Abstract
Chronic diabetic foot ulcers (DFUs) are a significant complication of diabetes mellitus, often leading to amputation, increased morbidity, and a substantial financial burden. Even with the advancements in the treatment of DFU, the risk of amputation still exists, and this occurs due to the presence of gangrene and osteomyelitis. Nonhealing in a chronic DFU is due to decreased angiogenesis, granulation tissue formation, and extracellular matrix remodeling in the presence of persistent inflammation. During wound healing, the proliferation and migration of fibroblasts, smooth muscle cells, and keratinocytes play a critical role in extracellular matrix (ECM) remodeling, angiogenesis, and epithelialization. The molecular factors regulating the migration, proliferation, and differentiation of these cells are scarcely discussed in the literature. The literature review identifies the key factors influencing the proliferation, migration, and differentiation of fibroblasts, keratinocytes, and vascular smooth muscle cells (VSMCs), which are critical in wound healing. This is followed by a discussion on the various novel factors regulating the migration, proliferation, and differentiation of these cells but not in the context of wound healing; however, they may play a role. Using a network analysis, we examined the interactions between various factors, and the findings suggest that the novel factors identified may play a significant role in promoting angiogenesis, granulation tissue formation, and extracellular matrix remodeling during wound healing or DFU healing. However, these interactions warrant further investigation to establish their role alone or synergistically.
1. Introduction
Diabetes mellitus is a rapidly growing chronic disease affecting 422 million people worldwide [1]. Diabetes is a metabolic disease characterized by hyperglycemia. Type 1 and type 2 are the two major types of diabetes mellitus. Type I diabetes mellitus (DM I) is classified by a lack of proper insulin production in the pancreas due to the destruction of insulin-producing pancreatic beta cells and it is normally first presented in adolescence, while type 2 diabetes (DM II) is characterized by decreased insulin secretion from the pancreas or the inability to use the secreted insulin, termed insulin resistance, due to prolonged hyperglycemia. Roughly 15–25% of those diagnosed with diabetes will develop diabetic foot ulcers at some point in their lifetime, resulting in between 9 and 25 million patients worldwide [2,3]. According to the Centers for Disease Control and Prevention, 60–80% of all lower limb amputations in the United States were directly as a result of diabetes [4].
Diabetic foot ulcers (DFUs) are an example of a chronic wound or a wound that does not progress through the normal stages of wound healing and becomes halted in the inflammatory phase without progressing to the resolution phase. For a wound to properly heal it must progress through four distinct wound-healing stages, which are hemostasis, inflammation, proliferation, and remodeling [5]. Immediately after the wound occurs, hemostasis begins the cessation of bleeding from a blood vessel. The two major functions of hemostasis are the clotting and release of growth factors. During hemostasis, platelet activation results in an increased secretion of cytokines, including transforming growth factor-β (TGF-β) and platelet-derived growth factor (PDGF). This occurs to promote the chemotaxis of neutrophils and macrophages initiating the inflammatory phase [6,7], the prolongation of which results in a nonhealing state of DFU [8,9]. Due to the chronicity of the inflammation contributing to the nonhealing of DFU, the advancements in wound healing methods, including wound dressings to maintain moisture and control the exudate, off-loading the ulcer of necrotic tissue, medication, and infection prevention, chronic DFUs remain an expensive and deadly issue [10]. Thus, to promote wound healing, the first step should be to attenuate the inflammation and then promote the infiltration of fibroblast, keratinocytes, and endothelial cells for ECM synthesis and remodeling, followed by angiogenesis playing a critical role in wound healing [11,12].
The role of targeting inflammation and fibroblast phenotype change [8,10,13,14], using stem cells to promote angiogenesis [14], and various factors contributing to or regulating inflammation, angiogenesis, ECM remodeling, and wound healing have been discussed [11,12,15]; however, the factors regulating the recruitment of fibroblasts, endothelial cells, vascular smooth muscle cells, and keratinocytes to the site of injury are not yet well discussed. Growth factors play a critical role in regulating the proliferation and migration of various cell types, including keratinocytes, fibroblasts, endothelial cells, and vascular smooth muscle cells [16,17,18,19,20], inflammation, and angiogenesis. The results of the studies using growth factors to promote wound healing are encouraging but still there is a need to find better therapeutics. This review aims to first comprehensively describe wound healing and the growth factors discussed in the literature, followed by describing the novel factors, which have not been discussed in the literature in regard to wound healing, regulating the proliferation and migration of epithelial cells (keratinocytes), fibroblast, endothelial cells, and vascular smooth muscle cells (VSMCs). These novel factors may be used in combination with the existing treatment to improve wound healing strategies.
2. Normal Wound Healing
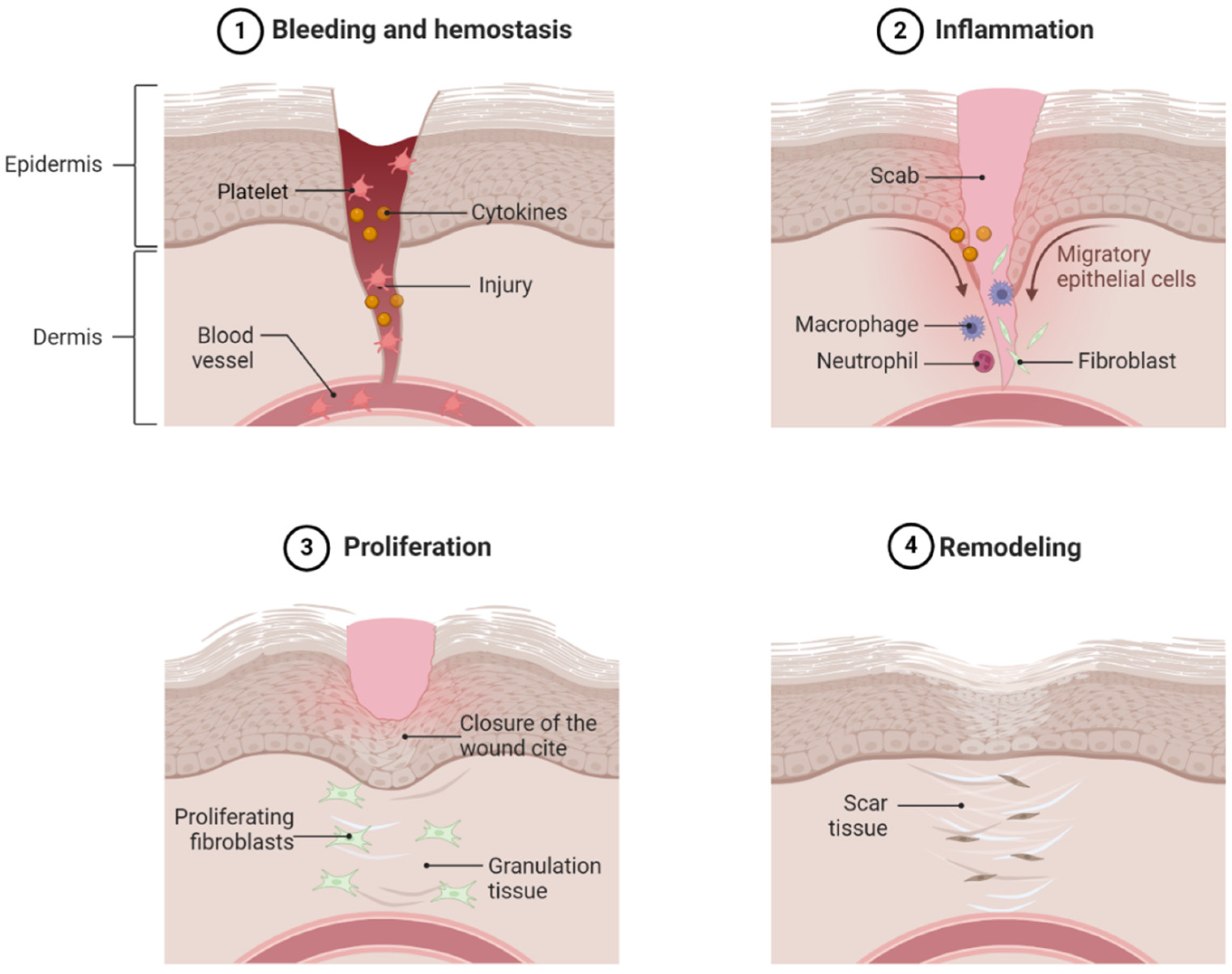
After hemostasis, the next phase of wound healing is the inflammatory phase (Figure 1). The inflammatory response is triggered by activated platelets, cytokines, and the products of hemostasis [21]. Many cells play a crucial role in the inflammatory process. In the early response, neutrophils arrive on the scene first. Their primary function is to kill various microbes to prevent infection. After the bacteria and debris have been cleaned by the neutrophils, they are removed from the wound site by apoptosis. Another key feature of the inflammation phase is the release of pro-inflammatory cytokines and chemokines at the wound site, which are crucial in the process of wound healing. Cytokines, such as interleukin (IL)-1, IL-6, and tumor necrosis factor (TNF)-α, work together to activate immune cells, regulate epithelial and fibroblast cells, and prevent infection [22].
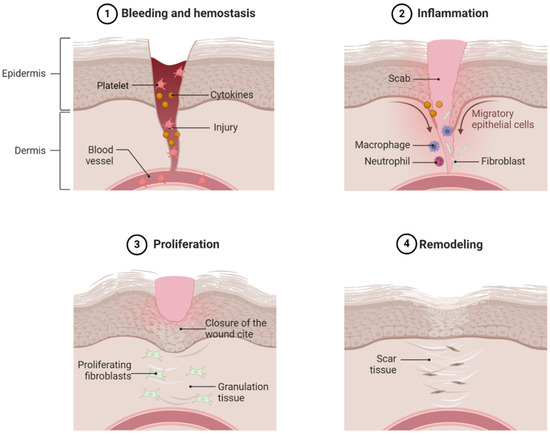
Figure 1.
Physiological wound healing. Normal dermal wound healing consists of four phases, namely hemostasis (1), inflammation (2), proliferation (3), and remodeling (4). Alteration in any event, persistent inflammation, decreased fibroblast and keratinocyte infiltration in the wound area, and decreased ECM remodeling and angiogenesis contribute to nonhealing diabetic foot ulcers. Hyperglycemia is a major underlying pathophysiology of these events.
The major focal point of the proliferative phase after the inflammation phase is the closure of the wound surface and the restoration of skin function (Figure 1). During proliferation, wound closure is achieved through keratinocyte activation, resulting in the formation of a protective epithelial barrier [23]. The preexisting keratinocytes at the wound edge begin to generate more cells to cover the wound. These keratinocytes rebuild the basal membrane through protein secretion. During the process of proliferation, the wound is replaced by granulation tissues, a complex of collagen bundles, composed of macrophages, granulocytes, fibroblasts, and blood vessels [24]. One cell that plays a key role in the formation of granulation tissue is the fibroblast. Fibroblasts, unique spindle-shaped cells, are mesenchymal-derived cells that produce collagen and the extracellular matrix (ECM). They play a diverse role in intracellular wound healing, primarily functioning to promote angiogenesis and construct and reshape the ECM [25].
The final step in wound healing is the remodeling phase (Figure 1). After the development of granulation tissues in the proliferation phase, the remodeling phase begins, where the wound matrix breakdown is relegated by fibroblasts. The goal of this is to achieve tensile strength and recover normal tissue structures. This stage is also critical for the recovery of the “normal” tissue appearance. This results from a decreased secretion of pro-inflammatory cytokines and an increased secretion of anti-inflammatory cytokines, such as IL-10 or transforming growth factor (TGF)-β1, helping in tissue remodeling. Collagen synthesis during ECM formation and remodeling is regulated by a plethora of growth factors, such as TGF-β1 and fibroblast growth factor (FGF), which have a strong effect on genic expression [24,26,27]. Another key feature of the remodeling stage is the selective reduction of angiogenesis, which is crucial in proper wound healing [28]. During wound healing, interactions from fibroblasts, keratinocytes, and endothelial cells utilize paracrine effects to regulate wound healing.
3. Role of Keratinocytes, Epithelial Cells, and Fibroblasts in Wound Healing
Three major cell types that play critical roles in wound healing are fibroblasts, keratinocytes, and VSMCs. Many studies have indicated that fibroblasts are essential in the treatment of diabetes and its complications. Studies have indicated that fibroblasts play many roles in health and diseases, beyond being just the immune-neutral cells they were originally classified as. Their key feature in wound healing is to construct and reshape the ECM. They detect injury and pathological stimulation to activate and regulate immune responses [25]. Further, the fibroblast heterogeneity and the effect of the wound microenvironment on fibroblasts also play a critical role in wound healing [12]. One study identified that during wound re-epithelialization, fibroblasts may migrate to the epicenter of the wound. Interactions between fibroblasts, keratinocytes, and endothelial cells utilize paracrine effects to regulate wound healing. One major characteristic of fibroblasts is their ability to change phenotype, altering their functionality. In the presence of a diabetic ulcer, the wound microenvironment is altered, due to the secretion of various cytokines (TGF-β, IL-6, IL-8), resulting in phenotypic changes in fibroblasts, affecting wound healing [12]. Fibroblast proliferation and migration are negatively regulated by ECM proteins [29].
Key fibroblast phenotypes are quiescent fibroblasts, myofibroblasts, and secretory fibroblasts. Quiescent fibroblasts, the largest population of dermal fibroblasts (anti-CD34+, anti-HSP47+, anti-SFA+, S100A4+), are common during the normal physiological state and the fibrosis phase. During the inflammation and resolution phases of wound healing, fibroblasts activate and are transdifferentiated to myofibroblasts (α-SMA+, fibronectin+, cadherin+, n-caldesmon−, smoothelin−, and desmin−) with the characteristics of both fibroblasts and smooth muscle cells [12]. These myofibroblasts contribute to wound healing by promoting ECM remodeling and tissue formation. During the early stages of wound healing, myofibroblasts actively migrate toward the wound bed to mediate ECM deposition and remodeling. The transitioning of fibroblasts to myofibroblasts, and then to quiescent fibroblasts, is controlled by both cellular and molecular mediators [30]. Studies show that the regulation of cytokine levels and fibroblast phenotype in the wound microenvironment may lead to a significant increase in the wound healing of diabetic foot ulcers [12]. Myofibroblasts also contribute to wound contraction through the activation of contractile signaling pathways involving non-muscle myosin II, ACTB, and ACTG1 [31].
Secretory fibroblast (CD 40+) density significantly increases in diabetic foot ulcers, which contributes to chronic inflammation [13]. The literature indicates that decreased populations of myofibroblasts and increased populations of secretory fibroblasts may lead to the nonhealing of DFUs. The phenotype shift to CD-40+ results in an increased secretion of IL-6 and IL-8. The increased expression of IL-8 has been associated with nonhealing DFUs. This correlation indicates that phenotype shifts to secretory fibroblasts play a role in nonhealing DFUs [30]. Thus, decreasing the secretory fibroblast population in a nonhealing DFU may result in amplified wound healing by attenuating the levels of pro-inflammatory cytokines and decreasing the phenotypic switch of the fibroblasts. Additionally, the role of angiogenic (FSP1+/CD31−/CD45−) and angiostatic (TSP1+) fibroblasts with their role in cardiac remodeling after infarction has been discussed in the literature but their role in the context of DFU healing is not well established [12].
Keratinocytes are the most predominant epithelial cell type [32]. They play crucial roles in normal skin function, such as the origination of the basal layer, keratin production, lipid secretion, and calcium absorption. Keratinocytes play a key role in wound healing, restoring the epithelial barrier. Wound microenvironment cues, such as cytokines, growth factors, and chemokines, give rise to the different cellular states of keratinocytes. Keratinocytes secrete cytokines and chemokines to recruit, activate, and regulate immune cells. dsRNA, coupled with the cytokines present at the wound site, activates dsRNA-sensing receptors in keratinocytes, resulting in a positive feedback loop, which leads to an immune response. Keratinocytes also express Atp-binding cassette sub family A member 12 (ABCA12) and transglutaminase 1 (TGM1), which are genes that are essential for skin repair and maintenance [33].
Keratinocyte fibroblast interactions have also been shown to play an important role in wound healing. The evidence suggests that keratinocytes promote the synthesis of growth factors by fibroblasts, which, in turn, stimulate keratinocyte proliferation in a double paracrine manner [34]. This interplay results in a positive feedback mechanism that is critical for effective wound repair. One factor that is upregulated in keratinocytes as a result of skin injury is transforming growth factor alpha (TGF-α). Predominantly expressed in keratinocytes, TGF-α exerts potent autocrine effects, significantly enhancing keratinocyte activity. High TGF-α expression has also been shown to be analogous with keratinocyte hyperproliferation [34]. Keratinocyte and fibroblast interactions have been shown to contribute to re-epithelialization. Epidermal keratinocytes have been hypothesized to alter fibroblast activity via cytokine connective tissue growth factor (CTGF). CTGF is a regulatory protein, which promotes the proliferation of mesenchymal cells, such as fibroblasts [35]. Transforming growth factor β1 (TGF-β1) stimulates the expression of CTGF, though CTGF may also act independently. Both TGF-β1 and CTGF are upregulated during wound healing and may be overexpressed in different fibrotic conditions [35].
The migration of keratinocytes is another essential component of wound healing, facilitating the restoration of the disrupted physical barrier between the wound site and the external environment. The reformation of the epidermis is achieved through the stratification of keratinocytes, which are interconnected via adherens junctions. These junctions are crucial, as they anchor the actin cytoskeletons and plasma membranes of adjacent cells [36]. The presence of diabetes results in a long-term, high-glucose environment, negatively affecting wound healing. Patients with diabetes have reduced levels of proliferation and migration of keratinocytes. This decreased proliferation and migration of keratinocytes results in insufficient re-epithelization of the wound, negatively altering the process of wound healing [25].
The most abundant cell type in vessels is VSMCs, which have a high plasticity. When a vessel injury occurs, VSMCs differentiate cell types from synthetic VSMCs to have increased migration and proliferation capabilities [37]. VSMCs can also change the phenotype, and the data indicate that the phenotype shift of VSMCs might be impacted by proinflammatory cytokines released by M1 macrophages. VSCM plasticity and phenotype shift have been shown to play a role in the regulation of atherosclerosis, plaque progression, vessel wall inflammation, and adverse remodeling [38]. Research indicates that the migration of VSMCs to the wound site may be a result of exposure to growth factors. Injury plays a key role in the growth dynamics of SMCs, increasing the rates of migration and proliferation to aid in wound healing [39].
Vascular endothelial cells and vascular lymphatics also play curtail roles in wound healing. In normal tissue, vascular endothelial cells regulate blood flow, control the permeability of the vessel wall, and maintain the fluidity of the blood. In response to the inflammatory phase of an injury, the recruitment of neutrophils activates endothelial cells. Vascular endothelial cells undergo phenotype changes, aiding in many phases of the inflammatory process [40]. There are two major types of endothelial activation: type 1 activation, which is independent of new gene expression, and type 2 activation, which is slower and depends on gene expression. Type 1 activation is controlled by ligands binding to G-protein coupled receptors, while type 2 activation is a sustained activation, mediated by tumor necrosis factor [40]. The restoration of skin function in wound healing is aided by the process of lymphangiogenesis; the formation of new lymphatic vessels [41]. Current research aims to identify the relationship between lymphangiogenesis and diabetic wound healing. Lymphangiogenesis has been shown to play a role in the inflammatory response. The loss of lymphatic vessels may induce inflammatory environments, which results in delays in wound healing [42].
4. Factors Regulating the Proliferation and Migration of Keratinocytes, Fibroblasts, and Smooth Muscle Cells
Growth factors have been shown to play a critical role in regulating various molecular aspects during wound healing, including the proliferation and migration of keratinocytes, fibroblasts, and VSMCs in normal physiological healing and diabetic foot ulcer healing (Table 1). The studies [16,17,18,19,20] performed using an in vitro model and an in vivo model with an animal model suggest the important roles of growth factors in promoting wound healing in DFU; however, only a few clinical trials have been conducted using these growth factors to show enhanced DFU healing in human patients (listed in Table 2).

Table 1.
Role of growth factors in regulating various mechanisms during wound healing.
Insulin-like growth factor 1 (IGF-1) is a peptide hormone that stimulates the proliferation of keratinocytes. It initiates cell spreading and membrane protrusion. IGF-1 stimulates phosphatidylinositol-3-kinase, which is necessary to induce cell-shape changes in keratinocytes [43]. IGF-1 levels are decreased at the wound site, indicating that its absence may play a role in delayed wound healing [44]. IGF1 promotes keratinocyte migration, promoting epithelialization and the contraction of the wound bed [45]. The beneficial role of IGF-1 is supported by increased wound healing with IGF-1 via the activation of IGF1R, promoting wound re-epithelialization, the epithelial tissue area, granulation tissue formation, and angiogenesis [46,47].
Epidermal growth factor (EGF), a polypeptide involved in epithelial maturation, initiates the mitogen-activated protein kinase (MAPK) pathway, and together with IGF-1, it can influence keratinocyte migration, which determines the wound epithelialization speed [43]. EGF significantly improves the healing rate of DFUs; however, it is important to regulate blood sugar during treatment with EGF [48,49]. EGF increases the number of fibroblasts and angiogenesis to promote wound healing [50]. EGF treatment decreases the expression of inflammatory mediators and increases the expression of the mediators involved in cell proliferation, angiogenesis, and ECM secretion [51]. The potential therapeutic role of EGF in enhancing wound healing and the decreased incidence of amputation has been documented [52]. These results suggest that EGF promoted DFU healing but most of the studies have described the topical use of EGF and more studies are warranted on the interstitial or intradermal use of EGF [53].
Fibroblast growth factor (FGF) is a polypeptide growth factor, with 23 different variants. FGF stimulates the proliferation of fibroblasts and angiogenesis, contributing to the formation of granulating tissues. FGF subtypes, such as aFGF, bFGF, and FGF 15/19, have been shown to affect the healing of wounds in diabetic conditions [54]. Further, a significant reduction in cure time and increased healing response after topical FGF application [53] support the notion that FGF has a beneficial effect on DFU healing by promoting angiogenesis, granulation tissue formation, re-epithelialization, and the detoxification of reactive oxygen species involving fibroblasts and keratinocytes and ERK2, Nrf2, Nrf3, and peroxiredoxin-6 signaling [16]. These studies suggest that FGF enhances wound healing by keratinocytes and fibroblast proliferation, contributing to angiogenesis, ECM formation and remodeling, and re-epithelialization [55,56,57]; however, an RCT concluded with low-quality evidence that EGF, platelet-rich plasma, and PDGF therapy promote wound healing, but no comment was related to FGF [58]. Thus, more clinical trials/studies are warranted on the efficacy of FGF.
Transforming growth factor (TGF)-β, a multifunctional cytokine, is expressed by most of the tissue and cell type. During acute wound healing, TGF-β is secreted by keratinocytes, suppressing the secretion of TGF-β from fibroblasts, which is required to revert the keratinocytes to their basal phenotype that is dose-dependent. TGF-β promotes keratinocyte proliferation, migration, differentiation, and the phenotypic switch, involving integrins and Smad signaling [59,60]. Further, the interaction between keratinocytes and fibroblasts is a must for skin homeostasis and wound healing [61]. Among the various isoforms of TGF-β, TGF-β3 induces regenerative characteristics in dermal fibroblasts [62], while TGF-β1 promotes proliferation and migration during wound healing [63]. TGF-β1 maintains skin homeostasis by inhibiting keratinocyte proliferation, regulating keratinocyte differentiation, and regulating the functions of keratinocytes and fibroblasts among other cells during wound healing. TGF-β1 promotes keratinocyte proliferation and migration during wound healing to promote re-epithelialization and fibroblast recruitment to the wound site [64,65]. These studies suggest that TGF-β plays a critical role in keratinocyte and fibroblast proliferation and migration during wound healing [59,64,66].
Platelet-derived growth factor-BB (PDGF-BB) is another growth factor that is one of the first released in the wound environment by activated platelets/degranulation, monocytes/macrophages, fibroblasts, and endothelial cells. PDGF-BB promotes granulation tissue formation and re-epithelialization by promoting fibroblast and keratinocyte proliferation and migration, contributing to enhanced wound healing when delivered in combination with VEGF, EGF, and IGF [67]. Additionally, PDGF also regulates mitogenic and chemotactic activity in fibroblasts and smooth muscle cells and the proliferation and differentiation of endothelial cells. This suggests that PDGF contributes to promoting wound healing by promoting ECM synthesis and angiogenesis [68]. This is supported by the findings of increased collagen deposition, angiogenesis, and enhanced wound healing using a PDGF-BB-derived hydrogel via stimulated fibroblast proliferation and migration [69,70]. The role of PDGF in promoting wound healing is further supported by the fact that in the initial phases of wound healing, PDGFRα signals the proliferation of fibroblast progenitors for fibroblast proliferation, while in the later phase, the downregulation of PDGFRα contributes to fibroblast differentiation into myofibroblasts that are necessary for ECM [71].
The studies discussed above suggest an important role of various growth factors in regulating fibroblast and keratinocyte proliferation and migration contributing to enhanced wound healing. This notion is also supported by various other studies (Table 2), as well as a meta-analysis of eight randomized clinical trials [48] concluding that EGF promotes wound healing in DFU. Another meta-analysis and systemic review including 281 studies concluded that the growth factors EGF, FGF, and GM-CSF increase the healing rate of acute skin wounds and decreased scar scores [72]. However, among all these growth factors, only PDGF has been approved by the FDA (Regranex; becaplermin gel, a topical gel for chronic wound) [73] for the treatment of chronic wounds and diabetic neuropathic ulcers. The various aspects of the failure of growth factors to be approved for wound healing have been discussed elsewhere [74].
It is important to note that only a few studies have performed a clinical trial using these growth factors promoting wound healing in DFU; however, there is indirect evidence to show the role of various growth factors in promoting wound healing in DFU. For instance, significantly increased IGF-1 levels in healed DFU after hyperbaric oxygen therapy suggest the role of IGF1 in promoting wound healing in DFU [75]. Other studies involving human patients (marked with *) have been listed in Table 2. These growth factors showed improved wound healing in DFU, but among EGF and PDGF, only PDGF received an FDA approval for DFU treatment, as mentioned above.

Table 2.
Recent evidence to support the use of growth factors in promoting wound healing.
Table 2.
Recent evidence to support the use of growth factors in promoting wound healing.
| Growth Factors | Type of Study/Model | Outcome of the Study | Mechanism/Observation |
|---|---|---|---|
| IGF1 [76] | HUVEC cells and C57BL/6 mice | Promotes angiogenesis, attenuates inflammation, and enhances wound healing | Via Ras/PI3K/IKK/NF-κB signaling pathways |
| IGF-1 with HBOT [75] | 48 patients with DFU treated with HBOT | HBOT increases IGF-1 expression and promotes wound healing | Increased IGF-1 with HBOT |
| IGF1 + Silk Fibroin [47] | Cg-Dock7m +/+ Leprdb/JNarI female mice (db/db) | Promotes wound healing in diabetic mice | Increased the epithelial tissue area and microvessel formation |
| * hEGF [77] | Randomized clinical trial of 61 human patients with DFU | 0.04% [wt/wt] hEGF is more effective in promoting wound healing and reducing healing time compared with 0.02% [wt/wt] hEGF | The effects of Actovegin plus 0.02% (wt/wt) hEGF and Actovegin plus 0.04% (wt/wt) hEGF on DFU healing was evaluated |
| * EGF-CMC [78] | Randomized clinical trial of 25 human patients with DMII and DFU | EGF-CMC is better in the colonization of strains producing less biofilm compared with CMC alone | Colonization of wounds by strains with lower biofilm formation abilities |
| * EGF-NPWT [79] | Retrospective study of 286 DFU patients | Decreases the rate of amputations in DFU | EGF alone or in combination with NPWT reduces amputation numbers |
| Human rFGF [80] | Primary human dermal fibroblasts | FGF improves fibroblast proliferation | Chemoattractant for fibroblasts |
| FGF2 [81] | C57BL6/J mice | Promotes epithelial–mesenchymal transition by downregulated E-cadherin and upregulated vimentin expression | Increased keratinocyte proliferation and migration |
| FGF2 [82] | Primary human dermal fibroblasts C57Bl/6 NRj female mice | FGF2 promotes proliferation and migration, and improves wound healing | Dermal fibroblast (DF)-derived extracellular vesicles (EVs) stabilize FGF2 |
| * PDGF [83] | 922 patients with a full-thickness diabetic neurotrophic foot ulcer | 100 μg/g PDGF significantly increases complete healing. PDGF also decreases the time to complete healing by 30%. | Enhanced wound healing with PDGF gels |
| * recombinant human PDGF [84] | 28 patients with DM II and chronic DFU | PDGF enhances DFU wound healing | rhPDGF 0.01% gel at a dose of 2.2 µg/cm2/day for 12 weeks |
| * PDGF-B (GAM501) Phase 1/2 clinical trial | 15 patients with nonhealing neuropathic diabetic foot ulcers | GAM501 was safe and well tolerated. GAM501 enhances wound healing in DFU. | Four administrations of GAM501 at 1-week intervals (locally covering the wound area) were administered to patients |
| Peptide-derived PDGF-BB [85] | L929 fibroblast cells Wistar Albino Rats | Promotes wound healing | Increased fibroblast migration. Increased granular tissue formation, re-epithelialization, angiogenesis, and collagen formation. |
| Peptide-derived from PDGF-BB [86] | Multiple cell lines BALB/c female mice | Promotes wound healing | Increased cell proliferation (fibroblasts and keratinocytes) |
| EVs with IFG and TGF-β [87] | Multiple cell lines Human patients | Promotes wound healing | Increased fibroblasts, endothelial cells, and mesenchymal stem cell proliferation. Activation of Akt and ERK. |
| VEGF-A, PDGF-BB, HB-EGF [67] | NOD mouse model | Promotes wound healing in type 1 DM | Changes the cellular milieu of the wound environment |
| VEGF-A and FGF1 [88] | B6.BKS(D)-Leprdb/J (db/db mice) | Improves wound healing | Increased neovascularization |
| mesoglycan/VEGF [89] | C57Bl6 mice | Increased deposition of granulation tissue | Induces angiogenesis. Increased fibroblasts recruitment. |
Insulin-like growth factor (IGF)1, human umbilical vein endothelial cells (HUVECs), phosphoinositide 3-kinases (PI3Ks), nuclear factor kappa B (NF-κB), epidermal growth factor-loaded carboxymethylcellulose (EGF-CMC), negative pressure wound therapy (NPWT), diabetic foot ulcer (DFU), fibroblast growth factor (FGF), platelet-derived growth factor (PDGF), transforming growth factor beta (TGF β), vascular endothelial growth factor (VEGF), hyperbaric oxygen therapy (HBOT), and heparin-binding EGF-like growth factor (HB-EGF). * indicates the growth factors investigated in human patients to promote wound healing.
Vascular smooth muscle cells (VSMCs) contribute to angiogenesis during wound healing and proliferation and the migration of VSMCs towards the wound site is important and is regulated by various factors. The phenotypic switch of VSMCs, which affects both proliferation and migration, plays a critical role during wound healing, angiogenesis, vessel remodeling, and tissue regeneration [11,12,28,90,91]. In type 2 diabetes, mitochondrial Ca2+ regulates the proliferation of VSMCs [92]. VSMC proliferation is regulated by SRSF1 (serine/arginine-rich splicing factor 1) after vascular injury [93] and the NF-κB p65/microRNA-17/RB pathway activation during inflammation [94]. MMP-2 and MMP-9 regulate VSMC migration and proliferation by degrading the matrix and non-matrix substrates [95]. The proliferation and migration of VSMCs are also regulated by various growth factors and cytokines, including angiotensin II, basic FGF, EGF, IGF-1, IL-6, IL-1β, PDGF (mainly PDGF-BB isoform), TGF-β1, VEGF secreted by ECs, and TNF-α [96,97,98,99]. TGF-β inhibits PDGF-induced VSMC proliferation via cyclin D1 downregulation, involving Akt-dependent Smad signaling after vascular injury [100], while basic FGF stimulates quiescent VSMCs and enhances proliferation and migration [101]. Antioxidant protein peroxiredoxin 1 promotes VSMC proliferation and migration involving TLR-4 [102]. These studies suggest that the proliferation and migration of VSMCs are regulated not only by growth factors but also by mediators involved in inflammation and oxidative stress, the factors playing a critical role in DFU pathogenesis.
The proliferation and migration of keratinocytes, fibroblasts, and VSMCs play a critical role in DFU healing and these are regulated by various factors. However, despite knowing these facts, there is still a need for better treatment strategies to promote healing, because of the risk of amputation due to chronic nonhealing ulcers. This suggests the need to delineate other factors, in addition to these, which regulate the proliferation and migration of keratinocytes, fibroblasts, and VSMCs, because targeting these novel factors may have therapeutic potential. We searched the literature and have listed such factors in the next section.
5. Novel Factors Regulating the Proliferation and Migration of Keratinocytes, Fibroblasts, and VSMCs
IL-17 secreted by CD4+ and CD8+ T cells and γδ T cells plays a critical role in the proliferation and migration of epidermal keratinocytes [103], fibroblast proliferation, activation, function (ECM production), senescence, and differentiation [104], and stimulates smooth muscle cell migration involving MMP-9, p38 MAPK and ERK1/2-dependent NF-κB, and AP-1 activation [105]. IL-17 promotes VEGF-dependent angiogenesis in cancer cells [106] and increases the secretion of the angiogenic factors IL-6, IL-8, and VEGF [107]. IL-17 promotes keratinocyte proliferation and enhances wound healing in the early phase of healing while contributing to persistent inflammation and delayed wound healing [108,109]. IL-17 in conjugation with IL-22 delays wound healing in infected skin [110], promotes HIF-1α in wound healing [111], and promotes epithelialization, fibroblast differentiation to myofibroblasts, and keratinocyte activation [112] during wound healing. These studies suggested that IL-17 not only regulates the proliferation and migration of fibroblasts, keratinocytes, and VSMCs but is also involved in regulating inflammation and epithelialization.
Src-kinase-associated protein 2 (Skap2), which is expressed in immune cells, regulates cell migration and motility, immune cell function, and integrin signaling [113]. Integrin signaling is involved in the inhibition of dermal fibroblast migration under hyperglycemic conditions involving KRT17 [114]. In a newly diagnosed patient with type 1 diabetes, Skap2 regulates β-cell apoptosis, controls glucose levels [115], and regulates proliferation, migration, and chemotaxis in macrophage precursor cells. Skap2 is involved in inflammatory diseases and attenuates/prevents excess inflammation via TLR4-NF-κB pathway activation [116]. Skap2 is also involved in promoting angiogenesis by phosphatase and tensin homolog (PTEN) ubiquitination and degradation induction [117]. These findings suggest that Skap2 may play a role in wound healing by regulating angiogenesis, inflammation, and immune cell infiltration.
Phosphodiesterases (PDEs) regulate cyclic nucleotides, which are essential in VSMC proliferation and migration. The reduction of PDE1, by inhibition or deficiency, reduces SMC migration and proliferation. PDE1C has been shown to play a key role in regulating growth factor receptors. PDGF receptor beta is regulated by PDE1C [118]. The inhibition of PDE5 has proven beneficial in wound healing by preventing cGMP degradation and providing high tissue levels [119]. Further, the supplementation of serum with PDE4 inhibition promotes macrophage recruitment for efficient pathogen clearance, promoting wound healing [120]. PDE4 inhibition is also associated with TNF-α and neutrophil elastase-induced fibroblast-mediated collagen gel degradation [121]. These findings suggest that PDEs may be involved in the regulation of multiple mechanisms during wound healing and thus must be investigated.
Factors involved in the proliferation of keratinocytes, fibroblasts, and VSMCs that have yet to be observed regarding diabetic foot ulcers should be utilized in future studies. The N-terminal form of the amyloid precursor protein (sAPPalpha), an epidermal growth factor, promotes the proliferation, migration, and adhesion of keratinocytes [122]. Since amyloid precursor-like protein 2 (APLP2) expression is upregulated in healing the corneal epithelium and promotes healing [123], the possibilities of sAPPalpha promoting healing in wound healing should be investigated. This becomes more important in the context of DFU healing because amyloid presence is associated with poor glycemic control and a higher body mass index [124].
Elastic microfibril interface–located protein 1 (EMELIN1)-α4/α9 integrin is associated with dermal fibroblast and keratinocyte proliferation inhibition in healthy epithelial cells [125]. EMILIN1 is involved in ECM deposition in osteoblasts involving fibulin-4 [126] and ECM remodeling is involved in DFU healing. Further, EMILIN1, a component of elastic fibers that connects it with collagen fibers, which are both important in ECM formation, also regulates cell behavior, growth factor activity, and ECM assembly [127,128], thus the role of EMELIN1 should be investigated in the context of DFU healing. Further, an association of EMILIN1 with islet regeneration [129] supports its probable role in DFU healing by controlling hyperglycemia.
Neuralized E3 ubiquitin protein ligase 1 (NEURL1) is a positive regulator of the Notch pathway. It utilizes E3 ligase activity to promote ubiquitination [130]. Notch is a cell surface receptor involved in proliferation and cell signaling. Hyperglycemic environments play a role in the activation of the notch receptor and contribute to the increased secretion of pro-inflammatory cytokines from macrophages [131]. Since NEURL1 is a positive regulator of the Notch receptor and Notch signaling may be a therapeutic target in DFU healing, further research on the role of NEURL1 in diabetic foot ulcers and as a therapeutic target should be investigated. This notion is supported by the fact that increased Dll4-Notch1 signaling impairs wound healing in DFU [132]. Further, the regulation of adult β-cell proliferation and maturity by Notch signaling makes it worth investigating the role of NEURL1–Notch signaling in promoting DFU healing.
Shisa2 expression, an endoplasmic reticulum (ER) localized protein, is attenuated by Notch signaling [133]. The overexpression of Shisa2 inhibits the proliferation of myoblasts. Shisa2 was found to be upregulated and differentially expressed in type 1 diabetes [134] and may serve as a biomarker. Shisa2 is also a biomarker of pancreatic bud development [135], the organ related to diabetes. Shisa2 is a negative regulator of Wnt signaling and is related to the inflammatory response [136], as well as to ferroptosis in airway epithelium in patients with asthma [137]. Since inflammation plays a critical role in DFU pathogenesis and Wnt signaling is involved in DFU pathogenesis, the role of Shisa2 should be investigated in the context of DFU healing.
Cerebral cavernous malformation (CCM)2, which functions in the stress-activated p38 Mitogen-activated protein kinase (MAPK) signaling cascade, is an adaptor protein called malcavernin that strengthens endothelial cell junctions and stabilizes vessels. The loss of CCM2 knockdown reduces EC migration and attenuates wound healing [138] and endocardial growth factor in wound healing [139]. A paralog of CCM2 (CCM2L) delays wound healing in mice by attenuating angiogenesis [140], while in cardiac cells, CCM2L promotes cardiovascular cell growth [139]. Endothelial cells (ECs) with silenced CCM1 and CCM2 change their phenotype to enter a senescence-associated secretory phenotype (SASP), which ECs use to invade the ECM and attract surrounding wild-type ECs and immune cells [141]. Angiogenesis is important in wound healing and CCM proteins regulate VEGF-mediated angiogenesis via the β1 integrin-Klf2-Egfl7-signaling pathway [142]. It should be noted that CCM2 and CCM3 differentially regulate the response of ECs and angiogenesis [143] in central cavernous malformation. CCM2 regulates RhoA activity to maintain vascular integrity and the knockdown of CCM2 is associated with impaired EC migration [138]. These studies suggest that CCM proteins may play a critical role in angiogenesis during wound healing by regulating EC migration, which is involved along with VSMCs in angiogenesis. Investigating the role of CCM in DFU healing is important because long-term treatment with high glucose attenuated CCM1 expression [144].
Ladybird homeobox 1 (Lbx1), a protein-coding gene, is necessary in lateral muscle migration and regulates the response to lateral migration signals in limb development [145]. Lbx1 is involved in neuronal cell fate determination [146] and myoblast cell proliferation for myogenesis [147], suggesting its probable role in wound healing because myogenesis and arteriogenesis enhance wound healing [148] and myofibroblasts can be reprogrammed to adipocytes during wound repair [149]. Thus, an increased population of myofibroblasts at wound sites, whose proliferation and migration are regulated by Lbx1, may have a role in promoting wound healing though to be invested.
Shroom3, a PDZ-domain protein, identified in kidney tissues, is required in epithelial redifferentiation and repair. Shroom3 has also been shown to play a role in proliferation and myofibroblast activity [150]. Shroom3 regulates the epithelial cell shape, involving the apical positioning of the actomyosin network [151]. Shroom3 involves cytoskeletal architecture regulation and maintenance, is expressed in VSMCs, and spatially has a similar expression to α-smooth muscle actin (α-SMA). The downregulation of Shroom3 expression is associated with VSMC hypertrophy [152]. Not directly related to the proliferation and migration of fibroblasts, keratinocytes, and VSMCs; the regulation of myofibroblast activity, epithelial cell shape, and VSMC hypertrophy suggest that Shroom3 may play a role in wound healing but warrants in-depth investigation.
Strip2, a member of the striatin-interacting phosphatase and kinase complex, promotes the proliferation and migration of VSMCs involving the P38 MAPK-AKT-MMP-2 signaling pathway [153]. The silencing of Strip2 is associated with abnormalities in vascular and heart development [154]. Strip2 has been identified as a key regulator in the differentiation of embryonic stem cells (ESCs) [140,154]. Further, stem cells are beneficial to wound healing in DFUs, by enhancing wound healing and promoting angiogenesis [28]. Further, stem cells, whose differentiation is regulated by Strip2, play a critical role in the migration and proliferation of fibroblasts towards the wound site, promoting collagen production and skin wound healing [155].
The novel factors discussed above suggest that these factors play a role in the proliferation and migration of keratinocytes, fibroblasts, and VSMCs, and angiogenesis, vascular stability, and wound healing; however, their direct role in DFU healing is uncertain. Thus, there is a need to investigate their role in DFU healing and this should be the focus of future research to determine their potential role. Further, it should be confirmed how these factors alone or in combination may contribute to DFU healing from the perspective of previous studies. To find the interaction between these genes and their association with the various mechanisms involved in DFU healing, we performed a network analysis using the STRING network and Networkanalyst.ca, with the input genes discussed above.
6. Network Analysis
The network analysis using the STRING network revealed an interaction between various proteins, including TGFB1, THBS1, MMP2, EGF, IGF1, MMP9, TNF, PDE4A, PDE5A, IL17A, CXCL8, IL8, IL10, IL1B, CD40, PTEN, TLR4, IL6, and others, suggesting their interactive role. For instance, TGF-β1 is involved in keratinocyte proliferation, migration, differentiation, and phenotypic switch [59,60], TGF-β3 induces regenerative characteristics in dermal fibroblasts [62], and TGF-β1 promotes the proliferation and migration of fibroblasts during wound healing [63]. Thrombospondin-1 (THBS1), a matrix glycoprotein, activates TGF-β1 and affects fibrosis by promoting the proliferation and migration of hypertrophic scar fibroblasts [156]. MMP-2 and -9 stimulate keratinocyte, fibroblast, and endothelial cell proliferation and migration and promote new granulation tissue and wound healing [157]. Phosphodiesterase regulates the proliferation, migration, and differentiation of various cells, including epithelial cells, and plays a role in EMT [158], which in turn plays a critical role in DFU healing. The role of CD40 in DFU healing and fibroblast phenotype changes has been reported in our previous study [13]. Further, various cytokines, including IL-1β, TNF, IL-17, IL-22, and IL-8, and pattern recognition receptors (TLRs) play a critical role in keratinocyte proliferation, inflammation regulation, and immune response [159], all playing a critical role in wound healing. The role of cytokines during wound healing is further supported by their changing levels after electromagnetic field stimulation [160], which promote wound healing after injury [161]. An interaction between these factors suggests their regulatory role in wound healing.
However, the STRING networking did not reveal the interaction of APLP2, SHROOM3, SHISA2, NEURL1, LBX1, and NRP2 with others (Figure 2A). Thus, we searched each protein in the STRING network and found associations of these proteins with factors contributing to various mechanisms involved in wound healing.

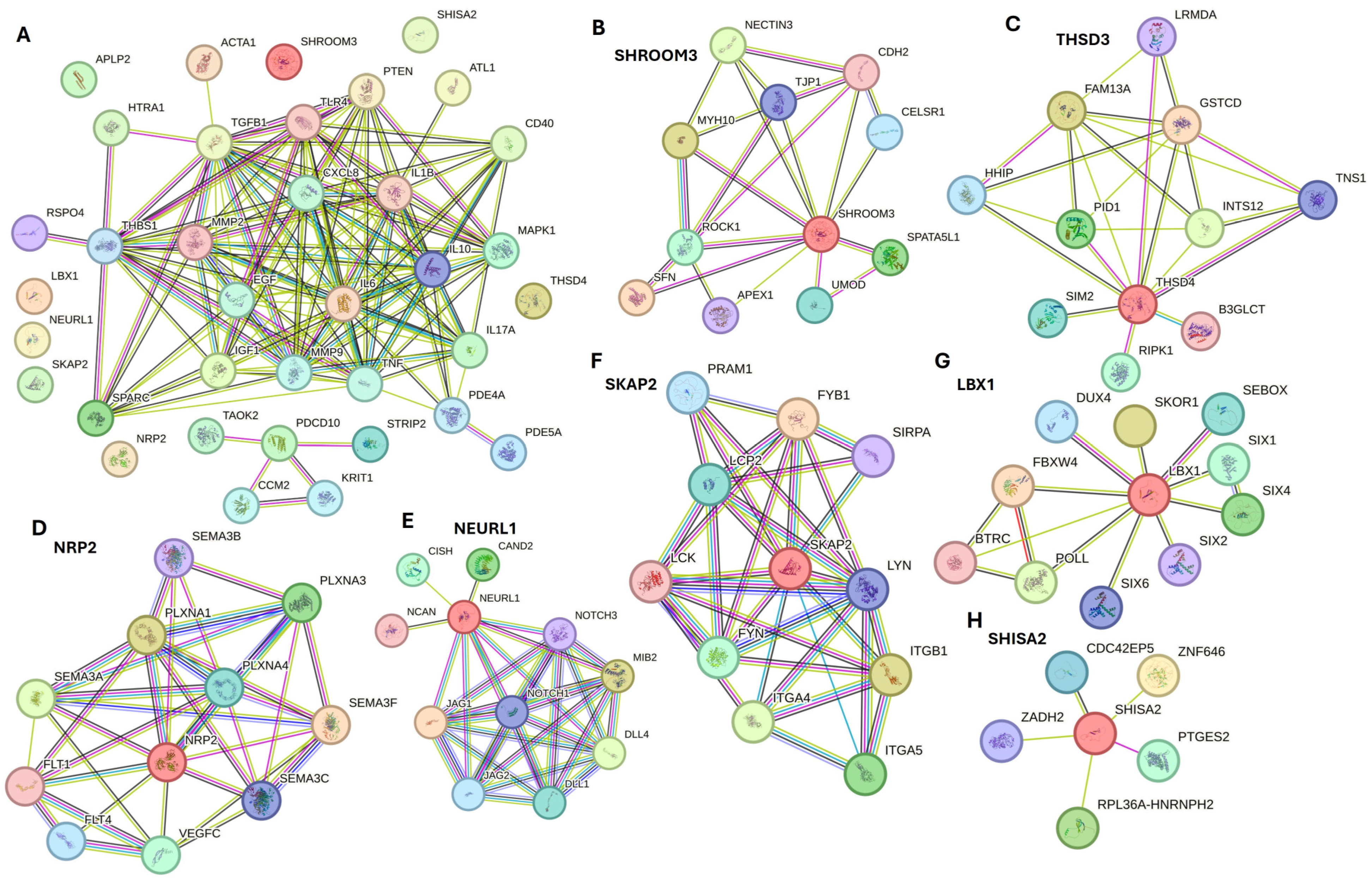
Figure 2.
Network analysis of various proteins using the Search Tool for the Retrieval of Interacting Genes/Proteins (STRING) network to predict functional associations between different proteins. (A) STRING network interaction of input gene list showing protein-protein interaction, (B) interactions for SHROOM3, (C) interactions for THSD3, (D) interactions for NRP2, (E) interactions for NEURL1, (F) interactions for SKAP2, (G) interactions for LBX1, and (H) interactions for SHISA2.
SHROOM3 (Figure 2B) showed an interaction with CDH2, which along with CDH11, plays a significant role in wound healing during the phenotypic change of fibroblasts to myofibroblasts, contributing to wound contraction and closure [162]. Thrombospondin type-1 domain-containing protein 4 (THSD4) (Figure 2C) was found to be upregulated with regeneration involving TGF-β signaling [163]. Neuropilin 2 (NRP2), a coreceptor that enhances human endothelial cell biological responses induced by VEGF-A and VEGF-C, showed an association of SEMA3F, SEMA3C, SEMA3A, SEMA3B, and VEGFC (Figure 2D), suggesting its involvement in regulating angiogenesis during DFU healing [11] and wound healing under hyperglycemic conditions [164]. Neuralized E3 Ubiquitin Protein Ligase 1 (NEURL1) (Figure 2E) showed an interaction with Notch1, Notch3, DLL1, and DLL4. NEURL1 accelerates wound healing in the corneal epithelium [165]. Notch signaling plays a critical role in cell differentiation, proliferation, and angiogenesis during DFU healing [132]. Src-kinase-associated protein 2 (SKAP2), an adaptor protein involved in integrin signaling, showed an interaction with ITGB1, ITGA5, and ITGA4 (Figure 2F), which are involved in diabetic ulcer healing [166,167]. LBX1 revealed interactions with SIX1, SIX2, SIX4, SIX6, and DUX4 (Figure 2G). The SIX family of genes/transcription factors are involved in the proliferation of muscle satellite cells [168] and regulate angiogenesis [169]. SHISA2 (Figure 2H) showed an interaction with zinc-binding alcohol dehydrogenase domain-containing 2 (ZADH2/PTGR3), which negatively regulates adipose/fat cell differentiation [170]. ZADH2 has been reported to have similar expression levels to VEGFA in gene expression studies [171], and since VEGFA is essential for angiogenesis, ZADH2 may also play a role.
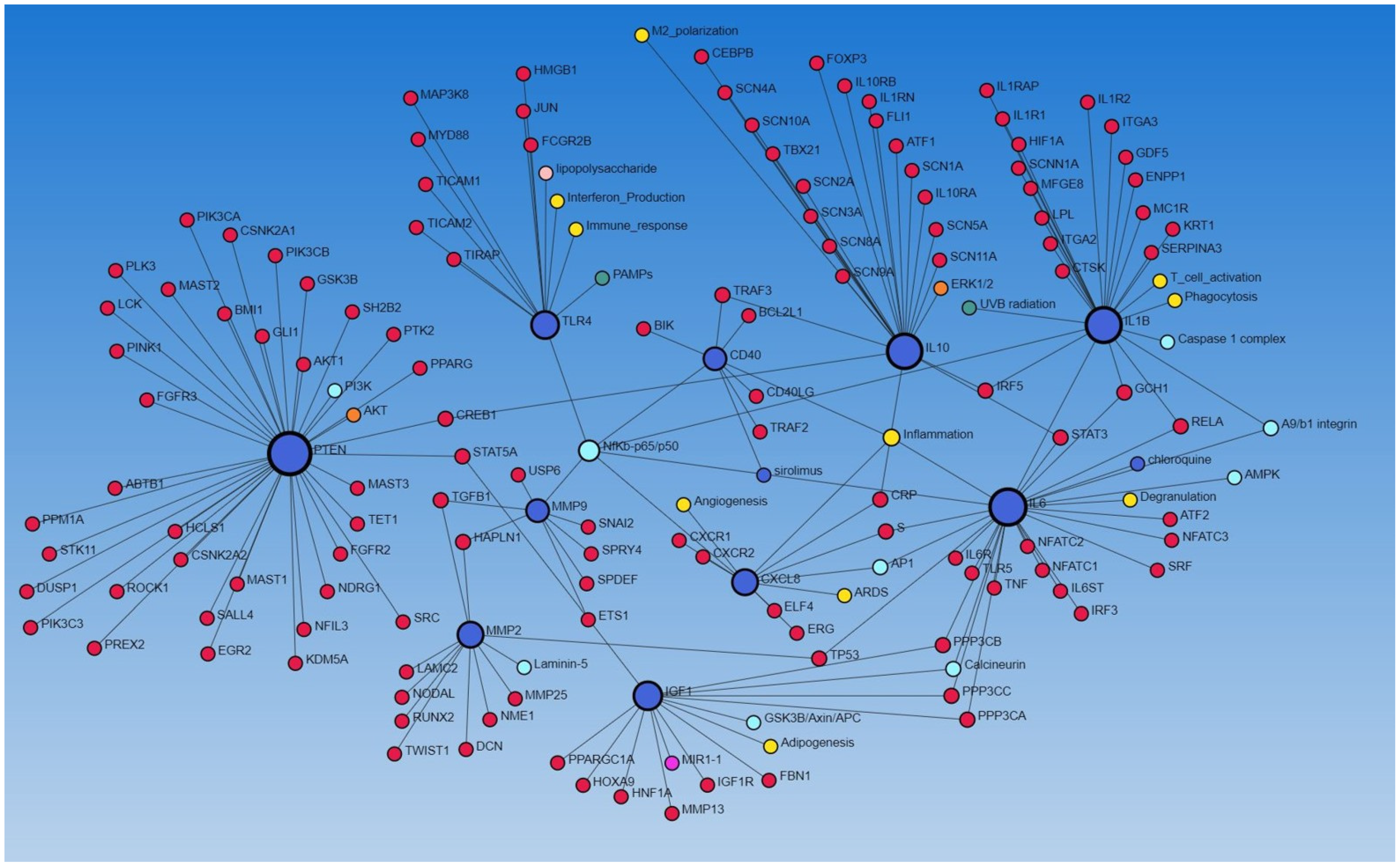
To further establish the interaction between various genes (encoding proteins), we performed a network analysis using NetworkAnalyst.ca (STRING and IMEx database). The network analysis using the STRING database revealed the interaction of IGF1, MMP2, MMP9, IL6, PTEN, TLR4, CD40, IL10, CXCL8, TP53, TGFB1, and IL1B (all involved in regulating wound healing as discussed above) with each other, as well as with other proteins, and the molecular/biological process, including angiogenesis, adipogenesis, inflammation, M2 differentiation, T-cell activation, phagocytosis, immune response, integrins, and interferon production (Figure 3), suggesting their role in diabetic ulcer healing. We have previously reported the increased expression of the CD40+ fibroblast population in DFU contributing to inflammation [13].
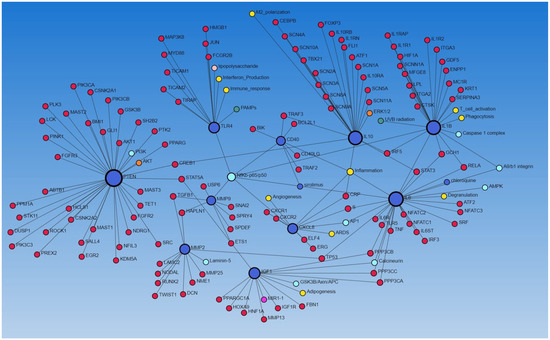
Figure 3.
Network analysis between different proteins using the STRING database via NetworkAnalyst.ca.
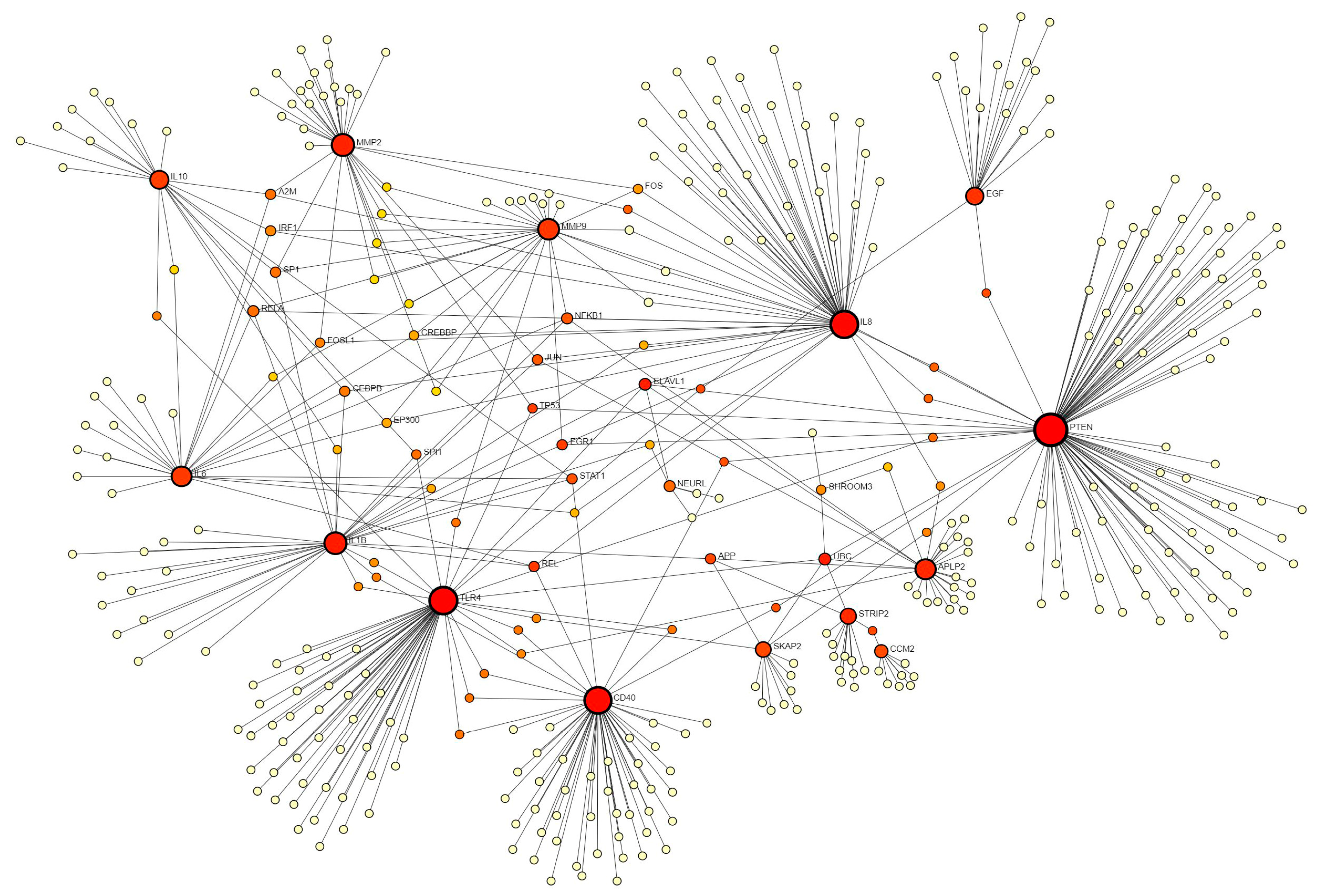
Using the STRING database did not reveal the interaction between various proteins (encoded by the genes discussed above) and hence we used the IMEx database. The analysis revealed an interaction between PTEN, EGF, IL8, MMP2, MMP9, APLP2, STRIP2, CCM2, IL6, IL1B, SPI1, STATs, IRF1, SP1, RELA, JUN, FOS, NFKB1, CEBPB, EP300, IL10, CD40, and TLR4 (Figure 4), playing a critical role in wound healing. The regulatory role of EGF, IL-8, MMP-2, MMP-9, IL1B, IL-10, CD40, and the TLRs has been discussed above. PTEN (phosphatase and tensin homologue) dephosphorylates PIP3 (phosphatidylinositol(3,4,5)-trisphosphate) and negatively regulates PI3Ks (phosphoinositide-3 kinases). PTEN inhibition promotes epithelial wound healing by significantly increasing the level of phosphorylated Akt (protein kinase B) [172] and keratinocyte proliferation [173]. Keratinocyte proliferation, adhesion, and migration are impaired by APP/APLP2 deficiency [122]. CCM2 gene codes for the protein malcavernin, which strengthens the interactions between the cells involved in blood vessel formation, thus its decreased expression may lead to leaky vessels or impaired angiogenesis [174]. STRIP2 interactions with other factors, including CCM2, UBC, SHROOM3, and APC, suggest its probable role during wound healing; however, the role of STRIP2 in wound healing in the existing literature is not defined.

Figure 4.
Network analysis using the IMEx database in Netwrokanalyst.ca.
The interaction between and with other proteins (Figure 4) and with some of the proteins that appeared in other networks (Figure 2 and Figure 3) suggests that the novel factors discussed above may play a role in the proliferation and migration of epithelial cells, fibroblasts, and VSMCs, along with other molecular mechanisms involved in the healing of diabetic foot ulcers. However, these interactions and their role alone or in combination in promoting DFU healing warrant investigations.
7. Translational Significance of Novel Factors and Factors in the Network Analysis
The description of the novel factors listed in Section 5 and Section 7 and their interactions with each other and the other factors involved in wound healing suggest their probable role in the pathogenesis of DFU and their suitability as therapeutic targets. However, it is important to relate these factors to diabetes to emphasize their role in DFU. For instance, IL-17 induces the expression of pro-inflammatory cytokines and chemokines, deteriorates β cell function, and induces insulin resistance in diabetes [175]. Skap2 regulates β-cell apoptosis and controls glucose levels [115]. PDE4 dysregulation plays a critical role in metabolic syndrome and PDE4 inhibitors attenuate diabetes symptoms and improve insulin resistance and hyperglycemia [176]. PDE5 inhibitors may act as insulin sensitizers [177]. Amyloid presence is associated with poor glycemic control and a higher body mass index [124]. APLP2 regulates glucose and insulin levels. Neuritin promotes neurite outgrowth and synapse maturation during neural development and regeneration and inhibits NEURL1, a regulator of Notch signaling [178]. Since diabetic neuropathy plays a critical role in DFU pathogenesis, the roles of NEURL1 and neuritin are worth investigating. Shisa2 was found to be upregulated and differentially expressed in type 1 diabetes [134] and may serve as a biomarker. Diabetes-induced hypermethylation inhibits Shroom3 and suppress neural tube closure [179]. Shroom3 also contributes to the maintenance of the glomerular filtration barrier integrity [180], which is altered in diabetes. An interaction of these factors (involved in diabetes pathogenesis) with PTEN, EGF, IL8, MMP2, MMP9, APLP2, STRIP2, CCM2, IL6, IL1B, SPI1, STATs, IRF1, SP1, RELA, JUN, FOS, NFKB1, CEBPB, EP300, IL10, CD40, and TLR4 plays a critical role in DFU pathogenesis, as discussed above, and the network analysis suggests that these factors may be therapeutic targets in DFU treatment. This notion is supported by the fact that targeting inflammation [181], ECM remodeling [182], and MMP-9 [183] to promote DFU healing have been suggested in the literature. Further, the network analysis showed an interaction between IL-8 and APLA2 and an increased expression of CXCL8 in nonhealing DFU, and IL-8 as a suitable target to promote healing in DFU has been proposed [8,184].
8. Conclusions
The proliferation, migration, and differentiation of fibroblasts, keratinocytes, and smooth muscle cells play a critical role in normal wound healing, as well as in the healing of diabetic foot ulcers (DFUs). However, in the diabetic wound environment, the normal progression of healing is disrupted due to persistent inflammation. The factors governing the proliferation and migration of these cells significantly influence the stages of wound healing and are crucial in understanding the mechanisms of DFU nonhealing. The novel factors discussed in this review highlight promising avenues identified through a network analysis, suggesting their potential role in promoting wound healing alone or in combination. However, to establish their role in wound healing, further research and clinical trials are warranted, mainly in the context of a hyperglycemic environment to establish better therapeutics for chronic nonhealing DFUs.
Author Contributions
Conceptualization, V.R.; research article curation, J.S.; writing—original draft preparation, J.S.; writing—review and editing, V.R.; visualization, V.R.; final approval, J.S. and V.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Dagogo-Jack, S. Diabetes Mellitus in Developing Countries and Underserved Communities; Springer: Cham, Switzerland, 2017. [Google Scholar]
- Goyal, R.; Singhal, M.; Jialal, I. Type 2 diabetes. In StatPearls [Internet]; StatPearls: Tampa/St. Petersburg, FL, USA, 2023. [Google Scholar]
- Martin, J.K.; Davis, B.L. Diabetic Foot Considerations Related to Plantar Pressures and Shear. Foot Ankle Clin. 2023, 28, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Singh, G.; Chawla, S. Amputation in Diabetic Patients. Med. J. Arm. Forces India 2006, 62, 36–39. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.a.; DiPietro, L.A. Factors affecting wound healing. J. Dent. Res. 2010, 89, 219–229. [Google Scholar] [CrossRef] [PubMed]
- LaPelusa, A.; Dave, H.D. Physiology, Hemostasis. In StatPearls [Internet]; StatPearls: Tampa/St. Petersburg, FL, USA, 2019. [Google Scholar]
- Almadani, Y.H.; Vorstenbosch, J.; Davison, P.G.; Murphy, A.M. Wound Healing: A Comprehensive Review. Semin. Plast. Surg. 2021, 35, 141–144. [Google Scholar] [CrossRef]
- Rai, V.; Moellmer, R.; Agrawal, D.K. The role of CXCL8 in chronic nonhealing diabetic foot ulcers and phenotypic changes in fibroblasts: A molecular perspective. Mol. Biol. Rep. 2022, 49, 1565–1572. [Google Scholar] [CrossRef]
- McCarty, S.M.; Percival, S.L. Proteases and Delayed Wound Healing. Adv. Wound Care 2013, 2, 438–447. [Google Scholar] [CrossRef]
- Shofler, D.; Rai, V.; Mansager, S.; Cramer, K.; Agrawal, D.K. Impact of resolvin mediators in the immunopathology of diabetes and wound healing. Expert. Rev. Clin. Immunol. 2021, 17, 681–690. [Google Scholar] [CrossRef]
- Rai, V.; Le, H.; Agrawal, D.K. Novel mediators regulating angiogenesis in diabetic foot ulcer healing. Can. J. Physiol. Pharmacol. 2023, 101, 488–501. [Google Scholar] [CrossRef]
- Rai, V.; Moellmer, R.; Agrawal, D.K. Role of fibroblast plasticity and heterogeneity in modulating angiogenesis and healing in the diabetic foot ulcer. Mol. Biol. Rep. 2023, 50, 1913–1929. [Google Scholar] [CrossRef]
- Littig, J.P.B.; Moellmer, R.; Estes, A.M.; Agrawal, D.K.; Rai, V. Increased Population of CD40+ Fibroblasts Is Associated with Impaired Wound Healing and Chronic Inflammation in Diabetic Foot Ulcers. J. Clin. Med. 2022, 11, 6335. [Google Scholar] [CrossRef]
- Littig, J.P.B.; Moellmer, R.; Agrawal, D.K.; Rai, V. Future applications of exosomes delivering resolvins and cytokines in facilitating diabetic foot ulcer healing. World J. Diabetes 2023, 14, 35–47. [Google Scholar] [CrossRef] [PubMed]
- Rai, V.; Agrawal, D.K. Role of Transcription Factors and MicroRNAs in Regulating Fibroblast Reprogramming in Wound Healing. J. Bioinform. Syst. Biol. 2023, 6, 110–120. [Google Scholar] [CrossRef]
- Zheng, S.Y.; Wan, X.X.; Kambey, P.A.; Luo, Y.; Hu, X.M.; Liu, Y.F.; Shan, J.Q.; Chen, Y.W.; Xiong, K. Therapeutic role of growth factors in treating diabetic wound. World J. Diabetes 2023, 14, 364–395. [Google Scholar] [CrossRef]
- Zarei, F.; Soleimaninejad, M. Role of growth factors and biomaterials in wound healing. Artif. Cells Nanomed. Biotechnol. 2018, 46, 906–911. [Google Scholar] [CrossRef]
- Greenhalgh, D.G. The role of growth factors in wound healing. J. Trauma Acute Care Surg. 1996, 41, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Li, J.F.; Duan, H.F.; Wu, C.T.; Zhang, D.J.; Deng, Y.; Yin, H.L.; Han, B.; Gong, H.C.; Wang, H.W.; Wang, Y.L. HGF accelerates wound healing by promoting the dedifferentiation of epidermal cells through beta1-integrin/ILK pathway. Biomed. Res. Int. 2013, 2013, 470418. [Google Scholar] [CrossRef] [PubMed]
- Bevan, D.; Gherardi, E.; Fan, T.P.; Edwards, D.; Warn, R. Diverse and potent activities of HGF/SF in skin wound repair. J. Pathol. 2004, 203, 831–838. [Google Scholar] [CrossRef]
- Strodtbeck, F. Physiology of wound healing. Newborn Infant Nurs. Rev. 2001, 1, 43–52. [Google Scholar] [CrossRef]
- Turabelidze, A.; Dipietro, L.A. Inflammation and wound healing. Endod. Top. 2011, 24, 26–38. [Google Scholar] [CrossRef]
- Gonzalez, A.C.d.O.; Costa, T.F.; Andrade, Z.d.A.; Medrado, A.R.A.P. Wound healing-A literature review. An. Bras. Dermatol. 2016, 91, 614–620. [Google Scholar] [CrossRef]
- Landen, N.X.; Li, D.; Stahle, M. Transition from inflammation to proliferation: A critical step during wound healing. Cell. Mol. Life Sci. 2016, 73, 3861–3885. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, Y.; He, W.; Mu, X.; Wu, X.; Deng, J.; Nie, X. Fibroblasts: Immunomodulatory factors in refractory diabetic wound healing. Front. Immunol. 2022, 13, 918223. [Google Scholar] [CrossRef]
- Singh, D.; Rai, V.; Agrawal, D.K. Regulation of Collagen I and Collagen III in Tissue Injury and Regeneration. Cardiol. Cardiovasc. Med. 2023, 7, 5–16. [Google Scholar] [CrossRef] [PubMed]
- Patel, P.; Rai, V.; Agrawal, D.K. Role of oncostatin-M in ECM remodeling and plaque vulnerability. Mol. Cell. Biochem. 2023, 478, 2451–2460. [Google Scholar] [CrossRef]
- Rai, V.; Moellmer, R.; Agrawal, D.K. Stem Cells and Angiogenesis: Implications and Limitations in Enhancing Chronic Diabetic Foot Ulcer Healing. Cells 2022, 11, 2287. [Google Scholar] [CrossRef]
- Rognoni, E.; Pisco, A.O.; Hiratsuka, T.; Sipila, K.H.; Belmonte, J.M.; Mobasseri, S.A.; Philippeos, C.; Dilao, R.; Watt, F.M. Fibroblast state switching orchestrates dermal maturation and wound healing. Mol. Syst. Biol. 2018, 14, e8174. [Google Scholar] [CrossRef]
- D’Urso, M.; Kurniawan, N.A. Mechanical and Physical Regulation of Fibroblast-Myofibroblast Transition: From Cellular Mechanoresponse to Tissue Pathology. Front. Bioeng. Biotechnol. 2020, 8, 609653. [Google Scholar] [CrossRef]
- Ibrahim, M.M.; Chen, L.; Bond, J.E.; Medina, M.A.; Ren, L.; Kokosis, G.; Selim, A.M.; Levinson, H. Myofibroblasts contribute to but are not necessary for wound contraction. Lab. Investig. 2015, 95, 1429–1438. [Google Scholar] [CrossRef] [PubMed]
- Yousef, H.; Alhajj, M.; Sharma, S. Anatomy, Skin (Integument), Epidermis. In StatPearls [Internet]; StatPearls: Tampa/St. Petersburg, FL, USA, 2017. [Google Scholar]
- Piipponen, M.; Li, D.; Landen, N.X. The Immune Functions of Keratinocytes in Skin Wound Healing. Int. J. Mol. Sci. 2020, 21, 8790. [Google Scholar] [CrossRef]
- Werner, S.; Krieg, T.; Smola, H. Keratinocyte-fibroblast interactions in wound healing. J. Investig. Dermatol. 2007, 127, 998–1008. [Google Scholar] [CrossRef]
- Nowinski, D.; Hoijer, P.; Engstrand, T.; Rubin, K.; Gerdin, B.; Ivarsson, M. Keratinocytes inhibit expression of connective tissue growth factor in fibroblasts in vitro by an interleukin-1alpha-dependent mechanism. J. Investig. Dermatol. 2002, 119, 449–455. [Google Scholar] [CrossRef]
- Nardini, J.T.; Chapnick, D.A.; Liu, X.; Bortz, D.M. Modeling keratinocyte wound healing dynamics: Cell-cell adhesion promotes sustained collective migration. J. Theor. Biol. 2016, 400, 103–117. [Google Scholar] [CrossRef] [PubMed]
- Cao, G.; Xuan, X.; Hu, J.; Zhang, R.; Jin, H.; Dong, H. How vascular smooth muscle cell phenotype switching contributes to vascular disease. Cell Commun. Signal 2022, 20, 180. [Google Scholar] [CrossRef] [PubMed]
- Rai, V.; Singh, H.; Agrawal, D.K. Targeting the Crosstalk of Immune Response and Vascular Smooth Muscle Cells Phenotype Switch for Arteriovenous Fistula Maturation. Int. J. Mol. Sci. 2022, 23, 12012. [Google Scholar] [CrossRef] [PubMed]
- Ammann, K.R.; DeCook, K.J.; Li, M.; Slepian, M.J. Migration versus proliferation as contributor to in vitro wound healing of vascular endothelial and smooth muscle cells. Exp. Cell Res. 2019, 376, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Pober, J.S.; Sessa, W.C. Evolving functions of endothelial cells in inflammation. Nat. Rev. Immunol. 2007, 7, 803–815. [Google Scholar] [CrossRef]
- Jiang, Y.; Perez-Moreno, M. Translational frontiers: Insight from lymphatics in skin regeneration. Front. Physiol. 2024, 15, 1347558. [Google Scholar] [CrossRef]
- Brunner, L.M.; He, Y.; Cousin, N.; Scholl, J.; Albin, L.K.; Schmucki, B.; Supersaxo, S.; Restivo, G.; Hafner, J.; Neri, D.; et al. Promotion of Lymphangiogenesis by Targeted Delivery of VEGF-C Improves Diabetic Wound Healing. Cells 2023, 12, 472. [Google Scholar] [CrossRef]
- Haase, I.; Evans, R.; Pofahl, R.; Watt, F.M. Regulation of keratinocyte shape, migration and wound epithelialization by IGF-1- and EGF-dependent signalling pathways. J. Cell Sci. 2003, 116, 3227–3238. [Google Scholar] [CrossRef]
- Blakytny, R.; Jude, E.B.; Martin Gibson, J.; Boulton, A.J.; Ferguson, M.W. Lack of insulin-like growth factor 1 (IGF1) in the basal keratinocyte layer of diabetic skin and diabetic foot ulcers. J. Pathol. 2000, 190, 589–594. [Google Scholar] [CrossRef]
- Garoufalia, Z.; Papadopetraki, A.; Karatza, E.; Vardakostas, D.; Philippou, A.; Kouraklis, G.; Mantas, D. Insulin-like growth factor-I and wound healing, a potential answer to non-healing wounds: A systematic review of the literature and future perspectives. Biomed. Rep. 2021, 15, 66. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.J.; Lu, M.C.; Chang, H.Y. Sustained Release of Insulin-Like Growth Factor-1 from Bombyx mori L. Silk Fibroin Delivery for Diabetic Wound Therapy. Int. J. Mol. Sci. 2021, 22, 6267. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.J.; Lu, M.C.; Chan, Y.C.; Huang, Y.F.; Chang, H.Y. An Insulin-like Growth Factor-1 Conjugated Bombyx mori Silk Fibroin Film for Diabetic Wound Healing: Fabrication, Physicochemical Property Characterization, and Dosage Optimization In Vitro and In Vivo. Pharmaceutics 2021, 13, 1459. [Google Scholar] [CrossRef] [PubMed]
- Rahim, F.; Yan, X.; Shah, J.A.; Bibi, N.; Khan, Z.U.; Nawaz, S.; Ming, Y. Epidermal growth factor outperforms placebo in the treatment of diabetic foot ulcer: A meta-analysis. F1000Res 2022, 11, 773. [Google Scholar] [CrossRef] [PubMed]
- Bui, T.Q.; Bui, Q.V.P.; Nemeth, D.; Hegyi, P.; Szakacs, Z.; Rumbus, Z.; Toth, B.; Emri, G.; Parniczky, A.; Sarlos, P.; et al. Epidermal Growth Factor is Effective in the Treatment of Diabetic Foot Ulcers: Meta-Analysis and Systematic Review. Int. J. Environ. Res. Public Health 2019, 16, 2584. [Google Scholar] [CrossRef]
- Zhang, J.; Hu, W.; Diao, Q.; Wang, Z.; Miao, J.; Chen, X.; Xue, Z. Therapeutic effect of the epidermal growth factor on diabetic foot ulcer and the underlying mechanisms. Exp. Ther. Med. 2019, 17, 1643–1648. [Google Scholar] [CrossRef]
- Berlanga-Acosta, J.; Camacho-Rodriguez, H.; Mendoza-Mari, Y.; Falcon-Cama, V.; Garcia-Ojalvo, A.; Herrera-Martinez, L.; Guillen-Nieto, G. Epidermal Growth Factor in Healing Diabetic Foot Ulcers: From Gene Expression to Tissue Healing and Systemic Biomarker Circulation. MEDICC Rev. 2020, 22, 24–31. [Google Scholar] [CrossRef]
- Cetinkaya, O.A.; Celik, S.U.; Erzincan, M.B.; Hazir, B.; Uncu, H. Intralesional epidermal growth factor application is a potential therapeutic strategy to improve diabetic foot ulcer healing and prevent amputation. Turk. J. Surg. 2020, 36, 15–22. [Google Scholar] [CrossRef]
- Wong, A.Y.W.; Hooi, N.M.F.; Yeo, B.S.Y.; Sultana, R.; Bee, Y.M.; Lee, A.; Tay, S.M. Improving Diabetic Wound Healing Outcomes with Topical Growth Factor Therapies: Systematic Review and Network Meta-analysis of Randomised-controlled Trials. J. Clin. Endocrinol. Metab. 2024, 109, e1642–e1651. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, Y.; Deng, J.; Li, W.; Nie, X. Fibroblast Growth Factor in Diabetic Foot Ulcer: Progress and Therapeutic Prospects. Front. Endocrinol. 2021, 12, 744868. [Google Scholar] [CrossRef]
- de Araujo, R.; Lobo, M.; Trindade, K.; Silva, D.F.; Pereira, N. Fibroblast Growth Factors: A Controlling Mechanism of Skin Aging. Skin. Pharmacol. Physiol. 2019, 32, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Seeger, M.A.; Paller, A.S. The Roles of Growth Factors in Keratinocyte Migration. Adv. Wound Care 2015, 4, 213–224. [Google Scholar] [CrossRef]
- Farooq, M.; Khan, A.W.; Kim, M.S.; Choi, S. The Role of Fibroblast Growth Factor (FGF) Signaling in Tissue Repair and Regeneration. Cells 2021, 10, 3242. [Google Scholar] [CrossRef]
- Thanigaimani, S.; Jin, H.; Ahmad, U.; Anbalagan, R.; Golledge, J. Comparative efficacy of growth factor therapy in healing diabetes-related foot ulcers: A network meta-analysis of randomized controlled trials. Diabetes Metab. Res. Rev. 2023, 39, e3670. [Google Scholar] [CrossRef] [PubMed]
- Liarte, S.; Bernabe-Garcia, A.; Nicolas, F.J. Role of TGF-beta in Skin Chronic Wounds: A Keratinocyte Perspective. Cells 2020, 9, 306. [Google Scholar] [CrossRef] [PubMed]
- Liarte, S.; Bernabe-Garcia, A.; Nicolas, F.J. Human Skin Keratinocytes on Sustained TGF-beta Stimulation Reveal Partial EMT Features and Weaken Growth Arrest Responses. Cells 2020, 9, 255. [Google Scholar] [CrossRef]
- Russo, B.; Brembilla, N.C.; Chizzolini, C. Interplay Between Keratinocytes and Fibroblasts: A Systematic Review Providing a New Angle for Understanding Skin Fibrotic Disorders. Front. Immunol. 2020, 11, 648. [Google Scholar] [CrossRef]
- Bukowska, J.; Kopcewicz, M.; Kur-Piotrowska, A.; Szostek-Mioduchowska, A.Z.; Walendzik, K.; Gawronska-Kozak, B. Effect of TGFbeta1, TGFbeta3 and keratinocyte conditioned media on functional characteristics of dermal fibroblasts derived from reparative (Balb/c) and regenerative (Foxn1 deficient; nude) mouse models. Cell Tissue Res. 2018, 374, 149–163. [Google Scholar] [CrossRef]
- Pakyari, M.; Farrokhi, A.; Maharlooei, M.K.; Ghahary, A. Critical Role of Transforming Growth Factor Beta in Different Phases of Wound Healing. Adv. Wound Care 2013, 2, 215–224. [Google Scholar] [CrossRef]
- Ramirez, H.; Patel, S.B.; Pastar, I. The Role of TGFbeta Signaling in Wound Epithelialization. Adv. Wound Care 2014, 3, 482–491. [Google Scholar] [CrossRef]
- Chong, D.L.W.; Trinder, S.; Labelle, M.; Rodriguez-Justo, M.; Hughes, S.; Holmes, A.M.; Scotton, C.J.; Porter, J.C. Platelet-derived transforming growth factor-beta1 promotes keratinocyte proliferation in cutaneous wound healing. J. Tissue Eng. Regen. Med. 2020, 14, 645–649. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, Y.; Li, N.; Teng, W.; Wang, M.; Zhang, Y.; Xiao, Z. TGF-beta1 promotes scar fibroblasts proliferation and transdifferentiation via up-regulating MicroRNA-21. Sci. Rep. 2016, 6, 32231. [Google Scholar] [CrossRef]
- White, M.J.V.; Briquez, P.S.; White, D.A.V.; Hubbell, J.A. VEGF-A, PDGF-BB and HB-EGF engineered for promiscuous super affinity to the extracellular matrix improve wound healing in a model of type 1 diabetes. NPJ Regen. Med. 2021, 6, 76. [Google Scholar] [CrossRef] [PubMed]
- Chawla, M.K. Role of Platelet-Derived Growth Factor-Elastin Like Polypeptide in Chronic Wound Healing. Master’s Thesis, Rutgers the State University of New Jersey, School of Graduate Studies, Newark, NJ, USA, 2020. [Google Scholar]
- Jian, K.; Yang, C.; Li, T.; Wu, X.; Shen, J.; Wei, J.; Yang, Z.; Yuan, D.; Zhao, M.; Shi, J. PDGF-BB-derived supramolecular hydrogel for promoting skin wound healing. J. Nanobiotechnol. 2022, 20, 201. [Google Scholar] [CrossRef]
- Thapa, R.K.; Margolis, D.J.; Kiick, K.L.; Sullivan, M.O. Enhanced wound healing via collagen-turnover-driven transfer of PDGF-BB gene in a murine wound model. ACS Appl. Bio Mater. 2020, 3, 3500–3517. [Google Scholar] [CrossRef]
- Yao, L.; Rathnakar, B.H.; Kwon, H.R.; Sakashita, H.; Kim, J.H.; Rackley, A.; Tomasek, J.J.; Berry, W.L.; Olson, L.E. Temporal control of PDGFRalpha regulates the fibroblast-to-myofibroblast transition in wound healing. Cell Rep. 2022, 40, 111192. [Google Scholar] [CrossRef]
- Wei, Y.; Li, J.; Huang, Y.; Lei, X.; Zhang, L.; Yin, M.; Deng, J.; Wang, X.; Fu, X.; Wu, J. The clinical effectiveness and safety of using epidermal growth factor, fibroblast growth factor and granulocyte-macrophage colony stimulating factor as therapeutics in acute skin wound healing: A systematic review and meta-analysis. Burn. Trauma 2022, 10, tkac002. [Google Scholar] [CrossRef]
- Mullin, J.A.; Rahmani, E.; Kiick, K.L.; Sullivan, M.O. Growth factors and growth factor gene therapies for treating chronic wounds. Bioeng. Transl. Med. 2024, 9, e10642. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Chang, C.; Levian, B.; Woodley, D.T.; Li, W. Why Are There So Few FDA-Approved Therapeutics for Wound Healing? Int. J. Mol. Sci. 2023, 24, 15109. [Google Scholar] [CrossRef]
- Aydin, F.; Kaya, A.; Karapinar, L.; Kumbaraci, M.; Imerci, A.; Karapinar, H.; Karakuzu, C.; Incesu, M. IGF-1 Increases with Hyperbaric Oxygen Therapy and Promotes Wound Healing in Diabetic Foot Ulcers. J. Diabetes Res. 2013, 2013, 567834. [Google Scholar] [CrossRef]
- Zhang, X.; Hu, F.; Li, J.; Chen, L.; Mao, Y.F.; Li, Q.B.; Nie, C.Y.; Lin, C.; Xiao, J. IGF-1 inhibits inflammation and accelerates angiogenesis via Ras/PI3K/IKK/NF-kappaB signaling pathways to promote wound healing. Eur. J. Pharm. Sci. 2024, 200, 106847. [Google Scholar] [CrossRef]
- Tsang, M.W.; Wong, W.K.; Hung, C.S.; Lai, K.M.; Tang, W.; Cheung, E.Y.; Kam, G.; Leung, L.; Chan, C.W.; Chu, C.M.; et al. Human epidermal growth factor enhances healing of diabetic foot ulcers. Diabetes Care 2003, 26, 1856–1861. [Google Scholar] [CrossRef]
- Pessanha, F.S.; Oliveira, B.; Oliveira, B.C.; Deutsch, G.; Teixeira, F.L.; Bokehi, L.C.; Calomino, M.A.; Rodrigues de Castilho, S.; Thire, R.; Teixeira, L.A.; et al. Effectiveness of Epidermal Growth Factor Loaded Carboxymethylcellulose (EGF-CMC) Hydrogel in Biofilm Formation in Wounds of Diabetic Patients: A Randomized Clinical Trial. Gels 2023, 9, 117. [Google Scholar] [CrossRef]
- Yasti, A.C.; Karaca, T.; Kendirci, M.; Akgun, A.E.; Sahiner, I.T.; Akin, M. Comparison of the Efficiency of Epidermal Growth Factor and Negative Pressure Wound Therapy in Diabetic Foot Patients. Int. J. Low. Extrem. Wounds 2023, 22, 93–102. [Google Scholar] [CrossRef]
- Benington, L.; Mo, J.; Li, M.; Rajan, G.; Locher, C.; Lim, L.Y. In Vitro Assessment of Wound-Healing Efficacy of Stabilized Basic Fibroblast Growth Factor (FGF-2) Solutions. Pharmaceuticals 2024, 17, 247. [Google Scholar] [CrossRef]
- Koike, Y.; Yozaki, M.; Utani, A.; Murota, H. Fibroblast growth factor 2 accelerates the epithelial-mesenchymal transition in keratinocytes during wound healing process. Sci. Rep. 2020, 10, 18545. [Google Scholar] [CrossRef] [PubMed]
- Petit, I.; Levy, A.; Estrach, S.; Feral, C.C.; Trentin, A.G.; Dingli, F.; Loew, D.; Qu, J.; Zhou, H.; Thery, C.; et al. Fibroblast growth factor-2 bound to specific dermal fibroblast-derived extracellular vesicles is protected from degradation. Sci. Rep. 2022, 12, 22131. [Google Scholar] [CrossRef] [PubMed]
- Steed, D.L. Clinical evaluation of recombinant human platelet-derived growth factor for the treatment of lower extremity ulcers. Plast. Reconstr. Surg. 2006, 117, 143S–149S; discussion 150S–151S. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, R.P.; Jhajharia, A.; Mohta, N.; Dogra, R.; Chaudhari, V.; Nayak, K.C. Use of a platelet-derived growth factor gel in chronic diabetic foot ulcers. Diabet. Foot 2009, 12, 80. [Google Scholar]
- Sadeghi-Ardebili, M.; Hasannia, S.; Dabirmanesh, B.; Khavari-Nejad, R.A. Functional characterization of the dimeric form of PDGF-derived fusion peptide fabricated based on theoretical arguments. Sci. Rep. 2024, 14, 1003. [Google Scholar] [CrossRef]
- Deptula, M.; Karpowicz, P.; Wardowska, A.; Sass, P.; Sosnowski, P.; Mieczkowska, A.; Filipowicz, N.; Dzierzynska, M.; Sawicka, J.; Nowicka, E.; et al. Development of a Peptide Derived from Platelet-Derived Growth Factor (PDGF-BB) into a Potential Drug Candidate for the Treatment of Wounds. Adv. Wound Care 2020, 9, 657–675. [Google Scholar] [CrossRef]
- Johnson, J.; Law, S.Q.K.; Shojaee, M.; Hall, A.S.; Bhuiyan, S.; Lim, M.B.L.; Silva, A.; Kong, K.J.W.; Schoppet, M.; Blyth, C.; et al. First-in-human clinical trial of allogeneic, platelet-derived extracellular vesicles as a potential therapeutic for delayed wound healing. J. Extracell. Vesicles 2023, 12, e12332. [Google Scholar] [CrossRef] [PubMed]
- Tejedor, S.; Wagberg, M.; Correia, C.; Avall, K.; Holtta, M.; Hultin, L.; Lerche, M.; Davies, N.; Bergenhem, N.; Snijder, A.; et al. The Combination of Vascular Endothelial Growth Factor A (VEGF-A) and Fibroblast Growth Factor 1 (FGF1) Modified mRNA Improves Wound Healing in Diabetic Mice: An Ex Vivo and In Vivo Investigation. Cells 2024, 13, 414. [Google Scholar] [CrossRef] [PubMed]
- Belvedere, R.; Novizio, N.; Morello, S.; Petrella, A. The combination of mesoglycan and VEGF promotes skin wound repair by enhancing the activation of endothelial cells and fibroblasts and their cross-talk. Sci. Rep. 2022, 12, 11041. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.C.; Gotlieb, A.I. Molecular basis of cardiovascular disease. In Molecular Pathology; Elsevier: Amsterdam, The Netherlands, 2009; pp. 227–245. [Google Scholar]
- Louis, S.F.; Zahradka, P. Vascular smooth muscle cell motility: From migration to invasion. Exp. Clin. Cardiol. 2010, 15, e75–e85. [Google Scholar]
- Koval, O.M.; Nguyen, E.K.; Mittauer, D.J.; Ait-Aissa, K.; Chinchankar, W.C.; Grumbach, I.M. Regulation of Smooth Muscle Cell Proliferation by Mitochondrial Ca2+ in Type 2 Diabetes. Int. J. Mol. Sci. 2023, 24, 12897. [Google Scholar] [CrossRef] [PubMed]
- Xie, N.; Chen, M.; Dai, R.; Zhang, Y.; Zhao, H.; Song, Z.; Zhang, L.; Li, Z.; Feng, Y.; Gao, H.; et al. SRSF1 promotes vascular smooth muscle cell proliferation through a Delta133p53/EGR1/KLF5 pathway. Nat. Commun. 2017, 8, 16016. [Google Scholar] [CrossRef]
- Yang, D.; Sun, C.; Zhang, J.; Lin, S.; Zhao, L.; Wang, L.; Lin, R.; Lv, J.; Xin, S. Proliferation of vascular smooth muscle cells under inflammation is regulated by NF-kappaB p65/microRNA-17/RB pathway activation. Int. J. Mol. Med. 2018, 41, 43–50. [Google Scholar] [CrossRef]
- Newby, A.C. Matrix metalloproteinases regulate migration, proliferation, and death of vascular smooth muscle cells by degrading matrix and non-matrix substrates. Cardiovasc. Res. 2006, 69, 614–624. [Google Scholar] [CrossRef]
- Gerthoffer, W.T. Mechanisms of vascular smooth muscle cell migration. Circ. Res. 2007, 100, 607–621. [Google Scholar] [CrossRef]
- Cucina, A.; Borrelli, V.; Randone, B.; Coluccia, P.; Sapienza, P.; Cavallaro, A. Vascular endothelial growth factor increases the migration and proliferation of smooth muscle cells through the mediation of growth factors released by endothelial cells. J. Surg. Res. 2003, 109, 16–23. [Google Scholar] [CrossRef] [PubMed]
- West-Livingston, L.N.; Ju, Y.M.; Velazquez, G.A.; Atala, A.; Lee, S.J. Role of Growth Factors in Smooth Muscle Cell Migration In Tissue Engineered Vascular Grafts. Ann. Vasc. Surg. 2022, 79, 397–398. [Google Scholar] [CrossRef]
- Zhou, J.; Shao, L.; Yu, J.; Huang, J.; Feng, Q. PDGF-BB promotes vascular smooth muscle cell migration by enhancing Pim-1 expression via inhibiting miR-214. Ann. Transl. Med. 2021, 9, 1728. [Google Scholar] [CrossRef] [PubMed]
- Martin-Garrido, A.; Williams, H.C.; Lee, M.; Seidel-Rogol, B.; Ci, X.; Dong, J.T.; Lassegue, B.; Martin, A.S.; Griendling, K.K. Transforming growth factor beta inhibits platelet derived growth factor-induced vascular smooth muscle cell proliferation via Akt-independent, Smad-mediated cyclin D1 downregulation. PLoS ONE 2013, 8, e79657. [Google Scholar] [CrossRef]
- Tsuji-Tamura, K.; Tamura, M. Basic fibroblast growth factor uniquely stimulates quiescent vascular smooth muscle cells and induces proliferation and dedifferentiation. FEBS Lett. 2022, 596, 1686–1699. [Google Scholar] [CrossRef]
- Zhu, Z.; Zheng, X.; Li, D.; Wang, T.; Xu, R.; Piao, H.; Liu, K. Prx1 promotes the proliferation and migration of vascular smooth muscle cells in a TLR4-dependent manner. Mol. Med. Rep. 2017, 15, 345–351. [Google Scholar] [CrossRef][Green Version]
- Furue, M.; Furue, K.; Tsuji, G.; Nakahara, T. Interleukin-17A and Keratinocytes in Psoriasis. Int. J. Mol. Sci. 2020, 21, 1275. [Google Scholar] [CrossRef]
- Sisto, M.; Lisi, S. Targeting Interleukin-17 as a Novel Treatment Option for Fibrotic Diseases. J. Clin. Med. 2023, 13, 164. [Google Scholar] [CrossRef]
- Cheng, G.; Wei, L.; Xiurong, W.; Xiangzhen, L.; Shiguang, Z.; Songbin, F. IL-17 stimulates migration of carotid artery vascular smooth muscle cells in an MMP-9 dependent manner via p38 MAPK and ERK1/2-dependent NF-kappaB and AP-1 activation. Cell Mol. Neurobiol. 2009, 29, 1161–1168. [Google Scholar] [CrossRef]
- Pan, B.; Shen, J.; Cao, J.; Zhou, Y.; Shang, L.; Jin, S.; Cao, S.; Che, D.; Liu, F.; Yu, Y. Interleukin-17 promotes angiogenesis by stimulating VEGF production of cancer cells via the STAT3/GIV signaling pathway in non-small-cell lung cancer. Sci. Rep. 2015, 5, 16053. [Google Scholar] [CrossRef]
- Huang, Q.; Duan, L.; Qian, X.; Fan, J.; Lv, Z.; Zhang, X.; Han, J.; Wu, F.; Guo, M.; Hu, G.; et al. IL-17 Promotes Angiogenic Factors IL-6, IL-8, and Vegf Production via Stat1 in Lung Adenocarcinoma. Sci. Rep. 2016, 6, 36551. [Google Scholar] [CrossRef] [PubMed]
- Hadian, Y.; Bagood, M.D.; Dahle, S.E.; Sood, A.; Isseroff, R.R. Interleukin-17: Potential Target for Chronic Wounds. Mediat. Inflamm. 2019, 2019, 1297675. [Google Scholar] [CrossRef] [PubMed]
- Mu, X.; Gu, R.; Tang, M.; Wu, X.; He, W.; Nie, X. IL-17 in wound repair: Bridging acute and chronic responses. Cell Commun. Signal 2024, 22, 288. [Google Scholar] [CrossRef] [PubMed]
- Lecron, J.C.; Charreau, S.; Jegou, J.F.; Salhi, N.; Petit-Paris, I.; Guignouard, E.; Burucoa, C.; Favot-Laforge, L.; Bodet, C.; Barra, A.; et al. IL-17 and IL-22 are pivotal cytokines to delay wound healing of S. aureus and P. aeruginosa infected skin. Front. Immunol. 2022, 13, 984016. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.; Huh, J.R. Cutting edge: Interleukin-17a prompts HIF1alpha for wound healing. Trends Immunol. 2022, 43, 861–863. [Google Scholar] [CrossRef]
- Wang, J.; Ding, X. IL-17 signaling in skin repair: Safeguarding metabolic adaptation of wound epithelial cells. Signal Transduct. Target. Ther. 2022, 7, 359. [Google Scholar] [CrossRef]
- Wilmink, M.; Spalinger, M.R. SKAP2-A Molecule at the Crossroads for Integrin Signalling and Immune Cell Migration and Function. Biomedicines 2023, 11, 2788. [Google Scholar] [CrossRef]
- Zhou, P.; Li, Y.; Zhang, S.; Chen, D.X.; Gao, R.; Qin, P.; Yang, C.; Li, Q. KRT17 from Keratinocytes with High Glucose Stimulation Inhibit Dermal Fibroblasts Migration Through Integrin alpha11. J. Endocr. Soc. 2024, 8, bvad176. [Google Scholar] [CrossRef]
- Floyel, T.; Meyerovich, K.; Prause, M.C.; Kaur, S.; Frorup, C.; Mortensen, H.B.; Nielsen, L.B.; Pociot, F.; Cardozo, A.K.; Storling, J. SKAP2, a Candidate Gene for Type 1 Diabetes, Regulates beta-Cell Apoptosis and Glycemic Control in Newly Diagnosed Patients. Diabetes 2021, 70, 464–476. [Google Scholar] [CrossRef]
- Takagane, K.; Umakoshi, M.; Itoh, G.; Kuriyama, S.; Goto, A.; Tanaka, M. SKAP2 suppresses inflammation-mediated tumorigenesis by regulating SHP-1 and SHP-2. Oncogene 2022, 41, 1087–1099. [Google Scholar] [CrossRef]
- Xie, X.; Cui, Q.; Jiang, T.; Zhao, Z.; Liu, Z.; Liu, J.; Yao, Q.; Wang, Y.; Dang, E.; Wang, G.; et al. A critical role of the endothelial S-phase kinase-associated protein 2/phosphatase and tensin homologue axis in angiogenesis and psoriasis. Br. J. Dermatol. 2024, 190, 244–257. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Nagel, D.J.; Zhou, Q.; Cygnar, K.D.; Zhao, H.; Li, F.; Pi, X.; Knight, P.A.; Yan, C. Role of cAMP-phosphodiesterase 1C signaling in regulating growth factor receptor stability, vascular smooth muscle cell growth, migration, and neointimal hyperplasia. Circ. Res. 2015, 116, 1120–1132. [Google Scholar] [CrossRef] [PubMed]
- Mas-Rosello, J.; Tenor, H.; Szabo, T.; Naef, R.; Sieber, S.; Gademann, K. Bifunctional Sildenafil Diazeniumdiolates Acting as Phosphodiesterase 5 Inhibitors and Nitric Oxide Donors- Towards Wound Healing. Chembiochem 2024, 25, e202300801. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.X.; Hsiung, T.C.; Weng, F.C.; Ding, S.L.; Wu, C.P.; Conti, M.; Chuang, T.H.; Catherine Jin, S.L. Synergistic effect of phosphodiesterase 4 inhibitor and serum on migration of endotoxin-stimulated macrophages. Innate Immun. 2018, 24, 501–512. [Google Scholar] [CrossRef] [PubMed]
- Kohyama, T.; Liu, X.; Zhu, Y.K.; Wen, F.Q.; Wang, H.J.; Fang, Q.; Kobayashi, T.; Rennard, S.I. Phosphodiesterase 4 inhibitor cilomilast inhibits fibroblast-mediated collagen gel degradation induced by tumor necrosis factor-alpha and neutrophil elastase. Am. J. Respir. Cell Mol. Biol. 2002, 27, 487–494. [Google Scholar] [CrossRef]
- Siemes, C.; Quast, T.; Kummer, C.; Wehner, S.; Kirfel, G.; Muller, U.; Herzog, V. Keratinocytes from APP/APLP2-deficient mice are impaired in proliferation, adhesion and migration in vitro. Exp. Cell Res. 2006, 312, 1939–1949. [Google Scholar] [CrossRef]
- Guo, J.; Thinakaran, G.; Guo, Y.; Sisodia, S.S.; Yu, F.X. A role for amyloid precursor-like protein 2 in corneal epithelial wound healing. Investig. Ophthalmol. Vis. Sci. 1998, 39, 292–300. [Google Scholar]
- Stanciu, G.D.; Bild, V.; Ababei, D.C.; Rusu, R.N.; Cobzaru, A.; Paduraru, L.; Bulea, D. Link Between Diabetes and Alzheimer’s Disease due to the Shared Amyloid Aggregation and Deposition Involving both Neurodegenerative Changes and Neurovascular Damages. J. Clin. Med. 2020, 9, 1713. [Google Scholar] [CrossRef]
- Danussi, C.; Petrucco, A.; Wassermann, B.; Pivetta, E.; Modica, T.M.; Del Bel Belluz, L.; Colombatti, A.; Spessotto, P. EMILIN1-alpha4/alpha9 integrin interaction inhibits dermal fibroblast and keratinocyte proliferation. J. Cell Biol. 2011, 195, 131–145. [Google Scholar] [CrossRef]
- Schiavinato, A.; Keene, D.R.; Imhof, T.; Doliana, R.; Sasaki, T.; Sengle, G. Fibulin-4 deposition requires EMILIN-1 in the extracellular matrix of osteoblasts. Sci. Rep. 2017, 7, 5526. [Google Scholar] [CrossRef]
- Gardeazabal, L.; Izeta, A. Elastin and collagen fibres in cutaneous wound healing. Exp. Dermatol. 2024, 33, e15052. [Google Scholar] [CrossRef]
- Imhof, T.; Korkmaz, Y.; Koch, M.; Sengle, G.; Schiavinato, A. EMILIN proteins are novel extracellular constituents of the dentin-pulp complex. Sci. Rep. 2020, 10, 15320. [Google Scholar] [CrossRef]
- Lavoie, J.R.; Creskey, M.M.; Muradia, G.; Bell, G.I.; Sherman, S.E.; Gao, J.; Stewart, D.J.; Cyr, T.D.; Hess, D.A.; Rosu-Myles, M. Brief Report: Elastin Microfibril Interface 1 and Integrin-Linked Protein Kinase Are Novel Markers of Islet Regenerative Function in Human Multipotent Mesenchymal Stromal Cells. Stem Cells 2016, 34, 2249–2255. [Google Scholar] [CrossRef][Green Version]
- Taal, K.; Tuvikene, J.; Rullinkov, G.; Piirsoo, M.; Sepp, M.; Neuman, T.; Tamme, R.; Timmusk, T. Neuralized family member NEURL1 is a ubiquitin ligase for the cGMP-specific phosphodiesterase 9A. Sci. Rep. 2019, 9, 7104. [Google Scholar] [CrossRef] [PubMed]
- Begum, F.; Keni, R.; Ahuja, T.N.; Beegum, F.; Nandakumar, K.; Shenoy, R.R. Notch signaling: A possible therapeutic target and its role in diabetic foot ulcers. Diabetes Metab. Syndr. 2022, 16, 102542. [Google Scholar] [CrossRef]
- Zheng, X.; Narayanan, S.; Sunkari, V.G.; Eliasson, S.; Botusan, I.R.; Grunler, J.; Catrina, A.I.; Radtke, F.; Xu, C.; Zhao, A.; et al. Triggering of a Dll4-Notch1 loop impairs wound healing in diabetes. Proc. Natl. Acad. Sci. USA 2019, 116, 6985–6994. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Wang, C.; Liu, X.; Kuang, S. Shisa2 regulates the fusion of muscle progenitors. Stem Cell Res. 2018, 31, 31–41. [Google Scholar] [CrossRef]
- Pujar, M.; Vastrad, B.; Kavatagimath, S.; Vastrad, C.; Kotturshetti, S. Identification of candidate biomarkers and pathways associated with type 1 diabetes mellitus using bioinformatics analysis. Sci. Rep. 2022, 12, 9157. [Google Scholar] [CrossRef] [PubMed]
- Sharon, N.; Vanderhooft, J.; Straubhaar, J.; Mueller, J.; Chawla, R.; Zhou, Q.; Engquist, E.N.; Trapnell, C.; Gifford, D.K.; Melton, D.A. Wnt Signaling Separates the Progenitor and Endocrine Compartments during Pancreas Development. Cell Rep. 2019, 27, 2281–2291.e2285. [Google Scholar] [CrossRef] [PubMed]
- Shehwana, H.; Ijaz, S.; Fatima, A.; Walton, S.; Sheikh, Z.I.; Haider, W.; Naz, S. Transcriptome Analysis of Host Inflammatory Responses to the Ectoparasitic Mite Sarcoptes scabiei var. hominis. Front. Immunol. 2021, 12, 778840. [Google Scholar] [CrossRef]
- Zheng, Y.; Fan, J.; Jiang, X. The role of ferroptosis-related genes in airway epithelial cells of asthmatic patients based on bioinformatics. Medicine 2023, 102, e33119. [Google Scholar] [CrossRef] [PubMed]
- Crose, L.E.S.; Hilder, T.L.; Sciaky, N.; Johnson, G.L. Cerebral cavernous malformation 2 protein promotes smad ubiquitin regulatory factor 1-mediated RhoA degradation in endothelial cells. J. Biol. Chem. 2009, 284, 13301–13305. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, S.P.; Nemade, H.; Cherianidou, A.; Peng, L.; Cruz-Molina, S.; Rada-Iglesias, A.; Sachinidis, A. Epigenetic mechanisms of Strip2 in differentiation of pluripotent stem cells. Cell Death Discov. 2022, 8, 447. [Google Scholar] [CrossRef]
- Zheng, X.; Xu, C.; Smith, A.O.; Stratman, A.N.; Zou, Z.; Kleaveland, B.; Yuan, L.; Didiku, C.; Sen, A.; Liu, X.; et al. Dynamic regulation of the cerebral cavernous malformation pathway controls vascular stability and growth. Dev. Cell 2012, 23, 342–355. [Google Scholar] [CrossRef] [PubMed]
- Vannier, D.R.; Shapeti, A.; Chuffart, F.; Planus, E.; Manet, S.; Rivier, P.; Destaing, O.; Albiges-Rizo, C.; Van Oosterwyck, H.; Faurobert, E. CCM2-deficient endothelial cells undergo a ROCK-dependent reprogramming into senescence-associated secretory phenotype. Angiogenesis 2021, 24, 843–860. [Google Scholar] [CrossRef]
- Renz, M.; Otten, C.; Faurobert, E.; Rudolph, F.; Zhu, Y.; Boulday, G.; Duchene, J.; Mickoleit, M.; Dietrich, A.C.; Ramspacher, C.; et al. Regulation of beta1 integrin-Klf2-mediated angiogenesis by CCM proteins. Dev. Cell 2015, 32, 181–190. [Google Scholar] [CrossRef]
- Zhu, Y.; Wu, Q.; Xu, J.F.; Miller, D.; Sandalcioglu, I.E.; Zhang, J.M.; Sure, U. Differential angiogenesis function of CCM2 and CCM3 in cerebral cavernous malformations. Neurosurg. Focus. 2010, 29, E1. [Google Scholar] [CrossRef]
- Fang, Z.; Sun, X.; Wang, X.; Ma, J.; Palaia, T.; Rana, U.; Miao, B.; Ragolia, L.; Hu, W.; Miao, Q.R. NOGOB receptor deficiency increases cerebrovascular permeability and hemorrhage via impairing histone acetylation-mediated CCM1/2 expression. J. Clin. Investig. 2022, 132, e151382. [Google Scholar] [CrossRef]
- Gross, M.K.; Moran-Rivard, L.; Velasquez, T.; Nakatsu, M.N.; Jagla, K.; Goulding, M. Lbx1 is required for muscle precursor migration along a lateral pathway into the limb. Development 2000, 127, 413–424. [Google Scholar] [CrossRef]
- Schinzel, F.; Seyfer, H.; Ebbers, L.; Nothwang, H.G. The Lbx1 lineage differentially contributes to inhibitory cell types of the dorsal cochlear nucleus, a cerebellum-like structure, and the cerebellum. J. Comp. Neurol. 2021, 529, 3032–3045. [Google Scholar] [CrossRef]
- Mennerich, D.; Braun, T. Activation of myogenesis by the homeobox gene Lbx1 requires cell proliferation. EMBO J. 2001, 20, 7174–7183. [Google Scholar] [CrossRef]
- Doukas, J.; Blease, K.; Craig, D.; Ma, C.; Chandler, L.A.; Sosnowski, B.A.; Pierce, G.F. Delivery of FGF genes to wound repair cells enhances arteriogenesis and myogenesis in skeletal muscle. Mol. Ther. 2002, 5, 517–527. [Google Scholar] [CrossRef] [PubMed]
- Teng, Y.Y.; Zou, M.L.; Zhou, X.J.; Wu, J.J.; Liu, S.Y.; Yuan, Z.D.; Jia, Y.; Zhang, K.W.; Li, X.; Ye, J.X.; et al. Novel prospects for scarless wound healing: The roles of myofibroblasts and adipocytes. J. Cell Mol. Med. 2022, 26, 5113–5121. [Google Scholar] [CrossRef]
- Li, A.; Cunanan, J.; Khalili, H.; Plageman, T.; Ask, K.; Khan, A.; Hunjan, A.; Drysdale, T.; Bridgewater, D. Shroom3, a Gene Associated with CKD, Modulates Epithelial Recovery after AKI. Kidney360 2022, 3, 51–62. [Google Scholar] [CrossRef]
- Hildebrand, J.D. Shroom regulates epithelial cell shape via the apical positioning of an actomyosin network. J. Cell Sci. 2005, 118, 5191–5203. [Google Scholar] [CrossRef]
- Sevilla-Perez, J.; Konigshoff, M.; Kwapiszewska, G.; Amarie, O.V.; Seeger, W.; Weissmann, N.; Schermuly, R.T.; Morty, R.E.; Eickelberg, O. Shroom expression is attenuated in pulmonary arterial hypertension. Eur. Respir. J. 2008, 32, 871–880. [Google Scholar] [CrossRef]
- Dai, L.; Zhou, J.; Li, T.; Qian, Y.; Jin, L.; Zhu, C.; Li, S. STRIP2 silencing inhibits vascular smooth muscle cell proliferation and migration via P38-AKT-MMP-2 signaling pathway. J. Cell Physiol. 2019, 234, 22463–22476. [Google Scholar] [CrossRef]
- Sabour, D.; Srinivasan, S.P.; Rohani, S.; Wagh, V.; Gaspar, J.A.; Panek, D.; Ardestani, M.A.; Doss, M.X.; Riet, N.; Abken, H.; et al. STRIP2 Is Indispensable for the Onset of Embryonic Stem Cell Differentiation. Mol. Ther. Methods Clin. Dev. 2017, 5, 116–129. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.; Ogino, R.; Yamakawa, S.; Suda, S.; Hayashida, K. Role and Function of Mesenchymal Stem Cells on Fibroblast in Cutaneous Wound Healing. Biomedicines 2022, 10, 1391. [Google Scholar] [CrossRef]
- Jiang, D.; Guo, B.; Lin, F.; Hui, Q.; Tao, K. Effect of THBS1 on the Biological Function of Hypertrophic Scar Fibroblasts. Biomed. Res. Int. 2020, 2020, 8605407. [Google Scholar] [CrossRef]
- Julovi, S.M.; McKelvey, K.; Minhas, N.; Chan, Y.A.; Xue, M.; Jackson, C.J. Involvement of PAR-2 in the Induction of Cell-Specific Matrix Metalloproteinase-2 by Activated Protein C in Cutaneous Wound Healing. Int. J. Mol. Sci. 2023, 25, 370. [Google Scholar] [CrossRef]
- Kolosionek, E.; Savai, R.; Ghofrani, H.A.; Weissmann, N.; Guenther, A.; Grimminger, F.; Seeger, W.; Banat, G.A.; Schermuly, R.T.; Pullamsetti, S.S. Expression and activity of phosphodiesterase isoforms during epithelial mesenchymal transition: The role of phosphodiesterase 4. Mol. Biol. Cell 2009, 20, 4751–4765. [Google Scholar] [CrossRef]
- Jiang, Y.; Tsoi, L.C.; Billi, A.C.; Ward, N.L.; Harms, P.W.; Zeng, C.; Maverakis, E.; Kahlenberg, J.M.; Gudjonsson, J.E. Cytokinocytes: The diverse contribution of keratinocytes to immune responses in skin. JCI Insight 2020, 5, e142067. [Google Scholar] [CrossRef]
- Costantini, E.; Aielli, L.; Serra, F.; De Dominicis, L.; Falasca, K.; Di Giovanni, P.; Reale, M. Evaluation of Cell Migration and Cytokines Expression Changes under the Radiofrequency Electromagnetic Field on Wound Healing In Vitro Model. Int. J. Mol. Sci. 2022, 23, 2205. [Google Scholar] [CrossRef]
- Mendoza-Mari, Y.; Rai, V.; Radwan, M.M.; Brazdzionis, J.; Connett, D.A.; Miulli, D.E.; Agrawal, D.K. Modulation of Inflammatory Response by Electromagnetic Field Stimulation in Traumatic Brain Injury in Yucatan Swine. J. Surg. Res. 2024, 7, 20–40. [Google Scholar] [CrossRef]
- Alimperti, S.; Andreadis, S.T. CDH2 and CDH11 act as regulators of stem cell fate decisions. Stem Cell Res. 2015, 14, 270–282. [Google Scholar] [CrossRef]
- Wang, Z.; Bai, Y.; Nie, H.; Xu, Q.; Yin, Z.; Zhang, Y.; Yin, X.; Yan, X. Molecular mechanisms of wound healing and regeneration of siphon in the Manila clam Ruditapes philippinarum revealed by transcriptomic analysis. Genomics 2021, 113, 1011–1025. [Google Scholar] [CrossRef]
- Lee, P.S.; Gao, N.; Dike, M.; Shkilnyy, O.; Me, R.; Zhang, Y.; Yu, F.X. Opposing Effects of Neuropilin-1 and -2 on Sensory Nerve Regeneration in Wounded Corneas: Role of Sema3C in Ameliorating Diabetic Neurotrophic Keratopathy. Diabetes 2019, 68, 807–818. [Google Scholar] [CrossRef]
- Jeong, W.Y.; Yoo, H.Y.; Kim, C.W. Neuregulin-1 accelerates corneal epithelial wound healing. Growth Factors 2017, 35, 225–233. [Google Scholar] [CrossRef]
- Wang, Z.; Wei, D.; Li, S.; Tang, Q.; Lu, G.; Gu, S.; Lu, L.; Liang, F.; Teng, J.; Lin, J.; et al. Healing mechanism of diabetic foot ulcers using single-cell RNA-sequencing. Ann. Transl. Med. 2023, 11, 210. [Google Scholar] [CrossRef]
- Ferroni, L.; Gardin, C.; Dalla Paola, L.; Campo, G.; Cimaglia, P.; Bellin, G.; Pinton, P.; Zavan, B. Characterization of Dermal Stem Cells of Diabetic Patients. Cells 2019, 8, 729. [Google Scholar] [CrossRef]
- Yajima, H.; Motohashi, N.; Ono, Y.; Sato, S.; Ikeda, K.; Masuda, S.; Yada, E.; Kanesaki, H.; Miyagoe-Suzuki, Y.; Takeda, S.; et al. Six family genes control the proliferation and differentiation of muscle satellite cells. Exp. Cell Res. 2010, 316, 2932–2944. [Google Scholar] [CrossRef]
- Rafiq, A.; Aashaq, S.; Jan, I.; Beigh, M.A. SIX1 transcription factor: A review of cellular functions and regulatory dynamics. Int. J. Biol. Macromol. 2021, 193, 1151–1164. [Google Scholar] [CrossRef]
- Yu, Y.H.; Chang, Y.C.; Su, T.H.; Nong, J.Y.; Li, C.C.; Chuang, L.M. Prostaglandin reductase-3 negatively modulates adipogenesis through regulation of PPARgamma activity. J. Lipid Res. 2013, 54, 2391–2399. [Google Scholar] [CrossRef] [PubMed]
- Gorenjak, V.; Vance, D.R.; Dade, S.; Stathopoulou, M.G.; Doherty, L.; Xie, T.; Murray, H.; Masson, C.; Lamont, J.; Fitzgerald, P.; et al. Epigenome-wide association study in healthy individuals identifies significant associations with DNA methylation and PBMC extract VEGF-A concentration. Clin. Epigenet. 2020, 12, 79. [Google Scholar] [CrossRef]
- Zhao, M. PTEN: A promising pharmacological target to enhance epithelial wound healing. Br. J. Pharmacol. 2007, 152, 1141–1144. [Google Scholar] [CrossRef] [PubMed]
- Leonard, M.K.; Kommagani, R.; Payal, V.; Mayo, L.D.; Shamma, H.N.; Kadakia, M.P. DeltaNp63alpha regulates keratinocyte proliferation by controlling PTEN expression and localization. Cell Death Differ. 2011, 18, 1924–1933. [Google Scholar] [CrossRef]
- Yang, L.; Wu, J.; Zhang, J. A Novel CCM2 Gene Mutation Associated with Cerebral Cavernous Malformation. Front. Neurol. 2020, 11, 70. [Google Scholar] [CrossRef]
- Elahi, R.; Nazari, M.; Mohammadi, V.; Esmaeilzadeh, K.; Esmaeilzadeh, A. IL-17 in type II diabetes mellitus (T2DM) immunopathogenesis and complications; molecular approaches. Mol. Immunol. 2024, 171, 66–76. [Google Scholar] [CrossRef]
- Ookawara, M.; Nio, Y. Phosphodiesterase 4 inhibitors in diabetic nephropathy. Cell. Signal 2022, 90, 110185. [Google Scholar] [CrossRef] [PubMed]
- Swiecicka, A. The efficacy of PDE5 inhibitors in diabetic patients. Andrology 2023, 11, 245–256. [Google Scholar] [CrossRef]
- Zhu, J.; Li, Y.; Zhong, C.; Zhu, M.; Zheng, Y.; Xiong, A.; Meng, P.; Shan, L.; Li, Y.; Huang, J. Neuritin affects the activity of neuralized-like 1 by promoting degradation and weakening its affinity for substrate. Acta Biochim. Biophys. Sin. 2023, 55, 1650–1658. [Google Scholar] [CrossRef]
- Wilde, J.J.; Petersen, J.R.; Niswander, L. Genetic, epigenetic, and environmental contributions to neural tube closure. Annu. Rev. Genet. 2014, 48, 583–611. [Google Scholar] [CrossRef]
- Yeo, N.C.; O’Meara, C.C.; Bonomo, J.A.; Veth, K.N.; Tomar, R.; Flister, M.J.; Drummond, I.A.; Bowden, D.W.; Freedman, B.I.; Lazar, J.; et al. Shroom3 contributes to the maintenance of the glomerular filtration barrier integrity. Genome Res. 2015, 25, 57–65. [Google Scholar] [CrossRef]
- Worsley, A.L.; Lui, D.H.; Ntow-Boahene, W.; Song, W.; Good, L.; Tsui, J. The importance of inflammation control for the treatment of chronic diabetic wounds. Int. Wound J. 2023, 20, 2346–2359. [Google Scholar] [CrossRef]
- Huang, Y.; Kyriakides, T.R. The role of extracellular matrix in the pathophysiology of diabetic wounds. Matrix Biol. Plus 2020, 6–7, 100037. [Google Scholar] [CrossRef]
- Chang, M. Targeting Matrix Metalloproteinase-9 for Therapeutic Intervention in Diabetic Foot Ulcers. ACS Pharmacol. Transl. Sci. 2024. [Google Scholar] [CrossRef]
- Mariottoni, P.; Jiang, S.W.; Prestwood, C.A.; Jain, V.; Suwanpradid, J.; Whitley, M.J.; Coates, M.; Brown, D.A.; Erdmann, D.; Corcoran, D.L.; et al. Single-Cell RNA Sequencing Reveals Cellular and Transcriptional Changes Associated with M1 Macrophage Polarization in Hidradenitis Suppurativa. Front. Med. 2021, 8, 665873. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).