Dendrimers—Novel Therapeutic Approaches for Alzheimer’s Disease
Abstract
:1. Introduction
2. Alzheimer’s Disease—Main Facts
3. Pathogenesis of Alzheimer’s Disease
4. Treatment of Alzheimer’s Disease
5. Main Facts about Dendrimers
6. Dendrimers in the Treatment of Alzheimer’s Disease
7. Dendrimers in Treatment of Other Diseases
8. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tiwari, S.; Atluri, V.; Kaushik, A.; Yndart, A.; Nair, M. Alzheimer’s disease: Pathogenesis, diagnostics, and therapeutics. Int. J. Nanomed. 2019, 14, 5541–5554. [Google Scholar] [CrossRef]
- Wong, W. Economic burden of Alzheimer disease and managed care considerations. Am. J. Manag. Care 2020, 26 (Suppl. S8), S177–S183. [Google Scholar] [CrossRef]
- Jia, J.; Wei, C.; Chen, S.; Li, F.; Tang, Y.; Qin, W.; Zhao, L.; Jin, H.; Xu, H.; Wang, F.; et al. The cost of Alzheimer’s disease in China and re-estimation of costs worldwide. Alzheimer’s Dement. 2018, 14, 483–491. [Google Scholar] [CrossRef]
- Masters, C.L.; Bateman, R.; Blennow, K.; Rowe, C.C.; Sperling, R.A.; Cummings, J.L. Alzheimer’s disease. Nat. Rev. Dis. Primers 2015, 1, 15056. [Google Scholar] [CrossRef]
- Jia, X.; Wang, Z.; Huang, F.; Su, C.; Du, W.; Jiang, H.; Wang, H.; Wang, J.; Wang, F.; Su, W.; et al. A comparison of the Mini-Mental State Examination (MMSE) with the Montreal Cognitive Assessment (MoCA) for mild cognitive impairment screening in Chinese middle-aged and older population: A cross-sectional study. BMC Psychiatry 2021, 21, 485. [Google Scholar] [CrossRef]
- Risacher, S.L.; Saykin, A.J. Neuroimaging in aging and neurologic diseases. Handb. Clin. Neurol. 2019, 167, 191–227. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Huang, W.; Su, L.; Xing, Y.; Jessen, F.; Sun, Y.; Shu, N.; Han, Y. Neuroimaging advances regarding subjective cognitive decline in preclinical Alzheimer’s disease. Mol. Neurodegener. 2020, 15, 55. [Google Scholar] [CrossRef]
- Prem Kumar, A.; Singh, N.; Nair, D.; Justin, A. Neuronal PET tracers for Alzheimer’s disease. Biochem. Biophys. Res. Commun. 2022, 587, 58–62. [Google Scholar] [CrossRef] [PubMed]
- Trejo-Lopez, J.A.; Yachnis, A.T.; Prokop, S. Neuropathology of Alzheimer’s Disease. Neurotherapeutics 2022, 19, 173–185. [Google Scholar] [CrossRef]
- Thal, D.R.; Rüb, U.; Orantes, M.; Braak, H. Phases of Aβ-deposition in the human brain and its relevance for the development of AD. Neurology 2002, 58, 1791–1800. [Google Scholar] [CrossRef]
- McCarron, M.O.; Nicoll, J.A.R. The high frequency of apolipoprotein E ϵ2 allele is specific for patients with cerebral amyloid angiopathy-related haemorrhage. Neurosci. Lett. 1998, 247, 45–48. [Google Scholar] [CrossRef] [PubMed]
- Sjölin, K.; Kultima, K.; Larsson, A.; Freyhult, E.; Zjukovskaja, C.; Alkass, K.; Burman, J. Distribution of five clinically important neuroglial proteins in the human brain. Mol. Brain 2022, 15, 52. [Google Scholar] [CrossRef]
- Wharton, S.B.; Minett, T.; Drew, D.; Forster, G.; Matthews, F.; Brayne, C.; Ince, P.G.; MRC Cognitive Function and Ageing Neuropathology Study Group. Epidemiological pathology of Tau in the ageing brain: Application of staging for neuropil threads (BrainNet Europe protocol) to the MRC cognitive function and ageing brain study. Acta Neuropathol. Commun. 2016, 4, 11. [Google Scholar] [CrossRef] [PubMed]
- Min, S.-W.; Cho, S.-H.; Zhou, Y.; Schroeder, S.; Haroutunian, V.; Seeley, W.W.; Huang, E.J.; Shen, Y.; Masliah, E.; Mukherjee, C.; et al. Acetylation of tau inhibits its degradation and contributes to tauopathy. Neuron 2010, 67, 953–966. [Google Scholar] [CrossRef] [PubMed]
- Grundke-Iqbal, I.; Iqbal, K.; Tung, Y.C.; Quinlan, M.; Wisniewski, H.M.; Binder, L.I. Abnormal phosphorylation of the microtubule-associated protein tau (tau) in Alzheimer cytoskeletal pathology. Proc. Natl. Acad. Sci. USA 1986, 83, 4913–4917. [Google Scholar] [CrossRef]
- Meraz-Ríos, M.A.; Lira-De León, K.I.; Campos-Peña, V.; de Anda-Hernández, M.A.; Mena-López, R. Tau oligomers and aggregation in Alzheimer’s disease. J. Neurochem. 2010, 112, 1353–1367. [Google Scholar] [CrossRef]
- Congdon, E.E.; Sigurdsson, E.M. Tau-targeting therapies for Alzheimer disease. Nat. Rev. Neurol. 2018, 14, 399–415. [Google Scholar] [CrossRef]
- Tönnies, E.; Trushina, E. Oxidative stress, synaptic dysfunction, and Alzheimer’s Disease. J. Alzheimer’s Dis. 2017, 57, 1105–1121. [Google Scholar] [CrossRef]
- Monzio Compagnoni, G.; di Fonzo, A.; Corti, S.; Comi, G.P.; Bresolin, N.; Masliah, E. The role of mitochondria in neurodegenerative diseases: The lesson from Alzheimer’s Disease and Parkinson’s Disease. Mol. Neurobiol. 2020, 57, 2959–2980. [Google Scholar] [CrossRef]
- Parker, W.D.; Filley, C.M.; Parks, J.K. Cytochrome oxidase deficiency in Alzheimer’s disease. Neurology 1990, 40, 1302. [Google Scholar] [CrossRef]
- Kish, S.J.; Bergeron, C.; Rajput, A.; Dozic, S.; Mastrogiacomo, F.; Chang, L.; Wilson, J.M.; DiStefano, L.M.; Nobrega, J.N. Brain cytochrome oxidase in Alzheimer’s Disease. J. Neurochem. 1992, 59, 776–779. [Google Scholar] [CrossRef]
- Szabados, T.; Dul, C.; Majtényi, K.; Hargitai, J.; Pénzes, Z.; Urbanics, R. A chronic Alzheimer’s model evoked by mitochondrial poison sodium azide for pharmacological investigations. Behav. Brain Res. 2004, 154, 31–40. [Google Scholar] [CrossRef]
- Höglinger, G.U.; Lannuzel, A.; Khondiker, M.E.; Michel, P.P.; Duyckaerts, C.; Féger, J.; Champy, P.; Prigent, A.; Medja, F.; Lombes, A.; et al. The mitochondrial complex I inhibitor rotenone triggers a cerebral tauopathy. J. Neurochem. 2005, 95, 930–939. [Google Scholar] [CrossRef]
- Escobar-Khondiker, M.; Höllerhage, M.; Muriel, M.-P.; Champy, P.; Bach, A.; Depienne, C.; Respondek, G.; Yamada, E.S.; Lannuzel, A.; Yagi, T.; et al. Annonacin, a natural mitochondrial complex I inhibitor, causes tau pathology in cultured neurons. J. Neurosci. 2007, 27, 7827–7837. [Google Scholar] [CrossRef]
- Pereira, C.; Santos, M.S.; Oliveira, C. Mitochondrial function impairment induced by amyloid β-peptide on PC12 cells. NeuroReport 1998, 9, 1749–1755. [Google Scholar] [CrossRef]
- Wang, X.; Su, B.; Lee, H.-G.; Li, X.; Perry, G.; Smith, M.A.; Zhu, X. Impaired balance of mitochondrial fission and fusion in Alzheimer’s Disease. J. Neurosci. 2009, 29, 9090–9103. [Google Scholar] [CrossRef] [PubMed]
- Sorrentino, V.; Romani, M.; Mouchiroud, L.; Beck, J.S.; Zhang, H.; D’Amico, D.; Moullan, N.; Potenza, F.; Schmid, A.W.; Rietsch, S.; et al. Enhancing mitochondrial proteostasis reduces amyloid-β proteotoxicity. Nature 2017, 552, 187–193. [Google Scholar] [CrossRef]
- Supnet, C.; Bezprozvanny, I. Neuronal calcium signaling, mitochondrial dysfunction, and Alzheimer’s Disease. J. Alzheimer’s Dis. 2010, 20 (Suppl. S2), S487–S498. [Google Scholar] [CrossRef] [PubMed]
- Bai, R.; Guo, J.; Ye, X.Y.; Xie, Y.; Xie, T. Oxidative stress: The core pathogenesis and mechanism of Alzheimer’s disease. Ageing Res. Rev. 2022, 77, 101619. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, S.; Puli, L.; Patil, C.R. Role of reactive oxygen species in the progression of Alzheimer’s disease. Drug Discov. Today 2021, 26, 794–803. [Google Scholar] [CrossRef]
- Dvorakova, M.; Höhler, B.; Vollerthun, R.; Fischbach, T.; Kummer, W. Macrophages: A major source of cytochrome b558 in the rat carotid body. Brain Res. 2000, 852, 349–354. [Google Scholar] [CrossRef]
- Geiszt, M.; Kapus, A.; Ligeti, E. Chronic granulomatous disease: More than the lack of superoxide? J. Leukoc. Biol. 2001, 69, 191–196. [Google Scholar] [CrossRef]
- Ginhoux, F.; Greter, M.; Leboeuf, M.; Nandi, S.; See, P.; Gokhan, S.; Mehler, M.F.; Conway, S.J.; Ng, L.G.; Stanley, E.R.; et al. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science 2010, 330, 841–845. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, K.; Kohsaka, S. Microglia: Activation and their significance in the central nervous system. J. Biochem. 2001, 130, 169–175. [Google Scholar] [CrossRef]
- Calkins, M.J.; Manczak, M.; Mao, P.; Shirendeb, U.; Reddy, P.H. Impaired mitochondrial biogenesis, defective axonal transport of mitochondria, abnormal mitochondrial dynamics and synaptic degeneration in a mouse model of Alzheimer’s disease. Hum. Mol. Genet. 2011, 20, 4515–4529. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.S.; Koh, S.H. Neuroinflammation in neurodegenerative disorders: The roles of microglia and astrocytes. Transl. Neurodegener. 2020, 9, 42. [Google Scholar] [CrossRef] [PubMed]
- Wolf, S.A.; Boddeke, H.W.G.M.; Kettenmann, H. Microglia in physiology and disease. Annu. Rev. Physiol. 2017, 79, 619–643. [Google Scholar] [CrossRef]
- Moser, V.A.; Pike, C.J. Obesity and sex interact in the regulation of Alzheimer’s disease. Neurosci. Biobehav. Rev. 2016, 67, 102–118. [Google Scholar] [CrossRef]
- Chakrabarti, M.; Haque, A.; Banik, N.L.; Nagarkatti, P.; Nagarkatti, M.; Ray, S.K. Estrogen receptor agonists for attenuation of neuroinflammation and neurodegeneration. Brain Res. Bull. 2014, 109, 22–31. [Google Scholar] [CrossRef]
- Justice, N.J. The relationship between stress and Alzheimer’s disease. Neurobiol. Stress 2018, 8, 127–133. [Google Scholar] [CrossRef]
- Bondy, S.C. Metal toxicity and neuroinflammation. Curr. Opin. Toxicol. 2021, 26, 8–13. [Google Scholar] [CrossRef]
- Nuzzo, D.; Picone, P.; Baldassano, S.; Caruana, L.; Messina, E.; Gammazza, A.; Cappello, F.; Mulè, F.; Carlo, M. Insulin resistance as common molecular denominator linking obesity to Alzheimer’s Disease. Curr. Alzheimer Res. 2015, 12, 723–735. [Google Scholar] [CrossRef] [PubMed]
- Bondi, M.W.; Edmonds, E.C.; Salmon, D.P. Alzheimer’s Disease: Past, present, and future. J. Int. Neuropsychol. Soc. 2017, 23, 818–831. [Google Scholar] [CrossRef]
- Grill, J.D.; Cummings, J.L. Current therapeutic targets for the treatment of Alzheimer’s disease. Expert Rev. Neurother. 2010, 10, 711–728. [Google Scholar] [CrossRef]
- Breijyeh, Z.; Karaman, R. Comprehensive review on Alzheimer’s Disease: Causes and treatment. Molecules 2020, 25, 5789. [Google Scholar] [CrossRef] [PubMed]
- Varadharajan, A.; Davis, A.D.; Ghosh, A.; Jagtap, T.; Xavier, A.; Menon, A.J.; Roy, D.; Gandhi, S.; Gregor, T. Guidelines for pharmacotherapy in Alzheimer’s disease—A primer on FDA-approved drugs. J. Neurosci. Rural Pract. 2023, 14, 566. [Google Scholar] [CrossRef] [PubMed]
- Kuns, B.; Rosani, A.; Varghese, D. Memantine; StatPearls Publishing: Treasure Island, FL, USA, 2023; Available online: http://europepmc.org/abstract/MED/29763201 (accessed on 2 May 2024).
- Wang, R.; Reddy, P.H. Role of glutamate and NMDA receptors in Alzheimer’s Disease. J. Alzheimer’s Dis. 2017, 57, 1041–1048. [Google Scholar] [CrossRef]
- Lee, D.; Slomkowski, M.; Hefting, N.; Chen, D.; Larsen, K.G.; Kohegyi, E.; Hobart, M.; Cummings, J.L.; Grossberg, G.T. Brexpiprazole for the treatment of agitation in Alzheimer Dementia. JAMA Neurol. 2023, 80, 1307. [Google Scholar] [CrossRef]
- van Dyck, C.H.; Swanson, C.J.; Aisen, P.; Bateman, R.J.; Chen, C.; Gee, M.; Kanekiyo, M.; Li, D.; Reyderman, L.; Cohen, S.; et al. Lecanemab in early Alzheimer’s Disease. N. Engl. J. Med. 2023, 388, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Nelson, S.E.; Lopez, O.L. Lecanemab for Alzheimer Disease. Neurology 2024, 102, e209265. [Google Scholar] [CrossRef]
- Huang, L.K.; Kuan, Y.C.; Lin, H.W.; Hu, C.J. Clinical trials of new drugs for Alzheimer disease: A 2020–2023 update. J. Biomed. Sci. 2023, 30, 83. [Google Scholar] [CrossRef]
- Kurkinen, M.T. Donanemab: Not two without a third. Adv. Clin. Exp. Med. 2023, 32, 1085–1087. [Google Scholar] [CrossRef]
- Söderberg, L.; Johannesson, M.; Nygren, P.; Laudon, H.; Eriksson, F.; Osswald, G.; Möller, C.; Lannfelt, L. Lecanemab, Aducanumab, and Gantenerumab—Binding profiles to different forms of amyloid-beta might explain efficacy and side effects in clinical trials for Alzheimer’s Disease. Neurotherapeutics 2023, 20, 195–206. [Google Scholar] [CrossRef] [PubMed]
- Crous-Bou, M.; Minguillón, C.; Gramunt, N.; Molinuevo, J.L. Alzheimer’s disease prevention: From risk factors to early intervention. Alzheimer’s Res. Ther. 2017, 9, 71. [Google Scholar] [CrossRef]
- Tomalia, D.A.; Baker, H.; Dewald, J.; Hall, M.; Kallos, G.; Martin, S.; Roeck, J.; Ryder, J.; Smith, P. Dendritic macromolecules: Synthesis of starburst dendrimers. Macromolecules 1986, 19, 2466–2468. [Google Scholar] [CrossRef]
- Dias, A.P.; da Silva Santos, S.; da Silva, J.V.; Parise-Filho, R.; Ferreira, E.I.; El Seoud, O.; Giarolla, J. Dendrimers in the context of nanomedicine. Int. J. Pharm. 2020, 573, 118814. [Google Scholar] [CrossRef]
- Arbez-Gindre, C.; Steele, B.R.; Micha-Screttas, M. Dendrimers in Alzheimer’s Disease: Recent approaches in multi-targeting strategies. Pharmaceutics 2023, 15, 898. [Google Scholar] [CrossRef]
- Villalonga-Barber, C.; Micha-Screttas, M.; Steele, B.; Georgopoulos, A.; Demetzos, C. Dendrimers as biopharmaceuticals: Synthesis and properties. Curr. Top. Med. Chem. 2008, 8, 1294–1309. [Google Scholar] [CrossRef] [PubMed]
- Bacha, K.; Chemotti, C.; Mbakidi, J.P.; Deleu, M.; Bouquillon, S. Dendrimers: Synthesis, encapsulation applications and specific interaction with the stratum corneum—A review. Macromol 2023, 3, 343–370. [Google Scholar] [CrossRef]
- Fox, L.J.; Richardson, R.M.; Briscoe, W.H. PAMAM dendrimer—Cell membrane interactions. Adv. Colloid Interface Sci. 2018, 257, 1–18. [Google Scholar] [CrossRef]
- Zhong, G.; Long, H.; Zhou, T.; Liu, Y.; Zhao, J.; Han, J.; Yang, X.; Yu, Y.; Chen, F.; Shi, S. Blood-brain barrier Permeable nanoparticles for Alzheimer’s disease treatment by selective mitophagy of microglia. Biomaterials 2022, 288, 121690. [Google Scholar] [CrossRef] [PubMed]
- Zibarov, A.; Oukhrib, A.; Aujard Catot, J.; Turrin, C.O.; Caminade, A.M. AB5 Derivatives of cyclotriphosphazene for the synthesis of dendrons and their applications. Molecules 2021, 26, 4017. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Naresh, K.; Chabre, Y.M.; Rej, R.; Saadeh, N.K.; Roy, R. “Onion peel” dendrimers: A straightforward synthetic approach towards highly diversified architectures. Polym. Chem. 2014, 5, 4321–4331. [Google Scholar] [CrossRef]
- Sandoval-Yañez, C.; Castro Rodriguez, C. Dendrimers: Amazing platforms for bioactive molecule delivery systems. Materials 2020, 13, 570. [Google Scholar] [CrossRef] [PubMed]
- Cruz, A.; Barbosa, J.; Antunes, P.; Bonifácio, V.D.B.; Pinto, S.N. A Glimpse into dendrimers integration in cancer imaging and theranostics. Int. J. Mol. Sci. 2023, 24, 5430. [Google Scholar] [CrossRef] [PubMed]
- Florendo, M.; Figacz, A.; Srinageshwar, B.; Sharma, A.; Swanson, D.; Dunbar, G.L.; Rossignol, J. Use of polyamidoamine dendrimers in brain diseases. Molecules 2018, 23, 2238. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Martínez, F.C.; Guerra, J.; Posadas, I.; Ceña, V. Barriers to non-viral vector-mediated gene delivery in the nervous system. Pharm. Res. 2011, 28, 1843–1858. [Google Scholar] [CrossRef] [PubMed]
- Gothwal, A.; Kumar, H.; Nakhate, K.T.; Ajazuddin; Dutta, A.; Borah, A.; Gupta, U. Lactoferrin coupled lower generation PAMAM dendrimers for brain targeted delivery of memantine in aluminum-chloride-induced Alzheimer’s Disease in mice. Bioconjugate Chem. 2019, 30, 2573–2583. [Google Scholar] [CrossRef] [PubMed]
- Janaszewska, A.; Lazniewska, J.; Trzepiński, P.; Marcinkowska, M.; Klajnert-Maculewicz, B. Cytotoxicity of dendrimers. Biomolecules 2019, 9, 330. [Google Scholar] [CrossRef]
- Klajnert, B.; Cortijo-Arellano, M.; Cladera, J.; Bryszewska, M. Influence of dendrimer’s structure on its activity against amyloid fibril formation. Biochem. Biophys. Res. Commun. 2006, 345, 21–28. [Google Scholar] [CrossRef]
- Aliev, G.; Ashraf, G.M.; Tarasov, V.V.; Chubarev, V.N.; Leszek, J.; Gasiorowski, K.; Makhmutova, A.; Baeesa, S.S.; Avila-Rodríguez, M.; Ustyugov, A.A.; et al. Alzheimer’s Disease—Future therapy based on dendrimers. Curr. Neuropharmacol. 2019, 17, 288–294. [Google Scholar] [CrossRef] [PubMed]
- Moreira, D.A.; Santos, S.D.; Leiro, V.; Pêgo, A.P. Dendrimers and derivatives as multifunctional nanotherapeutics for Alzheimer’s Disease. Pharmaceutics 2023, 15, 1054. [Google Scholar] [CrossRef]
- Wasiak, T.; Marcinkowska, M.; Pieszynski, I.; Zablocka, M.; Caminade, A.-M.; Majoral, J.-P.; Klajnert-Maculewicz, B. Cationic phosphorus dendrimers and therapy for Alzheimer’s disease. New J. Chem. 2015, 39, 4852–4859. [Google Scholar] [CrossRef]
- Albensi, B.C. Dysfunction of mitochondria: Implications for Alzheimer’s disease. Int. Rev. Neurobiol. 2019, 145, 13–27. [Google Scholar] [CrossRef]
- Swerdlow, R.H. Mitochondria and mitochondrial cascades in Alzheimer’s Disease. J. Alzheimer’s Dis. 2018, 62, 1403–1416. [Google Scholar] [CrossRef]
- Sharma, A.; Liaw, K.; Sharma, R.; Zhang, Z.; Kannan, S.; Kannan, R.M. Targeting mitochondrial dysfunction and oxidative stress in activated microglia using dendrimer-based therapeutics. Theranostics 2018, 8, 5529–5547. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.; Cho, Y.Y.; Shim, M.S.; Lee, J.Y.; Lee, H.S.; Kang, H.C. Mitochondria-targeted drug delivery in cancers. Biochim. Et Biophys. Acta (BBA)—Mol. Basis Dis. 2020, 1866, 165808. [Google Scholar] [CrossRef]
- Bielski, E.R.; Zhong, Q.; Brown, M.; da Rocha, S.R.P. Effect of the conjugation density of triphenylphosphonium cation on the mitochondrial targeting of poly(amidoamine) dendrimers. Mol. Pharm. 2015, 12, 3043–3053. [Google Scholar] [CrossRef]
- Igartúa, D.E.; Martinez, C.S.; Temprana, C.F.; Alonso, S.d.V.; Prieto, M.J. PAMAM dendrimers as a carbamazepine delivery system for neurodegenerative diseases: A biophysical and nanotoxicological characterization. Int. J. Pharm. 2018, 544, 191–202. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, L.; Wang, R.; Gao, Y.; Che, H.; Pan, Y.; Fu, P. Evaluating the Effectiveness of GTM-1, Rapamycin, and carbamazepine on autophagy and Alzheimer Disease. Med. Sci. Monit. 2017, 23, 801–808. [Google Scholar] [CrossRef]
- Romero, A.; Cacabelos, R.; Oset-Gasque, M.J.; Samadi, A.; Marco-Contelles, J. Novel tacrine-related drugs as potential candidates for the treatment of Alzheimer’s disease. Bioorganic Med. Chem. Lett. 2013, 23, 1916–1922. [Google Scholar] [CrossRef]
- Igartúa, D.E.; Martinez, C.S.; Alonso, S.d.V.; Prieto, M.J. Combined therapy for Alzheimer’s Disease: Tacrine and PAMAM dendrimers co-administration reduces the side effects of the drug without modifying its activity. AAPS PharmSciTech 2020, 21, 110. [Google Scholar] [CrossRef]
- Zhang, C.; Gu, Z.; Shen, L.; Liu, X.; Lin, H. In vivo evaluation and Alzheimer’s Disease treatment outcome of siRNA loaded dual targeting drug delivery system. Curr. Pharm. Biotechnol. 2019, 20, 56–62. [Google Scholar] [CrossRef]
- Alejska, M.; Kurzyńska-Kokorniak, A.; Broda, M.; Kierzek, R.; Figlerowicz, M. How RNA viruses exchange their genetic material. Acta Biochim. Pol. 2001, 48, 391–407. [Google Scholar] [CrossRef] [PubMed]
- Jafari, M.; Abolmaali, S.S.; Borandeh, S.; Najafi, H.; Zareshahrabadi, Z.; Koohi-Hosseinabadi, O.; Azarpira, N.; Zomorodian, K.; Tamaddon, A.M. Dendritic hybrid materials comprising polyhedral oligomeric silsesquioxane (POSS) and hyperbranched polyglycerol for effective antifungal drug delivery and therapy in systemic candidiasis. Nanoscale 2023, 15, 16163–16177. [Google Scholar] [CrossRef]
- Cunha, S.; Amaral, M.; Lobo, J.; Silva, A. Therapeutic strategies for Alzheimer’s and Parkinson’s Diseases by means of drug delivery systems. Curr. Med. Chem. 2016, 23, 3618–3631. [Google Scholar] [CrossRef] [PubMed]
- Ferrer-Lorente, R.; Lozano-Cruz, T.; Fernández-Carasa, I.; Miłowska, K.; de la Mata, F.J.; Bryszewska, M.; Consiglio, A.; Ortega, P.; Gómez, R.; Raya, A. Cationic carbosilane dendrimers prevent abnormal α-Synuclein accumulation in Parkinson’s Disease patient-specific dopamine neurons. Biomacromolecules 2021, 22, 4582–4591. [Google Scholar] [CrossRef] [PubMed]
- Klajnert, B.; Cortijo-Arellano, M.; Bryszewska, M.; Cladera, J. Influence of heparin and dendrimers on the aggregation of two amyloid peptides related to Alzheimer’s and prion diseases. Biochem. Biophys. Res. Commun. 2006, 339, 577–582. [Google Scholar] [CrossRef]
- Klajnert, B.; Cortijo-Arellano, M.; Cladera, J.; Majoral, J.P.; Caminade, A.M.; Bryszewska, M. Influence of phosphorus dendrimers on the aggregation of the prion peptide PrP 185–208. Biochem. Biophys. Res. Commun. 2007, 364, 20–25. [Google Scholar] [CrossRef]
- Klementieva, O.; Aso, E.; Filippini, D.; Benseny-Cases, N.; Carmona, M.; Juvés, S.; Appelhans, D.; Cladera, J.; Ferrer, I. Effect of Poly(propylene imine) Glycodendrimers on β-Amyloid aggregation in vitro and in APP/PS1 transgenic mice, as a model of brain amyloid deposition and Alzheimer’s Disease. Biomacromolecules 2013, 14, 3570–3580. [Google Scholar] [CrossRef]
- Rawding, P.A.; Bu, J.; Wang, J.; Kim, D.W.; Drelich, A.J.; Kim, Y.; Hong, S. Dendrimers for cancer immunotherapy: Avidity-based drug delivery vehicles for effective anti-tumor immune response. WIREs Nanomed. Nanobiotechnol. 2022, 14, 1752. [Google Scholar] [CrossRef]
- Hollingsworth, R.E.; Jansen, K. Turning the corner on therapeutic cancer vaccines. npj Vaccines 2019, 4, 7. [Google Scholar] [CrossRef]
- Aleanizy, F.S.; Alqahtani, F.Y.; Setó, S.; Khalil, N.; Aleshaiwi, L.; Alghamdi, M.; Alquadeib, B.; Alkahtani, H.; Aldarwesh, A.; Alqahtani, Q.H.; et al. Trastuzumab targeted neratinib loaded poly-amidoamine nendrimer nanocapsules for breast cancer therapy. Int. J. Nanomed. 2020, 15, 5433–5443. [Google Scholar] [CrossRef] [PubMed]
- An, H.; Deng, X.; Wang, F.; Xu, P.; Wang, N. Dendrimers as nanocarriers for the delivery of drugs obtained from natural products. Polymers 2023, 15, 2292. [Google Scholar] [CrossRef]
- Lazniewska, J.; Milowska, K.; Gabryelak, T. Dendrimers—Revolutionary drugs for infectious diseases. WIREs Nanomed. Nanobiotechnol. 2012, 4, 469–491. [Google Scholar] [CrossRef] [PubMed]
- Ortega, M.; Merino, A.G.; Fraile-Martínez, O.; Recio-Ruiz, J.; Pekarek, L.; Guijarro, L.G.; García-Honduvilla, N.; Álvarez-Mon, M.; Buján, J.; García-Gallego, S. Dendrimers and dendritic materials: From laboratory to medical practice in infectious diseases. Pharmaceutics 2020, 12, 874. [Google Scholar] [CrossRef]
- Caminade, A.M. Dendrimers, an emerging opportunity in personalized medicine? J. Pers. Med. 2022, 12, 1334. [Google Scholar] [CrossRef]
- Luczkowiak, J.; Muñoz, A.; Sánchez-Navarro, M.; Ribeiro-Viana, R.; Ginieis, A.; Illescas, B.M.; Martín, N.; Delgado, R.; Rojo, J. Glycosylated carbosilane dendrimers as potent inhibitors of Ebola virus infection. Bioconjug. Chem. 2013, 24, 1791–1801. [Google Scholar]
- Mintzer, M.A.; Grinstaff, M.W. Biomedical applications of dendrimers: A tutorial. Chem. Soc. Rev. 2011, 40, 173–190. [Google Scholar] [CrossRef]
- Wang, J.; Li, B.; Qiu, L.; Qiao, X.; Yang, H. Dendrimer-based drug delivery systems: History, challenges, and latest developments. J. Biol. Eng. 2022, 16, 18. [Google Scholar] [CrossRef]

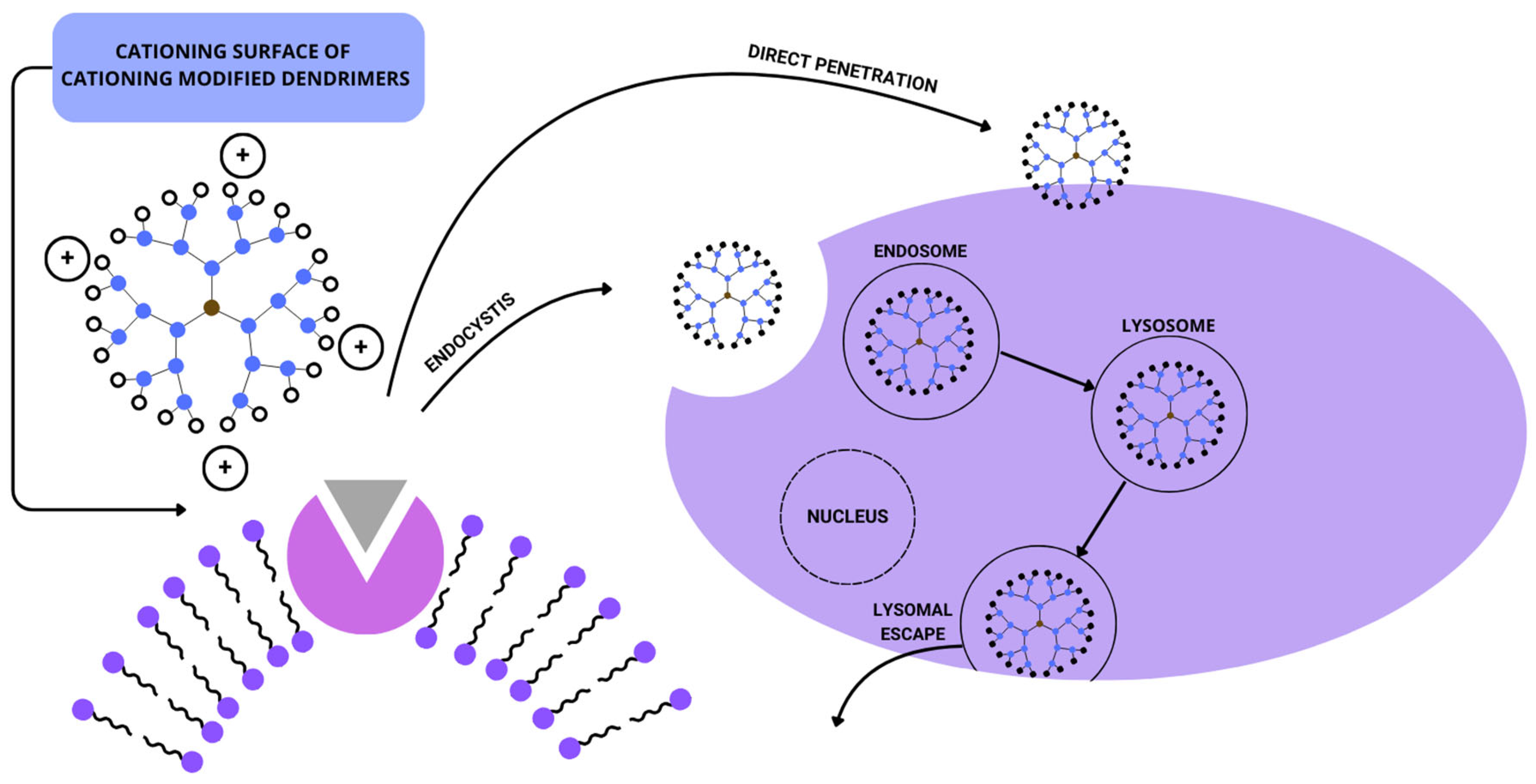
| Structure | Drug | Effects | Adverse Effects |
|---|---|---|---|
| cholinesterase enzyme inhibitor | Donepezil | Increases level of acetylcholinesterase in the synaptic gap [45] | nausea, weight loss, diarrhea, insomnia, vomiting, anorexia, asthenia [46] |
| Rivastigmine | nausea, weight loss, diarrhea, tremors, blurred vision, confusion [46] | ||
| Galantamine | nausea, weight loss, diarrhea, urinary retention, sinus bradycardia [46] | ||
| N-methyl-d-aspartate antagonist | Memantine | Slow neurotoxicity associated with neurodegenerative diseases [47] | dizziness, falls, agitation, headache, diarrhea, influenza-like symptoms [46] |
| Atypical antipsychotic drug | Brexipiprazole | Treatment of agitation associated with AD [46] | headache, nasopharyngitis, insomnia, urinary tract infection, dizziness [46] |
| Orexin receptor antagonist | Suvorexant | Treatment of sleep disturbances associated with AD [46] | somnolence, xerostomia, headache, dizziness, diarrhea, upper respiratory tract infection, dyspepsia, peripheral edema [46] |
| Monoclonal antibodies against amyloid-beta | Lecanemab | Binding amyloid-beta [52] | amyloid-related imaging abnormalities, mainly with edema [54] |
| Aducanumab | |||
| Donanemab |
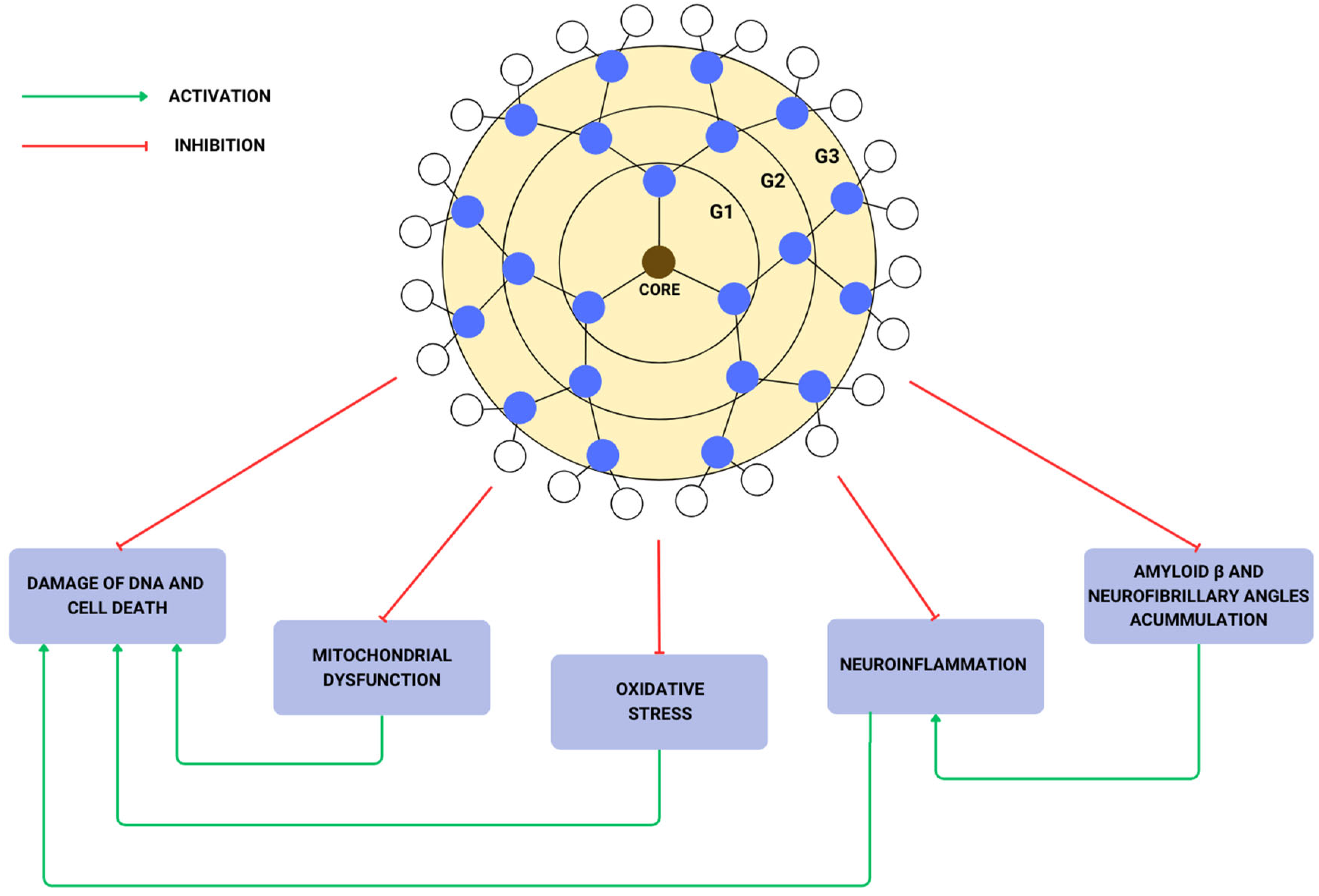
| Mechanism | |
|---|---|
| Amyloid fibrils | Dendrimers may inhibit amyloid formation by blocking their growth or breaking already existing ones [71] |
| Neurofibrillary tangles | Cationic phosphorus dendrimers induce aggregation of tau into amorphous structures instead of filamentous ones [72] |
| Mitochondrial dysfunction | Dendrimers may be potential nanocarriers and increase mitochondria targeting [58] |
| Drug-delivery system | Carbamazepine, due to its autophagy-enhancement effect, has the potential to reduce the amount of amyloid plaques in the hippocampus [81] |
| Tacrine is the most effective AChE inhibitor. Using dendrimers as DDS may reduce side effects [58] | |
| Prussian blue treats mitochondrial oxidative stress-induced damage [62] | |
| NL4-ApoA-I-siRNA suppresses BACE1, reducing Aβ generation [84] | |
| Polypropylene Imine (PPI) dendrimers can be functionalized with various bioactive molecules, such as antioxidants and metal chelators, to combat oxidative stress and metal ion dysregulation [85] | |
| Carbosilane dendrimers carry multiple therapeutic agents, including anti-inflammatory drugs and neuroprotective peptides directly to the central nervous system [86] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mroziak, M.; Kozłowski, G.; Kołodziejczyk, W.; Pszczołowska, M.; Walczak, K.; Beszłej, J.A.; Leszek, J. Dendrimers—Novel Therapeutic Approaches for Alzheimer’s Disease. Biomedicines 2024, 12, 1899. https://doi.org/10.3390/biomedicines12081899
Mroziak M, Kozłowski G, Kołodziejczyk W, Pszczołowska M, Walczak K, Beszłej JA, Leszek J. Dendrimers—Novel Therapeutic Approaches for Alzheimer’s Disease. Biomedicines. 2024; 12(8):1899. https://doi.org/10.3390/biomedicines12081899
Chicago/Turabian StyleMroziak, Magdalena, Gracjan Kozłowski, Weronika Kołodziejczyk, Magdalena Pszczołowska, Kamil Walczak, Jan Aleksander Beszłej, and Jerzy Leszek. 2024. "Dendrimers—Novel Therapeutic Approaches for Alzheimer’s Disease" Biomedicines 12, no. 8: 1899. https://doi.org/10.3390/biomedicines12081899





