Clinical Evaluation of Adrenal Incidentaloma: The Experience of a Referral Center
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mantero, F.; Terzolo, M.; Arnaldi, G.; Osella, G.; Masini, A.M.; Alı, A.; Giovagnetti, M.; Opocher, G.; Angeli, A.; Ndocrinology, E. A Survey on Adrenal Incidentaloma in Italy. J. Clin. Endocrinol. Metab. 2000, 85, 637–644. [Google Scholar] [CrossRef] [PubMed]
- Fassnacht, M.; Tsagarakis, S.; Terzolo, M.; Tabarin, A.; Sahdev, A.; Newell-Price, J.; Pelsma, I.; Marina, L.; Lorenz, K.; Bancos, I.; et al. European Society of Endocrinology Clinical Practice Guidelines on the Management of Adrenal Incidentalomas, in Collaboration with the European Network for the Study of Adrenal Tumors. Eur. J. Endocrinol. 2023, 189, 1–42. [Google Scholar] [CrossRef] [PubMed]
- Pivonello, R.; Isidori, A.M.; De Martino, M.C.; Newell-Price, J.; Biller, B.M.K.; Colao, A. Complications of Cushing’s Syndrome: State of the Art. Lancet Diabetes Endocrinol. 2016, 4, 611–629. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Vella, A. Glucose Metabolism in Cushing’s Syndrome. Curr. Opin. Endocrinol. Diabetes Obes. 2020, 27, 140–145. [Google Scholar] [CrossRef] [PubMed]
- Berr, C.M.; Stieg, M.R.; Deutschbein, T.; Quinkler, M.; Schmidmaier, R.; Osswald, A.; Reisch, N.; Ritzel, K.; Dimopoulou, C.; Fazel, J.; et al. Persistence of Myopathy in Cushing’s Syndrome: Evaluation of the German Cushing’s Registry. Eur. J. Endocrinol. 2017, 176, 737–746. [Google Scholar] [CrossRef] [PubMed]
- Delivanis, D.A.; Andrade, M.D.H.; Cortes, T.; Athimulam, S.; Khanna, A. Abnormal Body Composition in Patients with Adrenal Adenomas. Eur. J. Endocrinol. 2021, 185, 653–662. [Google Scholar] [CrossRef] [PubMed]
- Dekkers, O.M.; Horvath-Puho, E.; Jørgensen, J.O.L.; Cannegieter, S.C.; Ehrenstein, V.; Vandenbroucke, J.P.; Pereira, A.M.; Sørensen, H.T. Multisystem Morbidity and Mortality in Cushing’s Syndrome: A Cohort Study. J. Clin. Endocrinol. Metab. 2013, 98, 2277–2284. [Google Scholar] [CrossRef] [PubMed]
- Haas, A.V.; Hopkins, P.N.; Brown, N.J.; Pojoga, L.H.; Williams, J.S.; Adler, G.K.; Williams, G.H. Higher Urinary Cortisol Levels Associate with Increased Cardiovascular Risk. Endocr. Connect. 2019, 8, 634–640. [Google Scholar] [CrossRef]
- Petramala, L.; Olmati, F.; Concistrè, A.; Russo, R.; Mezzadri, M.; Soldini, M.; De Vincentis, G.; Iannucci, G.; De Toma, G.; Letizia, C. Cardiovascular and Metabolic Risk Factors in Patients with Subclinical Cushing. Endocrine 2020, 70, 150–163. [Google Scholar] [CrossRef]
- Fassnacht, M.; Arlt, W.; Bancos, I.; Dralle, H. Management of Adrenal Incidentalomas: European Society of Endocrinology Clinical Practice Guideline in Collaboration with the European Network for the Study of Adrenal Tumors. Eur. J. Endocrinol. 2016, 175, 1–34. [Google Scholar] [CrossRef] [PubMed]
- Morelli, V.; Scillitani, M.; Arosio, A.; Chiodini, I. Follow-up of Patients with Adrenal Incidentaloma, in Accordance with the European Society of Endocrinology Guidelines: Could We Be Safe? J. Endocrinol. Investig. 2016, 40, 331–333. [Google Scholar] [CrossRef]
- Nieman, L.K.; Biller, B.M.K.; Findling, J.W.; Murad, M.H.; Newell-Price, J.; Savage, M.O.; Tabarin, A. Treatment of Cushing’s Syndrome: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2015, 100, 2807–2831. [Google Scholar] [CrossRef] [PubMed]
- Deutschbein, T.; Reimondo, G.; Di Dalmazi, G.; Bancos, I.; Patrova, J.; Vassiliadi, D.A.; Nekić, A.B.; Margaritopoulos, D.; Dusek, T.; Maggio, R.; et al. Age-Dependent and Sex-Dependent Disparity in Mortality in Patients with Adrenal Incidentalomas and Autonomous Cortisol Secretion: An International, Retrospective, Cohort Study. Lancet Diabetes Endocrinol. 2022, 10, 499–508. [Google Scholar] [CrossRef]
- Prete, A.; Subramaniam, A.; Bancos, I.; Chortis, V.; Tsagarakis, S.; Lang, K. Cardiometabolic Disease Burden and Steroid Excretion in Benign Adrenal Tumors. Ann. Intern. Med. 2022, 175, 325–334. [Google Scholar] [CrossRef] [PubMed]
- Hong, A.R.; Kim, J.H.; Park, K.S.; Kim, K.Y.; Lee, J.H.; Kong, S.H. Optimal Follow-up Strategies for Adrenal Incidentalomas: Reappraisal of the 2016 ESE-ENSAT Guidelines in Real Clinical Practice. Eur. J. Endocrinol. 2017, 177, 475–483. [Google Scholar] [CrossRef]
- Papanastasiou, L.; Alexandraki, K.I.; Androulakis, I.I.; Fountoulakis, S.; Kounadi, T.; Markou, A.; Tsiavos, V.; Samara, C.; Papaioannou, T.G.; Piaditis, G.; et al. Concomitant Alterations of Metabolic Parameters, Cardiovascular Risk Factors and Altered Cortisol Secretion in Patients with Adrenal Incidentalomas during Prolonged Follow-Up. Clin. Endocrinol. 2017, 86, 488–498. [Google Scholar] [CrossRef] [PubMed]
- Araujo-Castro, M.; Cristina, M.A.; Lázaro, R.; Parra, P.; Martín, R.; Hernández, C.; Antonio, M.; Núñez, S.; Marazuela, M. Cardiometabolic pro Fi Le of Non-Functioning and Autonomous Cortisol-Secreting Adrenal Incidentalomas. Is the Cardiometabolic Risk Similar or Are There Differences? Endocrine 2019, 66, 650–659. [Google Scholar] [CrossRef] [PubMed]
- Comlekci, A.; Yener, S.; Ertilav, S. Adrenal Incidentaloma, Clinical, Metabolic, Follow-up Aspects: Single Centre Experience. Endocrine 2010, 37, 40–46. [Google Scholar] [CrossRef]
- Ceccato, F.; Tizianel, I.; Voltan, G.; Maggetto, G.; Boschin, I.M.; Quaia, E.; Scaroni, C. Attenuation Value in Adrenal Incidentalomas: A Longitudinal Study. Front. Endocrinol. 2021, 12, 794197. [Google Scholar] [CrossRef]
- Falcetta, P.; Orsolini, F.; Benelli, E.; Agretti, P.; Vitti, P.; Di, C.; Tonacchera, M. Clinical Features, Risk of Mass Enlargement, and Development of Endocrine Hyperfunction in Patients with Adrenal Incidentalomas: A Long-Term Follow-up Study. Endocrine 2020, 71, 178–188. [Google Scholar] [CrossRef]
- Bernini, G.P.; Moretti, A.; Oriandini, C.; Bardini, M.; Taurino, C.; Salvetti, A. Long-Term Morphological and Hormonal Follow-up in a Single Unit on 115 Patients with Adrenal Incidentalomas. Br. J. Cancer 2005, 92, 1104–1109. [Google Scholar] [CrossRef] [PubMed]
- Vassilatou, E.; Michalopoulou, S.; Tzavara, I. Hormonal Activity of Adrenal Incidentalomas: Results from a Long-Term Follow-up Study. Clin. Endocrinol. 2009, 70, 674–679. [Google Scholar] [CrossRef]
- Araujo-Castro, M.; Robles Lázaro, C.; Parra Ramírez, P.; García Centeno, R.; Gracia Gimeno, P.; Fernández-Ladreda, M.T.; Sampedro Núñez, M.A.; Marazuela, M.; Escobar-Morreale, H.F.; Valderrabano, P. Maximum adenoma diameter, regardless of uni- or bilaterality, is a risk factor for autonomous cortisol secretion in adrenal incidentalomas. J. Endocrinol. Investig. 2021, 44, 2349–2357. [Google Scholar] [CrossRef] [PubMed]
- Giordano, R.; Marinazzo, E.; Berardelli, R.; Picu, A.; Maccario, M.; Ghigo, E.; Arvat, E. Long-term morphological, hormonal, and clinical follow-up in a single unit on 118 patients with adrenal incidentalomas. Eur. J. Endocrinol. 2010, 162, 779–785. [Google Scholar] [CrossRef] [PubMed]
- Barzon, L.; Scaroni, C.; Sonino, N.; Fallo, F.; Paoletta, A.; Boscaro, M. Risk factors and long-term follow-up of adrenal incidentalomas. J. Clin. Endocrinol. Metab. 1999, 84, 520–526. [Google Scholar] [CrossRef]
- Goh, Z.; Phillips, I.; Hunt, P.J.; Soule, S.; Cawood, T.J. Three-year follow up of adrenal incidentalomas in a New Zealand centre. Intern. Med. J. 2020, 50, 350–356. [Google Scholar] [CrossRef]
- Anagnostis, P.; Efstathiadou, Z.; Polyzos, S.A.; Tsolakidou, K.; Litsas, I.D.; Panagiotou, A.; Kita, M. Long term follow-up of patients with adrenal incidentalomas--a single center experience and review of the literature. Exp. Clin. Endocrinol. Diabetes 2010, 118, 610–616. [Google Scholar] [CrossRef]
- Fagour, C.; Bardet, S.; Rohmer, V.; Arimone, Y.; Lecomte, P.; Valli, N.; Tabarin, A. Usefulness of adrenal scintigraphy in the follow-up of adrenocortical incidentalomas: A prospective multicenter study. Eur. J. Endocrinol. 2009, 160, 257–264. [Google Scholar] [CrossRef]
- Patrova, J.; Kjellman, M.; Wahrenberg, H.; Falhammar, H. Increased mortality in patients with adrenal incidentalomas and autonomous cortisol secretion: A 13-year retrospective study from one center. Endocrine 2017, 58, 267–275. [Google Scholar] [CrossRef]
- Aresta, C.; Favero, V.; Morelli, V.; Giovanelli, L.; Parazzoli, C.; Falchetti, A.; Pugliese, F.; Gennari, L.; Vescini, F.; Salcuni, A.; et al. Best Practice & Research Clinical Endocrinology & Metabolism Cardiovascular Complications of Mild Autonomous Cortisol Secretion. Best Pract. Res. Clin. Endocrinol. Metab. 2021, 35, 101494. [Google Scholar] [CrossRef]
- Li, D.; El Kawkgi, O.M.; Henriquez, A.F.; Bancos, I. Cardiovascular Risk and Mortality in Patients with Active and Treated Hypercortisolism. Gland. Surg. 2020, 9, 43–58. [Google Scholar] [CrossRef]
- Shibata, H.; Suzuki, H.; Maruyama, T.; Saruta, T. Gene Expression of Angiotensin II Receptor in Blood Cells of Cushing’s Syndrome. Hypertension 1995, 26, 1003–1010. [Google Scholar] [CrossRef] [PubMed]
- Kirilov, G.; Tomova, A.; Dakovska, L.; Kumanov, P.; Shinkov, A.; Alexandrov, A.S. Elevated Plasma Endothelin as an Additional Cardiovascular Risk Factor in Patients with Cushing’s Syndrome. Eur. J. Endocrinol. 2003, 149, 549–553. [Google Scholar] [CrossRef]
- Morgan, S.A.; Hassan-smith, Z.K.; Lavery, G.G.; Partners, B.H. MECHANISMS IN ENDOCRINOLOGY: Tissue-specific activation of cortisol in Cushing’s syndrome. Eur. J. Endocrinol. 2016, 175(2), 1–18. [Google Scholar] [CrossRef] [PubMed]
- Chapman, K.; Holmes, M.; Seckl, J. 11 Beta-Hydroxysteroid Dehydrogenases: Intracellular Gate-Keepers of Tissue Glucocorticoid Action. Physiol. Rev. 2013, 93, 1139–1206. [Google Scholar] [CrossRef]
- Favero, V.; Cremaschi, A.; Parazzoli, C.; Falchetti, A.; Gaudio, A.; Gennari, L.; Scillitani, A.; Vescini, F.; Morelli, V.; Aresta, C.; et al. Pathophysiology of Mild Hypercortisolism: From the Bench to the Bedside. Int. J. Mol. Sci. 2022, 23, 673. [Google Scholar] [CrossRef] [PubMed]
- Masserini, B.; Morelli, V.; Eller-Vainicher, C.; Zhukouskaya, V.; Cairoli, E.; Orsi, E.; Beck-Peccoz, P.; Spada, A.; Chiodini, I. Lipid Abnormalities in Patients with Adrenal Incidentalomas: Role of Subclinical Hypercortisolism and Impaired Glucose Metabolism. J. Endocrinol. Investig. 2015, 38, 623–628. [Google Scholar] [CrossRef]
- Di Dalmazi, G.; Vicennati, V.; Garelli, S.; Casadio, E.; Rinaldi, E.; Giampalma, E.; Mosconi, C.; Golfi, R. Cardiovascular Events and Mortality in Patients with Adrenal Incidentalomas That Are Either Non-Secreting or Associated with Intermediate Phenotype or Subclinical Cushing’s Syndrome: A 15-Year Retrospective Study. Lancet Diabetes Endocrinol. 2014, 2, 396–405. [Google Scholar] [CrossRef]
- Cassuto, H.; Kokhan, K.; Chakravarty, K.; Cohen, H.; Blum, B.; Olswang, Y.; Hakimi, P.; Xu, C.; Massillon, D.; Hanson, R.W.; et al. Glucocorticoids Regulate Transcription of the Gene for Phosphoenolpyruvate Carboxykinase in the Liver via an Extended Glucocorticoid Regulatory Unit. J. Biol. Chem. 2005, 280, 33873–33874. [Google Scholar] [CrossRef]
- Macfarlane, D.P.; Forbes, S.; Walker, B.R. Glucocorticoids and fatty acid metabolism in humans: Fuelling fat redistribution in the metabolic syndrome. J. Endocrinol. 2008, 197, 189–204. [Google Scholar] [CrossRef]
- Mazziotti, G.; Formenti, A.M.; Frara, S.; Maffezzoni, F.; Doga, M.; Giustina, A. Diabetes in Cushing Disease. Curr. Diabetes Rep. 2017, 17, 32. [Google Scholar] [CrossRef] [PubMed]
- Letizia, C.; Petramala, L.; Rosaria, C.; Di, T.; Chiappetta, C.; Zinnamosca, L.; Marinelli, C.; Iannucci, G.; Ciardi, A.; De Toma, G.; et al. Leptin and Adiponectin MRNA Expression from the Adipose Tissue Surrounding the Adrenal Neoplasia. J. Clin. Endocrinol. Metab. 2015, 100, 101–104. [Google Scholar] [CrossRef] [PubMed]
- Wallwork, C.J.; Parks, D.A.; Schimd-Schonnein, G.W. Xanthine oxidase activity in the dexamethasone-induced hypertensive rat. Microvasc. Res. 2003, 66, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Han, L.; Ma, R.; Saeed, K.; Xiong, H.; Klaassen, C.D.; Lu, Y.; Zhang, Y. Glucocorticoids Increase Renal Excretion of Urate in Mice by Downregulating Urate Transporter 1. Drug Metab. Dispos. 2019, 47, 1343–1351. [Google Scholar] [CrossRef]
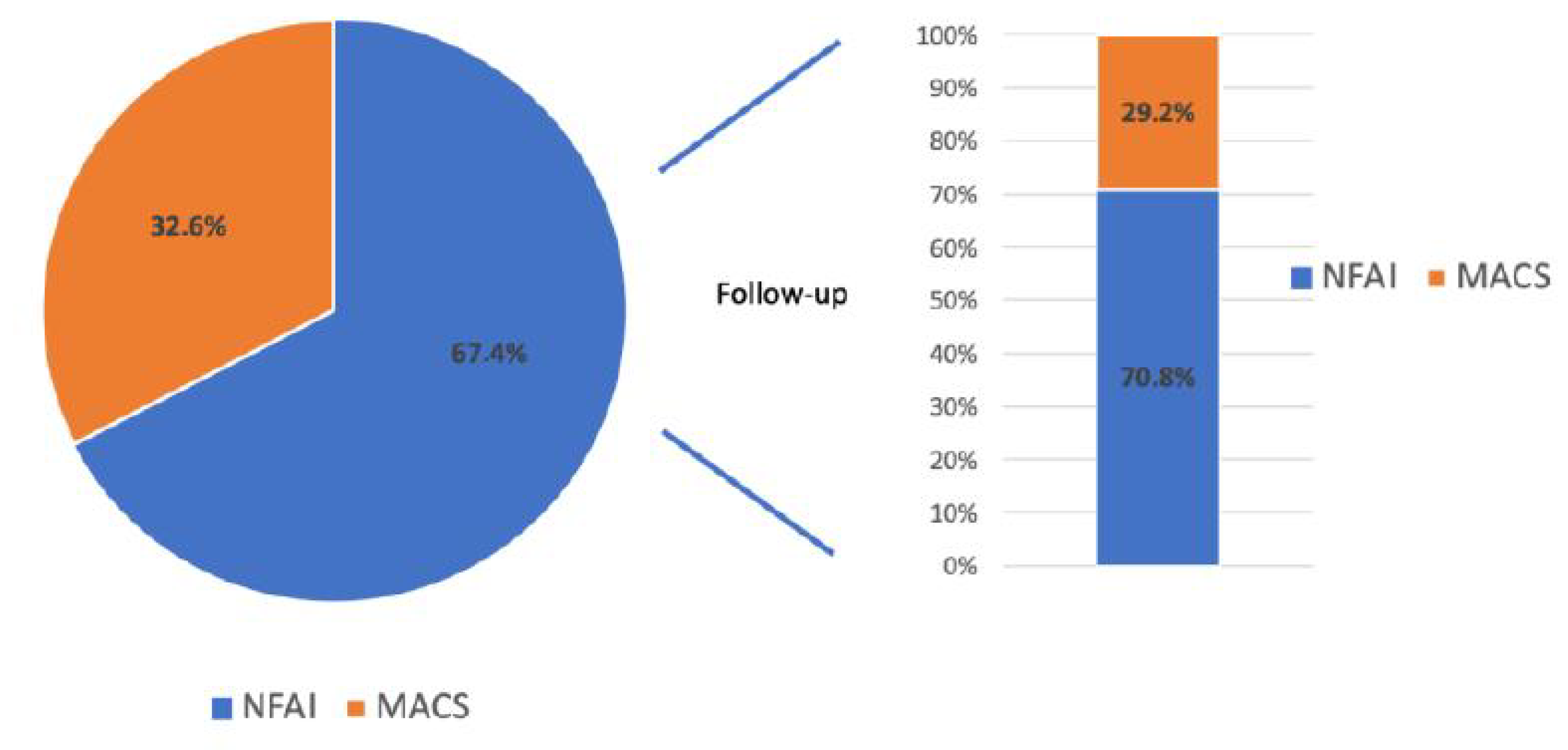
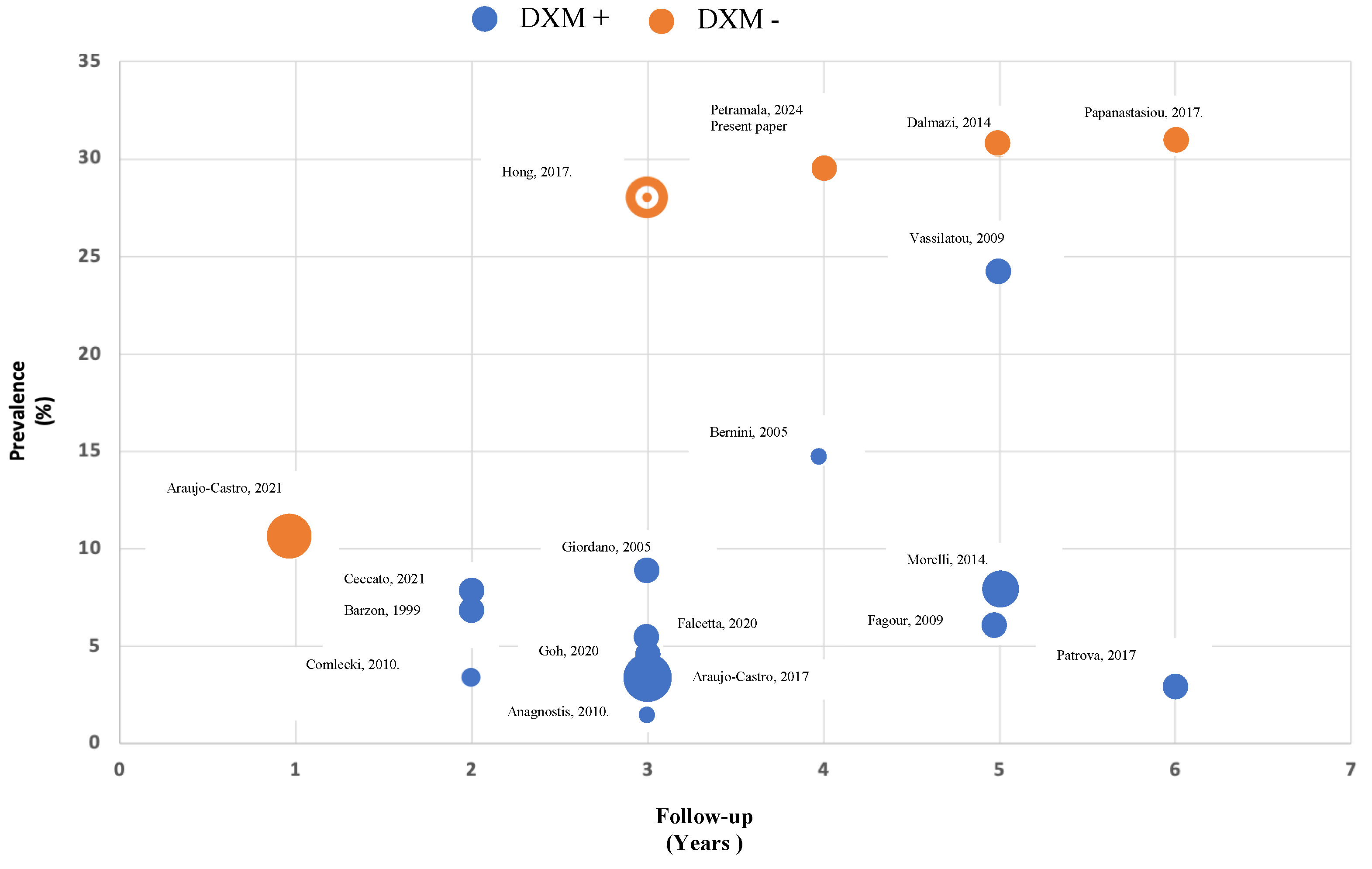
| Baseline | Age (Years) | Sex (M/F) | BMI (kg/m2) | WC (cm) | SBP (mmHg) | DBP (mmHg) | HR (bpm) |
|---|---|---|---|---|---|---|---|
| Allpatients (N = 132) | 61.7 ± 10.8 | 28/38 | 27.6 ± 3.9 | 98.8 ± 12.0 | 135.5 ± 15.3 | 82.2 ± 10.1 | 67.4 ± 9.7 |
| Negative DXM T0 (N = 89) | 61.6 ± 11.5 | 17/27 | 27.6 ± 4.4 | 98.7 ±1 3.0 | 135.8 ± 16.7 | 80.6 ± 9.9 | 68.4 ± 9.2 |
| Positive DXM T0 (N = 43) | 61.8 ± 9.4 | 11/11 | 27.7 ± 2.7 | 99.1 ± 10.3 | 134.9 ± 13.2 | 85.6 ± 8.47 | 65.5 ± 10.3 |
| p-value | ns | ns | ns | ns | ns | <0.05 | ns |
| Follow-up | Age (years) | Sex (M/F) | BMI (kg/m2) | WC (cm) | SBP (mmHg) | DBP (mmHg) | HR (bpm) |
| Negative DXM T1 (N = 63) | 64.6 ± 11.7 | 10/18 | 28.0 ± 4.7 | 100.9 ± 16.3 | 134.3 ± 14.8 | 81.6 ± 10.5 * | 65.6 ± 8.5 |
| Positive DXM T1 (N = 26) | 62.2 ± 8.62 | 7/9 | 27.9 ± 4.2 | 101.1 ± 11.7 | 134.3 ± 22.8 | 83.7 ± 9.7 * | 66.9 ± 11.7 |
| p-value | ns | ns | ns | ns | ns | <0.05 | ns |
| p-value Positive T0 DXM vs Positive T1 DXM | ns | ns | ns | ns | ns | ns | ns |
| Baseline | SBP-24 h (mmHg) | DBP-24 h (mmHg) | HR-24 h (bpm) | SBP-D (mmHg) | DBP-D (mmHg) | HR-D (bpm) | SBP-N (mmHg) | DBP-N (mmHg) | HR-N (bpm) | Dipper % |
|---|---|---|---|---|---|---|---|---|---|---|
| All patients (N = 132) | 130.3 ± 13.6 | 77.1 ± 10.0 | 73.5 ± 7.7 | 133.0 ± 14.3 | 80.0 ± 10.5 | 75.6 ± 7.7 | 121.3 ± 14.1 | 69.2 ± 9.3 | 66.4 ± 7.6 | 54 |
| Negative DXM T0 (N = 89) | 129.7 ± 15.3 | 75.9 ± 9.6 | 73.9 ± 7.9 | 132.1 ± 16.3 | 78.9 ± 10.3 | 76.5 ± 7.4 | 121.3±14.9 | 67.8 ± 8.5 | 66.6 ± 7.4 | 60 |
| Positive DXM T0 (N = 43) | 131.6 ± 10.8 | 79.7 ± 10 * | 72.9 ± 6.8 | 134.7 ± 0.6 | 81.9 ± 11.2 * | 72.4 ± 7.2 | 121.4 ± 13.0 | 72.4 ± 10.8 * | 66.1 ± 7.9 | 41 |
| p-value | ns | <0.05 | ns | ns | <0.05 | ns | ns | <0.05 | ns | ns |
| Follow-up | SBP-24 h (mmHg) | DBP-24h (mmHg) | HR-24 h (bpm) | SBP-D (mmHg) | DBP-D (mmHg) | HR-D (bpm) | SBP-N (mmHg) | DBP-N (mmHg) | HR-N (bpm) | Dipper % |
| Negative DXM T1 (N = 63) | 125.1 ± 11.7 | 71.3 ± 6.9 | 73.2 ± 7.4 | 127.5 ± 10.9 | 73.9 ± 7.3 | 74.5 ± 7.6 | 113.3 ± 26.5 | 64.5 ± 7.2 | 67.4 ± 8.6 | 50 |
| Positive DXM T1 (N = 26) | 126.8 ± 12.4 | 76.6 ± 8.8 * | 74.7 ± 8.9 | 129.6 ± 14.8 | 79.2 ± 8.9 * | 75.5 ± 9.2 | 117.8 ± 15.2 | 68.4 ± 8.9 * | 69.7 ± 11.1 | 25 |
| p-value | ns | <0.05 | ns | ns | <0.05 | ns | ns | <0.05 | ns | <0.05 |
| p-value Positive DXM T0 vs Positive DXM T1 | <0.05 | <0.05 | ns | ns | <0.05 | ns | ns | ns | ns | <0.05 |
| Baseline. | Plasma Cortisol (nmol/L) | Test DXM (nmol/L) | Plasma ACTH (pg/mL) | Urinary Free Cortisol (nmol/24 h) | Maximum Diameter Adrenal Adenoma (mm) |
|---|---|---|---|---|---|
| All patients (N = 132) | 434 ± 252 | 54.6 ± 85 | 11.1 ± 5.4 | 148 ± 102 | |
| Negative DXM T0 (N = 89) | 354 ± 224 | 20.1 ± 18 | 9.2 ± 2.3 | 134 ± 83 | 27.17 ± 7.41 |
| Positive DXM T0 (N = 43) | 519 ± 238 | 123.5 ± 23 | 12.2 ± 3.9 | 174 ± 50 | 27.48 ± 7.59 |
| p-value | <0.05 | <0.001 | ns | <0.02 | ns |
| Follow-up | Plasma cortisol (nmol/L) | Test DXM (nmol/L) | Plasma ACTH (pg/mL) | Urinary free cortisol (nmol/24 h) | Maximum diameter adrenal adenoma (mm) |
| Negative DXM T1 (N = 63) | 331 ± 172 | 24.2 ± 21 | 9.6 ± 3.4 | 125 ± 65 | 28.31 ± 8.81 |
| Positive DXM T1 (N = 26) | 407 ± 87 | 78 ± 22 | 9.9 ± 2.8 | 145 ± 76 | 28.74 ± 9.21 |
| p-value | <0.05 | <0.001 | ns | ns | ns |
| p-value Positive DXM T0 vs Positive DXM T1 | <0.05 | <0.05 | ns | ns | ns |
| Baseline | Creatinine (mg/dL) | Glycaemia (mg/dL) | LDL (mg/dL) | Triglycerides (mg/dL) | Uric acid (mg/dL) | μ-Albuminuria (24 h) |
|---|---|---|---|---|---|---|
| Allpatients (N = 132) | 0.89 ± 0.24 | 96.6 ± 19.9 | 113.5 ± 37.5 | 124.7 ± 52.9 | 5.0 ± 1.6 | 24.2 ± 15.2 |
| Negative DXM T0 (N = 89) | 0.85 ± 0.20 | 94.6 ± 19.8 | 114.1 ± 37.6 | 133.6 ± 55.8 | 4.7 ± 1.6 | 23.8 ± 15.0 |
| Positive DXM T0 (N = 43) | 0.99 ± 0.32 | 97.4 ± 18.7 | 115.4 ± 36.5 | 112.3 ± 41.4 | 5.4 ± 1.7 | 23.1 ± 10.8 |
| p-value | ns | <0.05 | ns | ns | <0.05 | ns |
| Follow-up | Creatinine (mg/dL) | Glycaemia (mg/dL) | LDL (mg/dL) | Triglycerides (mg/dL) | Uric acid (mg/dL) | μ-Albuminuria (24 h) |
| Negative DXM T1 (N = 63) | 0.92 ± 0.22 | 93.6 ± 17.7 | 98.6 ± 24.5 | 127.9 ± 59.8 | 4.8 ± 1.6 | 21.3 ± 48.7 |
| Positive DXM T1 (N = 26) | 0.84 ± 0.29 | 97.3 ± 26.4 | 94.1 ± 21.5 | 138.6 ± 100.1 | 5.2 ± 2.1 | 27.8 ± 55.6 |
| p-value | ns | <0.05 | ns | ns | <0.05 | ns |
| p-value Positive DXM T0 vs Positive DXM T1 | ns | ns | <0.03 | ns | ns | ns |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petramala, L.; Circosta, F.; Marino, L.; Palombi, E.; Costanzo, M.L.; Servello, A.; Galardo, G.; Letizia, C. Clinical Evaluation of Adrenal Incidentaloma: The Experience of a Referral Center. Biomedicines 2024, 12, 1910. https://doi.org/10.3390/biomedicines12081910
Petramala L, Circosta F, Marino L, Palombi E, Costanzo ML, Servello A, Galardo G, Letizia C. Clinical Evaluation of Adrenal Incidentaloma: The Experience of a Referral Center. Biomedicines. 2024; 12(8):1910. https://doi.org/10.3390/biomedicines12081910
Chicago/Turabian StylePetramala, Luigi, Francesco Circosta, Luca Marino, Edoardo Palombi, Maria Ludovica Costanzo, Adriana Servello, Gioacchino Galardo, and Claudio Letizia. 2024. "Clinical Evaluation of Adrenal Incidentaloma: The Experience of a Referral Center" Biomedicines 12, no. 8: 1910. https://doi.org/10.3390/biomedicines12081910
APA StylePetramala, L., Circosta, F., Marino, L., Palombi, E., Costanzo, M. L., Servello, A., Galardo, G., & Letizia, C. (2024). Clinical Evaluation of Adrenal Incidentaloma: The Experience of a Referral Center. Biomedicines, 12(8), 1910. https://doi.org/10.3390/biomedicines12081910






