Effect of Menopausal Hormone Therapy on Cellular Immunity Parameters and Cytokine Profile
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics
2.2. Subjects and Study Design
2.3. Isolation of Peripheral Blood Mononuclear Cells
2.4. Multiparametric Flow Cytometry Assay
2.5. Bio-Plex Multiplex Immunoassay
2.6. Statistical Analysis
3. Results
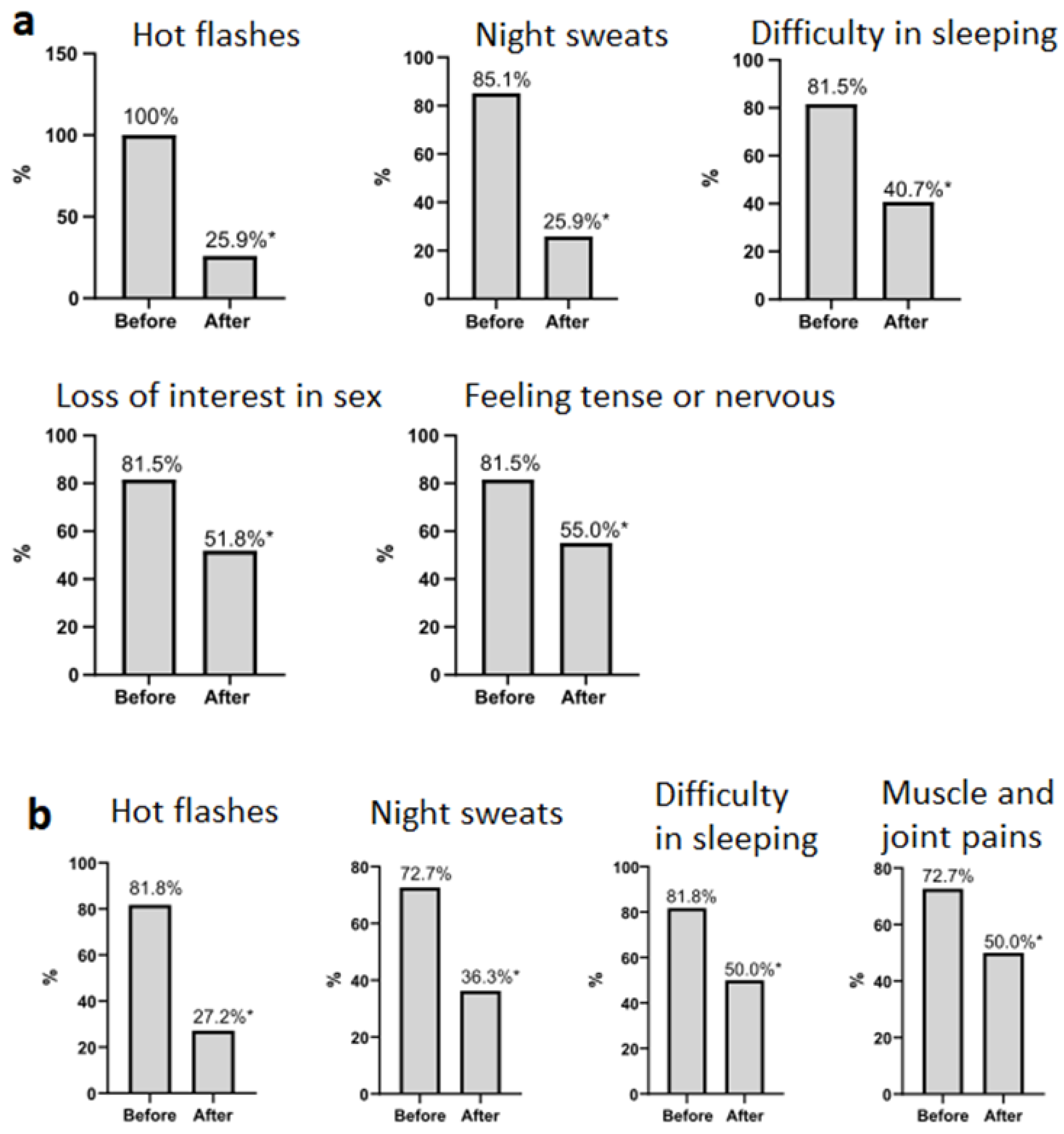
3.1. Patients’ Data
3.2. Changes in Cellular Immunity Parameters in Women during the Use of MHT
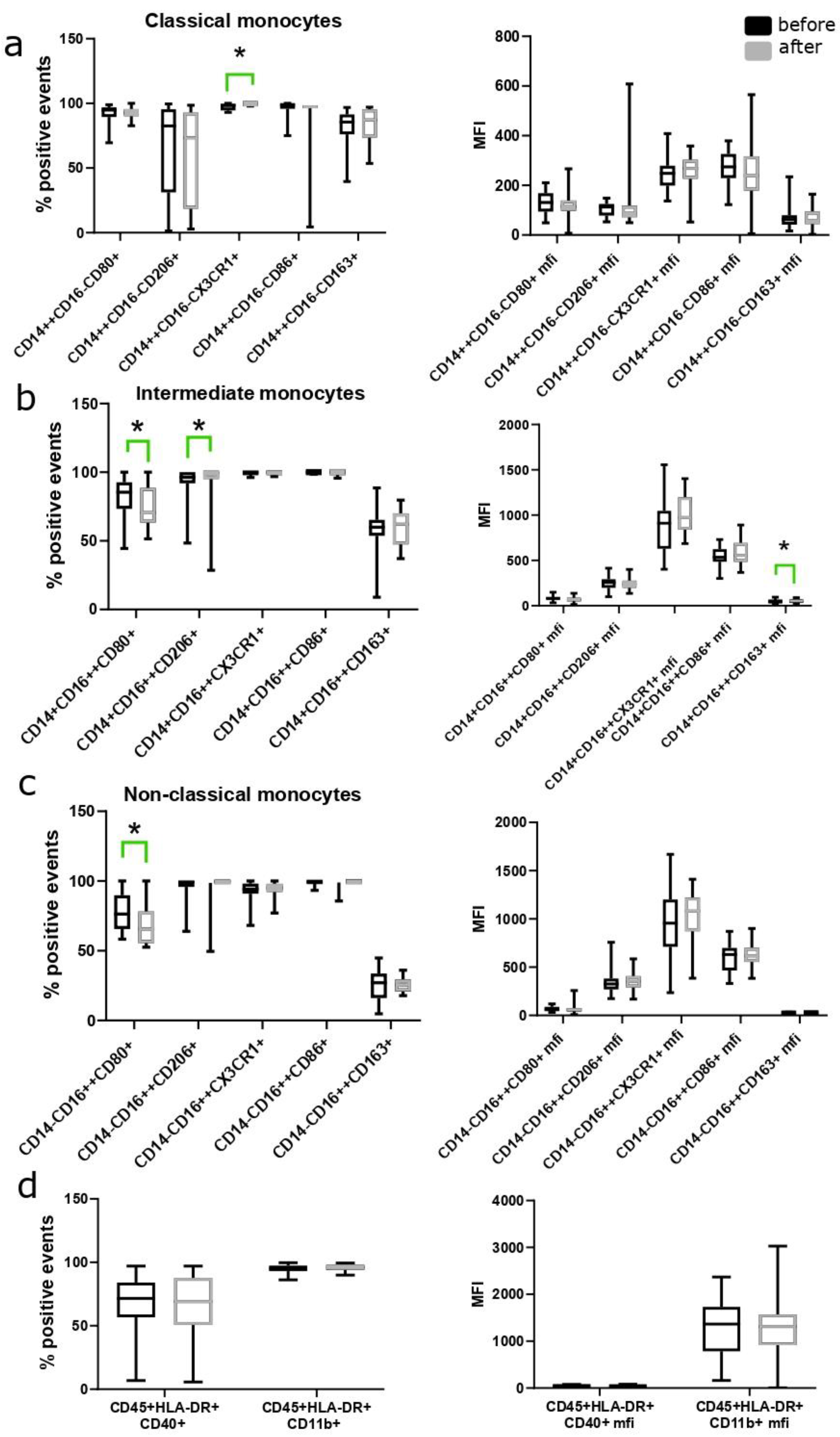
3.3. Changes in the Number of Monocyte Subpopulations during the Use of MHT
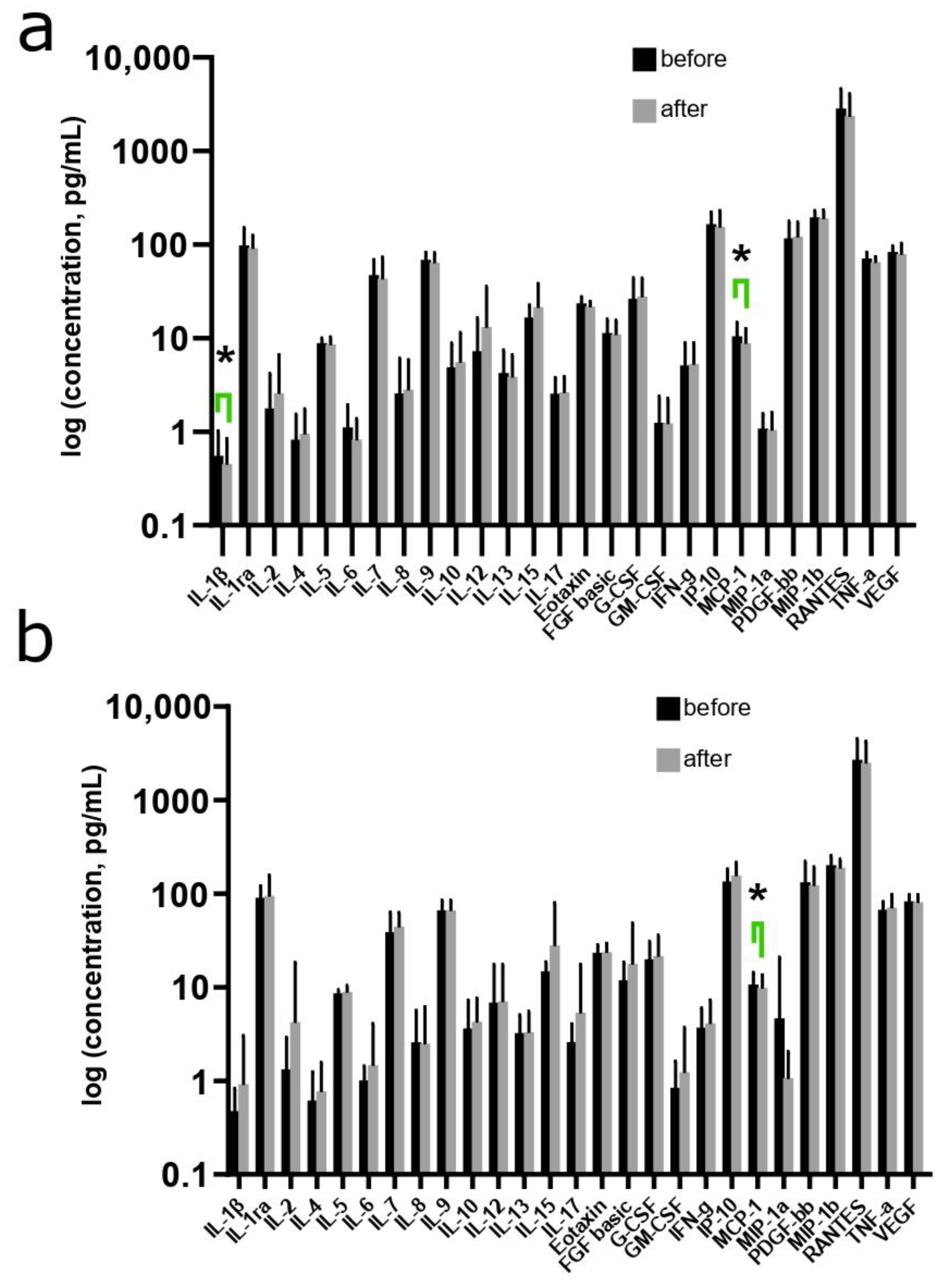
3.4. Changes in the Cytokine Profile in Women during the Use of MHT
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, Z.; Liang, Q.; Ren, Y.; Guo, C.; Ge, X.; Wang, L.; Cheng, Q.; Luo, P.; Zhang, Y.; Han, X. Immunosenescence: Molecular Mechanisms and Diseases. Signal Transduct. Target. Ther. 2023, 8, 200. [Google Scholar] [CrossRef] [PubMed]
- Ashley, N.T.; Demas, G.E. Neuroendocrine-Immune Circuits, Phenotypes, and Interactions. Horm. Behav. 2017, 87, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Tang, X.; Lv, X.; Meng, X.; Geng, L.; Zhong, Z.; Ding, Y.; Li, T.; Wan, Q. Age at Menarche and Risk of Ovarian Hyperstimulation Syndrome in Women Undergoing IVF/ICSI Cycles: A Retrospective Cohort Study. BMJ Open 2024, 14, e076867. [Google Scholar] [CrossRef] [PubMed]
- Kany, S.; Vollrath, J.T.; Relja, B. Cytokines in Inflammatory Disease. Int. J. Mol. Sci. 2019, 20, 6008. [Google Scholar] [CrossRef] [PubMed]
- Horváthová, M.; Ilavská, S.; Štefíková, K.; Szabová, M.; Krivošíková, Z.; Jahnová, E.; Tulinská, J.; Spustová, V.; Gajdoš, M. The Cell Surface Markers Expression in Postmenopausal Women and Relation to Obesity and Bone Status. Int. J. Environ. Res. Public Health 2017, 14, 751. [Google Scholar] [CrossRef] [PubMed]
- Vrachnis, N.; Zygouris, D.; Iliodromiti, Z.; Daniilidis, A.; Valsamakis, G.; Kalantaridou, S. Probing the Impact of Sex Steroids and Menopause-Related Sex Steroid Deprivation on Modulation of Immune Senescence. Maturitas 2014, 78, 174–178. [Google Scholar] [CrossRef] [PubMed]
- Deguchi, K.; Kamada, M.; Irahara, M.; Maegawa, M.; Yamamoto, S.; Ohmoto, Y.; Murata, K.; Yasui, T.; Yamano, S.; Aono, T. Postmenopausal Changes in Production of Type 1 and Type 2 Cytokines and the Effects of Hormone Replacement Therapy. Menopause 2001, 8, 266–273. [Google Scholar] [CrossRef]
- Vural, P.; Akgul, C.; Canbaz, M. Effects of Hormone Replacement Therapy on Plasma Pro-Inflammatory and Anti-Inflammatory Cytokines and Some Bone Turnover Markers in Postmenopausal Women. Pharmacol. Res. 2006, 54, 298–302. [Google Scholar] [CrossRef]
- Stopińska-Głuszak, U.; Waligóra, J.; Grzela, T.; Głuszak, M.; Jóźwiak, J.; Radomski, D.; Roszkowski, P.I.; Malejczyk, J. Effect of Estrogen/Progesterone Hormone Replacement Therapy on Natural Killer Cell Cytotoxicity and Immunoregulatory Cytokine Release by Peripheral Blood Mononuclear Cells of Postmenopausal Women. J. Reprod. Immunol. 2006, 69, 65–75. [Google Scholar] [CrossRef]
- Shah, L.; Elshaikh, A.O.; Lee, R.; Joy Mathew, C.; Jose, M.T.; Cancarevic, I. Do Menopause and Aging Affect the Onset and Progression of Rheumatoid Arthritis and Systemic Lupus Erythematosus? Cureus 2020, 12, e10944. [Google Scholar] [CrossRef]
- Wang, Y.; Mishra, A.; Brinton, R.D. Transitions in Metabolic and Immune Systems from Pre-Menopause to Post-Menopause: Implications for Age-Associated Neurodegenerative Diseases. F1000Research 2020, 9, 68. [Google Scholar] [CrossRef]
- Liang, W.; Liu, H.; Zeng, Z.; Liang, Z.; Xie, H.; Li, W.; Xiong, L.; Liu, Z.; Chen, M.; Jie, H.; et al. KRT17 Promotes T-Lymphocyte Infiltration through the YTHDF2–CXCL10 Axis in Colorectal Cancer. Cancer Immunol. Res. 2023, 11, 875–894. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, P.; Singh, S.M.; Able, C.; Dumas, K.; Kohn, J.; Kohn, T.P.; Clifton, M. Safety of Vaginal Estrogen Therapy for Genitourinary Syndrome of Menopause in Women with a History of Breast Cancer. Obstet. Gynecol. 2023, 142, 660–668. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Lin, Y.; Qu, C.; Arndt, V.; Baurley, J.W.; Berndt, S.I.; Bien, S.A.; Bishop, D.T.; Brenner, H.; Buchanan, D.D.; et al. Genetic Risk Impacts the Association of Menopausal Hormone Therapy with Colorectal Cancer Risk. Br. J. Cancer 2024, 130, 1687–1696. [Google Scholar] [CrossRef] [PubMed]
- Nair, A.R.; Pillai, A.J.; Nair, N. Cardiovascular Changes in Menopause. Curr. Cardiol. Rev. 2021, 17, e230421187681. [Google Scholar] [CrossRef] [PubMed]
- Nappi, R.E.; Chedraui, P.; Lambrinoudaki, I.; Simoncini, T. Menopause: A Cardiometabolic Transition. Lancet Diabetes Endocrinol. 2022, 10, 442–456. [Google Scholar] [CrossRef] [PubMed]
- Averyanova, M.; Vishnyakova, P.; Yureneva, S.; Yakushevskaya, O.; Fatkhudinov, T.; Elchaninov, A.; Sukhikh, G. Sex Hormones and Immune System: Menopausal Hormone Therapy in the Context of COVID-19 Pandemic. Front. Immunol. 2022, 13, 928171. [Google Scholar] [CrossRef] [PubMed]
- Pertyńska-Marczewska, M.; Pertyński, T. Premenopausal and Postmenopausal Women during the COVID-19 Pandemic. Menopausal Rev. 2022, 21, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Ferretti, V.V.; Klersy, C.; Bruno, R.; Cutti, S.; Nappi, R.E. Men with COVID-19 Die. Women Survive. Maturitas 2022, 158, 34–36. [Google Scholar] [CrossRef]
- Greene, J.G. Constructing a Standard Climacteric Scale. Maturitas 1998, 29, 25–31. [Google Scholar] [CrossRef]
- Clinical Guidelines: Menopause and Climacteric State in Women. Available online: https://cr.minzdrav.gov.ru/recomend/117_2 (accessed on 14 August 2024).
- Eilertsen, A.L.; Sandvik, L.; Steinsvik, B.; Sandset, P.M. Differential Impact of Conventional-Dose and Low-Dose Postmenopausal Hormone Therapy, Tibolone and Raloxifene on C-Reactive Protein and Other Inflammatory Markers. J. Thromb. Haemost. 2008, 6, 928–934. [Google Scholar] [CrossRef] [PubMed]
- Wong, K.L.; Yeap, W.H.; Tai, J.J.Y.; Ong, S.M.; Dang, T.M.; Wong, S.C. The Three Human Monocyte Subsets: Implications for Health and Disease. Immunol. Res. 2012, 53, 41–57. [Google Scholar] [CrossRef] [PubMed]
- Hearps, A.C.; Martin, G.E.; Angelovich, T.A.; Cheng, W.-J.; Maisa, A.; Landay, A.L.; Jaworowski, A.; Crowe, S.M. Aging Is Associated with Chronic Innate Immune Activation and Dysregulation of Monocyte Phenotype and Function. Aging Cell 2012, 11, 867–875. [Google Scholar] [CrossRef] [PubMed]
- Luckheeram, R.V.; Zhou, R.; Verma, A.D.; Xia, B. CD4+ T Cells: Differentiation and Functions. Clin. Dev. Immunol. 2012, 2012, 925135. [Google Scholar] [CrossRef] [PubMed]
- Averyanova, M.V.; Yureneva, S.V.; Kiseleva, V.V.; Yakushevskaya, O.V.; Vishnyakova, P.A.; Iskusnykh, M.E.; Elchaninov, A.V.; Fatkhudinov, T.K. Impact of Menopausal Hormone Therapy on Immune System Parameters. Akusherstvo I Ginekol. Obstet. Gynecol. 2023, 68–77. [Google Scholar] [CrossRef]
- Han, A.; Kim, J.Y.; Kwak-Kim, J.; Lee, S.K. Menopause Is an Inflection Point of Age-Related Immune Changes in Women. J. Reprod. Immunol. 2021, 146, 103346. [Google Scholar] [CrossRef]
- Clark, R.A. Resident Memory T Cells in Human Health and Disease. Sci. Transl. Med. 2015, 7, 269rv1. [Google Scholar] [CrossRef]
- Seli, E.; Pehlivan, T.; Selam, B.; Garcia-Velasco, J.A.; Arici, A. Estradiol Down-Regulates MCP-1 Expression in Human Coronary Artery Endothelial Cells. Fertil. Steril. 2002, 77, 542–547. [Google Scholar] [CrossRef]
- Christodoulakos, G.E.; Lambrinoudaki, I.V.; Economou, E.V.; Papadias, C.; Vitoratos, N.; Panoulis, C.P.; Kouskouni, E.E.; Vlachou, S.A.; Creatsas, G.C. Circulating Chemoattractants RANTES, Negatively Related to Endogenous Androgens, and MCP-1 Are Differentially Suppressed by Hormone Therapy and Raloxifene. Atherosclerosis 2007, 193, 142–150. [Google Scholar] [CrossRef]
- Sumino, H.; Ichikawa, S.; Kasama, S.; Kumakura, H.; Takayama, Y.; Sakamaki, T.; Kurabayashi, M. Effect of Transdermal Hormone Replacement Therapy on Carotid Artery Wall Thickness and Levels of Vascular Inflammatory Markers in Postmenopausal Women. Hypertens. Res. 2005, 28, 579–584. [Google Scholar] [CrossRef]
- Yasui, T.; Saijo, A.; Uemura, H.; Matsuzaki, T.; Tsuchiya, N.; Yuzurihara, M.; Kase, Y.; Irahara, M. Effects of Oral and Transdermal Estrogen Therapies on Circulating Cytokines and Chemokines in Postmenopausal Women with Hysterectomy. Eur. J. Endocrinol. 2009, 161, 267–273. [Google Scholar] [CrossRef]
- Miller, A.P.; Feng, W.; Xing, D.; Weathington, N.M.; Blalock, J.E.; Chen, Y.-F.; Oparil, S. Estrogen Modulates Inflammatory Mediator Expression and Neutrophil Chemotaxis in Injured Arteries. Circulation 2004, 110, 1664–1669. [Google Scholar] [CrossRef]
- Averyanova, M.V.; Vishnyakova, P.A.; Vishnyakova, P.A.; Kiseleva, V.V.; Kiseleva, V.V.; Vtorushina, V.; Vtorushina, V.; Krechetova, L.V.; Krechetova, L.V.; Elchaninov, A.V.; et al. Inflammaging from the perspective of gynecological endocrinology. Starting point. Morphology 2024. [Google Scholar] [CrossRef]
- Pinke, K.H.; Calzavara, B.; Faria, P.F.; do Nascimento, M.P.P.; Venturini, J.; Lara, V.S. Proinflammatory Profile of in Vitro Monocytes in the Ageing Is Affected by Lymphocytes Presence. Immun. Ageing 2013, 10, 22. [Google Scholar] [CrossRef]
- Tani, A.; Yasui, T.; Matsui, S.; Kato, T.; Kunimi, K.; Tsuchiya, N.; Yuzurihara, M.; Kase, Y.; Irahara, M. Different Circulating Levels of Monocyte Chemoattractant Protein-1 and Interleukin-8 during the Menopausal Transition. Cytokine 2013, 62, 86–90. [Google Scholar] [CrossRef]
- Rea, I.M.; Gibson, D.S.; McGilligan, V.; McNerlan, S.E.; Alexander, H.D.; Ross, O.A. Age and Age-Related Diseases: Role of Inflammation Triggers and Cytokines. Front. Immunol. 2018, 9, 586. [Google Scholar] [CrossRef]
- Seli, E.; Kayisli, U.A.; Selam, B.; Seli, M.; Arici, A. Estradiol Suppresses Vascular Monocyte Chemotactic Protein-1 Expression during Early Atherogenesis. Am. J. Obstet. Gynecol. 2002, 187, 1544–1549. [Google Scholar] [CrossRef]
- Caulin-Glaser, T.; García-Cardeña, G.; Sarrel, P.; Sessa, W.C.; Bender, J.R. 17β-Estradiol Regulation of Human Endothelial Cell Basal Nitric Oxide Release, Independent of Cytosolic Ca2+ Mobilization. Circ. Res. 1997, 81, 885–892. [Google Scholar] [CrossRef] [PubMed]
- Souza, C.L.S.E.; Barbosa, C.D.; Coelho, H.I.L.N.; Santos Júnior, M.N.; Barbosa, E.N.; Queiroz, É.C.; Teles, M.F.; Dos Santos, D.C.; Bittencourt, R.S.; Soares, T.d.J.; et al. Effects of 17β-Estradiol on Monocyte/Macrophage Response to Staphylococcus aureus: An In Vitro Study. Front. Cell. Infect. Microbiol. 2021, 11, 701391. [Google Scholar] [CrossRef] [PubMed]
- Stanojević, S.; Kovačević-Jovanović, V.; Dimitrijević, M.; Vujić, V.; Ćuruvija, I.; Blagojević, V.; Leposavić, G. Unopposed Estrogen Supplementation/Progesterone Deficiency in Post-Reproductive Age Affects the Secretory Profile of Resident Macrophages in a Tissue-Specific Manner in the Rat. Am. J. Reprod. Immunol. 2015, 74, 445–456. [Google Scholar] [CrossRef]
- Abrahamsen, B.; Bonnevie-Nielsen, V.; Ebbesen, E.N.; Gram, J.; Beck-Nielsen, H. Cytokines and Bone Loss in a 5-Year Longitudinal Study—Hormone Replacemen Therapy Suppresses Serum Soluble Interleukin-6 Receptor and Increases Interleukin-1-Receptor Antagonist: The Danish Osteoporosis Prevention Study. J. Bone Miner. Res. 2000, 15, 1545–1554. [Google Scholar] [CrossRef] [PubMed]
- Kovats, S. Estrogen Receptors Regulate Innate Immune Cells and Signaling Pathways. Cell. Immunol. 2015, 294, 63–69. [Google Scholar] [CrossRef]
- Kopper, N.W.; Gudeman, J.; Thompson, D.J. Transdermal Hormone Therapy in Postmenopausal Women: A Review of Metabolic Effects and Drug Delivery Technologies. Drug Des. Dev. Ther. 2009, 2, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Lakoski, S.G.; Herrington, D.M. Effects of Hormone Therapy on C-Reactive Protein and IL-6 in Postmenopausal Women: A Review Article. Climacteric 2005, 8, 317–326. [Google Scholar] [CrossRef]
- Goodman, W.A.; Bedoyan, S.M.; Havran, H.L.; Richardson, B.; Cameron, M.J.; Pizarro, T.T. Impaired Estrogen Signaling Underlies Regulatory T Cell Loss-of-Function in the Chronically Inflamed Intestine. Proc. Natl. Acad. Sci. USA 2020, 117, 17166–17176. [Google Scholar] [CrossRef]
- Rodenas, M.C.; Cabas, I.; Gómez-González, N.E.; Arizcun, M.; Meseguer, J.; Mulero, V.; García-Ayala, A. Estrogens Promote the Production of Natural Neutralizing Antibodies in Fish through G Protein-Coupled Estrogen Receptor 1. Front. Immunol. 2017, 8, 736. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, Y.; Chu, S.; Zu, Y.; Fox, S.; Mauvais-Jarvis, F. Effect of Menopausal Hormone Therapy on COVID-19 Severe Outcomes in Women—A Population-Based Study of the US National COVID Cohort Collaborative (N3C) Data. Maturitas 2023, 170, 39–41. [Google Scholar] [CrossRef]

| Index | Oral MHT (n = 27) | Transdermal MHT (n = 23) | p |
|---|---|---|---|
| Age, years a | 51.4 (3.8) | 50.2 (3.0) | 0.356 |
| BMI, kg/m2 a | 23.9 (2.76) | 24.0 (2.83) | 0.766 |
| Weight, kg a | 65.4 (7.61) | 65.6 (7.13) | 0.897 |
| smoking b | 4/27 (14.8%) | 3/23 (13.0%) | 0.907 |
| Duration of the period of absence of menstruation, months c | 13.0 (6.25; 27.0) | 13.0 (6.0; 33.0) | 0.811 |
| The sum of points on the Greene’s scale c | 20.0 (14.0; 26.5) | 19.5 (12.7; 27.2) | 0.865 |
| mild CS b | 6/27(22.2%) | 6/23 (26.0%) | 0.728 |
| CS moderate b | 6/27 (22.2%) | 6/23 (26.0%) | 0.728 |
| severe CS b | 15/27 (55.6%) | 10/23 (43.5%) | 0.547 |
| Index | Oral MHT (n = 27) | Transdermal MHT (n = 23) | ||||
|---|---|---|---|---|---|---|
| Baseline | 12 Weeks Later | p | Baseline | 12 Weeks Later | p | |
| Greene scale score * | 20.0 (14.0; 26.5) | 9.0 (4.5; 12.0) | 0.001 | 19.5 (12.7; 27.2) | 9.0 (5.0; 12.0) | 0.001 |
| Psycho-emotional symptoms (questions 1–11) * | 11.0 (7.0; 14.0) | 6.0 (2.0; 8.0) | 0.002 | 9.5 (6.75; 14.0) | 4.5 (2.0; 7.75) | 0.012 |
| Physical symptoms (questions 12–18) * | 3.0 (0.5; 7.5) | 1.0 (0.0; 3.00) | <0.001 | 3.0 (1.0; 6.25) | 1.0 (0.0; 3.00) | 0.022 |
| Vasomotor symptoms (question 19–20) * | 4.0 (2.5; 4.5) | 0.0 (0.0; 1.0) | <0.001 | 4.0 (2.0; 6.0) | 0.0 (0.0; 1.0) | 0.000 |
| Sexual symptoms (question 21) * | 2.0 (1.0; 2.5) | 1.0 (0.0; 2.0) | 0.001 | 2.0 (0.0; 2.0) | 1.0 (0.0; 1.0) | 0.001 |
| Index | Oral MHT (n = 27) | Transdermal MHT (n = 23) | ||||
|---|---|---|---|---|---|---|
| Baseline | After 3 Months | p | Baseline | After 3 Months | p | |
| Classical monocytes CD14++CD16− | 85.4 (81.4; 91.5) | 82.6 (74.0; 86.3) | 0.020 | 81.3 (76.0; 84.8) | 79.2 (75.6 82.8) | 0.484 |
| Non-classical Monocytes CD14−CD16++ | 7.29 (5.81; 9.88) | 7.34 (5.84; 9.62) | 0.5 | 8.61 (7.34; 9.60) | 8.59 (7.38; 10.1) | 0.131 |
| Intermediate monocytes CD14+CD16++ | 7.55 (5.55; 13.60) | 12.8 (8.30; 16.4) | 0.023 | 5.76 (4.79; 6.63) | 4.58 (2.79; 6.50) | 0.094 |
| T-helpers CD3+CD4+ | 64.8 (57.6; 69.5) | 63.0 (56.5; 70.3) | 0.387 | 65.5 (59.2; 70.9) | 66.0 (59.9; 72.0) | 0.005 |
| Cytotoxic T lymphocytes CD3+CD8+ | 30.6 (25.9; 40.0) | 33.7 (28.5; 40.7) | 0.22 | 28.2 (25.4; 36.0) | 29.3 (23.7; 37.6) | 0.101 |
| NK cells | 5.76 (4.48; 14.5) | 8.40 (3.60; 18.9) | 0.045 | 4.83 (3.57; 7.77) | 6.10 (2.85; 10.9) | 0.58 |
| B lymphocytes | 6.90 (2.96; 15.4) | 13.3 (3.56; 19.6) | 0.045 | 4.95 (2.87; 7.67) | 6.30 (2.00; 13.7) | 0.941 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Averyanova, M.; Yureneva, S.; Kiseleva, V.; Yakushevskaya, O.; Iskusnykh, M.; Pavlova, A.; Elchaninov, A.; Fatkhudinov, T.; Mikhanoshina, N.; Ivanets, T.; et al. Effect of Menopausal Hormone Therapy on Cellular Immunity Parameters and Cytokine Profile. Biomedicines 2024, 12, 1892. https://doi.org/10.3390/biomedicines12081892
Averyanova M, Yureneva S, Kiseleva V, Yakushevskaya O, Iskusnykh M, Pavlova A, Elchaninov A, Fatkhudinov T, Mikhanoshina N, Ivanets T, et al. Effect of Menopausal Hormone Therapy on Cellular Immunity Parameters and Cytokine Profile. Biomedicines. 2024; 12(8):1892. https://doi.org/10.3390/biomedicines12081892
Chicago/Turabian StyleAveryanova, Marina, Svetlana Yureneva, Viktoriia Kiseleva, Oksana Yakushevskaya, Marina Iskusnykh, Anna Pavlova, Andrey Elchaninov, Timur Fatkhudinov, Natalia Mikhanoshina, Tatiana Ivanets, and et al. 2024. "Effect of Menopausal Hormone Therapy on Cellular Immunity Parameters and Cytokine Profile" Biomedicines 12, no. 8: 1892. https://doi.org/10.3390/biomedicines12081892







