Rotational Thromboelastometric Profile in Early Sepsis: A Prospective Cohort Study
Abstract
:1. Introduction
2. Materials and Methods
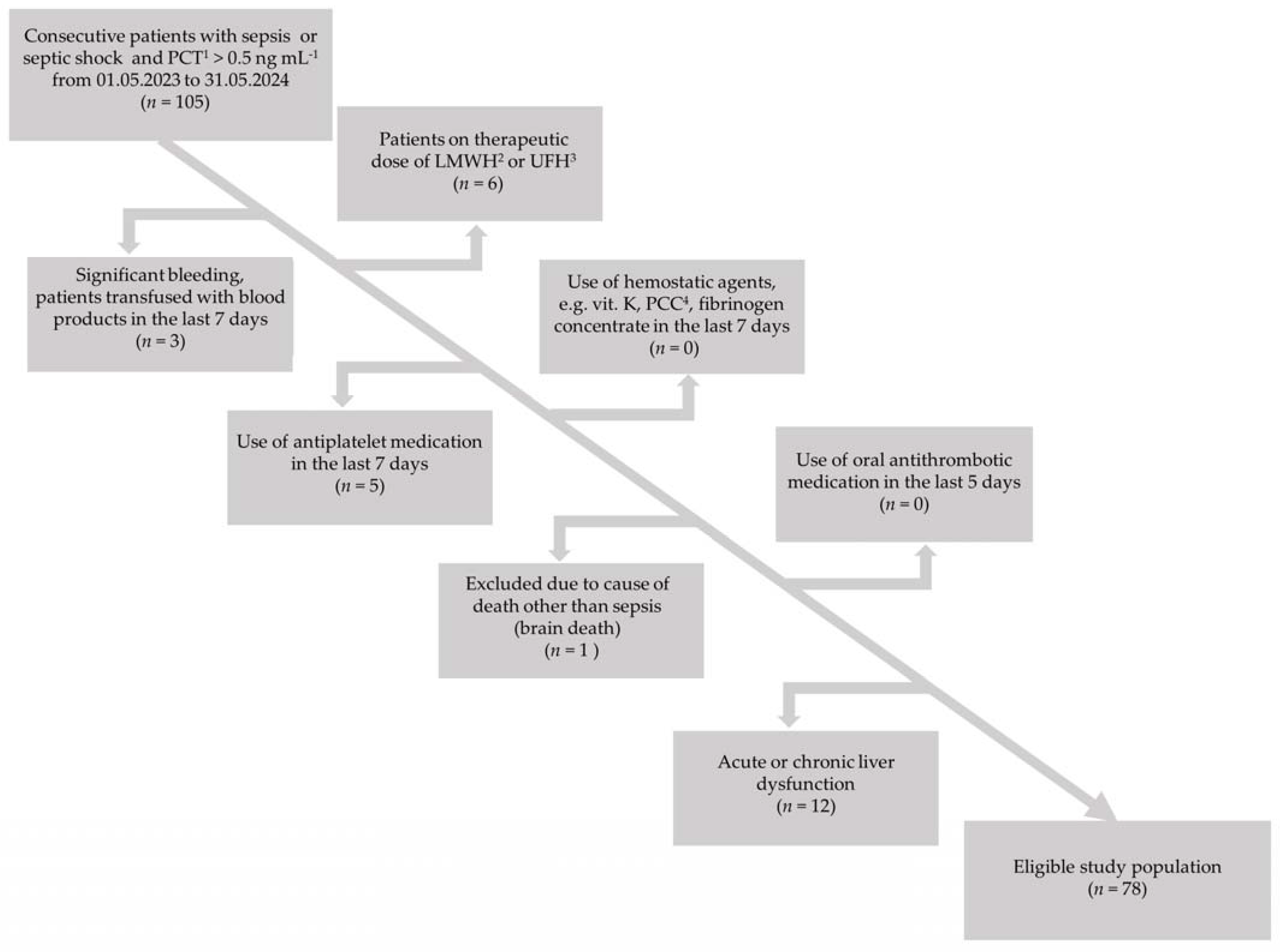
2.1. Clinical Data
2.2. Laboratory Data
Rotational Thromboelastometric Parameters
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.-D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef]
- Vincent, J.L.; Moreno, R.; Takala, J.; Willatts, S.; De Mendonça, A.; Bruining, H.; Reinhart, C.K.; Suter, P.M.; Thijs, L.G. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure: On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996, 22, 707–710. [Google Scholar] [CrossRef]
- Ferreira, F.L.; Bota, D.P.; Bross, A.; Mélot, C.; Vincent, J.-L. Serial Evaluation of the SOFA Score to Predict Outcome in Critically Ill Patients. JAMA 2001, 286, 1754–1758. [Google Scholar] [CrossRef]
- Palta, S.; Saroa, R.; Palta, A. Overview of the coagulation system. Indian J. Anaesth. 2014, 58, 515–523. [Google Scholar] [CrossRef]
- Walsh, T.S.; Stanworth, S.J.; Prescott, R.J.; Lee, R.J.; Watson, D.M.; Wyncoll, D. Prevalence, management, and outcomes of critically ill patients with prothrombin time prolongation in United Kingdom intensive care units*. Crit. Care Med. 2010, 38, 1939–1946. [Google Scholar] [CrossRef]
- Czempik, P.F.; Wiórek, A. Management Strategies in Septic Coagulopathy: A Review of the Current Literature. Healthcare 2023, 11, 227. [Google Scholar] [CrossRef] [PubMed]
- Levi, M.; Meijers, J.C. DIC: Which laboratory tests are most useful. Blood Rev. 2011, 25, 33–37. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, A.; Kelleher, A. Point-of-care coagulation testing. Contin. Educ. Anaesth. Crit. Care Pain 2012, 13, 12–16. [Google Scholar] [CrossRef]
- Evans, L.; Rhodes, A.; Alhazzani, W.; Antonelli, M.; Coopersmith, C.M.; French, C.; Machado, F.R.; Mcintyre, L.; Ostermann, M.; Prescott, H.C.; et al. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock 2021. Crit. Care Med. 2021, 49, e1063–e1143. [Google Scholar] [CrossRef] [PubMed]
- Egi, M.; Egi, M.; Ogura, H.; Ogura, H.; Yatabe, T.; Yatabe, T.; Atagi, K.; Atagi, K.; Inoue, S.; Inoue, S.; et al. The Japanese Clinical Practice Guidelines for Management of Sepsis and Septic Shock 2020 (J-SSCG 2020). J. Intensiv. Care 2021, 9, 53. [Google Scholar] [CrossRef]
- Samsudin, I.; Vasikaran, S.D. Clinical Utility and Measurement of Procalcitonin. Clin. Biochem. Rev. 2017, 38, 59–68. [Google Scholar] [PubMed]
- World Health Organization. WHO Handbook for Reporting Results of Cancer Treatment, 48th ed.; World Health Organization: Geneva, Switzerland, 1979; Available online: https://iris.who.int/handle/10665/37200 (accessed on 13 July 2024).
- Iba, T.; Di Nisio, M.; Levy, J.H.; Kitamura, N.; Thachil, J. New criteria for sepsis-induced coagulopathy (SIC) following the revised sepsis definition: A retrospective analysis of a nationwide survey. BMJ Open 2017, 7, e017046. [Google Scholar] [CrossRef] [PubMed]
- Taylor, F.B., Jr.; Toh, C.H.; Hoots, W.K.; Wada, H.; Levi, M.; Scientific Subcommittee on Disseminated Intravascular Co-agulation (DIC) of the International Society on Thrombosis and Haemostasis (ISTH). Towards definition, clinical and la-boratory criteria, and a scoring system for disseminated intravascular coagulation. Thromb. Haemost. 2001, 86, 1327–1330. [Google Scholar] [CrossRef] [PubMed]
- Gao, T.-Y.; Yang, W.-C.M.; Zhou, F.-H.; Song, Q.M. Analysis of D-dimer cut-off values for overt DIC diagnosis in exertional heat illness. Medicine 2020, 99, e23831. [Google Scholar] [CrossRef] [PubMed]
- Q.F.A. Thrombin (Bovine)—0020301800 (2 mL)/0020301700 (5 mL), Printed Insert Sheet: 303725, Revision: R10, Issued: 06/2017, C.O.: 481068. Instrumentation Laboratory Company: Bedford, MA, USA, 2017; Available online: https://www.biosystemsne.com.br/files/product/16186982506024-00020301800hemosilq.f.athrombinbovine2ml.pdf (accessed on 13 July 2024).
- Wolberg, A.S. Fibrinogen and fibrin: Synthesis, structure, and function in health and disease. J. Thromb. Haemost. 2023, 21, 3005–3015. [Google Scholar] [CrossRef] [PubMed]
- Mori, K.; Tsujita, Y.; Yamane, T.; Eguchi, Y. Decreasing Plasma Fibrinogen Levels in the Intensive Care Unit Are Associated with High Mortality Rates In Patients With Sepsis-Induced Coagulopathy. Clin. Appl. Thromb. 2022, 28. [Google Scholar] [CrossRef] [PubMed]
- Fiuza, C.; Bustin, M.; Talwar, S.; Tropea, M.; Gerstenberger, E.; Shelhamer, J.H.; Suffredini, A.F. Inflammation-promoting activity of HMGB1 on human microvascular endothelial cells. Blood 2003, 101, 2652–2660. [Google Scholar] [CrossRef]
- Madoiwa, S.; Nunomiya, S.; Ono, T.; Shintani, Y.; Ohmori, T.; Mimuro, J.; Sakata, Y. Plasminogen Activator Inhibitor 1 Promotes a Poor Prognosis in Sepsis-Induced Disseminated Intravascular Coagulation. Int. J. Hematol. 2006, 84, 398–405. [Google Scholar] [CrossRef]
- Scărlătescu, E.; Lancé, M.D.; White, N.J.; Tomescu, D.R. Thromboelastometric prediction of mortality using the kinetics of clot growth in critically ill septic patients. Blood Coagul. Fibrinolysis 2018, 29, 533–539. [Google Scholar] [CrossRef]
- Asakura, H. Classifying types of disseminated intravascular coagulation: Clinical and animal models. J. Intensiv. Care 2014, 2, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Iba, T.; Umemura, Y.; Wada, H.; Levy, J.H. Roles of Coagulation Abnormalities and Microthrombosis in Sepsis: Pathophysiology, Diagnosis, and Treatment. Arch. Med Res. 2021, 52, 788–797. [Google Scholar] [CrossRef]
- Hotchkiss, R.S.; Karl, I.E. The Pathophysiology and Treatment of Sepsis. N. Engl. J. Med. 2003, 348, 138–150. [Google Scholar] [CrossRef] [PubMed]
- Schultz, M.; van der Poll, T.; Levi, M. Sepsis and Thrombosis. Semin. Thromb. Hemost. 2013, 39, 559–566. [Google Scholar] [CrossRef]
- Giustozzi, M.; Ehrlinder, H.; Bongiovanni, D.; Borovac, J.A.; Guerreiro, R.A.; Gąsecka, A.; Papakonstantinou, P.E.; Parker, W.A. Coagulopathy and sepsis: Pathophysiology, clinical manifestations and treatment. Blood Rev. 2021, 50, 100864. [Google Scholar] [CrossRef] [PubMed]
- Storey, R.F.; Thomas, M.R. The role of platelets in inflammation. Thromb. Haemost. 2015, 114, 449–458. [Google Scholar] [CrossRef] [PubMed]
- Akinosoglou, K.; Theodoraki, S.; Xanthopoulou, I.; Perperis, A.; Gkavogianni, T.; Pistiki, A.; Giamarellos-Bourboulis, E.; A Gogos, C. Platelet reactivity in sepsis syndrome: Results from the PRESS study. Eur. J. Clin. Microbiol. Infect. Dis. 2017, 36, 2503–2512. [Google Scholar] [CrossRef]
- Lead: J Chan, D Bootland, R Kong Prompt card by: J Castle Version 6.0 For review: Oct 2023. Available online: https://www.bsuh.nhs.uk/library/wp-content/uploads/sites/8/2021/12/ROTEM-interpretation.pdf (accessed on 13 July 2024).
| Parameter | Median Value, IQR 1 | Reference Range |
|---|---|---|
| Procalcitonin [ng mL−1] | 3.51 (1.12–18.30) | <0.5 |
| C-reactive protein [mg L−1] | 176 (117–286) | <5.0 |
| Creatinine [mg dL−1] | 1.12 (0.67–1.60) | 0.67–1.17 |
| Estimated GFR 2 [mL min−1] | 60 (37–60) | >60 |
| Blood urea nitrogen [mg dL−1] | 28.99 (17.69–42.81) | 7.90–20.00 |
| Urea [mg dL−1] | 61.3 (38.8–91.5) | 17.1–49.2 |
| Bilirubin [mg dL-−1] | 0.60 (0.31–0.86) | 0.30–1.00 |
| White blood cells [× 103 µL−1] | 11.1 (7.1–17.9) | 4.0-10.0 |
| Hemoglobin [g L−1] | 106.0 (85.0–123.0) | 135.0–165.0 |
| Hematocrit [%] | 31.9 (26.4–35.6) | 40–53 |
| Platelets [× 103 µL−1] | 213 (159–283) | 130-400 |
| Prothrombin time [s] | 13.9 (12.8–16.4) | 9.4–12.5 |
| International normalized ratio | 1.15 (1.06–1.37) | 0.80–1.20 |
| Quick index [%] | 76.0 (58.8–86.0) | 80–120 |
| Activated partial thromboplastin time [s] | 32.5 (30.3–37.3) | 25.4–36.9 |
| Thrombin time [s] | 15.7 (14.3–16.8) | 10.3–16.6 |
| Fibrinogen [mg dL−1] | 523 (389–640) | 200–393 |
| D-dimers [ng mL−1] | 2613 (1716–5651) | <500 |
| Parameter | Median Value, IQR 1 | Reference Range |
|---|---|---|
| INTEM CT 2 [s] | 195 (177–211) | 100–240 |
| INTEM CFT 3 [s] | 59 (49–70) | 30–110 |
| INTEM alpha angle [°] | 78 (76–80) | 70–83 |
| INTEM A10 4 [mm] | 65 (60–69) | 44–66 |
| INTEM A20 5 [mm] | 70 (65–74) | 50–71 |
| INTEM MCF 6 [mm] | 70 (66–75) | 50–72 |
| INTEM LI30 7 [%] | 100 (99–100) | 94–100 |
| INTEM LI45 8 [%] | 97 (95–99) | - |
| INTEM ML 9 [%] | 5 (2–8) | 0–15 |
| EXTEM CT [s] | 77 (69–88) | 38–79 |
| EXTEM CFT [s] | 59 (48–70) | 34–159 |
| EXTEM alpha angle [°] | 78 (77–80) | 63–83 |
| EXTEM A10 [mm] | 65 (60–71) | 43–65 |
| EXTEM A20 [mm] | 69 (65–73) | 50–71 |
| EXTEM MCF [mm] | 70 (67–74) | 50–72 |
| EXTEM LI30 [%] | 99 (98–100) | 94–100 |
| EXTEM LI45 [%] | 96 (91–98) | - |
| EXTEM ML [%] | 8 (4–14) | 0–15 |
| FIBTEM CT [s] | 73 (65–82) | 38–62 |
| FIBTEM CFT [s] | 114 (65–312) | - |
| FIBTEM alpha angle [°] | 77 (74–79) | - |
| FIBTEM A10 [mm] | 24 (20–31) | 7–23 |
| FIBTEM A20 [mm] | 25 (21–33) | 8–24 |
| FIBTEM MCF [mm] | 26.5 (22.0–34.0) | 9–25 |
| FIBTEM LI30 [%] | 100 (100–100) | - |
| FIBTEM LI45 [%] | 100 (100–100) | - |
| FIBTEM ML [%] | 0 (0–1) | - |
| APTEM CT [s] | 74 (68–89) | - |
| APTEM CFT [s] | 60 (48.0–70) | - |
| APTEM alpha angle [°] | 78 (77–80) | - |
| APTEM A10 [mm] | 65 (60–70) | - |
| APTEM A20 [mm] | 70 (66–74) | - |
| APTEM MCF [mm] | 71 (67–75) | - |
| APTEM LI30 [%] | 100 (99–100) | - |
| APTEM LI45 [%] | 97 (94–99) | - |
| APTEM ML [%] | 6 (3–8) | - |
| Parameter | Survivors (n = 50/64%) | Non-Survivors (n = 28/36%) | p 1 |
|---|---|---|---|
| INTEM CT 2 [s] | 192 (177–207) | 197 (187–229) | 0.177 |
| INTEM CFT 3 [s] | 58 (48–70) | 62 (54–71) | 0.396 |
| INTEM alpha angle [°] | 79 (76–80) | 78 (76–80) | 0.526 |
| INTEM A10 4 [mm] | 65 (61–71) | 64 (59–68) | 0.255 |
| INTEM A20 5 [mm] | 70 (65–75) | 70 (65–72) | 0.327 |
| INTEM MCF 6 [mm] | 70 (66–75) | 70 (66–73) | 0.573 |
| INTEM LI30 7 [%] | 99 (99–100) | 100 (100–100) | 0.007 |
| INTEM LI45 8 [%] | 96 (94–98) | 99 (96–100) | <0.001 |
| INTEM ML 9 [%] | 6 (4–8) | 2 (1–6) | <0.001 |
| EXTEM CT [s] | 76 (70–86) | 77 (68–91) | 0.971 |
| EXTEM CFT [s] | 58 (48–68) | 64 (51–75) | 0.193 |
| EXTEM alpha angle [°] | 79 (77–80) | 78 (76–80) | 0.132 |
| EXTEM A10 [mm] | 66 (61–72) | 64 (59–68) | 0.139 |
| EXTEM A20 [mm] | 70 (65–74) | 68 (63–72) | 0.172 |
| EXTEM MCF [mm] | 71 (67–75) | 69 (67–73) | 0.169 |
| EXTEM LI30 [%] | 99 (96–100) | 100 (99–100) | 0.062 |
| EXTEM LI45 [%] | 95 (90–97) | 97 (95–100) | 0.003 |
| EXTEM ML [%] | 10 (6–17) | 5 (2–8) | <0.001 |
| FIBTEM CT [s] | 72 (65–80) | 75 (66–85) | 0.283 |
| FIBTEM CFT [s] | 114 (66–312) | 117 (61–298) | 0.923 |
| FIBTEM alpha angle [°] | 77 (74–78) | 77 (74–80) | 0.279 |
| FIBTEM A10 [mm] | 24 (19–31) | 23 (21–30) | 0.736 |
| FIBTEM A20 [mm] | 26 (20–34) | 25 (23–31) | 0.661 |
| FIBTEM MCF [mm] | 26 (20–34) | 27 (24–34) | 0.417 |
| FIBTEM LI30 [%] | 100 (100–100) | 100 (100–100) | 0.367 |
| FIBTEM LI45 [%] | 100 (100–100) | 100 (100–100) | 0.073 |
| FIBTEM ML [%] | 0 (0–2) | 0 (0–0) | 0.045 |
| APTEM CT [s] | 73 (65–88) | 77 (69–91) | 0.336 |
| APTEM CFT [s] | 56 (48–69) | 62 (53–74) | 0.177 |
| APTEM alpha angle [°] | 79 (77–81) | 78 (76–80) | 0.169 |
| APTEM A10 [mm] | 66 (60–72) | 64 (59–69) | 0.307 |
| APTEM A20 [mm] | 70 (66–75) | 70 (65–74) | 0.386 |
| APTEM MCF [mm] | 71 (67–76) | 70 (67–74) | 0.539 |
| APTEM LI30 [%] | 100 (99–100) | 100 (100–100) | 0.014 |
| APTEM LI45 [%] | 96 (94–97) | 98 (97–100) | 0.004 |
| APTEM ML [%] | 7 (5–9) | 3 (1–6) | 0.001 |
| Parameter | AUROC 1 (95% CI 2) | Cut-Off Value | p 3 |
|---|---|---|---|
| INTEM LI30 4 [%] | 0.665 (0.548–0.769) | >99 | 0.002 |
| INTEM LI45 5 [%] | 0.735 (0.622–0.829) | >98 | <0.001 |
| INTEM ML 6 [%] | 0.744 (0.633–0.836) | ≤2 | <0.001 |
| EXTEM LI45 [%] | 0.707 (0.592–0.805) | >94 | 0.002 |
| EXTEM ML [%] | 0.729 (0.617–0.824) | ≤7 | <0.001 |
| FIBTEM ML [%] | 0.610 (0.492–0.720) | ≤1 | 0.025 |
| APTEM LI30 [%] | 0.642 (0.524–0.749) | >99 | 0.005 |
| APTEM LI45 [%] | 0.702 (0.585–0.802) | >97 | 0.002 |
| APTEM ML [%] | 0.724 (0.611–0.820) | ≤2 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Czempik, P.F.; Wiórek, A. Rotational Thromboelastometric Profile in Early Sepsis: A Prospective Cohort Study. Biomedicines 2024, 12, 1880. https://doi.org/10.3390/biomedicines12081880
Czempik PF, Wiórek A. Rotational Thromboelastometric Profile in Early Sepsis: A Prospective Cohort Study. Biomedicines. 2024; 12(8):1880. https://doi.org/10.3390/biomedicines12081880
Chicago/Turabian StyleCzempik, Piotr F., and Agnieszka Wiórek. 2024. "Rotational Thromboelastometric Profile in Early Sepsis: A Prospective Cohort Study" Biomedicines 12, no. 8: 1880. https://doi.org/10.3390/biomedicines12081880







