero, F.; Elorza, A.; Torres, P.; Vandermeulen, L.; Amezaga, A.J.; Ferreiro-Iglesias, R.; Holvoet, T.; et al. The Impact of Vedolizumab and Ustekinumab on Articular Extra-Intestinal Manifestations in Inflammatory Bowel Disease Patients: A Real-Life Multicentre Cohort Study. J. Crohn’s Colitis 2022, 16, 1676–1686. [Google Scholar] [CrossRef] [PubMed]
- Rubin, D.T.; Reinisch, W.; Greuter, T.; Kotze, P.G.; Pinheiro, M.; Mundayat, R.; Maller, E.; Fellmann, M.; Lawendy, N.; Modesto, I.; et al. Extraintestinal manifestations at baseline, and the effect of tofacitinib, in patients with moderate to severe ulcerative colitis. Ther. Adv. Gastroenterol. 2021, 14, 17562848211005708. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wan, Z.; Jin, R.; Xu, T.; Ouyang, Y.; Wang, B.; Ruan, G.; Bai, X. Tofacitinib for extraintestinal manifestations of inflammatory bowel disease: A literature review. Int. Immunopharmacol. 2022, 105, 108517. [Google Scholar] [CrossRef]
- Admin, S. European Crohn’s and Colitis Organisation—ECCO—OP33 Effect of Upadacitinib (UPA) Treatment on Extraintestinal Manifestations (EIMs) in Patients with Moderate-to-Severe Ulcerative Colitis (UC): Results from the UPA Phase 3 Programme. Available online: https://www.ecco-ibd.eu/publications/congress-abstracts/item/op33-effect-of-upadacitinib-upa-treatment-on-extraintestinal-manifestations-eims-in-patients-with-moderate-to-severe-ulcerative-colitis-uc-results-from-the-upa-phase-3-programme.html (accessed on 5 September 2023).
- Webers, C.; Ortolan, A.; Sepriano, A.; Falzon, L.; Baraliakos, X.; Landewé, R.B.M.; Ramiro, S.; van der Heijde, D.; Nikiphorou, E. Efficacy and safety of biological DMARDs: A systematic literature review informing the 2022 update of the ASAS-EULAR recommendations for the management of axial spondyloarthritis. Ann. Rheum. Dis. 2023, 82, 130–141. [Google Scholar] [CrossRef] [PubMed]
- Barreiro-de-Acosta, M.; Lorenzo, A.; Domínguez-Muñoz, J.E. Efficacy of adalimumab for the treatment of extraintestinal manifestations of Crohn’s disease. Rev. Esp. Enferm. Dig. 2012, 104, 468–472. [Google Scholar] [CrossRef] [PubMed]
- Generini, S.; Giacomelli, R.; Fedi, R.; Fulminis, A.; Pignone, A.; Frieri, G.; Del Rosso, A.; Viscido, A.; Galletti, B.; Fazzi, M.; et al. Infliximab in spondyloarthropathy associated with Crohn’s disease: An open study on the efficacy of inducing and maintaining remission of musculoskeletal and gut manifestations. Ann. Rheum. Dis. 2004, 63, 1664–1669. [Google Scholar] [CrossRef]
- Luchetti, M.M.; Benfaremo, D.; Ciccia, F.; Bolognini, L.; Ciferri, M.; Farinelli, A.; Rossini, M.; Mosca, P.; Triolo, G.; Gabrielli, A. Adalimumab efficacy in enteropathic spondyloarthritis: A 12-mo observational multidisciplinary study. World J. Gastroenterol. 2017, 23, 7139–7149. [Google Scholar] [CrossRef]
- Rispo, A.; Scarpa, R.; Di Girolamo, E.; Cozzolino, A.; Lembo, G.; Atteno, M.; De Falco, T.; Lo Presti, M.; Castiglione, F. Infliximab in the treatment of extra-intestinal manifestations of Crohn’s disease. Scand. J. Rheumatol. 2005, 34, 387–391. [Google Scholar] [CrossRef]
- Iriarte, A.; Zaera, C.; Bachiller-Corral, J.; López-Sanromán, A. Inflammatory bowel disease as a paradoxical effect of anti-TNF alpha therapy. Gastroenterol. Hepatol. 2017, 40, 117–121. [Google Scholar] [CrossRef]
- Deodhar, A.; Sliwinska-Stanczyk, P.; Xu, H.; Baraliakos, X.; Gensler, L.S.; Fleishaker, D.; Wang, L.; Wu, J.; Menon, S.; Wang, C.; et al. Tofacitinib for the treatment of ankylosing spondylitis: A phase III, randomised, double-blind, placebo-controlled study. Ann. Rheum. Dis. 2021, 80, 1004–1013. [Google Scholar] [CrossRef]
- van der Heijde, D.; Baraliakos, X.; Sieper, J.; Deodhar, A.; Inman, R.D.; Kameda, H.; Zeng, X.; Sui, Y.; Bu, X.; Pangan, A.L.; et al. Efficacy and safety of upadacitinib for active ankylosing spondylitis refractory to biological therapy: A double-blind, randomised, placebo-controlled phase 3 trial. Ann. Rheum. Dis. 2022, 81, 1515–1523. [Google Scholar] [CrossRef] [PubMed]
- Dubash, S.; Marianayagam, T.; Tinazzi, I.; Al-Araimi, T.; Pagnoux, C.; Weizman, A.V.; Richette, P.; Tran Minh, M.-L.; Allez, M.; Singh, A.; et al. Emergence of severe spondyloarthropathy-related entheseal pathology following successful vedolizumab therapy for inflammatory bowel disease. Rheumatology 2019, 58, 963–968. [Google Scholar] [CrossRef] [PubMed]
- Macaluso, F.S.; Fries, W.; Viola, A.; Costantino, G.; Muscianisi, M.; Cappello, M.; Guida, L.; Giuffrida, E.; Magnano, A.; Pluchino, D.; et al. Effectiveness of Ustekinumab on Crohn’s Disease Associated Spondyloarthropathy: Real-World Data from the Sicilian Network for Inflammatory Bowel Diseases (SN-IBD). Expert Opin. Biol. Ther. 2020, 20, 1381–1384. [Google Scholar] [CrossRef] [PubMed]
- Clayton, T.H.; Walker, B.P.; Stables, G.I. Treatment of chronic erythema nodosum with infliximab. Clin. Exp. Dermatol. 2006, 31, 823–824. [Google Scholar] [CrossRef] [PubMed]
- Biemans, V.B.C.; van der Meulen-de Jong, A.E.; van der Woude, C.J.; Löwenberg, M.; Dijkstra, G.; Oldenburg, B.; de Boer, N.K.H.; van der Marel, S.; Bodelier, A.G.L.; Jansen, J.M.; et al. Ustekinumab for Crohn’s Disease: Results of the ICC Registry, a Nationwide Prospective Observational Cohort Study. J. Crohn’s Colitis 2020, 14, 33–45. [Google Scholar] [CrossRef] [PubMed]
- Pagani, K.; Lukac, D.; Bhukhan, A.; McGee, J.S. Cutaneous Manifestations of Inflammatory Bowel Disease: A Basic Overview. Am. J. Clin. Dermatol. 2022, 23, 481–497. [Google Scholar] [CrossRef] [PubMed]
- Maverakis, E.; Marzano, A.V.; Le, S.T.; Callen, J.P.; Brüggen, M.-C.; Guenova, E.; Dissemond, J.; Shinkai, K.; Langan, S.M. Pyoderma gangrenosum. Nat. Rev. Dis. Primer 2020, 6, 81. [Google Scholar] [CrossRef]
- Funayama, Y.; Kumagai, E.; Takahashi, K.-I.; Fukushima, K.; Sasaki, I. Early diagnosis and early corticosteroid administration improves healing of peristomal pyoderma gangrenosum in inflammatory bowel disease. Dis. Colon Rectum 2009, 52, 311–314. [Google Scholar] [CrossRef]
- Phillips, F.M.; Verstockt, B.; Sebastian, S.; Ribaldone, D.; Vavricka, S.; Katsanos, K.; Slattery, E.; de Suray, N.; Flores, C.; Fries, W.; et al. Inflammatory Cutaneous Lesions in Inflammatory Bowel Disease Treated with Vedolizumab or Ustekinumab: An ECCO CONFER Multicentre Case Series. J. Crohn’s Colitis 2020, 14, 1488–1493. [Google Scholar] [CrossRef]
- Vavricka, S.R.; Galván, J.A.; Dawson, H.; Soltermann, A.; Biedermann, L.; Scharl, M.; Schoepfer, A.M.; Rogler, G.; Prinz Vavricka, M.B.; Terracciano, L.; et al. Expression Patterns of TNFα, MAdCAM1, and STAT3 in Intestinal and Skin Manifestations of Inflammatory Bowel Disease. J. Crohn’s Colitis 2018, 12, 347–354. [Google Scholar] [CrossRef]
- Thomas, A.S.; Lin, P. Ocular manifestations of inflammatory bowel disease. Curr. Opin. Ophthalmol. 2016, 27, 552–560. [Google Scholar] [CrossRef]
- Singh, R.B.; Sinha, S.; Saini, C.; Elbasiony, E.; Thakur, S.; Agarwal, A. Recent advances in the management of non-infectious posterior uveitis. Int. Ophthalmol. 2020, 40, 3187–3207. [Google Scholar] [CrossRef] [PubMed]
- Jaffe, G.J.; Dick, A.D.; Brézin, A.P.; Nguyen, Q.D.; Thorne, J.E.; Kestelyn, P.; Barisani-Asenbauer, T.; Franco, P.; Heiligenhaus, A.; Scales, D.; et al. Adalimumab in Patients with Active Noninfectious Uveitis. N. Engl. J. Med. 2016, 375, 932–943. [Google Scholar] [CrossRef]
- Nguyen, Q.D.; Merrill, P.T.; Jaffe, G.J.; Dick, A.D.; Kurup, S.K.; Sheppard, J.; Schlaen, A.; Pavesio, C.; Cimino, L.; Van Calster, J.; et al. Adalimumab for prevention of uveitic flare in patients with inactive non-infectious uveitis controlled by corticosteroids (VISUAL II): A multicentre, double-masked, randomised, placebo-controlled phase 3 trial. Lancet 2016, 388, 1183–1192. [Google Scholar] [CrossRef] [PubMed]
- Suhler, E.B.; Adán, A.; Brézin, A.P.; Fortin, E.; Goto, H.; Jaffe, G.J.; Kaburaki, T.; Kramer, M.; Lim, L.L.; Muccioli, C.; et al. Safety and Efficacy of Adalimumab in Patients with Noninfectious Uveitis in an Ongoing Open-Label Study: VISUAL III. Ophthalmology 2018, 125, 1075–1087. [Google Scholar] [CrossRef]
- Mugheddu, C.; Atzori, L.; Del Piano, M.; Lappi, A.; Pau, M.; Murgia, S.; Zucca, I.; Rongioletti, F. Successful ustekinumab treatment of noninfectious uveitis and concomitant severe psoriatic arthritis and plaque psoriasis. Dermatol. Ther. 2017, 30, e12527. [Google Scholar] [CrossRef]
- Paley, M.A.; Karacal, H.; Rao, P.K.; Margolis, T.P.; Miner, J.J. Tofacitinib for refractory uveitis and scleritis. Am. J. Ophthalmol. Case Rep. 2019, 13, 53–55. [Google Scholar] [CrossRef]
- Williams, C.P.R.; Browning, A.C.; Sleep, T.J.; Webber, S.K.; McGill, J.I. A randomised, double-blind trial of topical ketorolac vs artificial tears for the treatment of episcleritis. Eye 2005, 19, 739–742. [Google Scholar] [CrossRef]
- Mintz, R.; Feller, E.R.; Bahr, R.L.; Shah, S.A. Ocular manifestations of inflammatory bowel disease. Inflamm. Bowel Dis. 2004, 10, 135–139. [Google Scholar] [CrossRef]
- Juillerat, P.; Manz, M.; Sauter, B.; Zeitz, J.; Vavricka, S.R. Swiss IBDnet, an official working group of the Swiss Society of Gastroenterology Therapies in Inflammatory Bowel Disease Patients with Extraintestinal Manifestations. Digestion 2020, 101 (Suppl. S1), 83–97. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Aty, A.; Gupta, A.; Del Priore, L.; Kombo, N. Management of noninfectious scleritis. Ther. Adv. Ophthalmol. 2022, 14, 25158414211070879. [Google Scholar] [CrossRef] [PubMed]
- Caron, B.; Peyrin-Biroulet, L.; Pariente, B.; Bouhnik, Y.; Seksik, P.; Bouguen, G.; Caillo, L.; Laharie, D.; Carbonnel, F.; Altwegg, R.; et al. Vedolizumab Therapy is Ineffective for Primary Sclerosing Cholangitis in Patients with Inflammatory Bowel Disease: A GETAID Multicentre Cohort Study. J. Crohn’s Colitis 2019, 13, 1239–1247. [Google Scholar] [CrossRef] [PubMed]
- Tse, C.S.; Loftus, E.V.; Raffals, L.E.; Gossard, A.A.; Lightner, A.L. Effects of vedolizumab, adalimumab and infliximab on biliary inflammation in individuals with primary sclerosing cholangitis and inflammatory bowel disease. Aliment. Pharmacol. Ther. 2018, 48, 190–195. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Li, Z.; Zeng, X.; Lin, Y.; Xie, W.-F. Ursodeoxycholic acid in primary sclerosing cholangitis: Meta-analysis of randomized controlled trials. Hepatol. Res. 2009, 39, 865–873. [Google Scholar] [CrossRef] [PubMed]
- Boonstra, K.; Weersma, R.K.; van Erpecum, K.J.; Rauws, E.A.; Spanier, B.W.M.; Poen, A.C.; van Nieuwkerk, K.M.; Drenth, J.P.; Witteman, B.J.; Tuynman, H.A.; et al. Population-based epidemiology, malignancy risk, and outcome of primary sclerosing cholangitis. Hepatology 2013, 58, 2045–2055. [Google Scholar] [CrossRef] [PubMed]
- Adam, R.; Karam, V.; Cailliez, V.; O Grady, J.G.; Mirza, D.; Cherqui, D.; Klempnauer, J.; Salizzoni, M.; Pratschke, J.; Jamieson, N.; et al. 2018 Annual Report of the European Liver Transplant Registry (ELTR)—50-year evolution of liver transplantation. Transpl. Int. 2018, 31, 1293–1317. [Google Scholar] [CrossRef]
- Lohse, A.W.; Sebode, M.; Jørgensen, M.H.; Ytting, H.; Karlsen, T.H.; Kelly, D.; Manns, M.P.; Vesterhus, M.; European Reference Network on Hepatological Diseases (ERN RARE-LIVER); International Autoimmune Hepatitis Group (IAIHG). Second-line and third-line therapy for autoimmune hepatitis: A position statement from the European Reference Network on Hepatological Diseases and the International Autoimmune Hepatitis Group. J. Hepatol. 2020, 73, 1496–1506. [Google Scholar] [CrossRef]
- Weiler-Normann, C.; Schramm, C.; Quaas, A.; Wiegard, C.; Glaubke, C.; Pannicke, N.; Möller, S.; Lohse, A.W. Infliximab as a rescue treatment in difficult-to-treat autoimmune hepatitis. J. Hepatol. 2013, 58, 529–534. [Google Scholar] [CrossRef]
- Mihai, I.R.; Burlui, A.M.; Rezus, I.I.; Mihai, C.; Macovei, L.A.; Cardoneanu, A.; Gavrilescu, O.; Dranga, M.; Rezus, E. Inflammatory Bowel Disease as a Paradoxical Reaction to Anti-TNF-α Treatment—A Review. Life 2023, 13, 1779. [Google Scholar] [CrossRef]
- Jostins, L.; Ripke, S.; Weersma, R.K.; Duerr, R.H.; McGovern, D.P.; Hui, K.Y.; Lee, J.C.; Philip Schumm, L.; Sharma, Y.; Anderson, C.A.; et al. Host–microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature 2012, 491, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Fiorino, G.; Danese, S.; Pariente, B.; Allez, M. Paradoxical immune-mediated inflammation in inflammatory bowel disease patients receiving anti-TNF-α agents. Autoimmun. Rev. 2014, 13, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Afzali, A.; Wheat, C.L.; Hu, J.K.; Olerud, J.E.; Lee, S.D. The association of psoriasiform rash with anti-tumor necrosis factor (anti-TNF) therapy in inflammatory bowel disease: A single academic center case series. J. Crohn’s Colitis 2014, 8, 480–488. [Google Scholar] [CrossRef] [PubMed]
- Revankar, R.; Patel, H.; Rojas, M.; Walsh, S.; McGee, J.S. Systematic review of TNFα-induced paradoxical psoriasis: Treatment outcomes of switching to alternative biologic therapies in inflammatory bowel disease patients. J. Dermatol. Treat. 2023, 34, 2133533. [Google Scholar] [CrossRef] [PubMed]
- Puig, L. Paradoxical Reactions: Anti-Tumor Necrosis Factor Alpha Agents, Ustekinumab, Secukinumab, Ixekizumab, and Others. In Adverse Reactions to Biologics; Karger Publishers: Basel, Switzerland, 2017. [Google Scholar] [CrossRef]
- Din, S.; Selinger, C.P.; Black, C.J.; Ford, A.C. Systematic review with network meta-analysis: Risk of Herpes zoster with biological therapies and small molecules in inflammatory bowel disease. Aliment. Pharmacol. Ther. 2023, 57, 666–675. [Google Scholar] [CrossRef] [PubMed]
- Panés, J.; Sandborn, W.J.; Schreiber, S.; Sands, B.E.; Vermeire, S.; D’Haens, G.; Panaccione, R.; Higgins, P.D.R.; Colombel, J.-F.; Feagan, B.G.; et al. Tofacitinib for induction and maintenance therapy of Crohn’s disease: Results of two phase IIb randomised placebo-controlled trials. Gut 2017, 66, 1049–1059. [Google Scholar] [CrossRef] [PubMed]
- Dupré, A.; Collins, M.; Nocturne, G.; Carbonnel, F.; Mariette, X.; Seror, R. Articular manifestations in patients with inflammatory bowel disease treated with vedolizumab. Rheumatology 2020, 59, 3275–3283. [Google Scholar] [CrossRef] [PubMed]
- García, M.J.; Pascual, M.; Del Pozo, C.; Díaz-González, A.; Castro, B.; Rasines, L.; Crespo, J.; Rivero, M. Impact of immune-mediated diseases in inflammatory bowel disease and implications in therapeutic approach. Sci. Rep. 2020, 10, 10731. [Google Scholar] [CrossRef] [PubMed]
- Algaba, A.; Guerra, I.; Ricart, E.; Iglesias, E.; Mañosa, M.; Gisbert, J.P.; Guardiola, J.; Mínguez, M.; Castro, B.; de Francisco, R.; et al. Extraintestinal Manifestations in Patients with Inflammatory Bowel Disease: Study Based on the ENEIDA Registry. Dig. Dis. Sci. 2021, 66, 2014–2023. [Google Scholar] [CrossRef]
- Guillo, L.; D’Amico, F.; Serrero, M.; Angioi, K.; Loeuille, D.; Costanzo, A.; Danese, S.; Peyrin-Biroulet, L. Assessment of extraintestinal manifestations in inflammatory bowel diseases: A systematic review and a proposed guide for clinical trials. United Eur. Gastroenterol. J. 2020, 8, 1013–1030. [Google Scholar] [CrossRef]
- Janssen Cilag, S.A.S. Effectiveness of Ustekinumab in Patients Suffering from Inflammatory Bowel Disease (Crohn’s Disease or Ulcerative Colitis) with Extra-Intestinal Manifestations or Immune-Mediated Inflammatory Diseases in a Real-World Setting. clinicaltrials.gov, Clinical Trial Registration NCT03606499, October 2023. Available online: https://clinicaltrials.gov/study/NCT03606499 (accessed on 1 January 2024).

| Genetic Risk Factors between Inflammatory Bowel Disease and Extraintestinal Manifestations | |
|---|---|
| Ankylosing spondylitis [15] | IL23R |
| Sacroiliitis [13] | NOD2/CARD15 |
| Erythema nodosum [16] | PTGER4, ITGAL, SOCS5, CD207, ITGB3, rs6828740 |
| Pyoderma gangrenosum [16] | IL8RA, PRDM1, USP15, TIMP3 |
| Uveitis [14] | NOD2/CARD15 |
| Primary sclerosing cholangitis [1] | SOCS1, JAK2, STAT3, TYK2, UBASSH3A, BCL2L11, FOXO1, IRF1 |
| System Involved | Disease | Synchronous with IBD | Treatment That Should Be Considered | Treatment That May Be Considered | Treatment That Cannot Be Recommended |
|---|---|---|---|---|---|
| Musculoskeletal EIM 13% (12.9–13.7) | Ankylosing spondylitis | no | Anti TNF-α | JAKi | vedolizumab, ustekinumab |
| Sacroiliitis | no | Anti TNF-α | JAKi | vedolizumab, ustekinumab | |
| Peripheral arthritis type 1 | yes | Anti TNF-α, JAKi, ustekinumab | vedolizumab | - | |
| Peripheral arthritis type 2 | no | Anti TNF-α, JAKi, ustekinumab | vedolizumab | - | |
| Skin EIM 5% (4.7–5.2) | Erythema nodosum | yes | - | Anti TNF-α | vedolizumab |
| Pyoderma gangrenosum | yes or no | systemic steroids | Anti TNF-α, JAKi, ustekinumab | vedolizumab | |
| Ocular EIM 2.1% (1.9–2.2) | Uveitis | yes or no | topical steroids, anti TNF-α (100%) | JAKi, ustekinumab | vedolizumab |
| Episcleritis | yes | topical lubricants | NSAIDs, corticosteroids | - | |
| Scleritis | yes or no | COX-2 inhibitors, topical NSAIDs, systemic corticosteroids | MTX, AZA, mycophenolate mofetil, calcineurin inhibitors, IFX | - | |
| Hepatobiliary EIM 0.75% (0.6–0.8) | Primary sclerosing cholangitis | no | - | Ursodeoxycholic acid | - |
| Autoimmune hepatitis | yes or no | Systemic steroids, AZA | infliximab | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Faggiani, I.; Fanizza, J.; D’Amico, F.; Allocca, M.; Zilli, A.; Parigi, T.L.; Barchi, A.; Danese, S.; Furfaro, F. Extraintestinal Manifestations in Inflammatory Bowel Disease: From Pathophysiology to Treatment. Biomedicines 2024, 12, 1839. https://doi.org/10.3390/biomedicines12081839
Faggiani I, Fanizza J, D’Amico F, Allocca M, Zilli A, Parigi TL, Barchi A, Danese S, Furfaro F. Extraintestinal Manifestations in Inflammatory Bowel Disease: From Pathophysiology to Treatment. Biomedicines. 2024; 12(8):1839. https://doi.org/10.3390/biomedicines12081839
Chicago/Turabian StyleFaggiani, Ilaria, Jacopo Fanizza, Ferdinando D’Amico, Mariangela Allocca, Alessandra Zilli, Tommaso Lorenzo Parigi, Alberto Barchi, Silvio Danese, and Federica Furfaro. 2024. "Extraintestinal Manifestations in Inflammatory Bowel Disease: From Pathophysiology to Treatment" Biomedicines 12, no. 8: 1839. https://doi.org/10.3390/biomedicines12081839
APA StyleFaggiani, I., Fanizza, J., D’Amico, F., Allocca, M., Zilli, A., Parigi, T. L., Barchi, A., Danese, S., & Furfaro, F. (2024). Extraintestinal Manifestations in Inflammatory Bowel Disease: From Pathophysiology to Treatment. Biomedicines, 12(8), 1839. https://doi.org/10.3390/biomedicines12081839






