Causal Relationship between Aging and Anorexia Nervosa: A White-Matter-Microstructure-Mediated Mendelian Randomization Analysis
Abstract
:1. Introduction
2. Materials and Methods
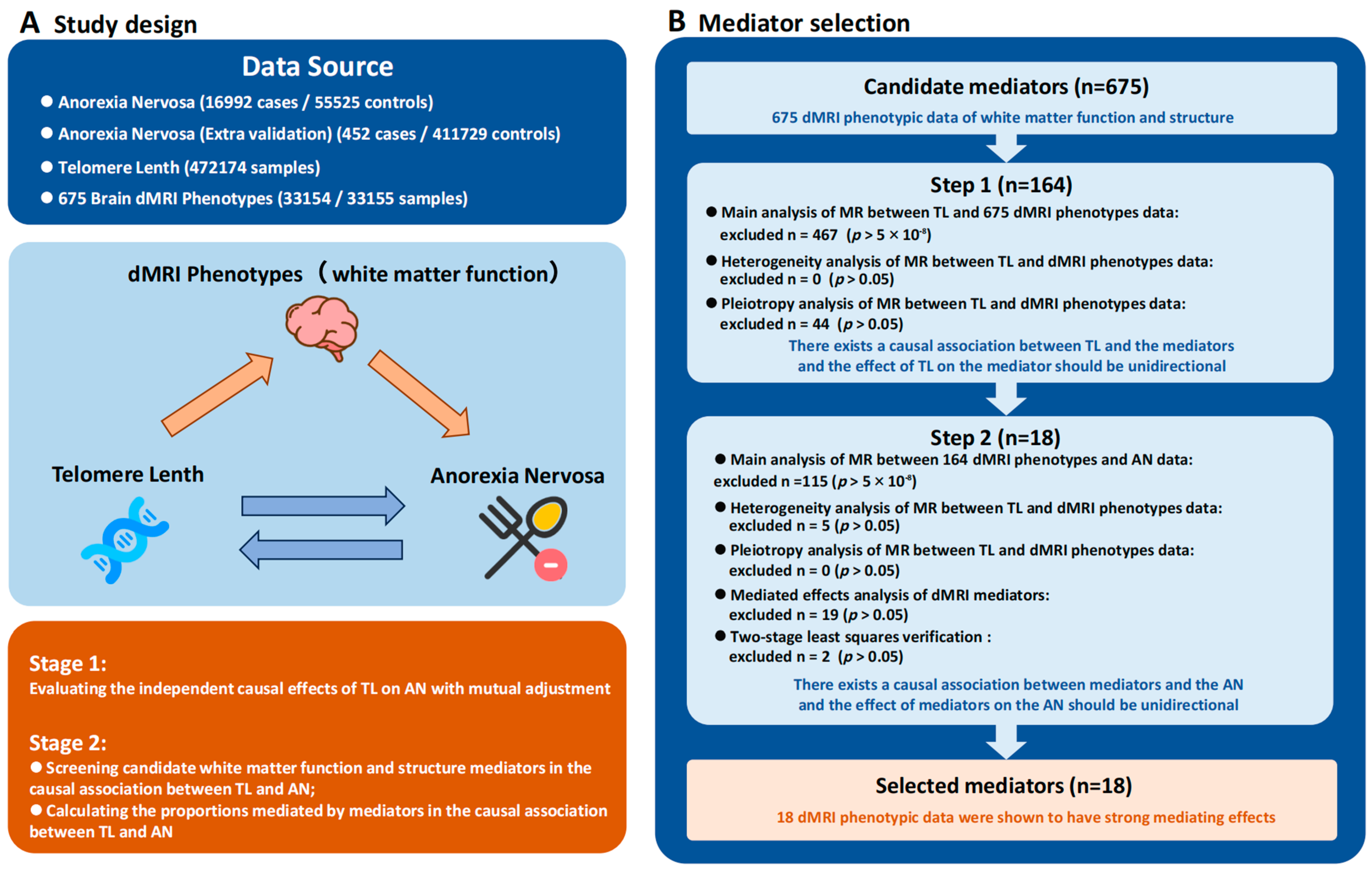
2.1. Study Design and Data Source
2.2. IVs Selection
2.3. MR Analysis
2.4. Sensitivity Analysis
2.5. Evaluation of Mediated Effects
3. Results
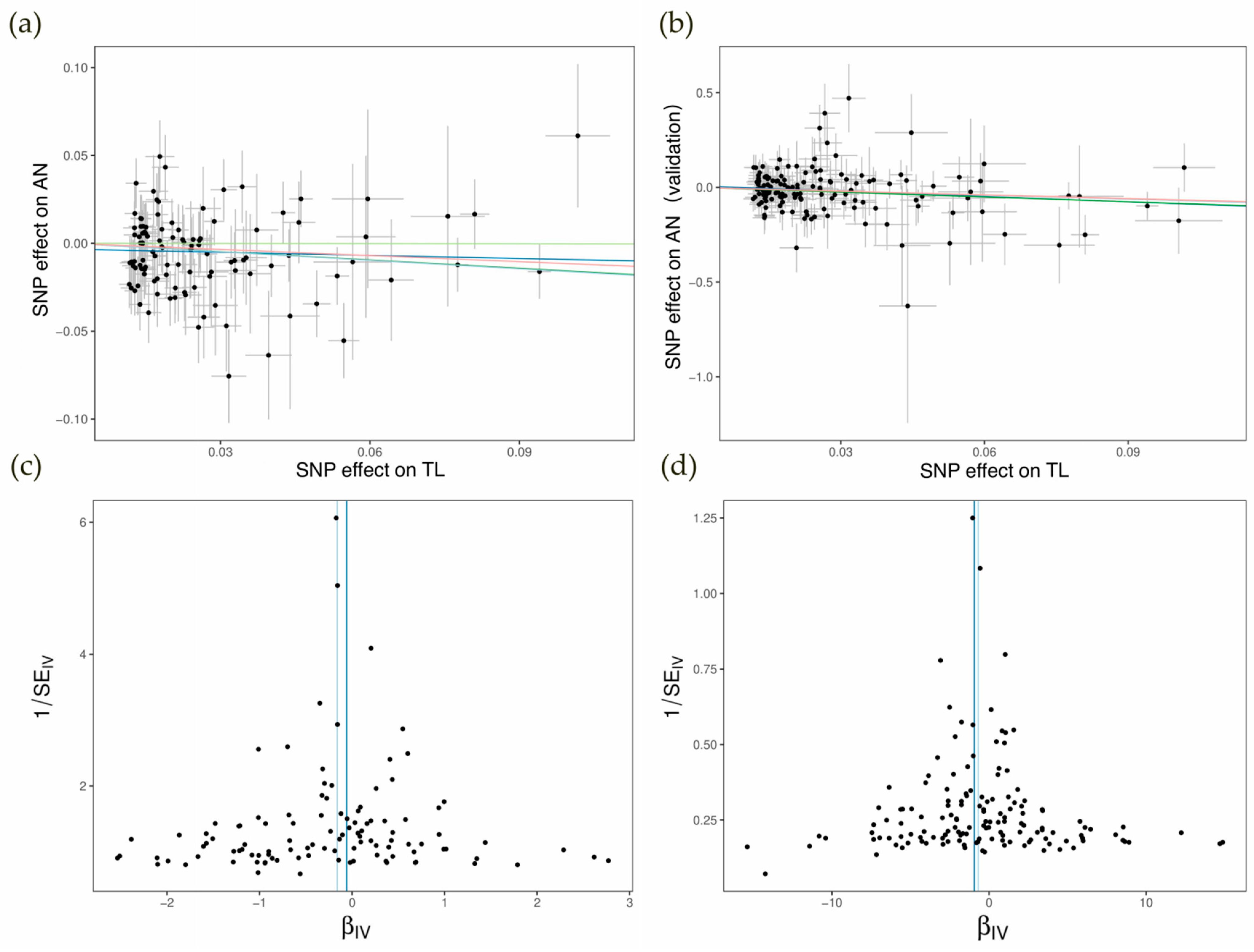
3.1. Direct MR Analysis and Sensitivity Analysis
3.2. Two-Step MR Analysis
3.3. Two-Step Sensitivity Analysis
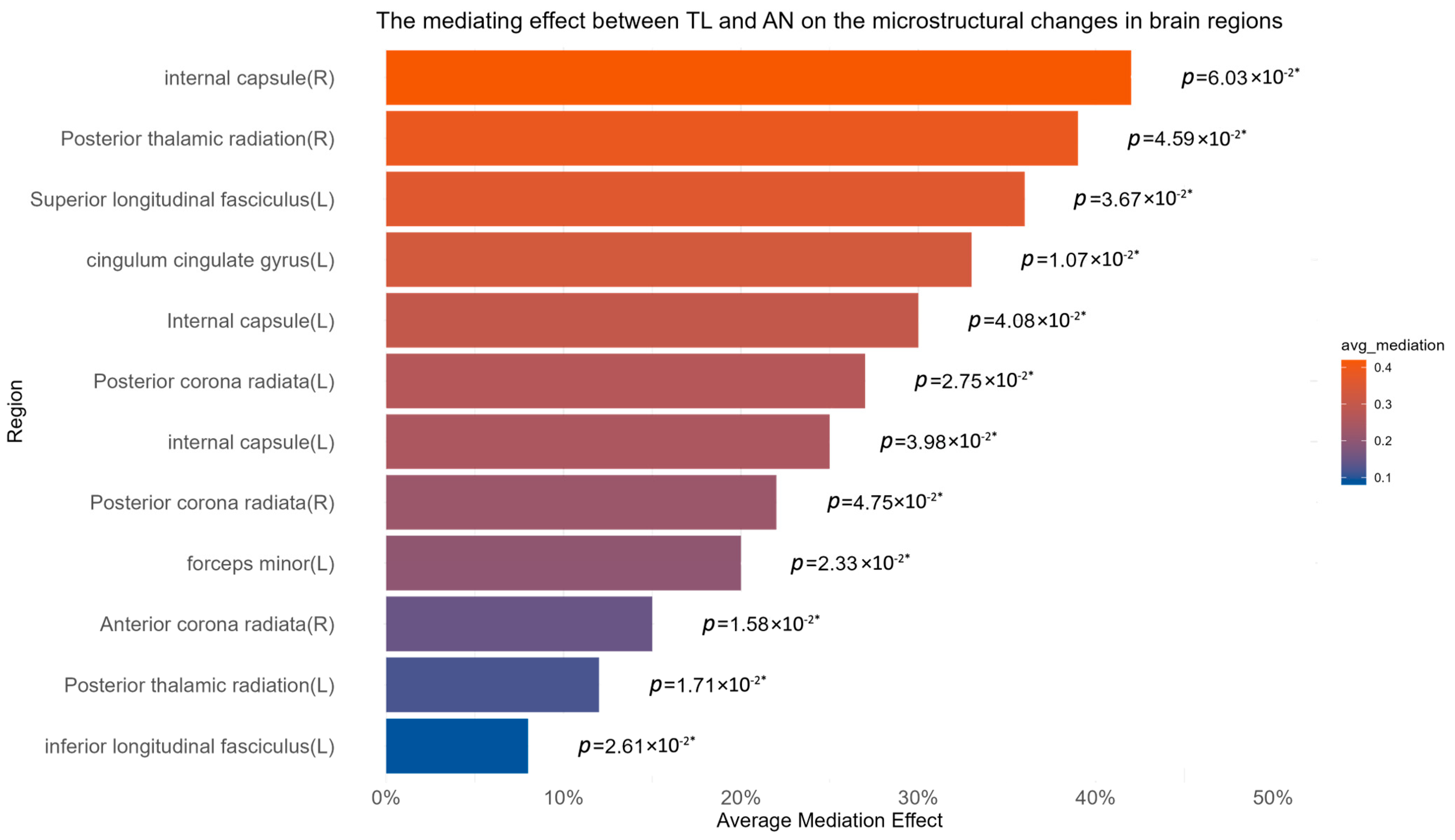
3.4. Calculation of Mediation Effect
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Name of Parameter | Brief Description | Common Interpretation |
|---|---|---|
| Fractional anisotropy (FA) | A scalar value describing the degree of anisotropy of a diffusion process, ranging from 0 (isotropic diffusion, no directionality) to 1 (highly anisotropic diffusion, strong directionality). | Commonly used to assess white matter integrity. Higher FA values are associated with greater fiber density, axonal diameter, and myelination. Lower FA values can indicate white matter damage or degeneration [102]. |
| Intra-Cellular Volume Fraction (ICVF) | The fraction of water diffusion restricted within intra-neurite compartments, reflecting neuronal density and dendritic complexity. | Changes in ICVF can reflect pathological conditions such as neuroinflammation or neurodegeneration. Higher ICVF values suggest greater cellular density, which can be indicative of healthy neural tissue [103]. |
| Isotropic Volume Fraction (ISOVF) | The fraction of water diffusion that is isotropic or hindered within tissues. | Higher ISOVF values may indicate increased tissue damage, edema, or isotropic diffusion due to reduced tissue integrity [104]. |
| Principal eigenvalue or axial diffusivity (L1) | The magnitude of water diffusion along the primary direction within a voxel. | Changes in axial diffusivity can indicate axonal injury or alterations in myelin sheath integrity, providing specific information on white matter pathology [105]. |
| Secondary eigenvalue (L2) | The magnitude of water diffusion perpendicular to the principal direction. | Secondary eigenvalue changes can reflect alterations in tissue integrity and may be indicative of more diffuse tissue damage, particularly in regions with complex fiber orientations [105]. |
| Tertiary eigenvalue (L3) | The magnitude of water diffusion in the direction with the least diffusion. | Like the secondary eigenvalue, changes in the tertiary eigenvalue can help characterize the microstructural complexity of the tissue, especially in areas with crossing fibers [105]. |
| Mean diffusivity (MD) | The average magnitude of water diffusion within a voxel, irrespective of direction. | Increased MD values are generally associated with tissue pathology such as cell swelling, edema, or decreased cell density, while decreased MD may suggest increased tissue organization or coherence [106]. |
| Mode of Anisotropy (MO) | The shape of diffusion tensor ellipsoids within tissues. | Different modes of anisotropy (e.g., planar, linear, spherical) provide insights into tissue microstructure changes and organization, aiding in the characterization of white matter tracts and pathology [102]. |
| Orientation Dispersion (OD) | The variability in fiber orientation within a voxel. | Higher orientation dispersion values indicate less coherent fiber orientations within a voxel, which may occur in regions with crossing fibers or disrupted white matter architecture [107]. |
References
- Mitchell, J.E.; Peterson, C.B. Anorexia Nervosa. N. Engl. J. Med. 2020, 382, 1343–1351. [Google Scholar] [CrossRef] [PubMed]
- Schaumberg, K.; Welch, E.; Breithaupt, L.; Hübel, C.; Baker, J.H.; Munn-Chernoff, M.A.; Yilmaz, Z.; Ehrlich, S.; Mustelin, L.; Ghaderi, A.; et al. The Science Behind the Academy for Eating Disorders’ Nine Truths about Eating Disorders. Eur. Eat. Disord. Rev. 2017, 25, 432–450. [Google Scholar] [CrossRef] [PubMed]
- Pike, K.M.; Dunne, P.E. The Rise of Eating Disorders in Asia: A Review. J. Eat. Disord. 2015, 3, 33. [Google Scholar] [CrossRef] [PubMed]
- Ulfvebrand, S.; Birgegård, A.; Norring, C.; Högdahl, L.; Von Hausswolff-Juhlin, Y. Psychiatric Comorbidity in Women and Men with Eating Disorders Results from a Large Clinical Database. Psychiatry Res. 2015, 230, 294–299. [Google Scholar] [CrossRef] [PubMed]
- Mehler, P.S.; Blalock, D.V.; Walden, K.; Kaur, S.; McBride, J.; Walsh, K.; Watts, J. Medical Findings in 1026 Consecutive Adult Inpatient–Residential Eating Disordered Patients. Int. J. Eat. Disord. 2018, 51, 305–313. [Google Scholar] [CrossRef] [PubMed]
- E Mitchell, J.; Crow, S. Medical complications of anorexia nervosa and bulimia nervosa. Curr. Opin. Psychiatry 2006, 19, 438–443. [Google Scholar] [CrossRef] [PubMed]
- Kertes, D.A.; Clendinen, C.; Duan, K.; Rabinowitz, J.A.; Browning, C.; Kvam, P. The Social Environment Matters for Telomere Length and Internalizing Problems during Adolescence. J. Youth Adolesc. 2023, 53, 21–35. [Google Scholar] [CrossRef] [PubMed]
- Bulik, C.M.; Coleman, J.R.I.; Hardaway, J.A.; Breithaupt, L.; Watson, H.J.; Bryant, C.D.; Breen, G. Genetics and neurobiology of eating disorders. Nat. Neurosci. 2022, 25, 543–554. [Google Scholar] [CrossRef]
- Duncan, L.; Yilmaz, Z.; Gaspar, H.; Walters, R.; Goldstein, J.; Anttila, V.; Bulik-Sullivan, B.; Ripke, S.; Eating Disorders Working Group of the Psychiatric Genomics Consortium; Thornton, L.; et al. Significant Locus and Metabolic Genetic Correlations Revealed in Genome-Wide Association Study of Anorexia Nervosa. Am. J. Psychiatry 2017, 174, 850–858. [Google Scholar] [CrossRef]
- Revy, P.; Kannengiesser, C.; Bertuch, A.A. Genetics of Human Telomere Biology Disorders. Nat. Rev. Genet. 2023, 24, 86–108. [Google Scholar] [CrossRef]
- Aguado, J.; d’Adda Di Fagagna, F.; Wolvetang, E. Telomere Transcription in Ageing. Ageing Res. Rev. 2020, 62, 101115. [Google Scholar] [CrossRef]
- Shay, J.W.; Wright, W.E. Telomeres and Telomerase: Three Decades of Progress. Nat. Rev. Genet. 2019, 20, 299–309. [Google Scholar] [CrossRef]
- Jiang, H.; Schiffer, E.; Song, Z.; Wang, J.; Zürbig, P.; Thedieck, K.; Moes, S.; Bantel, H.; Saal, N.; Jantos, J.; et al. Proteins Induced by Telomere Dysfunction and DNA Damage Represent Biomarkers of Human Aging and Disease. Proc. Natl. Acad. Sci. 2008, 105, 11299–11304. [Google Scholar] [CrossRef] [PubMed]
- Rossiello, F.; Jurk, D.; Passos, J.F.; di Fagagna, F.D. Telomere dysfunction in ageing and age-related diseases. Nat. Cell Biol. 2022, 24, 135–147. [Google Scholar] [CrossRef]
- Herrmann, M.; Pusceddu, I.; März, W.; Herrmann, W. Telomere biology and age-related diseases. Clin Chem Lab Med. 2018, 56, 1210–1222. [Google Scholar] [CrossRef]
- Cawthon, R.M.; Smith, K.R.; O’Brien, E.; Sivatchenko, A.; Kerber, R.A. Association between telomere length in blood and mortality in people aged 60 years or older. Lancet 2003, 361, 393–395. [Google Scholar] [CrossRef]
- King, K.S.; Kozlitina, J.; Rosenberg, R.N.; Peshock, R.M.; McColl, R.W.; Garcia, C.K. Effect of Leukocyte Telomere Length on Total and Regional Brain Volumes in a Large Population-Based Cohort. JAMA Neurol. 2014, 71, 1247–1254. [Google Scholar] [CrossRef] [PubMed]
- Wikgren, M.; Karlsson, T.; Söderlund, H.; Nordin, A.; Roos, G.; Nilsson, L.-G.; Adolfsson, R.; Norrback, K.-F. Shorter telomere length is linked to brain atrophy and white matter hyperintensities. Age and Ageing. 2013, 43, 212–217. [Google Scholar] [CrossRef]
- Fields, R.D. White matter in learning, cognition and psychiatric disorders. Trends Neurosci. 2008, 31, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Magioncalda, P.; Martino, M.; Conio, B.; Piaggio, N.; Teodorescu, R.; Escelsior, A.; Marozzi, V.; Rocchi, G.; Roccatagliata, L.; Northoff, G.; et al. Patterns of microstructural white matter abnormalities and their impact on cognitive dysfunction in the various phases of type I bipolar disorder. J. Affect. Disord. 2016, 193, 39–50. [Google Scholar] [CrossRef]
- Mueller, B.A.; Lim, K.O.; Hemmy, L.; Camchong, J. Diffusion MRI and its Role in Neuropsychology. Neuropsychol. Rev. 2015, 25, 250–271. [Google Scholar] [CrossRef]
- Le Bihan, D.; Johansen-Berg, H. Diffusion MRI at 25: Exploring brain tissue structure and function. NeuroImage 2011, 61, 324–341. [Google Scholar] [CrossRef] [PubMed]
- Dubois, J.; Alison, M.; Counsell, S.J.; Hertz-Pannier, L.; Hüppi, P.S.; Benders, M.J. MRI of the Neonatal Brain: A Review of Methodological Challenges and Neuroscientific Advances. J. Magn. Reson. Imaging 2020, 53, 1318–1343. [Google Scholar] [CrossRef]
- Smith, S.M.; Jenkinson, M.; Johansen-Berg, H.; Rueckert, D.; Nichols, T.E.; Mackay, C.E.; Watkins, K.E.; Ciccarelli, O.; Cader, M.Z.; Matthews, P.M.; et al. Tract-based spatial statistics: Voxelwise analysis of multi-subject diffusion data. NeuroImage 2006, 31, 1487–1505. [Google Scholar] [CrossRef]
- Jenkinson, M.; Beckmann, C.F.; Behrens, T.E.; Woolrich, M.W.; Smith, S.M. FSL. NeuroImage 2012, 62, 782–790. [Google Scholar] [CrossRef] [PubMed]
- Via, E.; Zalesky, A.; Sánchez, I.; Forcano, L.; Harrison, B.J.; Pujol, J.; Fernández-Aranda, F.; Menchón, J.M.; Soriano-Mas, C.; Cardoner, N.; et al. Disruption of brain white matter microstructure in women with anorexia nervosa. J. Psychiatry Neurosci. 2014, 39, 367–375. [Google Scholar] [CrossRef] [PubMed]
- Lawlor, D.A.; Harbord, R.M.; Sterne, J.A.C.; Timpson, N.; Smith, G.D. Mendelian randomization: Using genes as instruments for making causal inferences in epidemiology. Stat. Med. 2008, 27, 1133–1163. [Google Scholar] [CrossRef]
- Larsson, S.C.; Mason, A.M.; Bäck, M.; Klarin, D.; Damrauer, S.M.; Million, V.P.; Burgess, S. Genetic predisposition to smoking in relation to 14 cardiovascular diseases. Eur. Heart J. 2020, 41, 4092–4097. [Google Scholar] [CrossRef]
- Khera, A.V.; Chaffin, M.; Wade, K.H.; Zahid, S.; Brancale, J.; Xia, R.; Natarajan, P. Polygenic prediction of weight and obesity trajectories from birth to adulthood. Cell 2019, 177, 587–596. [Google Scholar] [CrossRef]
- Skrivankova, V.W.; Richmond, R.C.; Woolf, B.A.R.; Davies, N.M.; Swanson, S.A.; VanderWeele, T.J.; Timpson, N.J.; Higgins, J.P.T.; Dimou, N.; Langenberg, C.; et al. Strengthening the reporting of observational studies in epidemiology using mendelian randomisation (STROBE-MR): Explanation and elaboration. BMJ 2021, 375, n2233. [Google Scholar] [CrossRef]
- Codd, V.; Wang, Q.; Allara, E.; Musicha, C.; Kaptoge, S.; Stoma, S.; Jiang, T.; Hamby, S.E.; Braund, P.S.; Bountziouka, V.; et al. Polygenic basis and biomedical consequences of telomere length variation. Nat. Genet. 2021, 53, 1425–1433. [Google Scholar] [CrossRef] [PubMed]
- Watson, H.J.; Yilmaz, Z.; Thornton, L.M.; Hübel, C.; Coleman, J.R.I.; Gaspar, H.A.; Bryois, J.; Hinney, A.; Leppä, V.M.; Mattheisen, M.; et al. Genome-Wide Association Study Identifies Eight Risk Loci and Implicates Metabo-Psychiatric Origins for Anorexia Nervosa. Nat. Genet. 2019, 51, 1207–1214. [Google Scholar] [CrossRef]
- Kurki, M.I.; Karjalainen, J.; Palta, P.; Sipilä, T.P.; Kristiansson, K.; Donner, K.M.; Reeve, M.P.; Laivuori, H.; Aavikko, M.; Kaunisto, M.A.; et al. FinnGen provides genetic insights from a well-phenotyped isolated population. Nature 2023, 613, 508–518. [Google Scholar] [CrossRef]
- Lin, S.-H.; Brown, D.W.; Machiela, M.J. LDtrait: An Online Tool for Identifying Published Phenotype Associations in Linkage Disequilibrium. Cancer Res. 2020, 80, 3443–3446. [Google Scholar] [CrossRef] [PubMed]
- Sollis, E.; Mosaku, A.; Abid, A.; Buniello, A.; Cerezo, M.; Gil, L.; Groza, T.; Güneş, O.; Hall, P.; Hayhurst, J.; et al. The NHGRI-EBI GWAS Catalog: Knowledgebase and deposition resource. Nucleic Acids Res. 2022, 51, D977–D985. [Google Scholar] [CrossRef] [PubMed]
- Burgess, S.; Thompson, S.G. CRP CHD Genetics Collaboration Avoiding bias from weak instruments in Mendelian randomization studies. Leuk. Res. 2011, 40, 755–764. [Google Scholar] [CrossRef]
- Hemani, G.; Zheng, J.; Elsworth, B.; Wade, K.H.; Haberland, V.; Baird, D.; Laurin, C.; Burgess, S.; Bowden, J.; Langdon, R.; et al. The MR-Base platform supports systematic causal inference across the human phenome. eLife 2018, 7, e34408. [Google Scholar] [CrossRef]
- Deng, Y.-T.; Ou, Y.-N.; Wu, B.-S.; Yang, Y.-X.; Jiang, Y.; Huang, Y.-Y.; Liu, Y.; Tan, L.; Dong, Q.; Suckling, J.; et al. Identifying causal genes for depression via integration of the proteome and transcriptome from brain and blood. Mol. Psychiatry 2022, 27, 2849–2857. [Google Scholar] [CrossRef]
- A Brion, M.-J.; Shakhbazov, K.; Visscher, P.M. Calculating statistical power in Mendelian randomization studies. Leuk. Res. 2013, 42, 1497–1501. [Google Scholar] [CrossRef]
- Burgess, S.; Thompson, S.G. Interpreting findings from Mendelian randomization using the MR-Egger method. Eur. J. Epidemiol. 2017, 32, 377–389. [Google Scholar] [CrossRef]
- Pierce, B.L.; Burgess, S. Efficient Design for Mendelian Randomization Studies: Subsample and 2-Sample Instrumental Variable Estimators. Am. J. Epidemiol. 2013, 178, 1177–1184. [Google Scholar] [CrossRef]
- Schmidt, A.F.; Dudbridge, F. Mendelian Randomization with Egger Pleiotropy Correction and Weakly Informative Bayesian Priors. Int. J. Epidemiol. 2018, 47, 1217–1228. [Google Scholar] [CrossRef] [PubMed]
- Vaiserman, A.; Krasnienkov, D. Telomere Length as a Marker of Biological Age: State-of-the-Art, Open Issues, and Future Perspectives. Front. Genet. 2021, 11. [Google Scholar] [CrossRef] [PubMed]
- Turner, K.J.; Vasu, V.; Griffin, D.K. Telomere Biology and Human Phenotype. Cells 2019, 8, 73. [Google Scholar] [CrossRef]
- Mrcpsych, E.D.; Ryan, K.M.; Mrcpsych, C.O.; McLoughlin, D.M. Telomere length in patients with anorexia nervosa. Psychiatry Res. Commun. 2022, 2. [Google Scholar] [CrossRef]
- Hori, M.; Maekawa, T.; Kamiya, K.; Hagiwara, A.; Goto, M.; Takemura, M.Y.; Fujita, S.; Andica, C.; Kamagata, K.; Cohen-Adad, J.; et al. Advanced Diffusion MR Imaging for Multiple Sclerosis in the Brain and Spinal Cord. Magn. Reson. Med. Sci. 2022, 21, 58–70. [Google Scholar] [CrossRef] [PubMed]
- Costallat, B.L.; Ferreira, D.M.; Lapa, A.T.; Rittner, L.; Costallat, L.T.L.; Appenzeller, S. Brain diffusion tensor MRI in systematic lupus erythematosus: A systematic review. Autoimmun. Rev. 2018, 17, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Okudzhava, L.; Heldmann, M.; Münte, T.F. A systematic review of diffusion tensor imaging studies in obesity. Obes. Rev. 2021, 23, e13388. [Google Scholar] [CrossRef] [PubMed]
- Bergamino, M.; Walsh, R.R.; Stokes, A.M. Free-water diffusion tensor imaging improves the accuracy and sensitivity of white matter analysis in Alzheimer’s disease. Sci. Rep. 2021, 11, 6990. [Google Scholar] [CrossRef]
- Kamagata, K.; Andica, C.; Kato, A.; Saito, Y.; Uchida, W.; Hatano, T.; Lukies, M.; Ogawa, T.; Takeshige-Amano, H.; Akashi, T.; et al. Diffusion Magnetic Resonance Imaging-Based Biomarkers for Neurodegenerative Diseases. Int. J. Mol. Sci. 2021, 22, 5216. [Google Scholar] [CrossRef]
- Vaher, K.; Galdi, P.; Cabez, M.B.; Sullivan, G.; Stoye, D.Q.; Quigley, A.J.; Thrippleton, M.J.; Bogaert, D.; E Bastin, M.; Cox, S.R.; et al. General factors of white matter microstructure from DTI and NODDI in the developing brain. NeuroImage 2022, 254, 119169. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, S.; Takemura, H.; Horiguchi, H.; Miyazaki, A.; Matsumoto, K.; Masuda, Y.; Yoshikawa, K.; Nakano, T. Multi-Contrast Magnetic Resonance Imaging of Visual White Matter Pathways in Patients with Glaucoma. Investig. Opthalmology Vis. Sci. 2022, 63, 29. [Google Scholar] [CrossRef] [PubMed]
- Kamiya, K.; Hori, M.; Aoki, S. NODDI in clinical research. J. Neurosci. Methods. 2020, 346, 108908. [Google Scholar] [CrossRef]
- Lewis, S. Telomeres and depression. Nat. Rev. Neurosci. 2014, 15, 632. [Google Scholar] [CrossRef]
- Anitha, A.; Thanseem, I.; Vasu, M.M.; Viswambharan, V.; Poovathinal, S.A. Telomeres in Neurological Disorders. Adv. Clin. Chem. 2019, 90, 81–132. [Google Scholar] [PubMed]
- Osler, M.; Bendix, L.; Rask, L.; Rod, N.H. Stressful life events and leucocyte telomere length: Do lifestyle factors, somatic and mental health, or low grade inflammation mediate this relationship? Results from a cohort of Danish men born in 1953. Brain Behav. Immun. 2016, 58, 248–253. [Google Scholar] [CrossRef]
- M, A.; Smyth, A.M.; M, S.; Lawrie, S.M. The Neuroimmunology of Schizophrenia. Clin. Psychopharmacol. Neurosci. 2013, 11, 107–117. [Google Scholar] [CrossRef]
- Gotlib, I.H.; LeMoult, J.; Colich, N.L.; Foland-Ross, L.C.; Hallmayer, J.; Joormann, J.; Lin, J.; Wolkowitz, O.M. Telomere length and cortisol reactivity in children of depressed mothers. Mol. Psychiatry 2014, 20, 615–620. [Google Scholar] [CrossRef] [PubMed]
- Uziel, O.; Dickstein, H.; Beery, E.; Lewis, Y.; Loewenthal, R.; Uziel, E.; Shochat, Z.; Weizman, A.; Stein, D. Differences in Telomere Length between Adolescent Females with Anorexia Nervosa Restricting Type and Anorexia Nervosa Binge-Purge Type. Nutrients 2023, 15, 2596. [Google Scholar] [CrossRef]
- Sudo, Y.; Ota, J.; Takamura, T.; Kamashita, R.; Hamatani, S.; Numata, N.; Chhatkuli, R.B.; Yoshida, T.; Takahashi, J.; Kitagawa, H.; et al. Comprehensive elucidation of resting-state functional connectivity in anorexia nervosa by a multicenter cross-sectional study. Psychol. Med. 2024, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Prather, A.A.; Gurfein, B.; Moran, P.; Daubenmier, J.; Acree, M.; Bacchetti, P.; Sinclair, E.; Lin, J.; Blackburn, E.; Hecht, F.M.; et al. Tired telomeres: Poor global sleep quality, perceived stress, and telomere length in immune cell subsets in obese men and women. Brain Behav. Immun. 2014, 47, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.; Hou, Y.; Xu, C. Leucocyte telomere length, brain volume and risk of dementia: A prospective cohort study. Gen. Psychiatry 2023, 36, e101120. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, E.G.; Epel, E.S.; Lin, J.; Blackburn, E.H.; Rasgon, N.L. Relationship Between Leukocyte Telomere Length, Telomerase Activity, and Hippocampal Volume in Early Aging. JAMA Neurol. 2014, 71, 921–923. [Google Scholar] [CrossRef] [PubMed]
- Gampawar, P.; Schmidt, R.; Schmidt, H. Telomere length and brain aging: A systematic review and meta-analysis. Ageing Res. Rev. 2022, 80, 101679. [Google Scholar] [CrossRef]
- Koh, S.-H.; Choi, S.H.; Jeong, J.H.; Jang, J.-W.; Park, K.W.; Kim, E.-J.; Kim, H.J.; Hong, J.Y.; Yoon, S.J.; Yoon, B.; et al. Telomere shortening reflecting physical aging is associated with cognitive decline and dementia conversion in mild cognitive impairment due to Alzheimer’s disease. Aging 2020, 12, 4407–4423. [Google Scholar] [CrossRef] [PubMed]
- A Pathak, G.; Wendt, F.R.; Levey, D.F.; Mecca, A.P.; van Dyck, C.H.; Gelernter, J.; Polimanti, R. Plei-otropic effects of telomere length loci with brain morphology and brain tissue expression. Hum. Mol. Genet. 2021, 30, 1360–1370. [Google Scholar] [CrossRef]
- Whittemore, K.; Derevyanko, A.; Martinez, P.; Serrano, R.; Pumarola, M.; Bosch, F.; Blasco, M.A. Telomerase gene therapy ameliorates the effects of neurodegeneration associated to short telomeres in mice. Aging 2019, 11, 2916–2948. [Google Scholar] [CrossRef] [PubMed]
- Staffaroni, A.M.; Tosun, D.; Lin, J.; Elahi, F.M.; Casaletto, K.B.; Wynn, M.J.; Patel, N.; Neuhaus, J.; Walters, S.M.; Epel, E.S.; et al. Telomere attrition is associated with declines in medial temporal lobe volume and white matter microstructure in functionally independent older adults. Neurobiol. Aging 2018, 69, 68–75. [Google Scholar] [CrossRef]
- Luo, X.; Ruan, Z.; Liu, L. Causal relationship between telomere length and epilepsy: A bidirectional Mendelian randomization study. Epilepsia Open. 2023, 8, 1432–1439. [Google Scholar] [CrossRef]
- Puhlmann, L.M.C.; Valk, S.L.; Engert, V.; Bernhardt, B.C.; Lin, J.; Epel, E.S.; Vrticka, P.; Singer, T. Association of Short-term Change in Leukocyte Telomere Length with Cortical Thickness and Outcomes of Mental Training among Healthy Adults. JAMA Netw. Open. 2019, 2, e199687. [Google Scholar] [CrossRef]
- Miller, K.L.; Alfaro-Almagro, F.; Bangerter, N.K.; Thomas, D.L.; Yacoub, E.; Xu, J.; Bartsch, A.J.; Jbabdi, S.; Sotiropoulos, S.N.; Andersson, J.L.R.; et al. Multimodal population brain imaging in the UK Biobank prospective epidemiological study. Nat. Neurosci. 2016, 19, 1523–1536. [Google Scholar] [CrossRef]
- Matsumoto, N.; Sasao, T. An Autopsy Case of Autoimmune Hemolytic Anemia. Acta Patholigica Jpn. 1971, 21, 541–551. [Google Scholar] [CrossRef]
- Zheng, L.; Wang, Y.; Ma, J.; Wang, M.; Liu, Y.; Li, J.; Li, T.; Zhang, L. Machine learning research based on diffusion tensor images to distinguish between anorexia nervosa and bulimia nervosa. Front. Psychiatry 2024, 14, 1326271. [Google Scholar] [CrossRef]
- Brooks, S.J.; Barker, G.J.; O’Daly, O.G.; Brammer, M.; Williams, S.C.; Benedict, C.; Schiöth, H.B.; Treasure, J.; Campbell, I.C. Restraint of appetite and reduced regional brain volumes in anorexia nervosa: A voxel-based morphometric study. BMC Psychiatry 2011, 11, 179. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Wang, W.; Su, X.; Kemp, G.J.; Yang, X.; Su, J.; Tan, Q.; Zhao, Y.; Sun, H.; Yue, Q.; et al. Psychoradiological investigations of gray matter alterations in patients with anorexia nervosa. Transl. Psychiatry 2018, 8, 277. [Google Scholar] [CrossRef] [PubMed]
- Tose, K.; Takamura, T.; Isobe, M.; Hirano, Y.; Sato, Y.; Kodama, N.; Yoshihara, K.; Maikusa, N.; Moriguchi, Y.; Noda, T.; et al. Systematic reduction of gray matter volume in anorexia nervosa, but relative enlargement with clinical symptoms in the prefrontal and posterior insular cortices: A multicenter neuroimaging study. Mol. Psychiatry 2024, 29, 891–901. [Google Scholar] [CrossRef]
- Frank, G.K.; Shott, M.E.; Hagman, J.O.; Mittal, V.A. Alterations in Brain Structures Related to Taste Reward Circuitry in Ill and Recovered Anorexia Nervosa and in Bulimia Nervosa. Am. J. Psychiatry 2013, 170, 1152–1160. [Google Scholar] [CrossRef]
- Frank, G.K.; Shott, M.E.; Hagman, J.O.; Yang, T.T. Localized Brain Volume and White Matter Integrity Alterations in Adolescent Anorexia Nervosa. J. Am. Acad. Child Adolesc. Psychiatry 2013, 52, 1066–1075.e5. [Google Scholar] [CrossRef]
- Van den Eynde, F.; Suda, M.; Broadbent, H.; Guillaume, S.; Van den Eynde, M.; Steiger, H.; Israel, M.; Berlim, M.; Giampietro, V.; Simmons, A.; et al. Structural Magnetic Resonance Imaging in Eating Disorders: A Systematic Review of Voxel-Based Morphometry Studies. Eur. Eat. Disord. Rev. 2011, 20, 94–105. [Google Scholar] [CrossRef] [PubMed]
- Mühlau, M.; Gaser, C.; Ilg, R.; Conrad, B.; Leibl, C.; Cebulla, M.H.; Backmund, H.; Gerlinghoff, M.; Lommer, P.; Schnebel, A.; et al. Gray Matter Decrease of the Anterior Cingulate Cortex in Anorexia Nervosa. Am. J. Psychiatry 2007, 164, 1850–1857. [Google Scholar] [CrossRef]
- Fornito, A.; Bullmore, E.T. Connectomic Intermediate Phenotypes for Psychiatric Disorders. Front. Psychiatry 2012, 3, 19800. [Google Scholar] [CrossRef] [PubMed]
- Fornito, A.; Zalesky, A.; Pantelis, C.; Bullmore, E.T. Schizophrenia, neuroimaging and connectomics. NeuroImage 2012, 62, 2296–2314. [Google Scholar] [CrossRef]
- Frieling, H.; Fischer, J.; Wilhelm, J.; Engelhorn, T.; Bleich, S.; Hillemacher, T.; Dörfler, A.; Kornhuber, J.; de Zwaan, M.; Peschel, T. Microstructural abnormalities of the posterior thalamic radiation and the mediodorsal thalamic nuclei in females with anorexia nervosa—A voxel based diffusion tensor imaging (DTI) study. J. Psychiatr. Res. 2012, 46, 1237–1242. [Google Scholar] [CrossRef]
- Gaudio, S.; Quattrocchi, C.C. Neural basis of a multidimensional model of body image distortion in anorexia nervosa. Neurosci. Biobehav. Rev. 2012, 36, 1839–1847. [Google Scholar] [CrossRef]
- Devue, C.; Brédart, S. The neural correlates of visual self-recognition. Conscious. Cogn. 2011, 20, 40–51. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Li, T.; Yang, Y.; Wang, X.; Luo, T.; Shan, Y.; Zhu, Z.; Xiong, D.; Hauberg, M.E.; Bendl, J.; et al. Common genetic variation influencing human white matter microstructure. Science 2021, 372, eabf3736. [Google Scholar] [CrossRef]
- Burgess, S.; Bowden, J.; Fall, T.; Ingelsson, E.; Thompson, S.G. Sensitivity Analyses for Robust Causal Inference from Mendelian Randomization Analyses with Multiple Genetic Variants. Epidemiology 2017, 28, 30–42. [Google Scholar] [CrossRef]
- Kou, N.; Zhou, W.; He, Y.; Ying, X.; Chai, S.; Fei, T.; Fu, W.; Huang, J.; Liu, H. A Mendelian Randomization Analysis to Expose the Causal Effect of IL-18 on Osteoporosis Based on Genome-Wide Association Study Data. Front. Bioeng. Biotechnol. 2020, 8, 201. [Google Scholar] [CrossRef]
- Gormley, M.; Dudding, T.; Sanderson, E.; Martin, R.M.; Thomas, S.; Tyrrell, J.; Ness, A.R.; Brennan, P.; Munafò, M.; Pring, M.; et al. A multivariable Mendelian randomization analysis investigating smoking and alcohol consumption in oral and oropharyngeal cancer. Nat. Commun. 2020, 11, 6071. [Google Scholar] [CrossRef] [PubMed]
- Savla, J.; Neeland, I.J. The Pros and Cons of Mendelian Randomization Studies to Evaluate Emerging Cardiovascular Risk Factors. Curr. Cardiovasc. Risk Rep. 2018, 12, 1–9. [Google Scholar] [CrossRef]
- VanderWeele, T.J.; Tchetgen, E.J.T.; Cornelis, M.; Kraft, P. Methodological Challenges in Mendelian Randomization. Epidemiology 2014, 25, 427–435. [Google Scholar] [CrossRef] [PubMed]
- Smith, G.D.; Ebrahim, S. Mendelian randomization: Prospects, potentials, and limitations. Int. J. Epidemiol. 2004, 33, 30–42. [Google Scholar] [CrossRef]
- Drobinin, V.; Van Gestel, H.; Helmick, C.A.; Schmidt, M.H.; Bowen, C.V.; Uher, R. Reliability of multimodal MRI brain measures in youth at risk for mental illness. Brain Behav. 2020, 10, e01609. [Google Scholar] [CrossRef] [PubMed]
- Carrión-Castillo, A.; Boeckx, C. Insights into the genetic architecture of cerebellar lobules derived from the UK Biobank. Sci. Rep. 2024, 14, 9488. [Google Scholar] [CrossRef] [PubMed]
- Smeland, O.B.; Frei, O.; Fan, C.-C.; Shadrin, A.; Dale, A.M.; Andreassen, O.A. The emerging pattern of shared polygenic architecture of psychiatric disorders, conceptual and methodological challenges. Psychiatr. Genet. 2019, 29, 152–159. [Google Scholar] [CrossRef]
- Grigoroiu-Serbanescu, M.; Magureanu, S.; Milea, S.; Dobrescu, I.; Marinescu, E. Modest familial aggregation of eating disorders in restrictive anorexia nervosa with adolescent onset in a Romanian sample. Eur. Child Adolesc. Psychiatry 2003, 12, i47–i53. [Google Scholar] [CrossRef] [PubMed]
- Booij, L.; Steiger, H. Applying epigenetic science to the understanding of eating disorders: A promising paradigm for research and practice. Curr. Opin. Psychiatry 2020, 33, 515–520. [Google Scholar] [CrossRef] [PubMed]
- Hübel, C.; Marzi, S.J.; Breen, G.; Bulik, C.M. Epigenetics in eating disorders: A systematic review. Mol. Psychiatry 2019, 24, 901–915. [Google Scholar] [CrossRef]
- Kular, L.; Kular, S. Epigenetics applied to psychiatry: Clinical opportunities and future challenges. Psychiatry Clin. Neurosci. 2018, 72, 195–211. [Google Scholar] [CrossRef]
- Fan, Y.; Støving, R.K.; Ibraim, S.B.; Hyötyläinen, T.; Thirion, F.; Arora, T.; Lyu, L.; Stankevic, E.; Hansen, T.H.; Déchelotte, P.; et al. The gut microbiota contributes to the pathogenesis of anorexia nervosa in humans and mice. Nat. Microbiol. 2023, 8, 787–802. [Google Scholar] [CrossRef]
- Lu, T.; Forgetta, V.; Greenwood, C.M.; Zhou, S.; Richards, J.B. Circulating Proteins Influencing Psychiatric Disease: A Mendelian Randomization Study. Biol. Psychiatry 2022, 93, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Podwalski, P.; Szczygieł, K.; Tyburski, E.; Sagan, L.; Misiak, B.; Samochowiec, J. Magnetic resonance diffusion tensor imaging in psychiatry: A narrative review of its potential role in diagnosis. Pharmacol. Rep. 2021, 73, 43–56. [Google Scholar] [CrossRef] [PubMed]
- Garic, D.; Yeh, F.-C.; Graziano, P.; Dick, A.S. In vivo restricted diffusion imaging (RDI) is sensitive to differences in axonal density in typical children and adults. Anat. Embryol. 2021, 226, 2689–2705. [Google Scholar] [CrossRef]
- Lin, J.-H.; Chen, X.-H.; Wu, Y.; Cao, Y.-B.; Chen, H.-J.; Huang, N.-X. Altered isotropic volume fraction in gray matter after sleep deprivation and its association with visuospatial memory: A neurite orientation dispersion and density imaging study. Front. Neurosci. 2023, 17. [Google Scholar] [CrossRef] [PubMed]
- O’donnell, L.J.; Westin, C.-F. An Introduction to Diffusion Tensor Image Analysis. Neurosurg. Clin. N. Am. 2011, 22, 185–196. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, H.; Kawashima, R. Mean Diffusivity in the Dopaminergic System and Neural Differences Related to Dopaminergic System. Curr. Neuropharmacol. 2018, 16, 460–474. [Google Scholar] [CrossRef]
- Fukutomi, H.; Glasser, M.F.; Murata, K.; Akasaka, T.; Fujimoto, K.; Yamamoto, T.; Autio, J.A.; Okada, T.; Togashi, K.; Zhang, H.; et al. Diffusion Tensor Model links to Neurite Orientation Dispersion and Density Imaging at high b-value in Cerebral Cortical Gray Matter. Sci. Rep. 2019, 9, 12246. [Google Scholar] [CrossRef] [PubMed]

| Method | Test | Validation | ||||||
|---|---|---|---|---|---|---|---|---|
| Nsnp | p-Value | OR | 95%CI | Nsnp | p-Value | OR | 95%CI | |
| MR–Egger | 109 | 6.35 × 10−1 | 9.43 × 10−1 | 0.74–1.20 | 144 | 4.90 × 10−2 | 3.92 × 10−1 | 0.16–0.99 |
| Weighted median | 109 | 1.21 × 10−1 | 8.54 × 10−1 | 0.70–1.04 | 144 | 4.94 × 10−2 | 4.31 × 10−1 | 0.19–1.00 |
| IVW | 109 | 1.74 × 10−2 a | 8.51 × 10−1 | 0.75–0.97 | 144 | 7.36 × 10−3 b | 5.02 × 10−1 | 0.30–0.83 |
| Simple mode | 109 | 9.89 × 10−1 | 9.97 × 10−1 | 0.67–1.48 | 144 | 4.48 × 10−1 | 5.18 × 10−1 | 0.10–2.82 |
| Weighted mode | 109 | 2.91 × 10−1 | 8.91 × 10−1 | 0.72–1.10 | 144 | 2.13 × 10−1 | 5.18 × 10−1 | 0.19–1.45 |
| dMRI Phenotypes | Step1 | Step2 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Region | Phenotypes | Methods | Ppe | MRSr | phet | pple | psteiger | phet | pple | psteiger |
| Internal capsule (L) | FA | TBSS | 4.68 × 10−8 | 2.04 × 10−1 | 3.40 × 10−1 | 6.10 × 10−2 | 1.37 × 10−65 | 2.23 × 10−1 | 9.19 × 10−1 | 1.19 × 10−55 |
| Internal capsule (R) | FA | TBSS | 8.12 × 10−7 | 1.69 × 10−1 | 4.44 × 10−1 | 6.25 × 10−2 | 2.50 × 10−71 | 5.33 × 10−1 | 9.64 × 10−1 | 3.80 × 10−31 |
| Cingulum cingulate gyrus (L) | FA | TBSS | 1.67 × 10−3 | 7.46 × 10−2 | 9.75 × 10−1 | 2.71 × 10−1 | 7.05 × 10−77 | 1.23 × 10−1 | 9.45 × 10−1 | 6.87 × 10−41 |
| Cingulum cingulate gyrus (L) | FA | PXTB | 6.54 × 10−4 | 8.39 × 10−2 | 8.57 × 10−1 | 8.69 × 10−1 | 3.86 × 10−80 | 6.87 × 10−1 | 8.89 × 10−1 | 5.56 × 10−28 |
| Forceps minor (L) | FA | PXTB | 8.81 × 10−4 | 8.19 × 10−2 | 1.59 × 10−1 | 1.13 × 10−1 | 1.01 × 10−64 | 5.85 × 10−1 | 8.31 × 10−1 | 6.40 × 10−127 |
| Internal capsule (L) | MD | TBSS | 1.84 × 10−3 | 7.22 × 10−2 | 2.27 × 10−1 | 9.32 × 10−2 | 4.10 × 10−57 | 4.43 × 10−1 | 9.69 × 10−1 | 1.78 × 10−97 |
| Anterior corona radiata (R) | MD | TBSS | 2.80 × 10−2 | 3.77 × 10−2 | 8.16 × 10−1 | 3.40 × 10−1 | 5.94 × 10−65 | 7.11 × 10−1 | 8.50 × 10−1 | 4.17 × 10−147 |
| Posterior corona radiata (R) | MD | TBSS | 3.94 × 10−4 | 9.52 × 10−2 | 8.97 × 10−1 | 8.95 × 10−1 | 6.33 × 10−67 | 8.03 × 10−2 | 5.57 × 10−1 | 2.48 × 10−85 |
| Posterior thalamic radiation (R) | L1 | TBSS | 6.02 × 10−3 | 5.84 × 10−2 | 4.05 × 10−1 | 1.15 × 10−1 | 1.71 × 10−63 | 1.19 × 10−1 | 9.15 × 10−1 | 1.76 × 10−33 |
| Superior longitudinal fasciculus (L) | L1 | TBSS | 5.45 × 10−5 | 1.24 × 10−1 | 7.23 × 10−1 | 9.67 × 10−1 | 8.76 × 10−68 | 5.62 × 10−2 | 6.35 × 10−1 | 4.48 × 10−68 |
| Internal capsule (L) | L2 | TBSS | 7.95 × 10−7 | 1.72 × 10−1 | 5.22 × 10−1 | 5.52 × 10−2 | 4.45 × 10−63 | 2.97 × 10−1 | 7.89 × 10−1 | 2.34 × 10−49 |
| Posterior corona radiata (R) | L2 | TBSS | 1.27 × 10−5 | 1.39 × 10−1 | 3.17 × 10−1 | 7.28 × 10−1 | 4.34 × 10−63 | 8.45 × 10−1 | 9.72 × 10−1 | 5.04 × 10−79 |
| Posterior corona radiata (L) | L2 | TBSS | 4.00 × 10−4 | 9.43 × 10−2 | 3.73 × 10−1 | 3.41 × 10−1 | 1.91 × 10−61 | 6.40 × 10−1 | 7.81 × 10−1 | 2.18 × 10−84 |
| Internal capsule (L) | L3 | TBSS | 4.16 × 10−4 | 9.17 × 10−2 | 6.08 × 10−1 | 8.96 × 10−2 | 6.53 × 10−70 | 1.46 × 10−1 | 6.29 × 10−1 | 2.26 × 10−84 |
| Internal capsule (L) | ICVF | TBSS | 2.14 × 10−5 | 1.33 × 10−1 | 2.80 × 10−1 | 9.49 × 10−2 | 1.25 × 10−58 | 2.25 × 10−1 | 8.52 × 10−2 | 1.66 × 10−233 |
| Posterior corona radiata (R) | ICVF | TBSS | 1.72 × 10−2 | 4.26 × 10−2 | 6.93 × 10−1 | 7.17 × 10−1 | 2.80 × 10−70 | 4.45 × 10−1 | 7.87 × 10−1 | 3.60 × 10−147 |
| inferior longitudinal fasciculus (L) | ICVF | PXTB | 2.13 × 10−4 | 1.03 × 10−1 | 6.40 × 10−1 | 5.90 × 10−2 | 4.33 × 10−63 | 1.95 × 10−1 | 6.90 × 10−1 | 7.95 × 10−259 |
| Posterior thalamic radiation (L) | ICVF | PXTB | 1.05 × 10−3 | 8.40 × 10−2 | 7.33 × 10−1 | 5.29 × 10−2 | 1.28 × 10−64 | 9.23 × 10−2 | 2.79 × 10−1 | 2.02 × 10−183 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiu, H.; Shi, M.; Zhong, Z.; Hu, H.; Sang, H.; Zhou, M.; Feng, Z. Causal Relationship between Aging and Anorexia Nervosa: A White-Matter-Microstructure-Mediated Mendelian Randomization Analysis. Biomedicines 2024, 12, 1874. https://doi.org/10.3390/biomedicines12081874
Qiu H, Shi M, Zhong Z, Hu H, Sang H, Zhou M, Feng Z. Causal Relationship between Aging and Anorexia Nervosa: A White-Matter-Microstructure-Mediated Mendelian Randomization Analysis. Biomedicines. 2024; 12(8):1874. https://doi.org/10.3390/biomedicines12081874
Chicago/Turabian StyleQiu, Haoyuan, Miao Shi, Zicheng Zhong, Haoran Hu, Hunini Sang, Meijuan Zhou, and Zhijun Feng. 2024. "Causal Relationship between Aging and Anorexia Nervosa: A White-Matter-Microstructure-Mediated Mendelian Randomization Analysis" Biomedicines 12, no. 8: 1874. https://doi.org/10.3390/biomedicines12081874





