Review of T Helper 2-Type Inflammatory Diseases Following Immune Checkpoint Inhibitor Treatment
Abstract
1. Introduction
1.1. Programmed Death 1, Programmed Death Ligand 1 and Cytotoxic T-lymphocyte Associated Antigen 4
1.2. Immune-Related Adverse Events
1.3. Activation of CD4- and CD8-Positive T Cells and Suppression of Tregs Following Immune Checkpoint Inhibitor Treatment
1.4. Production of Autoantibodies Following Immune Checkpoint Inhibitor Treatment
1.5. Production of Cytokines Following Immune Checkpoint Inhibitor Treatment
1.6. Cutaneous Immune-Related Adverse Events
1.7. T Helper 1, 2, and 17 Immune Responses Following Immune Checkpoint Inhibitors Treatment
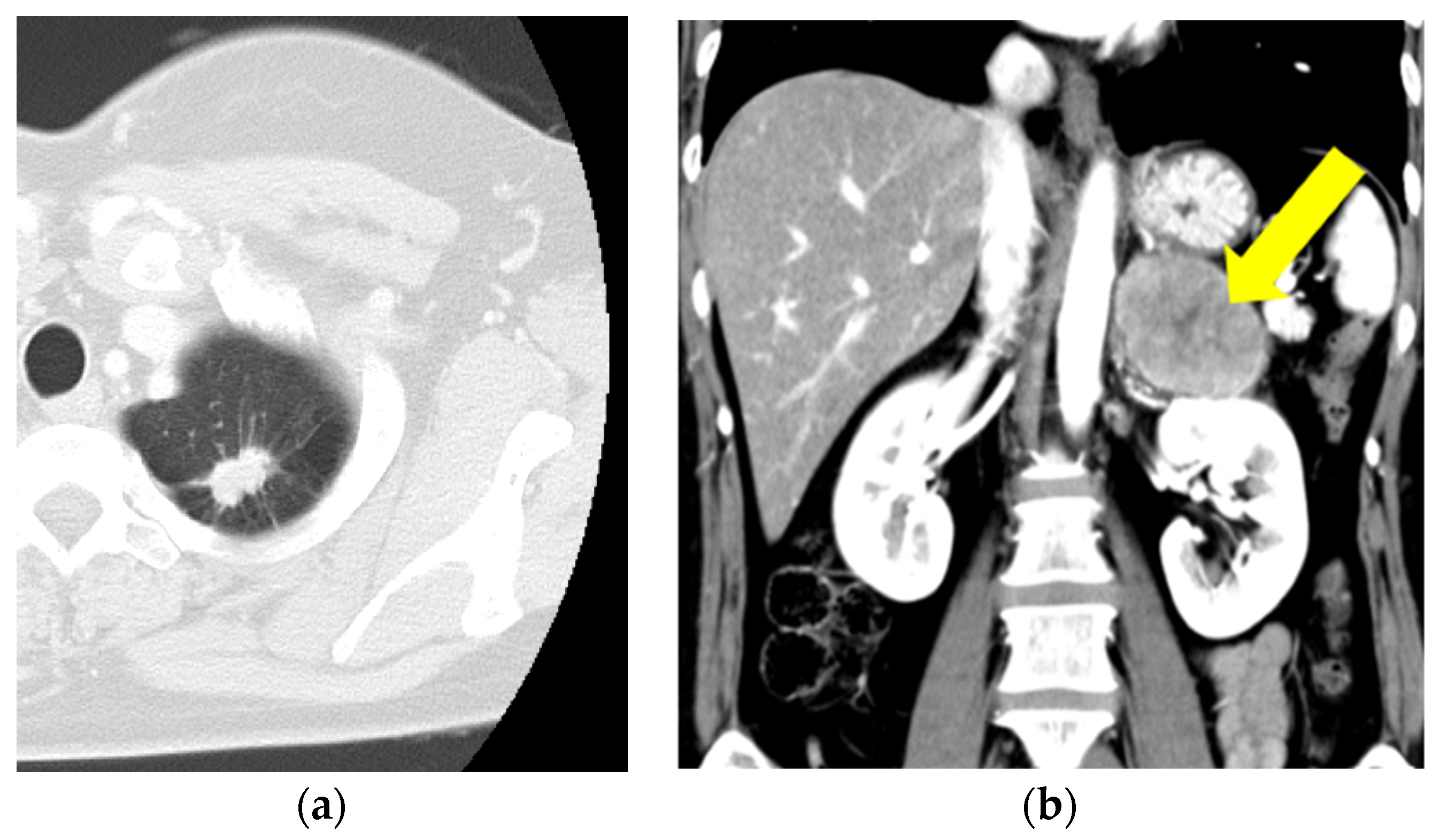
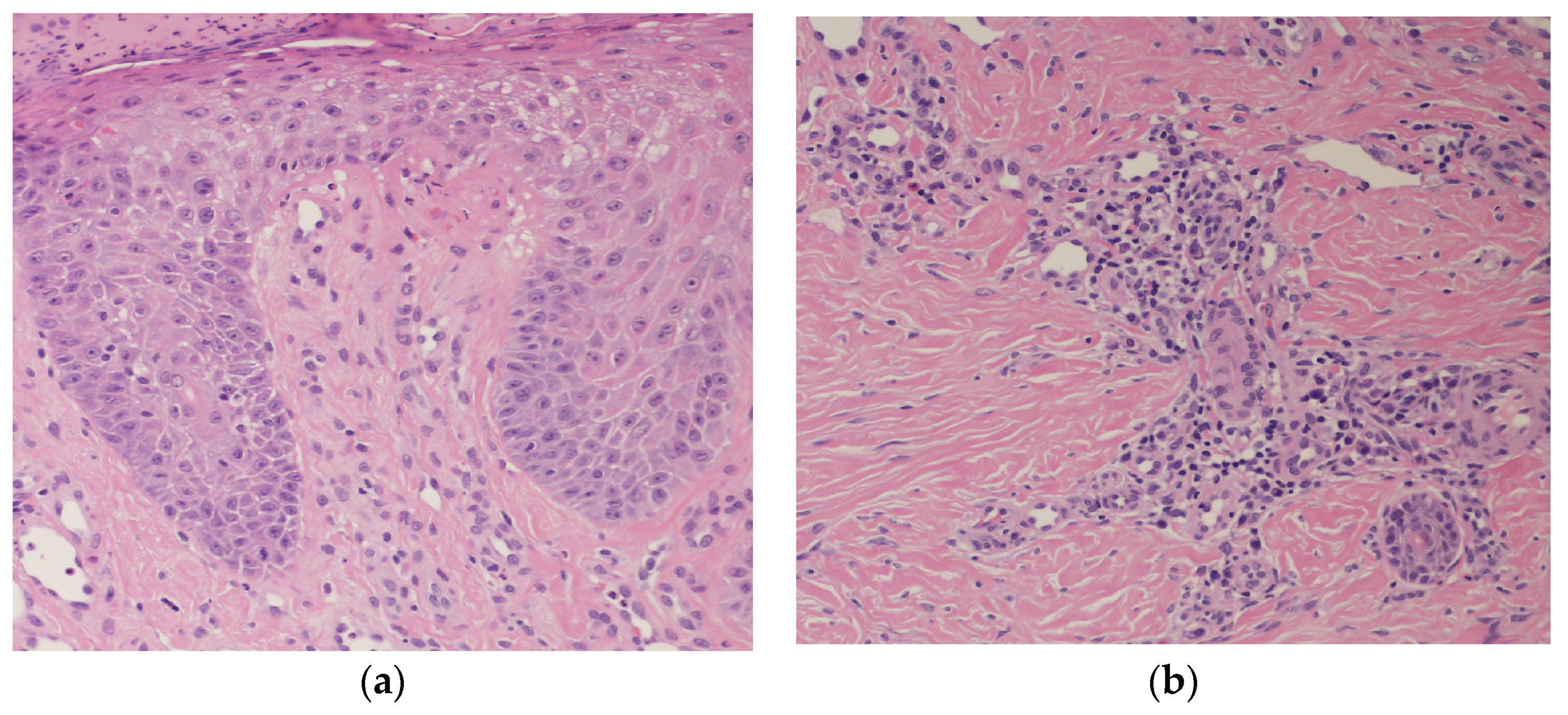
2. Case Presentation
3. Review
3.1. Eosinophilia
3.2. Eosinophilic Pneumonia
3.3. Eosinophilic Gastrointestinal Disorders (Enteritis, Esophagitis)
3.4. Allergic Bronchopulmonary Aspergillosis
3.5. Eosinophilic Bronchiolitis
3.6. Eosinophilic Granulomatosis with Polyangiitis
3.7. Asthma
3.8. Eosinophilic Fasciitis
3.9. Th2 Inflammatory Skin Diseases (PN, AD, BP, and EF)
3.9.1. Bullous Pemphigoid
3.9.2. Eosinophilic Folliculitis
3.9.3. Atopic Dermatitis
3.9.4. Prurigo Nodularis
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
List of Abbreviations
References
- Pardoll, D.M. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer 2012, 12, 252–264. [Google Scholar] [CrossRef] [PubMed]
- Gabrilovich, D.I.; Nagaraj, S. Myeloid-derived suppressor cells as regulators of the immune system. Nat. Rev. Immunol. 2009, 9, 162–174. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A.; Marchesi, F.; Malesci, A.; Laghi, L.; Allavena, P. Tumour-associated macrophages as treatment targets in oncology. Nat. Rev. Clin. Oncol. 2017, 14, 399–416. [Google Scholar] [CrossRef] [PubMed]
- Colegio, O.R.; Chu, N.Q.; Szabo, A.L.; Chu, T.; Rhebergen, A.M.; Jairam, V.; Cyrus, N.; Brokowski, C.E.; Eisenbarth, S.C.; Phillips, G.M.; et al. Functional polarization of tumour-associated macrophages by tumour-derived lactic acid. Nature 2014, 513, 559–563. [Google Scholar] [CrossRef] [PubMed]
- Seliger, B. Strategies of tumor immune evasion. BioDrugs 2005, 19, 347–354. [Google Scholar] [CrossRef]
- Sakaguchi, S.; Yamaguchi, T.; Nomura, T.; Ono, M. Regulatory T cells and immune tolerance. Cell 2008, 133, 775–787. [Google Scholar] [CrossRef] [PubMed]
- Ostrand-Rosenberg, S.; Sinha, P. Myeloid-derived suppressor cells: Linking inflammation and cancer. J. Immunol. 2009, 182, 4499–4506. [Google Scholar] [CrossRef] [PubMed]
- Marin-Acevedo, J.A.; Kimbrough, E.O.; Lou, Y. Next generation of immune checkpoint inhibitors and beyond. J. Hematol. Oncol. 2021, 14, 45. [Google Scholar] [CrossRef]
- Barclay, J.; Creswell, J.; León, J. Cancer immunotherapy and the PD-1/PD-L1 checkpoint pathway. Arch. Esp. Urol. 2018, 71, 393–399. [Google Scholar]
- Ribas, A.; Wolchok, J.D. Cancer immunotherapy using checkpoint blockade. Science 2018, 359, 1350–1355. [Google Scholar] [CrossRef]
- Jiang, X.; Wang, J.; Deng, X.; Xiong, F.; Ge, J.; Xiang, B.; Wu, X.; Ma, J.; Zhou, M.; Li, X.; et al. Role of the tumor microenvironment in PD-L1/PD-1-mediated tumor immune escape. Mol. Cancer 2019, 18, 10. [Google Scholar] [CrossRef] [PubMed]
- Selby, M.J.; Engelhardt, J.J.; Quigley, M.; Henning, K.A.; Chen, T.; Srinivasan, M.; Korman, A.J. Anti-CTLA-4 antibodies of IgG2a isotype enhance antitumor activity through reduction of intratumoral regulatory T cells. Cancer Immunol. Res. 2013, 1, 32–42. [Google Scholar] [CrossRef] [PubMed]
- Hargadon, K.M.; Johnson, C.E.; Williams, C.J. Immune checkpoint blockade therapy for cancer: An overview of FDA-approved immune checkpoint inhibitors. Int. Immunopharmacol. 2018, 62, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Rocha, M.; Correia de Sousa, J.; Salgado, M.; Araújo, A.; Pedroto, I. Management of gastrointestinal toxicity from immune checkpoint inhibitor. GE Port. J. Gastroenterol. 2019, 26, 268–274. [Google Scholar] [CrossRef] [PubMed]
- de Miguel, M.; Calvo, E. Clinical challenges of immune checkpoint inhibitors. Cancer Cell 2020, 38, 326–333. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.J.; Lee, H.J.; Farmer, J.R.; Reynolds, K.L. Mechanisms driving immune-related adverse events in cancer patients treated with immune checkpoint inhibitors. Curr. Cardiol. Rep. 2021, 23, 98. [Google Scholar] [CrossRef] [PubMed]
- Darnell, E.P.; Mooradian, M.J.; Baruch, E.N.; Yilmaz, M.; Reynolds, K.L. Immune-related adverse events (irAEs): Diagnosis, management, and clinical pearls. Curr. Oncol. Rep. 2020, 22, 39. [Google Scholar] [CrossRef] [PubMed]
- De Velasco, G.; Je, Y.; Bossé, D.; Awad, M.M.; Ott, P.A.; Moreira, R.B.; Schutz, F.; Bellmunt, J.; Sonpavde, G.P.; Hodi, F.S.; et al. Comprehensive meta-analysis of key immune-related adverse events from CTLA-4 and PD-1/PD-L1 inhibitors in cancer patients. Cancer Immunol. Res. 2017, 5, 312–318. [Google Scholar] [CrossRef] [PubMed]
- Puzanov, I.; Diab, A.; Abdallah, K.; Bingham, C.O.; Brogdon, C.; Dadu, R.; Hamad, L.; Kim, S.; Lacouture, M.E.; LeBoeuf, N.R.; et al. Managing toxicities associated with immune checkpoint inhibitors: Consensus recommendations from the society for immunotherapy of cancer (SITC) toxicity management working group. J. Immunother. Cancer 2017, 5, 95. [Google Scholar] [CrossRef]
- Esfahani, K.; Elkrief, A.; Calabrese, C.; Lapointe, R.; Hudson, M.; Routy, B.; Miller, W.H.; Calabrese, L. Moving towards personalized treatments of immune-related adverse events. Nat. Rev. Clin. Oncol. 2020, 17, 504–515. [Google Scholar] [CrossRef]
- Mahmood, S.S.; Fradley, M.G.; Cohen, J.V.; Nohria, A.; Reynolds, K.L.; Heinzerling, L.M.; Sullivan, R.J.; Damrongwatanasuk, R.; Chen, C.L.; Gupta, D.; et al. Myocarditis in patients treated with immune checkpoint inhibitors. J. Am. Coll. Cardiol. 2018, 71, 1755–1764. [Google Scholar] [CrossRef] [PubMed]
- Ibraheim, H.; Perucha, E.; Powell, N. Pathology of immune-mediated tissue lesions following treatment with immune checkpoint inhibitors. Rheumatology 2019, 58 (Suppl. S7), vii17–vii28. [Google Scholar] [CrossRef]
- Bamias, G.; Delladetsima, I.; Perdiki, M.; Siakavellas, S.I.; Goukos, D.; Papatheodoridis, G.V.; Daikos, G.L.; Gogas, H. Immunological characteristics of colitis associated with anti-CTLA-4 antibody therapy. Cancer Investig. 2017, 35, 443–455. [Google Scholar] [CrossRef] [PubMed]
- Suresh, K.; Naidoo, J.; Zhong, Q.; Xiong, Y.; Mammen, J.; de Flores, M.V.; Cappelli, L.; Balaji, A.; Palmer, T.; Forde, P.M.; et al. The alveolar immune cell landscape is dysregulated in checkpoint inhibitor pneumonitis. J. Clin. Investig. 2019, 129, 4305–4315. [Google Scholar] [CrossRef]
- Johnson, D.B.; Balko, J.M.; Compton, M.L.; Chalkias, S.; Gorham, J.; Xu, Y.; Hicks, M.; Puzanov, I.; Alexander, M.R.; Bloomer, T.L.; et al. Fulminant myocarditis with combination immune checkpoint blockade. N. Engl. J. Med. 2016, 375, 1749–1755. [Google Scholar] [CrossRef]
- Hughes, J.; Vudattu, N.; Sznol, M.; Gettinger, S.; Kluger, H.; Lupsa, B.; Herold, K.C. Precipitation of autoimmune diabetes with anti-PD-1 immunotherapy. Diabetes Care 2015, 38, e55–e57. [Google Scholar] [CrossRef]
- Curry, J.L.; Reuben, A.; Szczepaniak-Sloane, R.; Ning, J.; Milton, D.R.; Lee, C.H.; Hudgens, C.; George, S.; Torres-Cabala, C.; Johnson, D.; et al. Gene expression profiling of lichenoid dermatitis immune-related adverse event from immune checkpoint inhibitors reveals increased CD14+ and CD16+ monocytes driving an innate immune response. J. Cutan. Pathol. 2019, 46, 627–636. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, S.; Yang, F.; Qi, X.; Wang, X.; Guan, X.; Shen, C.; Duma, N.; Vera Aguilera, J.; Chintakuntlawar, A.; et al. Treatment-related adverse events of PD-1 and PD-L1 inhibitors in clinical trials: A systematic review and meta-analysis. JAMA Oncol. 2019, 5, 1008–1019. [Google Scholar] [CrossRef]
- Kaunitz, G.J.; Loss, M.; Rizvi, H.; Ravi, S.; Cuda, J.D.; Bleich, K.B.; Esandrio, J.; Sander, I.; Le, D.T.; Diaz, L.A., Jr.; et al. Cutaneous eruptions in patients receiving immune checkpoint blockade: Clinicopathologic analysis of the nonlichenoid histologic pattern. Am. J. Surg. Pathol. 2017, 41, 1381–1389. [Google Scholar] [CrossRef]
- Shi, V.J.; Rodic, N.; Gettinger, S.; Leventhal, J.S.; Neckman, J.P.; Girardi, M.; Bosenberg, M.; Choi, J.N. Clinical and histologic features of lichenoid mucocutaneous eruptions due to anti-programmed cell death 1 and anti-programmed cell death ligand 1 immunotherapy. JAMA Dermatol. 2016, 152, 1128–1136. [Google Scholar] [CrossRef] [PubMed]
- Okazaki, T.; Honjo, T. PD-1 and PD-1 ligands: From discovery to clinical application. Int. Immunol. 2007, 19, 813–824. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Zhu, G.; Tamada, K.; Chen, L. B7-H1, a third member of the B7 family, co-stimulates T-cell proliferation and interleukin-10 secretion. Nat. Med. 1999, 5, 1365–1369. [Google Scholar] [CrossRef]
- Latchman, Y.; Wood, C.R.; Chernova, T.; Chaudhary, D.; Borde, M.; Chernova, I.; Iwai, Y.; Long, A.J.; Brown, J.A.; Nunes, R.; et al. PD-L2 is a second ligand for PD-1 and inhibits T cell activation. Nat. Immunol. 2001, 2, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Tseng, S.Y.; Otsuji, M.; Gorski, K.; Huang, X.; Slansky, J.E.; Pai, S.I.; Shalabi, A.; Shin, T.; Pardoll, D.M.; Tsuchiya, H. B7-DC, a new dendritic cell molecule with potent costimulatory properties for T cells. J. Exp. Med. 2001, 193, 839–846. [Google Scholar] [CrossRef]
- Youngnak, P.; Kozono, Y.; Kozono, H.; Iwai, H.; Otsuki, N.; Jin, H.; Omura, K.; Yagita, H.; Pardoll, D.M.; Chen, L.; et al. Differential binding properties of B7-H1 and B7-DC to programmed death-1. Biochem. Biophys. Res. Commun. 2003, 307, 672–677. [Google Scholar] [CrossRef]
- Tanaka, R.; Ichimura, Y.; Kubota, N.; Saito, A.; Nakamura, Y.; Ishitsuka, Y.; Watanabe, R.; Fujisawa, Y.; Mizuno, S.; Takahashi, S.; et al. Differential involvement of programmed cell death ligands in skin immune responses. J. Investig. Dermatol. 2022, 142, 145–154.e8. [Google Scholar] [CrossRef]
- Reschke, R.; Shapiro, J.W.; Yu, J.; Rouhani, S.J.; Olson, D.J.; Zha, Y.; Gajewski, T.F. Checkpoint blockade-induced dermatitis and colitis are dominated by tissue-resident memory T cells and Th1/Tc1 cytokines. Cancer Immunol. Res. 2022, 10, 1167–1174. [Google Scholar] [CrossRef] [PubMed]
- Maillard, A.; Pastor, D.; Merat, R. Anti-PD-1-Induced hidradenitis suppurativa. Dermatopathology 2021, 8, 37–39. [Google Scholar] [CrossRef]
- Pach, J.J.; Mbonu, N.; Bhullar, S.; Cohen, J.M.; Leventhal, J.S. Immune checkpoint inhibitor-induced psoriasis: Diagnosis, management, and a review of cases. Dermatol. Clin. 2024, 42, 481–493. [Google Scholar] [CrossRef]
- Fukushi, S.; Yamasaki, K.; Aiba, S. Nuclear localization of activated STAT6 and STAT3 in epidermis of prurigo nodularis. Br. J. Dermatol. 2011, 165, 990–996. [Google Scholar] [CrossRef]
- Delyon, J.; Mateus, C.; Lefeuvre, D.; Lanoy, E.; Zitvogel, L.; Chaput, N.; Roy, S.; Eggermont, A.M.; Routier, E.; Robert, C. Experience in daily practice with ipilimumab for the treatment of patients with metastatic melanoma: An early increase in lymphocyte and eosinophil counts is associated with improved survival. Ann. Oncol. 2013, 24, 1697–1703. [Google Scholar] [CrossRef] [PubMed]
- Goetzl, E.J. Th2 cells in rapid immune responses and protective avoidance reactions. FASEB J. 2024, 38, e23485. [Google Scholar] [CrossRef]
- Kouro, T.; Takatsu, K. IL-5- and eosinophil-mediated inflammation: From discovery to therapy. Int. Immunol. 2009, 21, 1303–1309. [Google Scholar] [CrossRef]
- Bernard-Tessier, A.; Jeanville, P.; Champiat, S.; Lazarovici, J.; Voisin, A.L.; Mateus, C.; Lambotte, O.; Annereau, M.; Michot, J.M. Immune-related eosinophilia induced by anti-programmed death 1 or death-ligand 1 antibodies. Eur. J. Cancer 2017, 81, 135–137. [Google Scholar] [CrossRef]
- Weide, B.; Martens, A.; Hassel, J.C.; Berking, C.; Postow, M.A.; Bisschop, K.; Simeone, E.; Mangana, J.; Schilling, B.; Di Giacomo, A.M.; et al. Baseline biomarkers for outcome of melanoma patients treated with pembrolizumab. Clin. Cancer Res. 2016, 22, 5487–5496. [Google Scholar] [CrossRef]
- Carretero, R.; Sektioglu, I.M.; Garbi, N.; Salgado, O.C.; Beckhove, P.; Hämmerling, G.J. Eosinophils orchestrate cancer rejection by normalizing tumor vessels and enhancing infiltration of CD8(þ)T cells. Nat. Immunol. 2015, 16, 609–617. [Google Scholar] [CrossRef]
- Mato, N.; Bando, M.; Kusano, A.; Hirano, T.; Nakayama, M.; Uto, T.; Nakaya, T.; Yamasawa, H.; Sugiyama, Y. Clinical significance of interleukin 33 (IL-33) in patients with eosinophilic pneumonia. Allergol. Int. 2013, 62, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Allen, J.N.; Pacht, E.R.; Gadek, J.E.; Davis, W.B. Acute eosinophilic pneumonia as a reversible cause of noninfectious respiratory failure. N. Engl. J. Med. 1989, 321, 569–574. [Google Scholar] [CrossRef] [PubMed]
- De Giacomi, F.; Decker, P.A.; Vassallo, R.; Ryu, J.H. Acute eosinophilic pneumonia: Correlation of clinical characteristics with underlying cause. Chest 2017, 152, 379–385. [Google Scholar] [CrossRef]
- Bratke, K.; Fritz, L.; Nokodian, F.; Geißler, K.; Garbe, K.; Lommatzsch, M.; Virchow, J.C. Differential regulation of PD-1 and its ligands in allergic asthma. Clin. Exp. Allergy 2017, 47, 1417–1425. [Google Scholar] [CrossRef]
- van der Werf, N.; Redpath, S.A.; Azuma, M.; Yagita, H.; Taylor, M.D. Th2 cell-intrinsic hypo-responsiveness determines susceptibility to helminth infection. PLOS Pathog. 2013, 9, e1003215. [Google Scholar] [CrossRef]
- Zhang, Y.; Chung, Y.; Bishop, C.; Daugherty, B.; Chute, H.; Holst, P.; Kurahara, C.; Lott, F.; Sun, N.; Welcher, A.A.; et al. Regulation of T cell activation and tolerance by PDL2. Proc. Natl. Acad. Sci. USA 2006, 103, 11695–11700. [Google Scholar] [CrossRef]
- Jodai, T.; Yoshida, C.; Sato, R.; Kakiuchi, Y.; Sato, N.; Iyama, S.; Kimura, T.; Saruwatari, K.; Saeki, S.; Ichiyasu, H.; et al. A potential mechanism of the onset of acute eosinophilic pneumonia triggered by an anti-PD-1 immune checkpoint antibody in a lung cancer patient. Immun. Inflam. Dis. 2019, 7, 3–6. [Google Scholar] [CrossRef]
- Mohammed, A.; Tang, B.; Sadikot, S.; Barmaimon, G. Acute eosinophilic pneumonia induced by immune checkpoint inhibitor and anti-TIGIT therapy. Am. J. Case Rep. 2024, 25, e943740. [Google Scholar] [CrossRef]
- Yang, J.; Lagana, S.M.; Saenger, Y.M.; Carvajal, R.D. Dual checkpoint inhibitor-associated eosinophilic enteritis. J. Immunother. Cancer 2019, 7, 310. [Google Scholar] [CrossRef]
- Barnett, J.S.; Yu, K.K.; Rivera Rivera, X.; Bhatt, A. Severe dysphagia with eosinophilic esophagitis pattern of injury related to pembrolizumab therapy. ACG Case Rep. J. 2024, 11, e01252. [Google Scholar] [CrossRef]
- Nakamura, M.; Otsuka, T.; Kumagai, M.; Arisawa, T. Pembrolizumab-induced eosinophilic gastrointestinal disorders. Intern. Med. 2022, 61, 267–269. [Google Scholar] [CrossRef]
- Rothenberg, M.E. Eosinophilic gastrointestinal disorders (EGID). J. Allergy Clin. Immunol. 2004, 113, 11–28, quiz 29. [Google Scholar] [CrossRef]
- Caldwell, J.M.; Collins, M.H.; Stucke, E.M.; Putnam, P.E.; Franciosi, J.P.; Kushner, J.P.; Abonia, J.P.; Rothenberg, M.E. Histologic eosinophilic gastritis is a systemic disorder associated with blood and extragastric eosinophilia, TH2 immunity, and a unique gastric transcriptome. J. Allergy Clin. Immunol. 2014, 134, 1114–1124. [Google Scholar] [CrossRef]
- Zhang, M.; Li, Y. Eosinophilic gastroenteritis: A state-of-the-art review. J. Gastroenterol. Hepatol. 2017, 32, 64–72. [Google Scholar] [CrossRef]
- Muldoon, E.G.; Strek, M.E.; Patterson, K.C. Allergic and noninvasive infectious pulmonary aspergillosis syndromes. Clin. Chest Med. 2017, 38, 521–534. [Google Scholar] [CrossRef]
- Tracy, M.C.; Okorie, C.U.A.; Foley, E.A.; Moss, R.B. Allergic bronchopulmonary aspergillosis. J. Fungi 2016, 2, 17. [Google Scholar] [CrossRef]
- Donato, A.A.; Krol, R. Allergic bronchopulmonary aspergillosis presumably unmasked by PD-1 inhibition. BMJ Case Rep. 2019, 12, e227814. [Google Scholar] [CrossRef] [PubMed]
- Poletti, V. Eosinophilic bronchiolitis: Is it a new syndrome? Eur. Respir. J. 2013, 41, 1012–1013. [Google Scholar] [CrossRef]
- Tang, T.T.; Cheng, H.H.; Zhang, H.; Lin, X.L.; Huang, L.J.; Zhang, W.; Jiang, S.P. Hypereosinophilic obliterative bronchiolitis with an elevated level of serum CEA: A case report and a review of the literature. Eur. Rev. Med. Pharmacol. Sci. 2015, 19, 2634–2640. [Google Scholar]
- Tamura, A.; Hashimoto, M.; Hosoi, A.; Hojo, M. A case of eosinophilic bronchiolitis after the initiation of immune checkpoint inhibitor. Thorac. Cancer 2023, 14, 1894–1898. [Google Scholar] [CrossRef] [PubMed]
- Wakashin, H.; Hirose, K.; Maezawa, Y.; Kagami, S.I.; Suto, A.; Watanabe, N.; Saito, Y.; Hatano, M.; Tokuhisa, T.; Iwakura, Y.; et al. IL-23 and Th17 cells enhance Th2-cell-mediated eosinophilic airway inflammation in mice. Am. J. Respir. Crit. Care Med. 2008, 178, 1023–1032. [Google Scholar] [CrossRef]
- Dias, P.M.; Banerjee, G. The role of Th17/IL-17 on eosinophilic inflammation. J. Autoimmun. 2013, 40, 9–20. [Google Scholar] [CrossRef]
- Harada, M.; Naoi, H.; Yasuda, K.; Ito, Y.; Kagoo, N.; Kubota, T.; Ichijo, K.; Mochizuki, E.; Uehara, M.; Matsuura, S.; et al. Programmed cell death-1 blockade in kidney carcinoma may induce eosinophilic granulomatosis with polyangiitis: A case report. BMC Pulm. Med. 2021, 21, 6. [Google Scholar] [CrossRef]
- Milne, M.E.; Kimball, J.; Tarrant, T.K.; Al-Rohil, R.N.; Leverenz, D.L. The role of T helper Type 2 (Th2) cytokines in the pathogenesis of eosinophilic granulomatosis with polyangiitis (eGPA): An illustrative case and discussion. Curr. Allergy Asthma Rep. 2022, 22, 141–150. [Google Scholar] [CrossRef]
- Wechsler, M.E.; Akuthota, P.; Jayne, D.; Khoury, P.; Klion, A.; Langford, C.A.; Merkel, P.A.; Moosig, F.; Specks, U.; Cid, M.C.; et al. Mepolizumab or placebo for eosinophilic granulomatosis with polyangiitis. N. Engl. J. Med. 2017, 376, 1921–1932. [Google Scholar] [CrossRef] [PubMed]
- Maeno, K.; Fukuda, S.; Oguri, T.; Niimi, A. Nivolumab-induced asthma in a patient with non-small-cell lung cancer. Ann. Oncol. 2017, 28, 2891. [Google Scholar] [CrossRef] [PubMed]
- Price, D.B.; Rigazio, A.; Campbell, J.D.; Bleecker, E.R.; Corrigan, C.J.; Thomas, M.; Wenzel, S.E.; Wilson, A.M.; Small, M.B.; Gopalan, G.; et al. Blood eosinophil count and prospective annual asthma disease burden: A UK cohort study. Lancet Respir. Med. 2015, 3, 849–858. [Google Scholar] [CrossRef] [PubMed]
- Harada, T.; Uetani, N.; Inui, G.; Ishikawa, H.; Funaki, Y.; Takata, M.; Okazaki, R.; Yamaguchi, K.; Morita, M.; Kitatani, S.; et al. Pembrolizumab-induced asthma exacerbation with hypereosinophilia and elevated interleukin-5 in endometrial cancer: A case report. Respir. Med. Case Rep. 2024, 49, 102035. [Google Scholar] [CrossRef]
- Oflazoglu, E.; Swart, D.A.; Anders-Bartholo, P.; Jessup, H.K.; Norment, A.M.; Lawrence, W.A.; Brasel, K.; Tocker, J.E.; Horan, T.; Welcher, A.A.; et al. Paradoxical role of programmed death-1 ligand 2 in Th2 immune responses in vitro and in a mouse asthma model vivo. Eur. J. Immunol. 2004, 34, 3326–3336. [Google Scholar] [CrossRef]
- Kissoonsingh, P.; Sutton, B.; Iqbal, S.U.; Pallan, L.; Steven, N.; Khoja, L. Eosinophilic asthma secondary to adjuvant anti-PD-1 immune checkpoint inhibitor treatment in a melanoma patient. Case Rep. Oncol. Med. 2022, 2022, 2658136. [Google Scholar] [CrossRef]
- Xu, W.; Lian, B.; Cui, C.; Guo, J. The combination therapy with the cytotoxic T lymphocyte-associated antigen-4 and programmed death 1 antibody-induced asthma in a patient with advanced melanoma. J. Cancer Res. Ther. 2021, 17, 808–810. [Google Scholar] [CrossRef] [PubMed]
- Hayakawa, Y.; Kawaguchi, T.; Yamasaki, K.; Endo, M.; Komatsu, M.; Ishiguro, Y.; Murata, Y.; Yatera, K. Immune checkpoint inhibitor-induced asthma and chronic obstructive pulmonary disease overlap in patient with adenocarcinoma. Respirol. Case Rep. 2023, 11, e01222. [Google Scholar] [CrossRef] [PubMed]
- Hamada, K.; Yoshimura, K.; Oshinomi, K.; Hirasawa, Y.; Ariizumi, H.; Ohkuma, R.; Shida, M.; Kubota, Y.; Matsui, H.; Ishiguro, T.; et al. A case of bronchial asthma as an immune-related adverse event of pembrolizumab treatment for bladder cancer: A case report. Medicine 2022, 101, e28339. [Google Scholar] [CrossRef]
- Soto, F.; Torre-Sada, L.F.; Mott, F.E.; Kim, S.T.; Nurieva, R.; Shannon, V.R.; Faiz, S.A.; Casal, R.F.; Altan, M.; Lin, J.; et al. Sarcoidosis and airway disease after immune checkpoint inhibitor therapy: Case study and review of the literature. J. Immunother. Precis. Oncol. 2023, 6, 111–116. [Google Scholar] [CrossRef]
- Ihn, H. Eosinophilic fasciitis: From pathophysiology to treatment. Allergol. Int. 2019, 68, 437–439. [Google Scholar] [CrossRef] [PubMed]
- Haddad, H.; Sundaram, S.; Magro, C.; Gergis, U. Eosinophilic fasciitis as a paraneoplastic syndrome, a case report and review of the literature. Hematol. Oncol. Stem Cell Ther. 2014, 7, 90–92. [Google Scholar] [CrossRef] [PubMed]
- Viallard, J.F.; Taupin, J.L.; Ranchin, V.; Leng, B.; Pellegrin, J.L.; Moreau, J.F. Analysis of leukemia inhibitory factors, type 1 and type 2 cytokine production in patients with eosinophilic fasciitis. J. Rheumatol. 2001, 28, 75–80. [Google Scholar] [PubMed]
- Bui, A.N.; Nelson, C.A.; Lian, C.G.; Canales, A.L.; LeBoeuf, N.R. Eosinophilic fasciitis induced by nivolumab therapy managed without treatment interruption or systemic immunosuppression. JAAD Case Rep. 2020, 6, 693–696. [Google Scholar] [CrossRef]
- Pabón-Cartagena, G.; López, A.; Watts, E.; Alonso, N. Eosinophilic fasciitis in association with nivolumab: The importance of eosinophilia. JAAD Case Rep. 2020, 6, 1303–1306. [Google Scholar] [CrossRef]
- Salamaliki, C.; Solomou, E.E.; Liossis, S.C. Immune checkpoint inhibitor-associated scleroderma-like syndrome: A report of a pembrolizumab-induced “eosinophilic fasciitis-like” case and a review of the literature. Rheumatol. Ther. 2020, 7, 1045–1052. [Google Scholar] [CrossRef]
- Asdourian, M.S.; Shah, N.; Jacoby, T.V.; Reynolds, K.L.; Chen, S.T. Association of bullous pemphigoid with immune checkpoint inhibitor therapy in patients with cancer: A systematic review. JAMA Dermatol. 2022, 158, 933–941. [Google Scholar] [CrossRef]
- Tabatabaei-Panah, P.S.; Moravvej, H.; Alirajab, M.; Etaaty, A.; Geranmayeh, M.; Hosseine, F.; Khansari, A.; Mahdian, M.; Mirhashemi, M.; Parvizi, S.; et al. Association between TH2 cytokine gene polymorphisms and risk of bullous pemphigoid. Immunol. Investig. 2022, 51, 343–356. [Google Scholar] [CrossRef]
- Chen, F.; Wang, Y.; Chen, X.; Yang, N.; Li, L. Targeting interleukin 4 and interleukin 13: A novel therapeutic approach in bullous pemphigoid. Ann. Med. 2023, 55, 1156–1170. [Google Scholar] [CrossRef]
- Rossi, A.; Magri, F.; Caro, G.; Federico, A.; Fortuna, M.C.; Soda, G.; De Vincentiis, L.; Carlesimo, M. Eosinophilic folliculitis of the scalp associated with PD-1/PDL1 inhibitors. J. Cosmet. Dermatol. 2020, 19, 3367–3370. [Google Scholar] [CrossRef]
- Tolino, E.; Proietti, I.; Skroza, N.; Guardo, A.D.; Fraia, M.D.; Dybala, A.; Potenza, C. Atopic dermatitis onset in a melanoma patient under pembrolizumab therapy: A case of successful treatment with dupilumab. Indian J. Dermatol. 2024, 69, 268–269. [Google Scholar] [CrossRef]
- David Boothe, W.; Tarbox, J.A.; Tarbox, M.B. Atopic dermatitis: Pathophysiology. Adv. Exp. Med. Biol. 2017, 1027, 21–37. [Google Scholar] [CrossRef]
- Gooderham, M.J.; Hong, H.C.H.; Eshtiaghi, P.; Papp, K.A. Dupilumab: A review of its use in the treatment of atopic dermatitis. J. Am. Acad. Dermatol. 2018, 78 (Suppl. S1), S28–S36. [Google Scholar] [CrossRef]
- Fattore, D.; Panariello, L.; Annunziata, M.C.; Fabbrocini, G. Prurigo nodularis and pembrolizumab: A therapeutic challenge. Eur. J. Cancer 2019, 110, 8–10. [Google Scholar] [CrossRef]
- Pereira, M.P.; Steinke, S.; Zeidler, C.; Forner, C.; Riepe, C.; Augustin, M.; Bobko, S.; Dalgard, F.; Elberling, J.; Garcovich, S.; et al. European academy of dermatology and venereology European prurigo project: Expert consensus on the definition, classification and terminology of chronic prurigo. J. Eur. Acad. Dermatol. Venereol. 2018, 32, 1059–1065. [Google Scholar] [CrossRef]
- Capellero, S.; Erriquez, J.; Battistini, C.; Porporato, R.; Scotto, G.; Borella, F.; Di Renzo, M.F.; Valabrega, G.; Olivero, M. Ovarian cancer cells in ascites form aggregates that display a hybrid epithelial-mesenchymal phenotype and allows survival and proliferation of metastasizing cells. Int. J. Mol. Sci. 2022, 23, 833. [Google Scholar] [CrossRef] [PubMed]
- Yosipovitch, G.; Rosen, J.D.; Hashimoto, T. Itch: From mechanism to (novel) therapeutic approaches. J. Allergy Clin. Immunol. 2018, 142, 1375–1390. [Google Scholar] [CrossRef]
- Nakajima, S.; Yonekura, S.; Nakamizo, S.; Egawa, G.; Kabashima, K. Dupilumab as a novel treatment option for prurigo nodularis. J. Allergy Clin. Immunol. 2023, 152, 870–872. [Google Scholar] [CrossRef] [PubMed]
- Dermani, F.K.; Samadi, P.; Rahmani, G.; Kohlan, A.K.; Najafi, R. PD-1/PD-L1 immune checkpoint: Potential target for cancer therapy. J. Cell Physiol. 2019, 234, 1313–1325. [Google Scholar] [CrossRef]
- Han, M.; Ma, J.; Ouyang, S.; Wang, Y.; Zheng, T.; Lu, P.; Zheng, Z.; Zhao, W.; Li, H.; Wu, Y.; et al. The kinase p38α functions in dendritic cells to regulate Th2-cell differentiation and allergic inflammation. Cell Mol. Immunol. 2022, 19, 805–819. [Google Scholar] [CrossRef] [PubMed]
- Lacouture, M.E.; Goleva, E.; Shah, N.; Rotemberg, V.; Kraehenbuehl, L.; Ketosugbo, K.F.; Merghoub, T.; Maier, T.; Bang, A.; Gu, S.; et al. Immunologic profiling of immune-related cutaneous adverse events with checkpoint inhibitors reveals polarized actionable pathways. Clin. Cancer Res. 2024, 30, 2822–2834. [Google Scholar] [CrossRef] [PubMed]
- Kuo, A.M.S.; Gu, S.; Stoll, J.; Moy, A.P.; Dusza, S.W.; Gordon, A.; Haliasos, E.C.; Janjigian, Y.; Kraehenbuehl, L.; Quigley, E.A.; et al. Management of immune-related cutaneous adverse events with dupilumab. J. Immunother. Cancer 2023, 11, e007324. [Google Scholar] [CrossRef] [PubMed]
- Gambichler, T.; Susok, L. PD-1 blockade for disseminated Kaposi sarcoma in a patient with atopic dermatitis and chronic CD8 lymphopenia. Immunotherapy 2020, 12, 451–457. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mima, Y.; Ohtsuka, T.; Ebato, I.; Nakata, Y.; Tsujita, A.; Nakazato, Y.; Norimatsu, Y. Review of T Helper 2-Type Inflammatory Diseases Following Immune Checkpoint Inhibitor Treatment. Biomedicines 2024, 12, 1886. https://doi.org/10.3390/biomedicines12081886
Mima Y, Ohtsuka T, Ebato I, Nakata Y, Tsujita A, Nakazato Y, Norimatsu Y. Review of T Helper 2-Type Inflammatory Diseases Following Immune Checkpoint Inhibitor Treatment. Biomedicines. 2024; 12(8):1886. https://doi.org/10.3390/biomedicines12081886
Chicago/Turabian StyleMima, Yoshihito, Tsutomu Ohtsuka, Ippei Ebato, Yukihiro Nakata, Akihiro Tsujita, Yoshimasa Nakazato, and Yuta Norimatsu. 2024. "Review of T Helper 2-Type Inflammatory Diseases Following Immune Checkpoint Inhibitor Treatment" Biomedicines 12, no. 8: 1886. https://doi.org/10.3390/biomedicines12081886
APA StyleMima, Y., Ohtsuka, T., Ebato, I., Nakata, Y., Tsujita, A., Nakazato, Y., & Norimatsu, Y. (2024). Review of T Helper 2-Type Inflammatory Diseases Following Immune Checkpoint Inhibitor Treatment. Biomedicines, 12(8), 1886. https://doi.org/10.3390/biomedicines12081886




