Changes in Mitochondrial Function and Cell Death Patterns in Peripheral Blood Mononuclear Cells during Trastuzumab Treatment Following Doxorubicin Chemotherapy
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Patient Population
2.3. Data Collection
2.4. Cardiac Biomarkers
2.5. The Analysis of Mitochondrial Respiration, Oxidative Stress, and Cell Death in PBMCs
2.6. Echocardiography
2.7. Statistical Analysis
3. Results
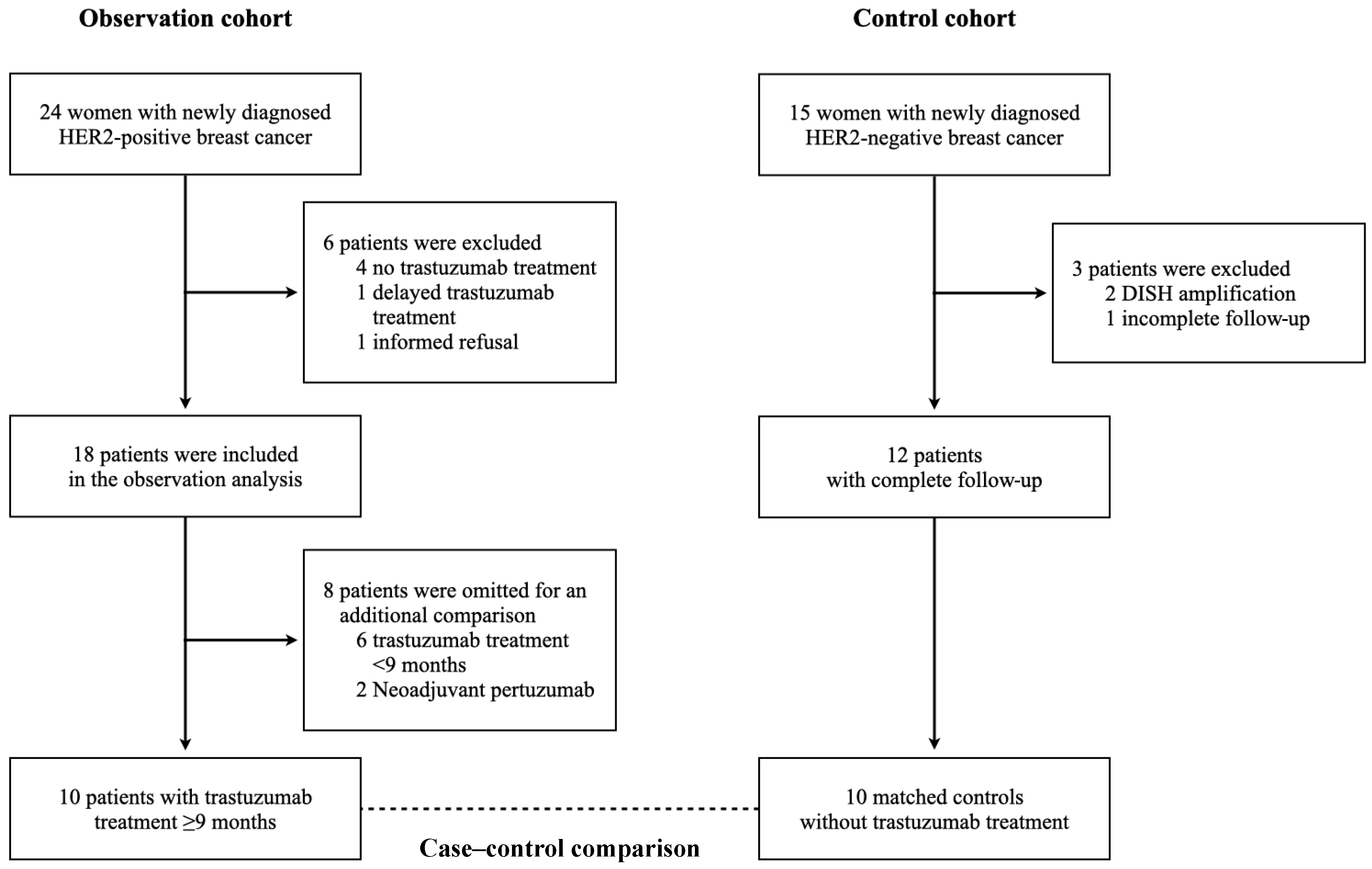
3.1. Patients
3.2. Clinical Data, Cardiac Biomarkers, and Echocardiography
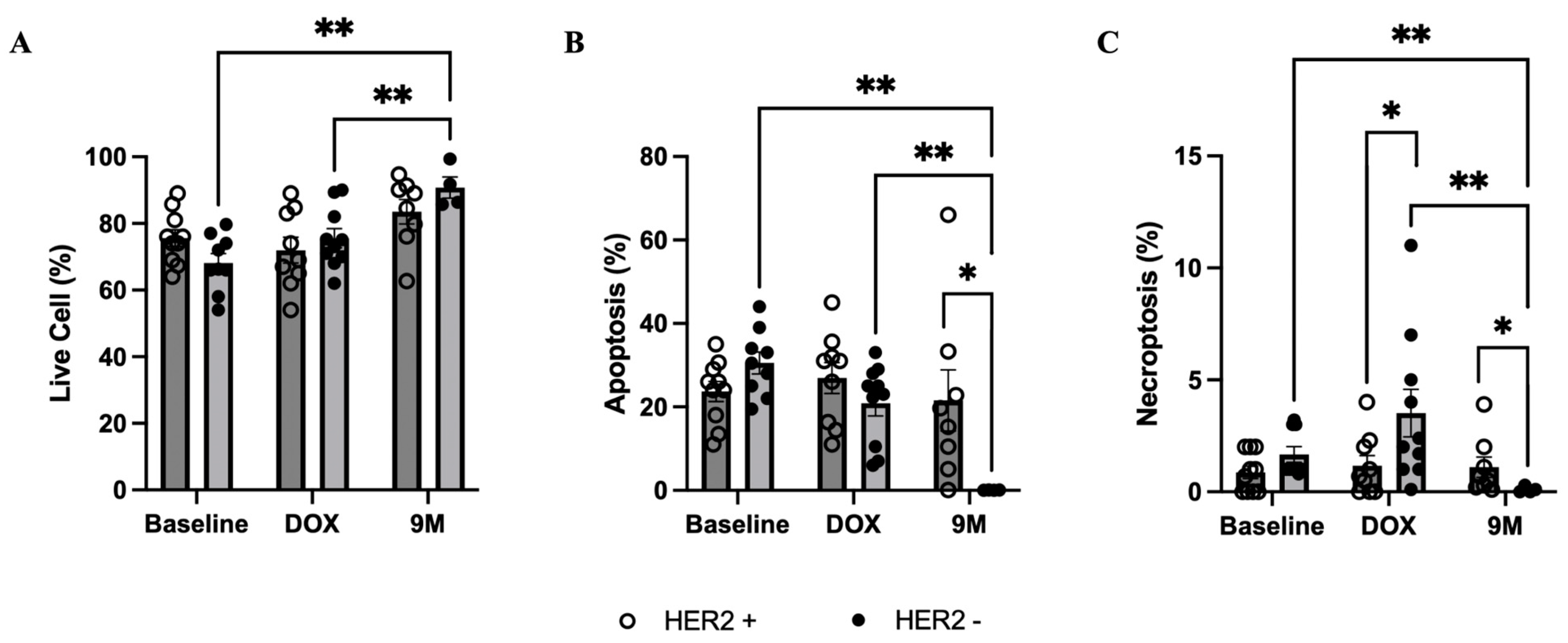
3.3. Mitochondrial Respiration, Oxidative Stress, and Cell Death in PBMCs
3.4. Case-Control Comparison
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Piccart-Gebhart, M.J.; Procter, M.; Leyland-Jones, B.; Goldhirsch, A.; Untch, M.; Smith, I.; Gianni, L.; Baselga, J.; Bell, R.; Jackisch, C.; et al. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N. Engl. J. Med. 2005, 353, 1659–1672. [Google Scholar] [CrossRef]
- Romond, E.H.; Perez, E.A.; Bryant, J.; Suman, V.J.; Geyer, C.E., Jr.; Davidson, N.E.; Tan-Chiu, E.; Martino, S.; Paik, S.; Kaufman, P.A.; et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N. Engl. J. Med. 2005, 353, 1673–1684. [Google Scholar] [CrossRef]
- Spielmann, M.; Roché, H.; Delozier, T.; Canon, J.L.; Romieu, G.; Bourgeois, H.; Extra, J.M.; Serin, D.; Kerbrat, P.; Machiels, J.P.; et al. Trastuzumab for patients with axillary-node-positive breast cancer: Results of the FNCLCC-PACS 04 trial. J. Clin. Oncol. 2009, 27, 6129–6134. [Google Scholar] [CrossRef]
- Slamon, D.J.; Leyland-Jones, B.; Shak, S.; Fuchs, H.; Paton, V.; Bajamonde, A.; Fleming, T.; Eiermann, W.; Wolter, J.; Pegram, M.; et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N. Engl. J. Med. 2001, 344, 783–792. [Google Scholar] [CrossRef]
- Marty, M.; Cognetti, F.; Maraninchi, D.; Snyder, R.; Mauriac, L.; Tubiana-Hulin, M.; Chan, S.; Grimes, D.; Antón, A.; Lluch, A.; et al. Randomized phase II trial of the efficacy and safety of trastuzumab combined with docetaxel in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer administered as first-line treatment: The M77001 study group. J. Clin. Oncol. 2005, 23, 4265–4274. [Google Scholar] [CrossRef] [PubMed]
- Grazette, L.P.; Boecker, W.; Matsui, T.; Semigran, M.; Force, T.L.; Hajjar, R.J.; Rosenzweig, A. Inhibition of ErbB2 causes mitochondrial dysfunction in cardiomyocytes: Implications for herceptin-induced cardiomyopathy. J. Am. Coll. Cardiol. 2004, 44, 2231–2238. [Google Scholar] [CrossRef]
- Rohrbach, S.; Muller-Werdan, U.; Werdan, K.; Koch, S.; Gellerich, N.F.; Holtz, J. Apoptosis-modulating interaction of the neuregulin/erbB pathway with anthracyclines in regulating Bcl-xS and Bcl-xL in cardiomyocytes. J. Mol. Cell. Cardiol. 2005, 38, 485–493. [Google Scholar] [CrossRef] [PubMed]
- Gordon, L.I.; Burke, M.A.; Singh, A.T.; Prachand, S.; Lieberman, E.D.; Sun, L.; Naik, T.J.; Prasad, S.V.; Ardehali, H. Blockade of the erbB2 receptor induces cardiomyocyte death through mitochondrial and reactive oxygen species-dependent pathways. J. Biol. Chem. 2009, 284, 2080–2087. [Google Scholar] [CrossRef]
- Ozturk, M.; Ozler, M.; Kurt, Y.G.; Ozturk, B.; Uysal, B.; Ersoz, N.; Yasar, M.; Demirbas, S.; Kurt, B.; Acikel, C.; et al. Efficacy of melatonin, mercaptoethylguanidine and 1400W in doxorubicin- and trastuzumab-induced cardiotoxicity. J. Pineal Res. 2011, 50, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Ewer, M.S.; Vooletich, M.T.; Durand, J.B.; Woods, M.L.; Davis, J.R.; Valero, V.; Lenihan, D.J. Reversibility of trastuzumab-related cardiotoxicity: New insights based on clinical course and response to medical treatment. J. Clin. Oncol. 2005, 23, 7820–7826. [Google Scholar] [CrossRef]
- Li, P.; Wang, B.; Sun, F.; Li, Y.; Li, Q.; Lang, H.; Zhao, Z.; Gao, P.; Zhao, Y.; Shang, Q.; et al. Mitochondrial respiratory dysfunctions of blood mononuclear cells link with cardiac disturbance in patients with early-stage heart failure. Sci. Rep. 2015, 5, 10229. [Google Scholar] [CrossRef]
- Shirakawa, R.; Yokota, T.; Nakajima, T.; Takada, S.; Yamane, M.; Furihata, T.; Maekawa, S.; Nambu, H.; Katayama, T.; Fukushima, A.; et al. Mitochondrial reactive oxygen species generation in blood cells is associated with disease severity and exercise intolerance in heart failure patients. Sci. Rep. 2019, 9, 14709. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Wang, D.D.; Qiu, Y.; Airhart, S.; Liu, Y.; Stempien-Otero, A.; O’Brien, K.D.; Tian, R. Boosting NAD level suppresses inflammatory activation of PBMCs in heart failure. J. Clin. Investig. 2020, 130, 6054–6063. [Google Scholar] [CrossRef] [PubMed]
- Petricevic, B.; Laengle, J.; Singer, J.; Sachet, M.; Fazekas, J.; Steger, G.; Bartsch, R.; Jensen-Jarolim, E.; Bergmann, M. Trastuzumab mediates antibody-dependent cell-mediated cytotoxicity and phagocytosis to the same extent in both adjuvant and metastatic HER2/neu breast cancer patients. J. Transl. Med. 2013, 11, 307. [Google Scholar] [CrossRef] [PubMed]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. Ann. Intern. Med. 2007, 147, 573–577. [Google Scholar] [CrossRef] [PubMed]
- Lyon, A.R.; López-Fernández, T.; Couch, L.S.; Asteggiano, R.; Aznar, M.C.; Bergler-Klein, J.; Boriani, G.; Cardinale, D.; Cordoba, R.; Cosyns, B.; et al. 2022 ESC Guidelines on cardio-oncology developed in collaboration with the European Hematology Association (EHA), the European Society for Therapeutic Radiology and Oncology (ESTRO) and the International Cardio-Oncology Society (IC-OS). Eur. Heart J. 2022, 43, 4229–4361. [Google Scholar] [CrossRef]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2015, 28, 1–39.e14. [Google Scholar] [CrossRef]
- Plana, J.C.; Galderisi, M.; Barac, A.; Ewer, M.S.; Ky, B.; Scherrer-Crosbie, M.; Ganame, J.; Sebag, I.A.; Agler, D.A.; Badano, L.P.; et al. Expert consensus for multimodality imaging evaluation of adult patients during and after cancer therapy: A report from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2014, 27, 911–939. [Google Scholar] [CrossRef]
- Zamorano, J.L.; Lancellotti, P.; Rodriguez Muñoz, D.; Aboyans, V.; Asteggiano, R.; Galderisi, M.; Habib, G.; Lenihan, D.J.; Lip, G.Y.H.; Lyon, A.R.; et al. 2016 ESC Position Paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC Committee for Practice Guidelines: The Task Force for cancer treatments and cardiovascular toxicity of the European Society of Cardiology (ESC). Eur. Heart J. 2016, 37, 2768–2801. [Google Scholar] [CrossRef] [PubMed]
- Christensen, I.B.; Abrahamsen, M.L.; Ribas, L.; Buch-Larsen, K.; Marina, D.; Andersson, M.; Larsen, S.; Schwarz, P.; Dela, F.; Gillberg, L. Peripheral blood mononuclear cells exhibit increased mitochondrial respiration after adjuvant chemo- and radiotherapy for early breast cancer. Cancer Med. 2023, 12, 16985–16996. [Google Scholar] [CrossRef] [PubMed]
- Lacourt, T.E.; Kavelaars, A.; Tripathy, D.; Heijnen, C.J. Associations between fatigue and cellular metabolism in breast cancer patients: A longitudinal study. Psychoneuroendocrinology 2022, 144, 105866. [Google Scholar] [CrossRef] [PubMed]
- Barron, C.C.; Alhussein, M.M.; Kaur, U.; Cosman, T.L.; Tyagi, N.K.; Brown, M.; Mukherjee, S.D.; Ellis, P.M.; Dhesy-Thind, S.; Leong, D.P. An evaluation of the safety of continuing trastuzumab despite overt left ventricular dysfunction. Curr. Oncol. 2019, 26, 240–246. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Gao, Y.; Li, H.; Wang, X.; Jin, M.; Shen, Z.; Yang, D.; Zhang, X.; Wei, Z.; Chen, Z.; et al. Association between oxidative stress, mitochondrial function of peripheral blood mononuclear cells and gastrointestinal cancers. J. Transl. Med. 2023, 21, 107. [Google Scholar] [CrossRef]
- Gaynor, N.; Blanco, A.; Madden, S.F.; Moran, B.; Fletcher, J.M.; Kaukonen, D.; Ramírez, J.S.; Eustace, A.J.; McDermott, M.S.J.; Canonici, A.; et al. Alterations in immune cell phenotype and cytotoxic capacity in HER2+ breast cancer patients receiving HER2-targeted neo-adjuvant therapy. Br. J. Cancer 2023, 129, 1022–1031. [Google Scholar] [CrossRef] [PubMed]




| Clinical Data | Observation Cohort | Case-Control Comparison | ||
|---|---|---|---|---|
| HER2 + (n = 18) | HER2 + (n = 10) | HER2 − (n = 10) | p-Value a | |
| Age (years) | 48.8 ± 9.1 | 46.5 ± 10.7 | 49.0 ± 7.5 | 0.55 |
| Height (cm) | 157.4 ± 6.0 | 156.6 ± 6.4 | 156.2 ± 6.8 | 0.89 |
| Weight (kg) | 59.9 ± 9.8 | 57.7 ± 7.6 | 57.0 ± 14.6 | 0.90 |
| Serum creatinine (mg/dL) | 0.70 ± 0.15 | 0.67 ± 0.14 | 0.70 ± 0.13 | 0.55 |
| Comorbidities (%) | ||||
| Hypertension | 2 (11.1) | 1 (10) | 1 (10) | 1.00 |
| Dyslipidemia | 6 (33.3) | 2 (20) | 2 (20) | 1.00 |
| Diabetes | 0 (0) | 0 (0) | 0 (0) | - |
| Atherosclerotic CVD | 0 (0) | 0 (0) | 0 (0) | - |
| Smoking history (%) | 0 (0) | 0 (0) | 0 (0) | - |
| Left-side cancer (%) | 8 (44.4) | 4 (40) | 5 (50) | 1.00 |
| Doxorubicin chemotherapy | ||||
| Total dose (mg/m2) | 223 ± 8 | 224 ± 9 | 222 ± 10 | 0.72 |
| Neoadjuvant setting (%) | 6 (33.3) | 4 (40) | 3 (30) | 1.00 |
| Trastuzumab treatment (%) | N/A | - | ||
| Subcutaneous administration | 9 (50) | 3 (30) | ||
| Neoadjuvant setting (combined with pertuzumab) | 2 (11.1) | 0 (0) | ||
| ≥9 months postoperative to treatment | 12 (66.7) | 10 (100) | ||
| Radiotherapy (%) | 16 (88.9) | 10 (100) | 5 (50) | 0.03 |
| Medications (%) | ||||
| ACEIs or ARBs | 2 (11.1) | 1 (10) | 0 (0) | - |
| BBs | 1 (5.6) | 0 (0) | 1 (10) | - |
| Statins | 5 (27.8) | 1 (10) | 0 (0) | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leemasawat, K.; Osataphan, N.; Apaijai, N.; Yanpiset, P.; Phrommintikul, A.; Somwangprasert, A.; Chattipakorn, S.C.; Chattipakorn, N. Changes in Mitochondrial Function and Cell Death Patterns in Peripheral Blood Mononuclear Cells during Trastuzumab Treatment Following Doxorubicin Chemotherapy. Biomedicines 2024, 12, 1970. https://doi.org/10.3390/biomedicines12091970
Leemasawat K, Osataphan N, Apaijai N, Yanpiset P, Phrommintikul A, Somwangprasert A, Chattipakorn SC, Chattipakorn N. Changes in Mitochondrial Function and Cell Death Patterns in Peripheral Blood Mononuclear Cells during Trastuzumab Treatment Following Doxorubicin Chemotherapy. Biomedicines. 2024; 12(9):1970. https://doi.org/10.3390/biomedicines12091970
Chicago/Turabian StyleLeemasawat, Krit, Nichanan Osataphan, Nattayaporn Apaijai, Panat Yanpiset, Arintaya Phrommintikul, Areewan Somwangprasert, Siriporn C. Chattipakorn, and Nipon Chattipakorn. 2024. "Changes in Mitochondrial Function and Cell Death Patterns in Peripheral Blood Mononuclear Cells during Trastuzumab Treatment Following Doxorubicin Chemotherapy" Biomedicines 12, no. 9: 1970. https://doi.org/10.3390/biomedicines12091970
APA StyleLeemasawat, K., Osataphan, N., Apaijai, N., Yanpiset, P., Phrommintikul, A., Somwangprasert, A., Chattipakorn, S. C., & Chattipakorn, N. (2024). Changes in Mitochondrial Function and Cell Death Patterns in Peripheral Blood Mononuclear Cells during Trastuzumab Treatment Following Doxorubicin Chemotherapy. Biomedicines, 12(9), 1970. https://doi.org/10.3390/biomedicines12091970







