Characterization of the Secretome from Spheroids of Adipose-Derived Stem Cells (SASCs) and Its Potential for Tissue Regeneration
Abstract
1. Introduction
2. Materials and Methods
2.1. Cells Extraction and Culture
2.2. Secretome Collection and Exosomes Extraction
2.3. Protein Extraction and Quantification
2.4. Exosomes Characterization
2.5. mRNA Extraction from Exosomes
2.6. miRNA Extraction from Exosomes
2.7. RNA Quantification
2.8. mRNA Reverse Transcription and Real-Time PCR
2.9. miRNA Reverse Transcription and Real-Time PCR
2.10. Wound-Healing Assay
2.11. Statistical Analysis
3. Results
3.1. Exosomes Characterization
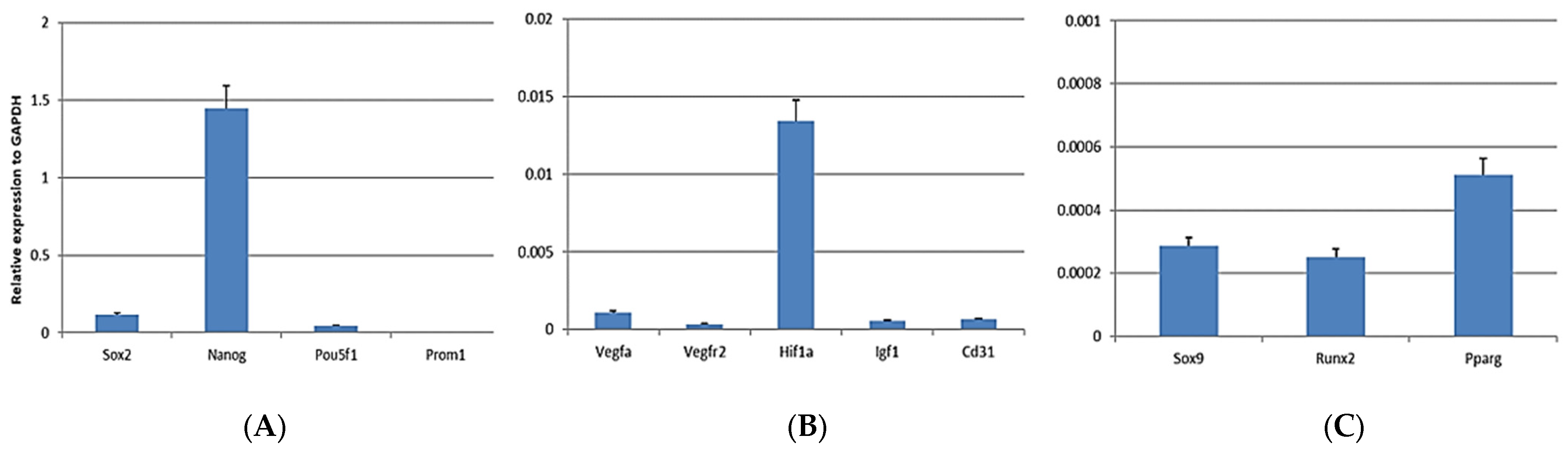
3.2. mRNA Analysis
3.3. miRNA Analysis
3.4. Wound-Healing Assay
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zakrzewski, W.; Dobrzyński, M.; Szymonowicz, M.; Rybak, Z. Stem cells: Past, present, and future. Stem Cell Res. Ther. 2019, 10, 68. [Google Scholar] [CrossRef]
- Inman, J.L.; Robertson, C.; Mott, J.D.; Bissell, M.J. Mammary gland development: Cell fate specification, stem cells and the microenvironment. Development 2015, 142, 1028–1042. [Google Scholar] [CrossRef]
- Takahashi, K.; Yamanaka, S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006, 126, 663–676. [Google Scholar] [CrossRef]
- Uccelli, A.; Moretta, L.; Pistoia, V. Mesenchymal stem cells in health and disease. Nat. Rev. Immunol. 2008, 8, 726–736. [Google Scholar] [CrossRef]
- Morrison, S.J.; Kimble, J. Asymmetric and symmetric stem-cell divisions in development and cancer. Nature 2006, 441, 1068–1074. [Google Scholar] [CrossRef]
- Friedenstein, A.J.; Chailakhjan, R.K.; Lalykina, K.S. The development of fibroblast colonies in monolayer cultures of guinea-pig bone marrow and spleen cells. Cell Tissue Kinet. 1970, 3, 393–403. [Google Scholar] [CrossRef]
- Wang, Y.; Fang, J.; Liu, B.; Shao, C.; Shi, Y. Reciprocal regulation of mesenchymal stem cells and immune responses. Cell Stem Cell 2022, 29, 1515–1530. [Google Scholar] [CrossRef]
- Zhou, J.; Shi, Y. Mesenchymal stem/stromal cells (MSCs): Origin, immune regulation, and clinical applications. Cell Mol. Immunol. 2023, 20, 555–557. [Google Scholar] [CrossRef]
- Khan, A.A.; Huat, T.J.; Al Mutery, A.; El-Serafi, A.T.; Kacem, H.H.; Abdallah, S.H.; Reza, M.F.; Abdullah, J.M.; Jaafar, H. Significant transcriptomic changes are associated with differentiation of bone marrow-derived mesenchymal stem cells into neural progenitor-like cells in the presence of bFGF and EGF. Cell Biosci. 2020, 10, 126. [Google Scholar] [CrossRef]
- Pittenger, M.F.; Mackay, A.M.; Beck, S.C.; Jaiswal, R.K.; Douglas, R.; Mosca, J.D.; Moorman, M.A.; Simonetti, D.W.; Craig, S.; Marshak, D.R. Multilineage potential of adult human mesenchymal stem cells. Science 1999, 284, 143–147. [Google Scholar] [CrossRef]
- Bae, K.S.; Park, J.B.; Kim, H.S.; Kim, D.S.; Park, D.J.; Kang, S.J. Neuron-like differentiation of bone marrow-derived mesenchymal stem cells. Yonsei Med. J. 2011, 52, 401–412. [Google Scholar] [CrossRef]
- Huang, W.H.; Chang, M.C.; Tsai, K.S.; Hung, M.C.; Chen, H.L.; Hung, S.C. Mesenchymal stem cells promote growth and angiogenesis of tumors in mice. Oncogene 2013, 32, 4343–4354. [Google Scholar] [CrossRef] [PubMed]
- Weiss, A.R.R.; Dahlke, M.H. Immunomodulation by Mesenchymal Stem Cells (MSCs): Mechanisms of Action of Living, Apoptotic, and Dead MSCs. Front. Immunol. 2019, 10, 1191. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.; Gebhart, N.; Richelson, E.; Brott, T.G.; Meschia, J.F.; Zubair, A.C. Mechanism of mesenchymal stem cell-induced neuron recovery and anti-inflammation. Cytotherapy 2014, 16, 1336–1344. [Google Scholar] [CrossRef] [PubMed]
- Kossl, J.; Bohacova, P.; Hermankova, B.; Javorkova, E.; Zajicova, A.; Holan, V. Antiapoptotic Properties of Mesenchymal Stem Cells in a Mouse Model of Corneal Inflammation. Stem Cells Dev. 2021, 30, 418–427. [Google Scholar] [CrossRef] [PubMed]
- Papazian, I.; Kyrargyri, V.; Evangelidou, M.; Voulgari-Kokota, A.; Probert, L. Mesenchymal Stem Cell Protection of Neurons against Glutamate Excitotoxicity Involves Reduction of NMDA-Triggered Calcium Responses and Surface GluR1, and Is Partly Mediated by TNF. Int. J. Mol. Sci. 2018, 19, 651. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Tredget, E.E.; Wu, P.Y.; Wu, Y. Paracrine factors of mesenchymal stem cells recruit macrophages and endothelial lineage cells and enhance wound healing. PLoS ONE 2008, 3, e1886. [Google Scholar] [CrossRef]
- Huang, W.; Lv, B.; Zeng, H.; Shi, D.; Liu, Y.; Chen, F.; Li, F.; Liu, X.; Zhu, R.; Yu, L.; et al. Paracrine Factors Secreted by MSCs Promote Astrocyte Survival Associated with GFAP Downregulation After Ischemic Stroke via p38 MAPK and JNK. J. Cell Physiol. 2015, 230, 2461–2475. [Google Scholar] [CrossRef] [PubMed]
- Kuchroo, P.; Dave, V.; Vijayan, A.; Viswanathan, C.; Ghosh, D. Paracrine factors secreted by umbilical cord-derived mesenchymal stem cells induce angiogenesis in vitro by a VEGF-independent pathway. Stem Cells Dev. 2015, 24, 437–450. [Google Scholar] [CrossRef]
- Kwon, H.M.; Hur, S.M.; Park, K.Y.; Kim, C.K.; Kim, Y.M.; Kim, H.S.; Shin, H.C.; Won, M.H.; Ha, K.S.; Kwon, Y.G.; et al. Multiple paracrine factors secreted by mesenchymal stem cells contribute to angiogenesis. Vascul Pharmacol. 2014, 63, 19–28. [Google Scholar] [CrossRef]
- Pankajakshan, D.; Agrawal, D.K. Mesenchymal Stem Cell Paracrine Factors in Vascular Repair and Regeneration. J. Biomed. Technol. Res. 2014, 1. [Google Scholar] [CrossRef] [PubMed]
- Miana, V.V.; González, E.A.P. Adipose tissue stem cells in regenerative medicine. Ecancermedicalscience 2018, 12, 822. [Google Scholar] [CrossRef]
- Krawczenko, A.; Klimczak, A. Adipose Tissue-Derived Mesenchymal Stem/Stromal Cells and Their Contribution to Angiogenic Processes in Tissue Regeneration. Int. J. Mol. Sci. 2022, 23, 2425. [Google Scholar] [CrossRef]
- Chen, H.; Seaman, L.; Liu, S.; Ried, T.; Rajapakse, I. Chromosome conformation and gene expression patterns differ profoundly in human fibroblasts grown in spheroids versus monolayers. Nucleus 2017, 8, 383–391. [Google Scholar] [CrossRef][Green Version]
- Jauković, A.; Abadjieva, D.; Trivanović, D.; Stoyanova, E.; Kostadinova, M.; Pashova, S.; Kestendjieva, S.; Kukolj, T.; Jeseta, M.; Kistanova, E.; et al. Specificity of 3D MSC Spheroids Microenvironment: Impact on MSC Behavior and Properties. Stem Cell Rev. Rep. 2020, 16, 853–875. [Google Scholar] [CrossRef]
- Koledova, Z. 3D Cell Culture: An Introduction; Springer Nature: Berlin, Germany, 2017. [Google Scholar]
- Langhans, S.A. Three-Dimensional. Front. Pharmacol. 2018, 9, 6. [Google Scholar]
- Duval, K.; Grover, H.; Han, L.-H.; Mou, Y.; Pegoraro, A.F.; Fredberg, J.; Zi, C. Modeling Physiological Events in 2D vs. 3D Cell Culture. Physiology 2017, 32, 266–277. [Google Scholar] [CrossRef]
- Kaiser, D. A microbial genetic journey. Annu. Rev. Microbiol. 2006, 60, 1–25. [Google Scholar] [CrossRef]
- Kapałczyńska, M.; Kolenda, T.; Przybyła, W.; Zajączkowska, M.; Teresiak, A.; Filas, V.; Ibbs, M.; Bliźniak, R.; Łuczewski, Ł.; Lamperska, K. 2D and 3D cell cultures—A comparison of different types of cancer cell cultures. Arch. Med. Sci. 2018, 14, 910–919. [Google Scholar] [CrossRef] [PubMed]
- Birgersdotter, A.; Sandberg, R.; Ernberg, I. Gene expression perturbation in vitro—A growing case for three-dimensional (3D) culture systems. Semin. Cancer Biol. 2005, 15, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Mueller-Klieser, W. Three-dimensional cell cultures: From molecular mechanisms to clinical applications. Am. J. Physiol. 1997, 273, C1109–C1123. [Google Scholar] [CrossRef] [PubMed]
- Benuck, M.; Marks, N. Differences in the degradation of hypothalamic releasing factors by rat and human serum. Life Sci. 1976, 19, 1271–1276. [Google Scholar] [CrossRef]
- Bartosh, T.J.; Ylöstalo, J.H.; Mohammadipoor, A.; Bazhanov, N.; Coble, K.; Claypool, K.; Lee, R.H.; Choi, H.; Prockop, D.J. Aggregation of human mesenchymal stromal cells (MSCs) into 3D spheroids enhances their antiinflammatory properties. Proc. Natl. Acad. Sci. USA 2010, 107, 13724–13729. [Google Scholar] [CrossRef] [PubMed]
- Di Stefano, A.B.; Urrata, V.; Trapani, M.; Moschella, F.; Cordova, A.; Toia, F. Systematic review on spheroids from adipose-derived stem cells: Spontaneous or artefact state? J. Cell. Physiol. 2022, 237, 4397–4411. [Google Scholar] [CrossRef]
- Redondo-Castro, E.; Cunningham, C.J.; Miller, J.; Brown, H.; Allan, S.M.; Pinteaux, E. Changes in the secretome of tridimensional spheroid-cultured human mesenchymal stem cells in vitro by interleukin-1 priming. Stem Cell Res. Ther. 2018, 9, 11. [Google Scholar] [CrossRef]
- Di Stefano, A.B.; Grisafi, F.; Perez-Alea, M.; Castiglia, M.; Di Simone, M.; Meraviglia, S.; Cordova, A.; Moschella, F.; Toia, F. Cell quality evaluation with gene expression analysis of spheroids (3D) and adherent (2D) adipose stem cells. Gene 2021, 768, 145269. [Google Scholar] [CrossRef] [PubMed]
- Di Stefano, A.; Leto Barone, A.; Giammona, A.; Apuzzo, T.; Moschella, P.; Di Franco, S.; Giunta, G.; Carmisciano, M.; Eleuteri, C.; Todaro, M.; et al. Identification and Expansion of Adipose Stem Cells with Enhanced Bone Regeneration Properties. J. Regen Med. 2015, 11, 2–3. [Google Scholar]
- Di Stefano, A.B.; Grisafi, F.; Castiglia, M.; Perez, A.; Montesano, L.; Gulino, A.; Toia, F.; Fanale, D.; Russo, A.; Moschella, F.; et al. Spheroids from adipose-derived stem cells exhibit an miRNA profile of highly undifferentiated cells. J. Cell Physiol. 2018, 233, 8778–8789. [Google Scholar] [CrossRef]
- Di Stefano, A.B.; Montesano, L.; Belmonte, B.; Gulino, A.; Gagliardo, C.; Florena, A.M.; Bilello, G.; Moschella, F.; Cordova, A.; Leto Barone, A.A.; et al. Human Spheroids from Adipose-Derived Stem Cells Induce Calvarial Bone Production in a Xenogeneic Rabbit Model. Ann. Plast. Surg. 2021, 86, 714–720. [Google Scholar] [CrossRef]
- Lee, G.H.; Kim, S.H.; Kang, A.; Takayama, S.; Lee, S.H.; Park, J.Y. Deformable L-shaped microwell array for trapping pairs of heterogeneous cells. J. Micromech. Microeng. 2015, 25, 035005. [Google Scholar] [CrossRef]
- Falchook, A.D.; Mayberry, R.I.; Poizner, H.; Burtis, D.B.; Doty, L.; Heilman, K.M. Sign language aphasia from a neurodegenerative disease. Neurocase 2013, 19, 434–444. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, M.; Priimagi, A.; Rochon, P. Effect of saturation on the diffraction efficiency of holographically recorded gratings in azopolymer films. Opt. Express 2009, 17, 844–849. [Google Scholar] [CrossRef] [PubMed]
- Skorik, V.I.; Malikova, T.M.; Boltovskaia, L.F.; Zelikson, B.M.; Safonova, E.S. Control of hypoxia using apneic oxygenation with extrapulmonary membrane elimination of CO2. Biull. Eksp. Biol. Med. 1987, 104, 162–164. [Google Scholar] [CrossRef] [PubMed]
- Otto, A.; Fontaine, J.; Tschirhart, E.; Fontaine, D.; Berkenboom, G. Rosuvastatin treatment protects against nitrate-induced oxidative stress in eNOS knockout mice: Implication of the NAD(P)H oxidase pathway. Br. J. Pharmacol. 2006, 148, 544–552. [Google Scholar] [CrossRef] [PubMed]
- Staines, A.G.; Sindelar, P.; Coughtrie, M.W.; Burchell, B. Farnesol is glucuronidated in human liver, kidney and intestine in vitro, and is a novel substrate for UGT2B7 and UGT1A1. Biochem. J. 2004, 384 Pt 3, 637–645. [Google Scholar] [CrossRef] [PubMed]
- McKay, R. Stem cells in the central nervous system. Science 1997, 276, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Driscoll, J.; Patel, T. The mesenchymal stem cell secretome as an acellular regenerative therapy for liver disease. J. Gastroenterol. 2019, 54, 763–773. [Google Scholar] [CrossRef] [PubMed]
- Hooper, A.T.; Butler, J.; Petit, I.; Rafii, S. Does N-cadherin regulate interaction of hematopoietic stem cells with their niches? Cell Stem Cell 2007, 1, 127–129. [Google Scholar] [CrossRef] [PubMed]
- Neupert, W.; Brunner, M. The protein import motor of mitochondria. Nat. Rev. Mol. Cell Biol. 2002, 3, 555–565. [Google Scholar] [CrossRef]
- Watt, T.T.; Martinez-Ramos, G.; Majumdar, D. Race/ethnicity, acculturation, and sex differences in the relationship between parental social support and children’s overweight and obesity. J. Health Care Poor Underserved 2012, 23, 1793–1805. [Google Scholar] [CrossRef]
- Gurung, S.; Perocheau, D.; Touramanidou, L.; Baruteau, J. The exosome journey: From biogenesis to uptake and intracellular signalling. Cell Commun. Signal 2021, 19, 47. [Google Scholar] [CrossRef] [PubMed]
- Tricarico, C.; Clancy, J.; D’Souza-Schorey, C. Biology and biogenesis of shed microvesicles. Small GTPases 2017, 8, 220–232. [Google Scholar] [CrossRef] [PubMed]
- Borges, F.T.; Reis, L.A.; Schor, N. Extracellular vesicles: Structure, function, and potential clinical uses in renal diseases. Braz. J. Med. Biol. Res. 2013, 46, 824–830. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Xie, F.; Wang, L.; Zhang, L.; Zhang, S.; Fang, M.; Zhou, F. The function and clinical application of extracellular vesicles in innate immune regulation. Cell Mol. Immunol. 2020, 17, 323–334. [Google Scholar] [CrossRef] [PubMed]
- Al-Shaibani, M.B.H. Three-dimensional cell culture (3DCC) improves secretion of signaling molecules of mesenchymal stem cells (MSCs). Biotechnol. Lett. 2022, 44, 143–155. [Google Scholar] [CrossRef] [PubMed]
- Miranda, J.P.; Camões, S.P.; Gaspar, M.M.; Rodrigues, J.S.; Carvalheiro, M.; Bárcia, R.N.; Cruz, P.; Cruz, H.; Simões, S.; Santos, J.M. The Secretome Derived From 3D-Cultured Umbilical Cord Tissue MSCs Counteracts Manifestations Typifying Rheumatoid Arthritis. Front. Immunol. 2019, 10, 18. [Google Scholar] [CrossRef] [PubMed]
- Toia, F.; Lo Presti, E.; Di Stefano, A.B.; Di Simone, M.; Trapani, M.; Corsale, A.M.; Picone, C.; Moschella, F.; Dieli, F.; Cordova, A.; et al. An analysis of the immunomodulatory properties of human spheroids from adipose-derived stem cells. Life Sci. 2023, 321, 121610. [Google Scholar] [CrossRef] [PubMed]
- Barbara Di Stefano, A.; Toia, F.; Urrata, V.; Trapani, M.; Montesano, L.; Cammarata, E.; Moschella, F.; Cordova, A. Spheroids of adipose derived stem cells show their potential in differentiating towards the angiogenic lineage. Gene 2023, 878, 147578. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Huang, Z.; Zhang, X.; Song, X.; Xiao, Y. Reflecting Size Differences of Exosomes by Using the Combination of Membrane-Targeting Viscosity Probe and Fluorescence Lifetime Imaging Microscopy. Anal. Chem. 2019, 91, 15308–15316. [Google Scholar] [CrossRef]
- Huang, Y.; Shen, X.J.; Zou, Q.; Wang, S.P.; Tang, S.M.; Zhang, G.Z. Biological functions of microRNAs: A review. J. Physiol. Biochem. 2011, 67, 129–139. [Google Scholar] [CrossRef]
- O’Brien, J.; Hayder, H.; Zayed, Y.; Peng, C. Overview of MicroRNA Biogenesis, Mechanisms of Actions, and Circulation. Front. Endocrinol. 2018, 9, 402. [Google Scholar] [CrossRef] [PubMed]
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef] [PubMed]
- Jeyaram, A.; Jay, S.M. Preservation and Storage Stability of Extracellular Vesicles for Therapeutic Applications. AAPS J. 2017, 20, 1. [Google Scholar] [CrossRef] [PubMed]
- Ziche, M.; Maglione, D.; Ribatti, D.; Morbidelli, L.; Lago, C.T.; Battisti, M.; Paoletti, I.; Barra, A.; Tucci, M.; Parise, G.; et al. Placenta growth factor-1 is chemotactic, mitogenic, and angiogenic. Lab. Investig. 1997, 76, 517–531. [Google Scholar] [PubMed]
- Cao, Z.; Xie, Y.; Yu, L.; Li, Y.; Wang, Y. Hepatocyte growth factor (HGF) and stem cell factor (SCF) maintained the stemness of human bone marrow mesenchymal stem cells (hBMSCs) during long-term expansion by preserving mitochondrial function via the PI3K/AKT, ERK1/2, and STAT3 signaling pathways. Stem Cell Res. Ther. 2020, 11, 329. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, S.; Morishita, R.; Nakamura, S.; Yamamoto, K.; Moriguchi, A.; Nagano, T.; Taiji, M.; Noguchi, H.; Matsumoto, K.; Nakamura, T.; et al. Potential role of hepatocyte growth factor, a novel angiogenic growth factor, in peripheral arterial disease: Downregulation of HGF in response to hypoxia in vascular cells. Circulation 1999, 100 (Suppl. S19), II301–II308. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Carlton, M.; Chen, X.; Kaur, N.; Ryan, H.; Parker, T.J.; Lin, Z.; Xiao, Y.; Zhou, Y. Effect of fibronectin, FGF-2, and BMP4 in the stemness maintenance of BMSCs and the metabolic and proteomic cues involved. Stem Cell Res. Ther. 2021, 12, 165. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Song, S.; Li, X.; Zhu, J.; Li, W.; Du, B.; Guo, Y.; Xi, X.; Han, R. Down-Regulation of Fibroblast Growth Factor 2 (FGF2) Contributes to the Premature Senescence of Mouse Embryonic Fibroblast. Med. Sci. Monit. 2020, 26, e920520. [Google Scholar] [CrossRef]
- Cavaillon, J.M. Pro- versus anti-inflammatory cytokines: Myth or reality. Cell. Mol. Biol. 2001, 47, 695–702. [Google Scholar]
- Gawlik-Rzemieniewska, N.; Bednarek, I. The role of NANOG transcriptional factor in the development of malignant phenotype of cancer cells. Cancer Biol. Ther. 2016, 17, 1–10. [Google Scholar] [CrossRef]
- Pitrone, M.; Pizzolanti, G.; Tomasello, L.; Coppola, A.; Morini, L.; Pantuso, G.; Ficarella, R.; Guarnotta, V.; Perrini, S.; Giorgino, F.; et al. NANOG Plays a Hierarchical Role in the Transcription Network Regulating the Pluripotency and Plasticity of Adipose Tissue-Derived Stem Cells. Int. J. Mol. Sci. 2017, 18, 1107. [Google Scholar] [CrossRef]
- Sun, Z.; Han, Q.; Zhu, Y.; Li, Z.; Chen, B.; Liao, L.; Bian, C.; Li, J.; Shao, C.; Zhao, R.C. NANOG has a role in mesenchymal stem cells’ immunomodulatory effect. Stem Cells Dev. 2011, 20, 1521–1528. [Google Scholar] [CrossRef]
- Yoon, D.S.; Kim, Y.H.; Jung, H.S.; Paik, S.; Lee, J.W. Importance of Sox2 in maintenance of cell proliferation and multipotency of mesenchymal stem cells in low-density culture. Cell Prolif. 2011, 44, 428–440. [Google Scholar] [CrossRef] [PubMed]
- Park, S.B.; Seo, K.W.; So, A.Y.; Seo, M.S.; Yu, K.R.; Kang, S.K.; Kang, K.S. SOX2 has a crucial role in the lineage determination and proliferation of mesenchymal stem cells through Dickkopf-1 and c-MYC. Cell Death Differ. 2012, 19, 534–545. [Google Scholar] [CrossRef]
- Niknejad, P.; Azizi, H.; Sojoudi, K. Protein and Gene Expression Analysis in Neonate and Adult Mouse Testicular Germ Cells by Immunohistochemistry and Immunocytochemistry. Cell Reprogram 2021, 23, 349–358. [Google Scholar] [CrossRef] [PubMed]
- Kong, R.; Gao, J.; Ji, L.; Zhao, D. MicroRNA-126 promotes proliferation, migration, invasion and endothelial differentiation while inhibits apoptosis and osteogenic differentiation of bone marrow-derived mesenchymal stem cells. Cell Cycle 2020, 19, 2119–2138. [Google Scholar] [CrossRef] [PubMed]
- Fish, J.E.; Santoro, M.M.; Morton, S.U.; Yu, S.; Yeh, R.F.; Wythe, J.D.; Ivey, K.N.; Bruneau, B.G.; Stainier, D.Y.; Srivastava, D. miR-126 regulates angiogenic signaling and vascular integrity. Dev. Cell 2008, 15, 272–284. [Google Scholar] [CrossRef]
- Shafei, S.; Khanmohammadi, M.; Ghanbari, H.; Nooshabadi, V.T.; Tafti, S.H.A.; Rabbani, S.; Kasaiyan, M.; Basiri, M.; Tavoosidana, G. Effectiveness of exosome mediated miR-126 and miR-146a delivery on cardiac tissue regeneration. Cell Tissue Res. 2022, 390, 71–92. [Google Scholar] [CrossRef]
- Shen, H.; Jiang, W.; Yu, Y.; Feng, Y.; Zhang, T.; Liu, Y.; Guo, L.; Zhou, N.; Huang, X. microRNA-146a mediates distraction osteogenesis via bone mesenchymal stem cell inflammatory response. Acta Histochem. 2022, 124, 151913. [Google Scholar] [CrossRef]
- Dull, K.; Fazekas, F.; Deák, D.; Kovács, D.; Póliska, S.; Szegedi, A.; Zouboulis, C.C.; Törőcsik, D. miR-146a modulates TLR1/2 and 4 induced inflammation and links it with proliferation and lipid production via the indirect regulation of GNG7 in human SZ95 sebocytes. Sci. Rep. 2021, 11, 21510. [Google Scholar] [CrossRef]
- Han, R.; Gao, J.; Wang, L.; Hao, P.; Chen, X.; Wang, Y.; Jiang, Z.; Jiang, L.; Wang, T.; Zhu, L.; et al. MicroRNA-146a negatively regulates inflammation via the IRAK1/TRAF6/NF-κB signaling pathway in dry eye. Sci. Rep. 2023, 13, 11192. [Google Scholar] [CrossRef] [PubMed]
- Taganov, K.D.; Boldin, M.P.; Chang, K.J.; Baltimore, D. NF-kappaB-dependent induction of microRNA miR-146, an inhibitor targeted to signaling proteins of innate immune responses. Proc. Natl. Acad. Sci. USA 2006, 103, 12481–12486. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.Y.; Deng, S.Y.; He, Y.B. Ni GX miR-451 inhibits cell growth migration angiogenesis in human osteosarcoma via down-regulating IL, 6R. Biochem. Biophys. Res. Commun. 2017, 482, 987–993. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, A.; Xiang, J.; Lv, Y.; Zhang, X. miR-451 acts as a suppressor of angiogenesis in hepatocellular carcinoma by targeting the IL-6R-STAT3 pathway. Oncol. Rep. 2016, 36, 1385–1392. [Google Scholar] [CrossRef] [PubMed]
- Pakravan, K.; Babashah, S.; Sadeghizadeh, M.; Mowla, S.J.; Mossahebi-Mohammadi, M.; Ataei, F.; Dana, N.; Javan, M. MicroRNA-100 shuttled by mesenchymal stem cell-derived exosomes suppresses in vitro angiogenesis through modulating the mTOR/HIF-1α/VEGF signaling axis in breast cancer cells. Cell Oncol. 2017, 40, 457–470. [Google Scholar] [CrossRef] [PubMed]
- Grundmann, S.; Hans, F.P.; Kinniry, S.; Heinke, J.; Helbing, T.; Bluhm, F.; Sluijter, J.P.; Hoefer, I.; Pasterkamp, G.; Bode, C.; et al. MicroRNA-100 regulates neovascularization by suppression of mammalian target of rapamycin in endothelial and vascular smooth muscle cells. Circulation 2011, 123, 999–1009. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Zhang, H.; Ding, W.; Fan, Z.; Ji, B.; Ding, C.; Ji, F.; Tang, H. miR-143 promotes angiogenesis and osteoblast differentiation by targeting HDAC7. Cell Death Dis. 2020, 11, 179. [Google Scholar] [CrossRef] [PubMed]
- Green, D.; Dalmay, T.; Fraser, W.D. Role of miR-140 in embryonic bone development and cancer. Clin. Sci. 2015, 129, 863–873. [Google Scholar] [CrossRef]
- Duan, L.; Liang, Y.; Xu, X.; Xiao, Y.; Wang, D. Recent progress on the role of miR-140 in cartilage matrix remodelling and its implications for osteoarthritis treatment. Arthritis Res. Ther. 2020, 22, 194. [Google Scholar] [CrossRef]
- Mahjoor, M.; Afkhami, H.; Najafi, M.; Nasr, A.; Khorrami, S. The role of microRNA-30c in targeting interleukin 6, as an inflammatory cytokine, in the mesenchymal stem cell: A therapeutic approach in colorectal cancer. J. Cancer Res. Clin. Oncol. 2023, 149, 3149–3160. [Google Scholar] [CrossRef]
- Sadeghi, S.; Tehrani, F.R.; Tahmasebi, S.; Shafiee, A.; Hashemi, S.M. Exosome engineering in cell therapy and drug delivery. Inflammopharmacology 2023, 31, 145–169. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Corbett, A.L.; Taatizadeh, E.; Tasnim, N.; Little, J.P.; Garnis, C.; Daugaard, M.; Guns, E.; Hoorfar, M.; Li, I.T.S. Challenges and opportunities in exosome research-Perspectives from biology, engineering, and cancer therapy. APL Bioeng. 2019, 3, 011503. [Google Scholar] [CrossRef] [PubMed]
- Hussen, B.M.; Faraj, G.S.H.; Rasul, M.F.; Hidayat, H.J.; Salihi, A.; Baniahmad, A.; Taheri, M.; Ghafouri-Frad, S. Strategies to overcome the main challenges of the use of exosomes as drug carrier for cancer therapy. Cancer Cell Int. 2022, 22, 323. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Urrata, V.; Toia, F.; Cammarata, E.; Franza, M.; Montesano, L.; Cordova, A.; Di Stefano, A.B. Characterization of the Secretome from Spheroids of Adipose-Derived Stem Cells (SASCs) and Its Potential for Tissue Regeneration. Biomedicines 2024, 12, 1842. https://doi.org/10.3390/biomedicines12081842
Urrata V, Toia F, Cammarata E, Franza M, Montesano L, Cordova A, Di Stefano AB. Characterization of the Secretome from Spheroids of Adipose-Derived Stem Cells (SASCs) and Its Potential for Tissue Regeneration. Biomedicines. 2024; 12(8):1842. https://doi.org/10.3390/biomedicines12081842
Chicago/Turabian StyleUrrata, Valentina, Francesca Toia, Emanuele Cammarata, Mara Franza, Luigi Montesano, Adriana Cordova, and Anna Barbara Di Stefano. 2024. "Characterization of the Secretome from Spheroids of Adipose-Derived Stem Cells (SASCs) and Its Potential for Tissue Regeneration" Biomedicines 12, no. 8: 1842. https://doi.org/10.3390/biomedicines12081842
APA StyleUrrata, V., Toia, F., Cammarata, E., Franza, M., Montesano, L., Cordova, A., & Di Stefano, A. B. (2024). Characterization of the Secretome from Spheroids of Adipose-Derived Stem Cells (SASCs) and Its Potential for Tissue Regeneration. Biomedicines, 12(8), 1842. https://doi.org/10.3390/biomedicines12081842






