Quantification of Ions in Human Urine—A Review for Clinical Laboratories
Abstract
1. Urine in Diagnosis—An Overview
2. Urine Analysis—A Summary
3. Ions in Urine as Biomarkers
4. Ions’ Quantification
4.1. Gravimetry
4.2. Titration
4.3. Flame Emission Specrtophotometry
4.4. Fluorimetry
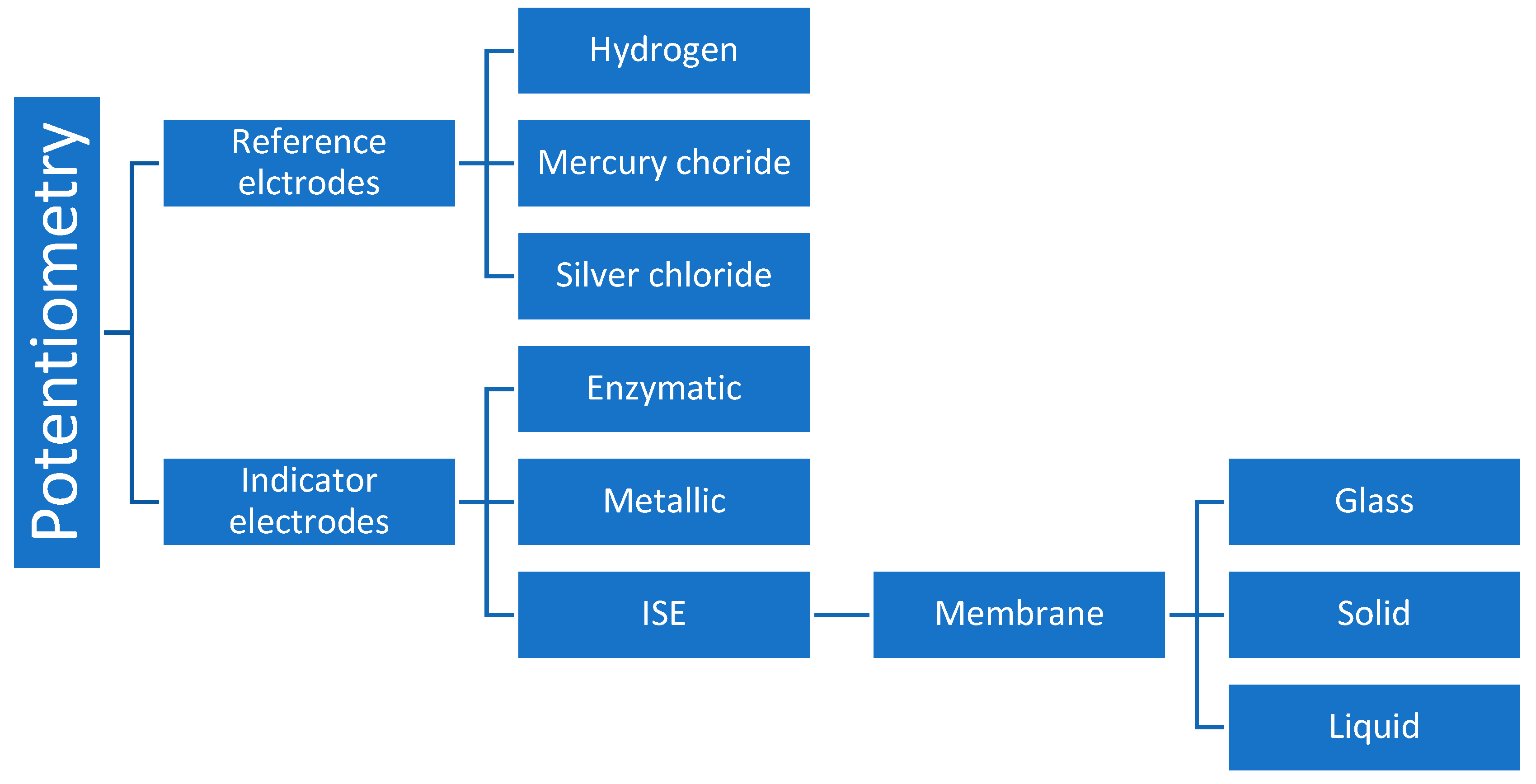
4.5. Potentiometry
4.6. Chromatography
4.7. Electrophoresis
4.8. Kinetic Colorimetric Tests
4.9. Enzymatic Colorimetric Assays
4.10. Flow Cytometry
4.11. Atomic Absorption
4.12. Plasma Emission Spectrometry
4.13. Paper-Based Devices
5. Discussion
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ATP | Adenosine triphosphate |
| EDTA | Ethylene-diamino-tetraacetic acid |
| G6P | Glucose-6-phosphate |
| NADP+ | Nicotinamide adenosine dinucleotide phosphate |
| AAS | Atomic absorption spectrometry |
References
- Edwards, J.G. The Formation of Urine. Arch. Intern. Med. 1940, 65, 800. [Google Scholar] [CrossRef]
- Eaton, D.C.; Pooler, J.P.; Vander, A. Basic Renal Processes for Sodium, Chloride, and Water. In Vander’s Renal Physiology; Eaton, D., Pooler, J., Eds.; McGraw Hill: New York, NY, USA, 2016. [Google Scholar]
- Chabner, D.-E. The Language of Medicine, 11th ed.; Elsevier: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Aslanian, N.L.; Babaian, L.A.; Eripian, G.Z.; Grigorian, D.Z. Rhythms of Electrolyte Excretion in Healthy People. Lab Delo 1989, 9, 21–23. [Google Scholar]
- Strasinger, S. Análisis De Orina Y De Los Líquidos Corporales, 6th ed.; Panamericana: Ciudad de México, Mexico, 2016. [Google Scholar]
- Henrique, F.; George, M. Indicações Para Prescrição Do Ionograma Médicos Do Sistema Nacional de Saúde Departamento Da Qualidade Na Saúde (Dqs@dgs.Pt). 2011. Available online: https://ordemdosmedicos.pt/wp-content/uploads/2017/09/Indicac%C3%B5es_para_Prescricao_do_Ionograma.pdf (accessed on 31 May 2022).
- Roche Diagnostics ISE Indirect Na-K-Cl ForGen.2. 2016.
- Chang, R. Química, 8th ed.; McGraw-Hill: New York, NY, USA, 2005. [Google Scholar]
- Roche Diagnostics Ca2. 2013.
- Ryan, M.F.; Barbour, H. Magnesium Measurement in Routine Clinical Practice. Ann. Clin. Biochem. 1998, 35, 449–459. [Google Scholar] [CrossRef] [PubMed]
- Swaminathan, R. Magnesium metabolism and its disorders. Clin. Biochem. Rev. 2003, 24, 47–66. [Google Scholar] [PubMed]
- Roche Diagnostics Manesium Gen.2. 2019.
- Rieben, W.K.; Van Slyke, D.D. Gravimetric determination of potassium as phospho-12-tungstate. J. Biol. Chem. 1944, 156, 765–776. [Google Scholar] [CrossRef]
- Richeys, B.; Cayley, D.S.; Mossing, M.C.; Kolka, C.; Anderson, C.F.; Farrar, T.C.; Record, M.T. Variability of the intracellular ionic environment of Escherichia coli. Differences between in vitro and in vivo effects of ion concentrations on protein-DNA interactions and gene expression. J. Biol. Chem. 1987, 262, 7157–7164. [Google Scholar]
- Schwarzenbach, G.; Biedermann, W.; Bangerter, F. Komplexone VI. Neue einfache Titriermethoden zur Bestimmung der Wasserhärte. Helv. Chim. Acta 1946, 29, 811–818. [Google Scholar] [CrossRef]
- Hulanicki, A.; Maj-Żurawska, M.; Glab, S. Titrimetry—Potentiometry. In Reference Module in Chemistry, Molecular Sciences and Chemical Engineering; Elsevier: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Loureiro, J.; Janz, J. Iodometric and Colorimetric Methods for the Estimation of Calcium in Serum Based on the Use of an Improved Permanganate Solution. Biochem. J. 1944, 38, 16. [Google Scholar] [CrossRef]
- Sen, B. Indirect complexometric titration of sodium and potassium with EDTA. Fresenius’ Z. Anal. Chem. 1957, 157, 2–6. [Google Scholar] [CrossRef]
- Da Glória, N.A.; Catani, R.A.; Matuo, T. Método do EDTA na determinação do cálcio e magnésio “trocável” do solo. An. Esc. Super. Agric. Luiz Queiroz 1964, 21, 219–228. [Google Scholar] [CrossRef]
- Hald, P.M. The flame photometer for the measurement of sodium and potassium in biological materials. J. Biol. Chem. 1946, 167, 499–510. [Google Scholar] [CrossRef]
- Ewing, C.C. Flame Photometric Determination of Magnesium in Biological Samples. Master’s Thesis, University of Tennessee, Knoxville, TN, USA, 1963. [Google Scholar]
- Haworth, F.; Cleaver, T.J. Flame-photometric determination of calcium and magnesium in vegetables. J. Sci. Food Agric. 1961, 12, 848–852. [Google Scholar] [CrossRef]
- Epstein, W.; Schultz, S.G. Cation Transport in Escherichia coli: V. Regulation of Cation Content. J. Gen. Physiol. 1965, 49, 221–234. [Google Scholar] [PubMed]
- Hill, J.B. Automated Fluorometric Method for Determination of Serum Calcium. Clin. Chem. 1965, 11, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Gosling, P. Analytical Reviews in Clinical Biochemistry: Calcium Measurement. Ann. Clin. Biochem. 1986, 23, 146–156. [Google Scholar] [CrossRef]
- Miyoshi, N.; Kimura, S.; Fukuda, M. A new method of determining intracellular free Ca2+ concentration using Quin2-fluorescence. Photochem. Photobiol. 1991, 53, 415–418. [Google Scholar] [CrossRef]
- Fernandes, J.C.B.; Kubota, L.T.; Neto, G.d.O. Eletrodos íon-seletivos: Histórico, mecanismo de resposta, seletividade e revisão dos conceitos. Quim. Nova 2001, 24, 120–130. [Google Scholar] [CrossRef]
- Lingenfelter, P.; Bartoszewicz, B.; Migdalski, J.; Sokalski, T.; Bućko, M.M.; Filipek, R.; Lewenstam, A. Reference Electrodes with Polymer-Based Membranes—Comprehensive Performance Characteristics. Membranes 2019, 9, 161. [Google Scholar] [CrossRef]
- Yaroshenko, I.; Kirsanov, D.; Kartsova, L.; Sidorova, A.; Borisova, I.; Legin, A. Determination of urine ionic composition with potentiometric multisensor system. Talanta 2015, 131, 556–561. [Google Scholar] [CrossRef] [PubMed]
- Blaz, T.; Baś, B.; Kupis, J.; Migdalski, J.; Lewenstam, A. Multielectrode potentiometry in a one-drop sample. Electrochem. Commun. 2013, 34, 181–184. [Google Scholar] [CrossRef]
- Isildak, Ö.; Özbek, O. Application of Potentiometric Sensors in Real Samples. Crit. Rev. Anal. Chem. 2021, 51, 218–231. [Google Scholar] [CrossRef]
- Marin, D.; Medicuti, F.; Teijeiro, C. An Electrochemistry Experiment: Hydrogen Evolution Reaction on Different Electrodes. J. Chem. Educ. 1994, 71, A277. [Google Scholar] [CrossRef]
- Guilbault, G.G.; Rohm, T.J. Ion-selective Electrodes and Enzyme Electrodes in Environmental and Clinical Studies. Int. J. Environ. Anal. Chem. 1975, 4, 51–64. [Google Scholar] [CrossRef]
- Eisenman, G. Similarities and differences between liquid and solid ion exchangers and their usefulness as ion specific electrodes. Anal. Chem. 1968, 40, 310–320. [Google Scholar] [CrossRef]
- Belyustin, A.A. The centenary of glass electrode: From Max Cremer to F. G. K. Baucke. J. Solid State Electrochem. 2010, 15, 47–65. [Google Scholar] [CrossRef]
- Bagg, J.; Nicholson, O.; Vinen, R. Biionic potentials of a liquid-membrane electrode selective toward calcium. J. Phys. Chem. 1971, 75, 2138–2143. [Google Scholar] [CrossRef]
- Pershina, L.V.; Grabeklis, A.R.; Isankina, L.N.; Skorb, E.V.; Nikolaev, K.G. Determination of sodium and potassium ions in patients with SARS-CoV-2 disease by ion-selective electrodes based on polyelectrolyte complexes as a pseudo-liquid contact phase. RSC Adv. 2021, 11, 36215–36221. [Google Scholar] [CrossRef] [PubMed]
- Anker, P.; Jenny, H.B.; Wuthier, U.; Asper, R.; Ammann, D.; Simon, W. Potentiometry of Na+ in undiluted serum and urine with use of an improved neutral carrier-based solvent polymeric membrane electrode. Clin. Chem. 1983, 29, 1508–1512. [Google Scholar] [CrossRef] [PubMed]
- Nelson, A.M.; Habibi, S.; DeLancey, J.O.L.; Ashton-Miller, J.A.; Burns, M.A. Electrochemical Sensing of Urinary Chloride Ion Concentration for Near Real-Time Monitoring. Biosensors 2023, 13, 331. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Enano, J.; Vélez, P.; Gil, M.; Jose-Cunilleras, E.; Bassols, A.; Martín, F. Characterization of electrolyte content in urine samples through a differential microfluidic sensor based on dumbbell-shaped defected ground structures. Int. J. Microw. Wirel. Technol. 2020, 12, 817–824. [Google Scholar] [CrossRef]
- Harvey, B.J.; Kernan, R.P. Intracellular ion activities in frog skin in relation to external sodium and effects of amiloride and/or ouabain. J. Physiol. 1984, 349, 501–517. [Google Scholar] [CrossRef]
- Miller, A.J. Ion-Selective Microelectrodes for Measurement of Intracellular Ion Concentrations. In Methods in Cell Biology; Academic Press: Cambridge, MA, USA, 1995. [Google Scholar]
- Coskun, O. Separation Tecniques: Chromatography. N. Clin. Istanb. 2016, 3, 156–160. [Google Scholar] [CrossRef]
- Yu, B.-S.; Yuan, Q.-G.; Nie, L.-H.; Yao, S.-Z. Ion chromatographic determination of calcium and magnesium cations in human saliva and urine with a piezoelectric detector. J. Pharm. Biomed. Anal. 2001, 25, 1027–1032. [Google Scholar] [CrossRef] [PubMed]
- Chapp, A.D.; Schum, S.; Behnke, J.E.; Hahka, T.; Huber, M.J.; Jiang, E.; Larson, R.A.; Shan, Z.; Chen, Q.-H. Measurement of cations, anions, and acetate in serum, urine, cerebrospinal fluid, and tissue by ion chromatography. Physiol. Rep. 2018, 6, e13666. [Google Scholar] [CrossRef] [PubMed]
- Hopsort, G.; Latapie, L.; Serrano, K.G.; Loubière, K.; Tzedakis, T. Deciphering the human urine matrix: A new approach to simultaneously quantify the main ions and organic compounds by ion chromatography/mass spectrometry (IC-MS). Anal. Bioanal. Chem. 2023, 415, 5337–5352. [Google Scholar] [CrossRef] [PubMed]
- Wallingford, R.A.; Ewing, A.G. Capillary Electrophoresis. Adv. Chromatogr. 1989, 29, 1–76. [Google Scholar]
- Rogacs, A.; Santiago, J.G. Temperature Effects on Electrophoresis. Anal. Chem. 2013, 85, 5103–5113. [Google Scholar] [CrossRef]
- Poboży, E.; Trojanowicz, M. Application of Capillary Electrophoresis for Determination of Inorganic Analytes in Waters. Molecules 2021, 26, 6972. [Google Scholar] [CrossRef] [PubMed]
- Timerbaev, A.R.; Hirokawa, T. Recent advances of transient isotachophoresis-capillary electrophoresis in the analysis of small ions from high-conductivity matrices. Electrophoresis 2006, 27, 323–340. [Google Scholar] [CrossRef] [PubMed]
- Fritz, J.S. Recent developments in the separation of inorganic and small organic ions by capillary electrophoresis. J. Chromatogr. A 2000, 884, 261–275. [Google Scholar] [CrossRef]
- Nussbaumer, S.; Fleury-Souverain, S.; Bouchoud, L.; Rudaz, S.; Bonnabry, P.; Veuthey, J.-L. Determination of potassium, sodium, calcium and magnesium in total parenteral nutrition formulations by capillary electrophoresis with contactless conductivity detection. J. Pharm. Biomed. Anal. 2010, 53, 130–136. [Google Scholar] [CrossRef]
- Lancioni, C.; Aspromonte, J.; Tascon, M.; Gagliardi, L.G. Development of a background electrolyte for the determination of inorganic cations in high ionic strength samples by capillary electrophoresis with indirect UV-absorption detection. J. Chromatogr. A 2021, 1645, 462091. [Google Scholar] [CrossRef] [PubMed]
- Yeasmin, S.; Ammanath, G.; Ali, Y.; Boehm, B.O.; Yildiz, U.H.; Palaniappan, A.; Liedberg, B. Colorimetric Urinalysis for On-Site Detection of Metabolic Biomarkers. ACS Appl. Mater. Interfaces 2020, 12, 31270–31281. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, G.M.; Silva, W.R.; Barreto, D.N.; Lamarca, R.S.; Lima Gomes, P.C.F.; Petruci, J.F.D.S.; Batista, A.D. Novel approaches for colorimetric measurements in analytical chemistry—A review. Anal. Chim. Acta 2020, 1135, 187–203. [Google Scholar] [CrossRef] [PubMed]
- Kessler, G.; Wolfman, M. An Automated Procedure for the Simultaneous Determination of Calcium and Phosphorus. Clin. Chem. 1964, 10, 686–703. [Google Scholar] [CrossRef] [PubMed]
- Ripoll, J.-P. Determination du calcium serique par une technique utilisant le bleu de methylthymol. Clin. Chim. Acta 1976, 72, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Kratochvil, B.; He, X.-W. A study of the Ca2+–Arsenazo III system and its application to the spectrophotometric determination of free calcium in solution. Can. J. Chem. 1990, 68, 1932–1936. [Google Scholar] [CrossRef]
- Bourguignon, C.; Dupuy, A.M.; Coste, T.; Michel, F.; Cristol, J.P. Evaluation of NM-BAPTA method for plasma total calcium measurement on Cobas 8000®. Clin. Biochem. 2014, 47, 636–639. [Google Scholar] [CrossRef] [PubMed]
- Lindstrom, F.; Diehl, H. Indicator for the Titration of Calcium Plus Magnesium with (Ethylenedinitrilo)tetraacetate. Anal. Chem. 1960, 32, 1123–1127. [Google Scholar] [CrossRef]
- McCoy, S.; Maclaren, N.K.; Gudat, J.C. Bilirubin interferes in the aca determination of Mg2+ in serum. Clin. Chem. 1983, 29, 1309. [Google Scholar] [CrossRef]
- Pollack, H. Micrurgical Studies in Cell Physiology. J. Gen. Physiol. 1928, 11, 539–545. [Google Scholar] [CrossRef]
- Jöbsis, F.; O’Connor, M. Calcium release and reabsorption in the sartorius muscle of the toad. Biochem. Biophys. Res. Commun. 1966, 25, 246–252. [Google Scholar] [CrossRef]
- Garcia, R.A.; Vanelli, C.P.; Junior, O.d.S.P.; Corrêa, J.O.D.A. Comparative analysis for strength serum sodium and potassium in three different methods: Flame photometry, ion-selective electrode (ISE) and colorimetric enzymatic. J. Clin. Lab. Anal. 2018, 32, e22594. [Google Scholar] [CrossRef] [PubMed]
- Kimura, S.; Iyama, S.; Yamaguchi, Y.; Hayashi, S.; Fushimi, R.; Amino, N. New enzymatic assay for calcium in serum. Clin. Chem. 1996, 42, 1202–1205. [Google Scholar] [CrossRef]
- Laerum, O.D.; Farsund, T. Clinical application of flow cytometry: A review. Cytometry 1981, 2, 1–13. [Google Scholar] [CrossRef] [PubMed]
- McKinnon, K.M. Flow Cytometry: An Overview. Curr. Protoc. Immunol. 2018, 120, 5.1.1–5.1.11. [Google Scholar] [CrossRef]
- Balkay, L.; Márián, T.; Emri, M.; Krasznai, Z.; Trón, L. Flow cytometric determination of intracellular free potassium concentration. Cytometry 1997, 28, 42–49. [Google Scholar] [CrossRef]
- Iatridou, H.; Foukaraki, E.; Kuhn, M.A.; Marcus, E.M.; Haugland, R.P.; Katerinopoulos, H.E. The Development of a New Family of Intracellular Calcium Probes. Cell Calcium 1994, 15, 190–198. [Google Scholar] [CrossRef] [PubMed]
- Posey, A.D.; Kawalekar, O.U.; June, C.H. Measurement of Intracellular Ions by Flow Cytometry. Curr. Protoc. Cytom. 2015, 72, 9.8.1–9.8.21. [Google Scholar] [CrossRef]
- Lagalante, A.F. Atomic Absorption Spectroscopy: A Tutorial Review*. Appl. Spectrosc. Rev. 2004, 34, 173–189. [Google Scholar] [CrossRef]
- Wood, C.; LeMoigne, J. Intraeellular Acid-Base Responses to Environmental Hyperoxia and Normoxie Recovery in Rainbow Trout. Respir. Physiol. 1991, 86, 91–113. [Google Scholar] [CrossRef]
- Willis, J.B. Determination of Calcium and Magnesium in Urine by Atomic Absorption Spectroscopy. Anal. Chem. 1961, 33, 556–559. [Google Scholar] [CrossRef]
- Sommer, M.J.; Rutman, M.G.; Wask-Rotter, E.; Wagoner, H.; Fritsche, E.T. Determination of Calcium in Serum Samples by AAS Using a Fuel Lean Flame; Varian Australia Pty. Ltd.: Mulgrave, VIC, Australia, 1995. [Google Scholar]
- Krejčová, A.; Černohorský, T.; Čurdová, E. Determination of sodium, potassium, magnesium and calcium in urine by inductively coupled plasma atomic emission spectrometry. The study of matrix effects. J. Anal. At. Spectrom. 2001, 16, 1002–1005. [Google Scholar] [CrossRef]
- Seller, H.G.; Sigel, A.; Sigel, H. Handbook on Metals in Clinical and Analytical Chemistry; Dekker, M., Ed.; Marcel Dekker: New York, NY, USA, 1994; ISBN 0824790944/9780824790943. [Google Scholar]
- Ghaderinezhad, F.; Koydemir, H.C.; Tseng, D.; Karinca, D.; Liang, K.; Ozcan, A.; Tasoglu, S. Sensing of electrolytes in urine using a miniaturized paper-based device. Sci. Rep. 2020, 10, 13620. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferrão, A.R.; Pestana, P.; Borges, L.; Palmeira-de-Oliveira, R.; Palmeira-de-Oliveira, A.; Martinez-de-Oliveira, J. Quantification of Ions in Human Urine—A Review for Clinical Laboratories. Biomedicines 2024, 12, 1848. https://doi.org/10.3390/biomedicines12081848
Ferrão AR, Pestana P, Borges L, Palmeira-de-Oliveira R, Palmeira-de-Oliveira A, Martinez-de-Oliveira J. Quantification of Ions in Human Urine—A Review for Clinical Laboratories. Biomedicines. 2024; 12(8):1848. https://doi.org/10.3390/biomedicines12081848
Chicago/Turabian StyleFerrão, Ana Rita, Paula Pestana, Lígia Borges, Rita Palmeira-de-Oliveira, Ana Palmeira-de-Oliveira, and José Martinez-de-Oliveira. 2024. "Quantification of Ions in Human Urine—A Review for Clinical Laboratories" Biomedicines 12, no. 8: 1848. https://doi.org/10.3390/biomedicines12081848
APA StyleFerrão, A. R., Pestana, P., Borges, L., Palmeira-de-Oliveira, R., Palmeira-de-Oliveira, A., & Martinez-de-Oliveira, J. (2024). Quantification of Ions in Human Urine—A Review for Clinical Laboratories. Biomedicines, 12(8), 1848. https://doi.org/10.3390/biomedicines12081848





