Abnormally High Expression of DNAJB6 Accelerates Malignant Progression of Lung Adenocarcinoma
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Collection
2.2. Survival and Mutation Analyses
2.3. Univariate and Multivariate Analyses for DNAJB6 and Nomogram Construction
2.4. Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) Pathway Enrichment
2.5. Tumor-Infiltrating Immune Cell Analysis
2.6. Immunohistochemical (IHC) Assay
2.7. Cell Culture
2.8. Transfection Assay
2.9. Lentivirus Transduction
2.10. Cell Proliferation, Cell Cycle, and Cell Colony Formation
2.11. WB
2.12. RNA-Seq
2.13. Nude Mouse Xenograft Model
2.14. Statistical Analysis
3. Results
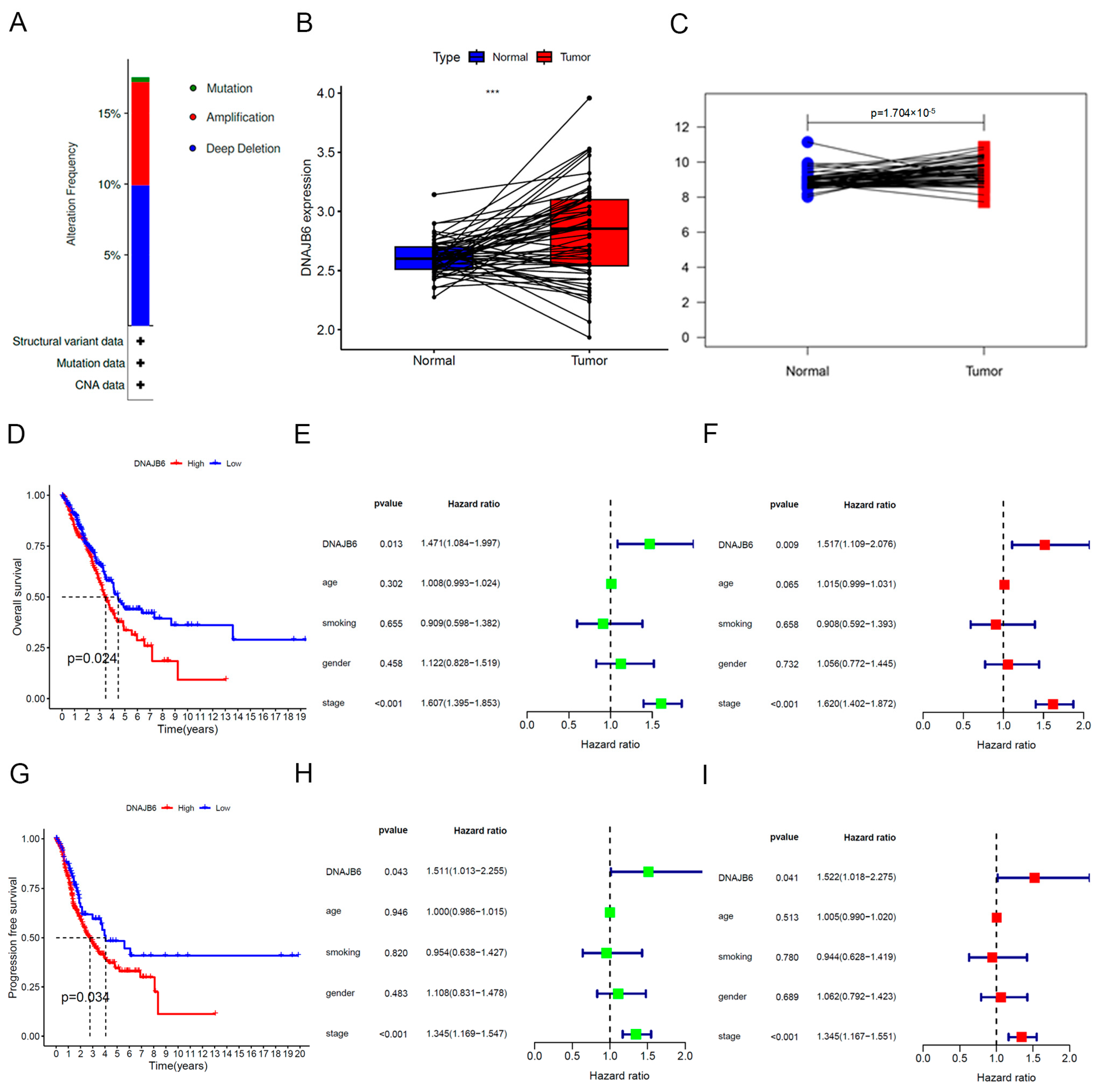
3.1. DNAJB6 Expression Is Abnormally Elevated in Several Human Cancers
3.2. DNAJB6 mRNA Levels Are Elevated in the LUAD-TCGA Dataset and Correlate with a Poor Prognosis
3.3. Establishment of a Nomogram Based on DNAJB6
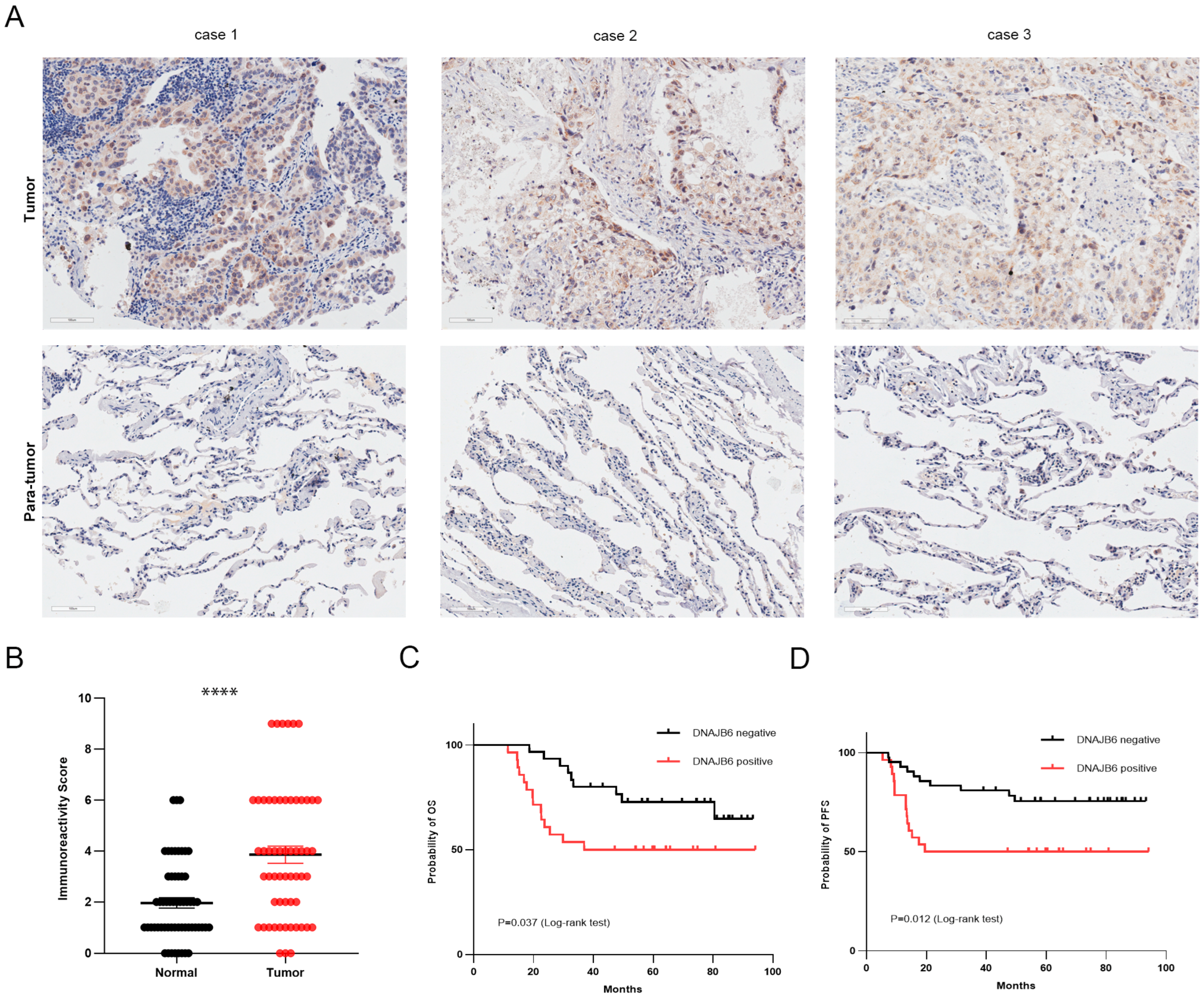
3.4. DNAJB6 Protein Levels Are Elevated in LUAD and Correlate with a Poor Prognosis in Clinical Samples
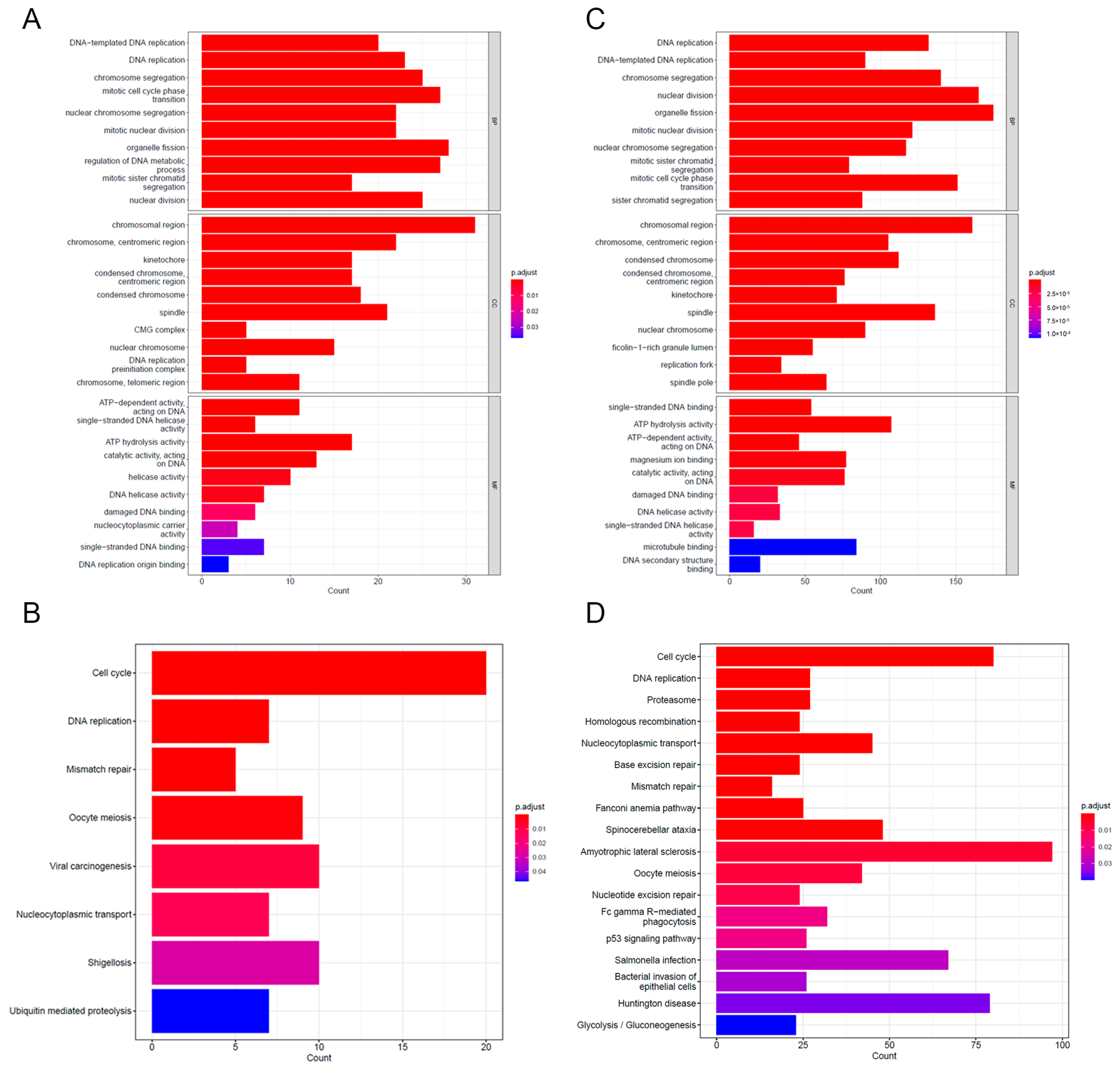
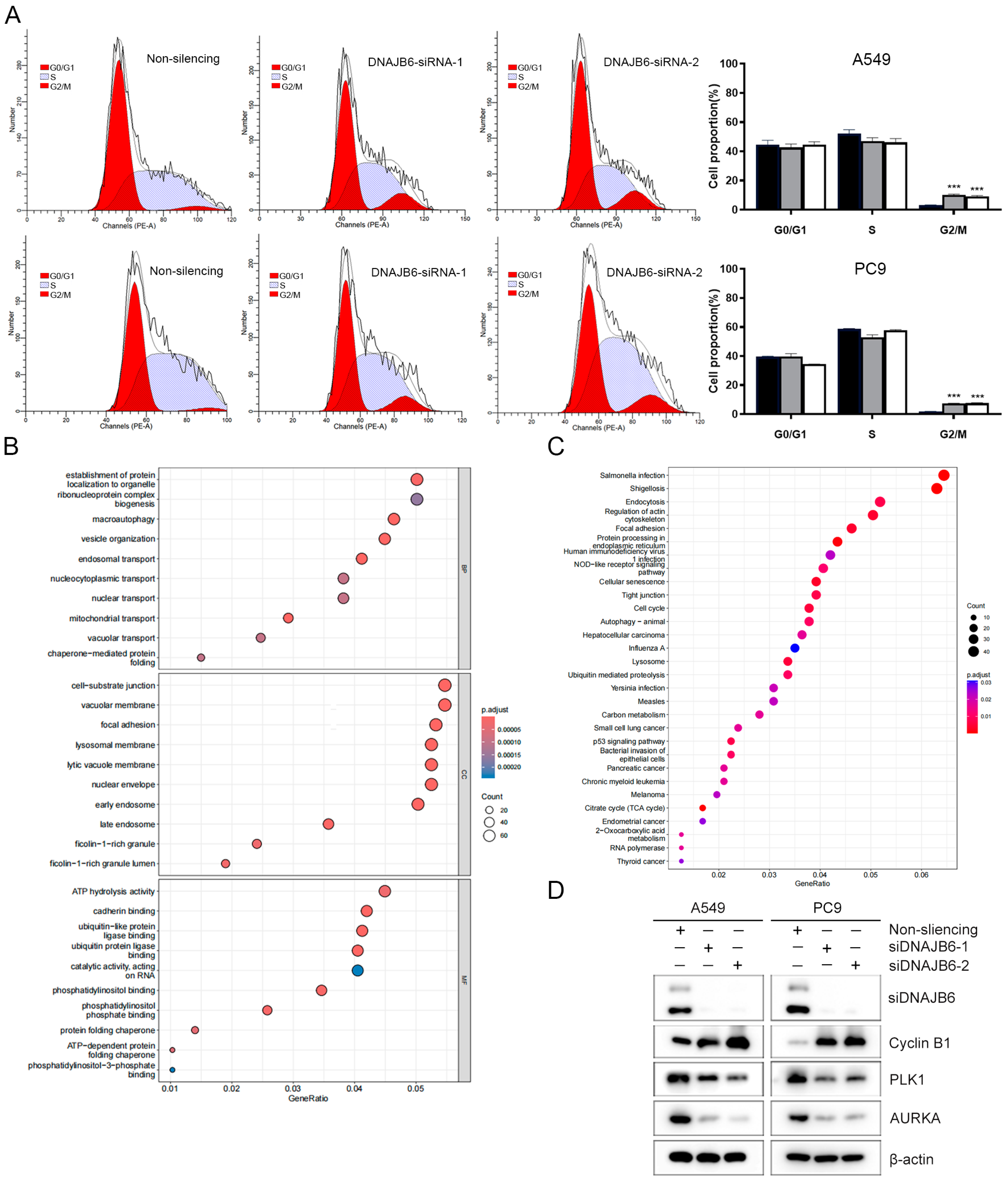
3.5. GO and KEGG Enrichment Analyses of DEGs
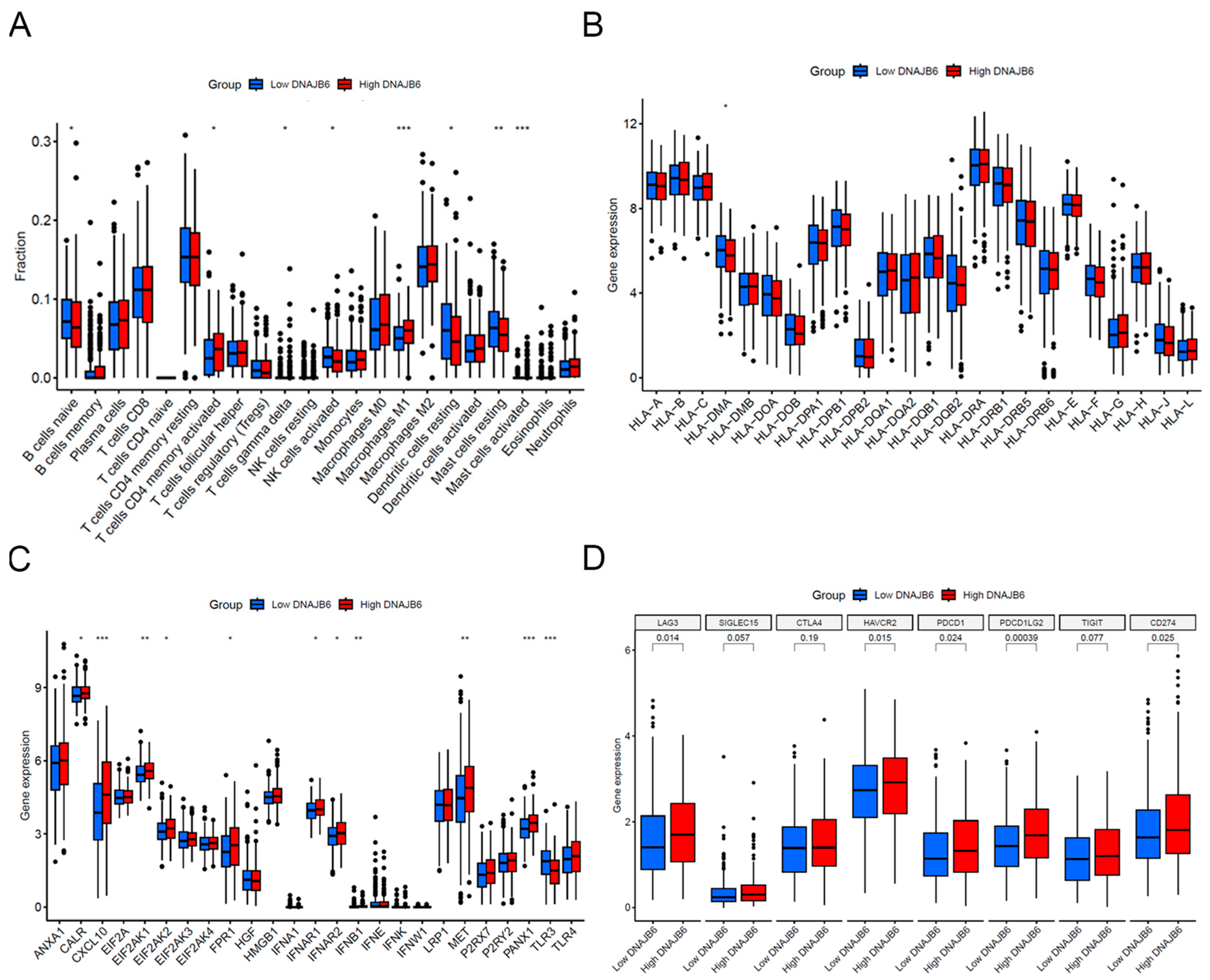
3.6. Immune Infiltration Analysis between Low and High DNAJB6 Expression Profiles
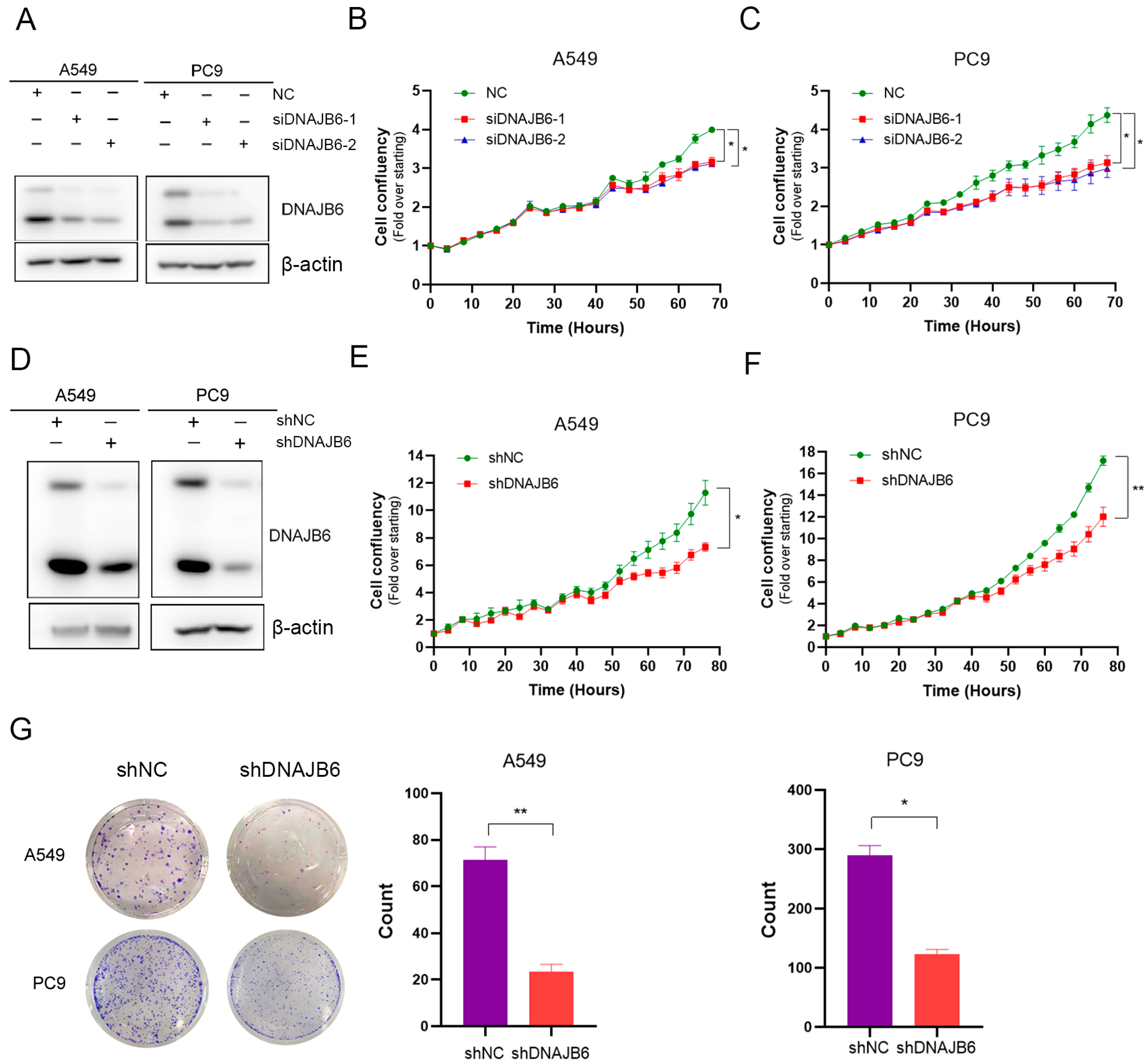
3.7. DNAJB6 Knockdown Inhibits LUAD Cell Proliferation In Vitro
3.8. DNAJB6 Knockdown Induces G2/M Phase Arrest in LUAD Cells
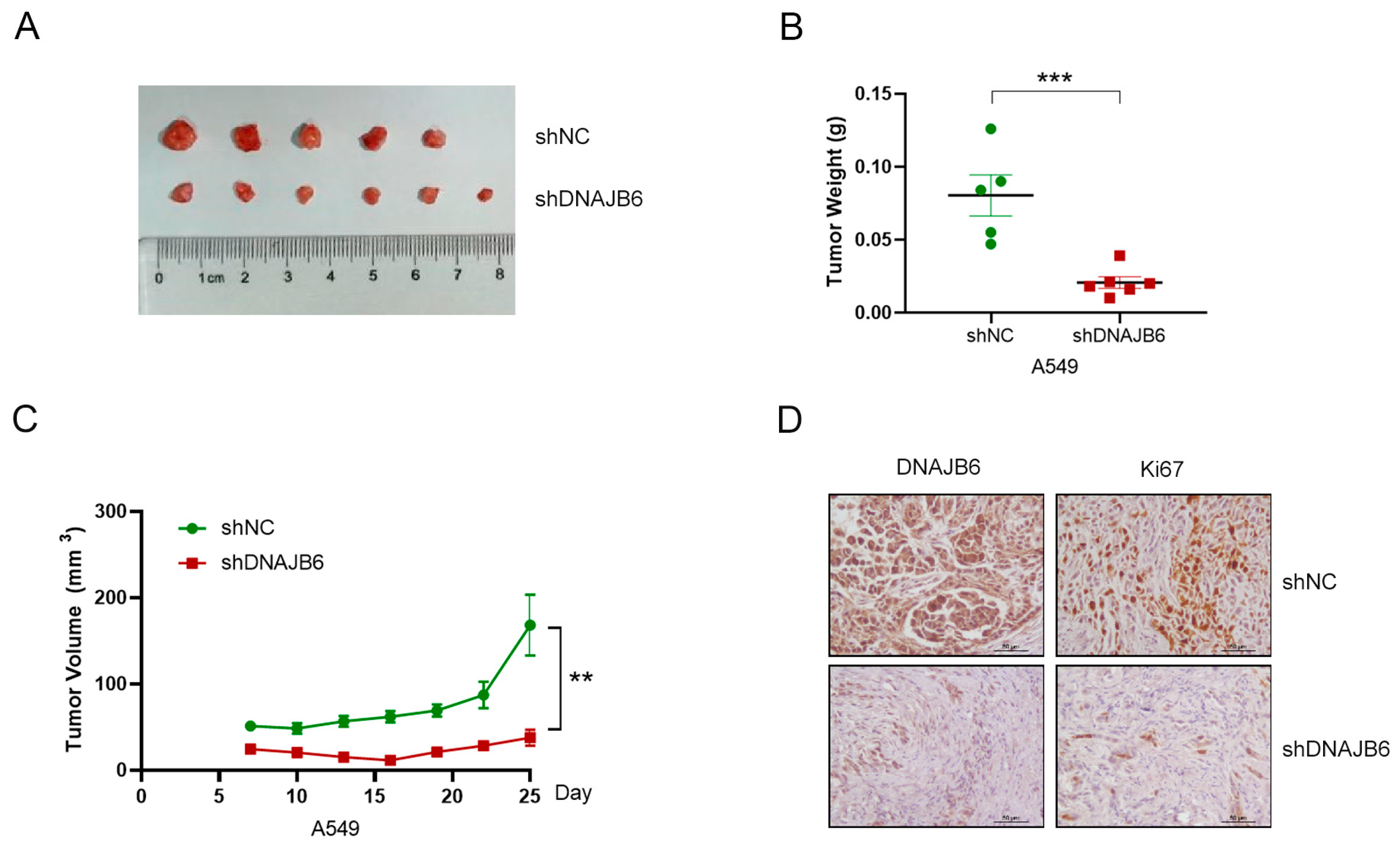
3.9. DNAJB6 Knockdown Suppresses LUAD Tumor Growth In Vivo
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| LUAD | Lung adenocarcinoma |
| LIHC | Liver hepatocellular carcinoma |
| LUSC | Lung squamous cell carcinoma |
| GO | Gene Ontology |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| HSPs | Heat shock proteins |
| WB | Western blotting |
| DEGs | Differentially expressed genes |
| TCGA | The Cancer Genome Atlas |
| IHC | Immunohistochemical |
| IRS | Immune response score |
| DAB | Diaminobenzidine |
| BRCA | Breast invasive carcinoma |
| CHOL | Cholangiocarcinoma |
| COAD | Colon adenocarcinoma |
| ESCA | Esophageal carcinoma |
| HNSC | Head and neck squamous cell carcinoma |
| NK | Natural killer |
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Radziszewska, A.; Karczmarek-Borowska, B.; Gradalska-Lampart, M.; Filip, A.A. Epidemiology, prevention and risk morbidity factors for lung cancer. Pol. Merkur. Lekarski. 2015, 38, 113–118. [Google Scholar]
- Bade, B.C.; Dela Cruz, C.S. Lung Cancer 2020: Epidemiology, Etiology, and Prevention. Clin. Chest Med. 2020, 41, 1–24. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, J.; Yuan, C.; Luo, Y.; Li, Y.; Dai, P.; Sun, W.; Zhang, N.; Ren, J.; Zhang, J.; et al. Establishment of the Prognostic Index Reflecting Tumor Immune Microenvironment of Lung Adenocarcinoma Based on Metabolism-Related Genes. J. Cancer 2020, 11, 7101–7115. [Google Scholar] [CrossRef]
- Wang, X.; Chen, M.; Zhou, J.; Zhang, X. HSP27, 70 and 90, anti-apoptotic proteins, in clinical cancer therapy (Review). Int. J. Oncol. 2014, 45, 18–30. [Google Scholar] [CrossRef] [PubMed]
- Jhaveri, K.; Taldone, T.; Modi, S.; Chiosis, G. Advances in the clinical development of heat shock protein 90 (Hsp90) inhibitors in cancers. Biochim. Biophys. Acta 2012, 1823, 742–755. [Google Scholar] [CrossRef] [PubMed]
- Kampinga, H.H.; Hageman, J.; Vos, M.J.; Kubota, H.; Tanguay, R.M.; Bruford, E.A.; Cheetham, M.E.; Chen, B.; Hightower, L.E. Guidelines for the nomenclature of the human heat shock proteins. Cell Stress. Chaperones 2009, 14, 105–111. [Google Scholar] [CrossRef]
- Lianos, G.D.; Alexiou, G.A.; Mangano, A.; Mangano, A.; Rausei, S.; Boni, L.; Dionigi, G.; Roukos, D.H. The role of heat shock proteins in cancer. Cancer Lett. 2015, 360, 114–118. [Google Scholar] [CrossRef]
- Izawa, I.; Nishizawa, M.; Ohtakara, K.; Ohtsuka, K.; Inada, H.; Inagaki, M. Identification of Mrj, a DnaJ/Hsp40 family protein, as a keratin 8/18 filament regulatory protein. J. Biol. Chem. 2000, 275, 34521–34527. [Google Scholar] [CrossRef]
- Kampinga, H.H.; Craig, E.A. The HSP70 chaperone machinery: J proteins as drivers of functional specificity. Nat. Rev. Mol. Cell Biol. 2010, 11, 579–592. [Google Scholar] [CrossRef]
- Diane, A.; Abunada, H.; Khattab, N.; Moin, A.S.M.; Butler, A.E.; Dehbi, M. Role of the DNAJ/HSP40 family in the pathogenesis of insulin resistance and type 2 diabetes. Ageing Res. Rev. 2021, 67, 101313. [Google Scholar] [CrossRef]
- Sterrenberg, J.N.; Blatch, G.L.; Edkins, A.L. Human DNAJ in cancer and stem cells. Cancer Lett. 2011, 312, 129–142. [Google Scholar] [CrossRef]
- Abayev-Avraham, M.; Salzberg, Y.; Gliksberg, D.; Oren-Suissa, M.; Rosenzweig, R. DNAJB6 mutants display toxic gain of function through unregulated interaction with Hsp70 chaperones. Nat. Commun. 2023, 14, 7066. [Google Scholar] [CrossRef]
- Ruggieri, A.; Saredi, S.; Zanotti, S.; Pasanisi, M.B.; Maggi, L.; Mora, M. DNAJB6 Myopathies: Focused Review on an Emerging and Expanding Group of Myopathies. Front. Mol. Biosci. 2016, 3, 63. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Long, P.A.; Bos, J.M.; Shih, Y.H.; Ma, X.; Sundsbak, R.S.; Chen, J.; Jiang, Y.; Zhao, L.; Hu, X.; et al. A modifier screen identifies DNAJB6 as a cardiomyopathy susceptibility gene. JCI Insight 2016, 1, e88797. [Google Scholar] [CrossRef]
- Yabe, I.; Tanino, M.; Yaguchi, H.; Takiyama, A.; Cai, H.; Kanno, H.; Takahashi, I.; Hayashi, Y.K.; Watanabe, M.; Takahashi, H.; et al. Pathology of frontotemporal dementia with limb girdle muscular dystrophy caused by a DNAJB6 mutation. Clin. Neurol. Neurosurg. 2014, 127, 10–12. [Google Scholar] [CrossRef] [PubMed]
- Bason, M.; Meister-Broekema, M.; Alberts, N.; Dijkers, P.; Bergink, S.; Sibon, O.C.M.; Kampinga, H.H. Astrocytic expression of the chaperone DNAJB6 results in non-cell autonomous protection in Huntington’s disease. Neurobiol. Dis. 2019, 124, 108–117. [Google Scholar] [CrossRef]
- Durrenberger, P.F.; Filiou, M.D.; Moran, L.B.; Michael, G.J.; Novoselov, S.; Cheetham, M.E.; Clark, P.; Pearce, R.K.; Graeber, M.B. DnaJB6 is present in the core of Lewy bodies and is highly up-regulated in parkinsonian astrocytes. J. Neurosci. Res. 2009, 87, 238–245. [Google Scholar] [CrossRef]
- Mansson, C.; Arosio, P.; Hussein, R.; Kampinga, H.H.; Hashem, R.M.; Boelens, W.C.; Dobson, C.M.; Knowles, T.P.; Linse, S.; Emanuelsson, C. Interaction of the molecular chaperone DNAJB6 with growing amyloid-beta 42 (Abeta42) aggregates leads to sub-stoichiometric inhibition of amyloid formation. J. Biol. Chem. 2014, 289, 31066–31076. [Google Scholar] [CrossRef]
- Ko, S.H.; Liau, Y.J.; Chi, Y.H.; Lai, M.J.; Chiang, Y.P.; Lu, C.Y.; Chang, L.Y.; Tarn, W.Y.; Huang, L.M. Interference of DNAJB6/MRJ Isoform Switch by Morpholino Inhibits Replication of HIV-1 and RSV. Mol. Ther. Nucleic Acids 2019, 14, 251–261. [Google Scholar] [CrossRef]
- Gao, J.; Aksoy, B.A.; Dogrusoz, U.; Dresdner, G.; Gross, B.; Sumer, S.O.; Sun, Y.; Jacobsen, A.; Sinha, R.; Larsson, E.; et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci. Signal. 2013, 6, pl1. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.C.; Wang, G.Z.; Wen, Z.S.; Zhou, Y.C.; Hu, Q.; Zhang, B.; Qu, L.W.; Gao, S.H.; Liu, J.; Ma, L.; et al. Systematic identification of CDC34 that functions to stabilize EGFR and promote lung carcinogenesis. EBioMedicine 2020, 53, 102689. [Google Scholar] [CrossRef]
- Qian, Y.; Galan-Cobo, A.; Guijarro, I.; Dang, M.; Molkentine, D.; Poteete, A.; Zhang, F.; Wang, Q.; Wang, J.; Parra, E.; et al. MCT4-dependent lactate secretion suppresses antitumor immunity in LKB1-deficient lung adenocarcinoma. Cancer Cell 2023, 41, 1363–1380.e7. [Google Scholar] [CrossRef]
- Gao, J.; Ao, Y.Q.; Zhang, L.X.; Deng, J.; Wang, S.; Wang, H.K.; Jiang, J.H.; Ding, J.Y. Exosomal circZNF451 restrains anti-PD1 treatment in lung adenocarcinoma via polarizing macrophages by complexing with TRIM56 and FXR1. J. Exp. Clin. Cancer Res. CR 2022, 41, 295. [Google Scholar] [CrossRef]
- Mitra, A.; Shevde, L.A.; Samant, R.S. Multi-faceted role of HSP40 in cancer. Clin. Exp. Metastasis 2009, 26, 559–567. [Google Scholar] [CrossRef]
- Mitra, A.; Fillmore, R.A.; Metge, B.J.; Rajesh, M.; Xi, Y.; King, J.; Ju, J.; Pannell, L.; Shevde, L.A.; Samant, R.S. Large isoform of MRJ (DNAJB6) reduces malignant activity of breast cancer. Breast Cancer Res. BCR 2008, 10, R22. [Google Scholar] [CrossRef]
- Mitra, A.; Rostas, J.W.; Dyess, D.L.; Shevde, L.A.; Samant, R.S. Micro-RNA-632 downregulates DNAJB6 in breast cancer. Lab. Investig. 2012, 92, 1310–1317. [Google Scholar] [CrossRef] [PubMed]
- Tien, J.F.; Mazloomian, A.; Cheng, S.G.; Hughes, C.S.; Chow, C.C.T.; Canapi, L.T.; Oloumi, A.; Trigo-Gonzalez, G.; Bashashati, A.; Xu, J.; et al. CDK12 regulates alternative last exon mRNA splicing and promotes breast cancer cell invasion. Nucleic Acids Res. 2017, 45, 6698–6716. [Google Scholar] [CrossRef]
- Yu, V.Z.; Wong, V.C.; Dai, W.; Ko, J.M.; Lam, A.K.; Chan, K.W.; Samant, R.S.; Lung, H.L.; Shuen, W.H.; Law, S.; et al. Nuclear Localization of DNAJB6 Is Associated with Survival of Patients with Esophageal Cancer and Reduces AKT Signaling and Proliferation of Cancer Cells. Gastroenterology 2015, 149, 1825–1836.e5. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.T.; Jiang, Y.Y.; Shang, L.; Shi, Z.Z.; Liang, J.W.; Wang, Z.; Zhang, Y.; Hao, J.J.; Jia, X.M.; Xu, X.; et al. Overexpression of DNAJB6 promotes colorectal cancer cell invasion through an IQGAP1/ERK-dependent signaling pathway. Mol. Carcinog. 2015, 54, 1205–1213. [Google Scholar] [CrossRef]
- Sezer, A.; Kilickap, S.; Gümüş, M.; Bondarenko, I.; Özgüroğlu, M.; Gogishvili, M.; Turk, H.M.; Cicin, I.; Bentsion, D.; Gladkov, O.; et al. Cemiplimab monotherapy for first-line treatment of advanced non-small-cell lung cancer with PD-L1 of at least 50%: A multicentre, open-label, global, phase 3, randomised, controlled trial. Lancet 2021, 397, 592–604. [Google Scholar] [CrossRef]
- Hellmann, M.D.; Paz-Ares, L.; Bernabe Caro, R.; Zurawski, B.; Kim, S.W.; Carcereny Costa, E.; Park, K.; Alexandru, A.; Lupinacci, L.; de la Mora Jimenez, E.; et al. Nivolumab plus Ipilimumab in Advanced Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2019, 381, 2020–2031. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, D.; Xiao, J.; Du, Y.; Zhang, L.; Qin, X. Abnormally High Expression of DNAJB6 Accelerates Malignant Progression of Lung Adenocarcinoma. Biomedicines 2024, 12, 1981. https://doi.org/10.3390/biomedicines12091981
Wang D, Xiao J, Du Y, Zhang L, Qin X. Abnormally High Expression of DNAJB6 Accelerates Malignant Progression of Lung Adenocarcinoma. Biomedicines. 2024; 12(9):1981. https://doi.org/10.3390/biomedicines12091981
Chicago/Turabian StyleWang, Di, Jiayu Xiao, Yang Du, Li Zhang, and Xuzhen Qin. 2024. "Abnormally High Expression of DNAJB6 Accelerates Malignant Progression of Lung Adenocarcinoma" Biomedicines 12, no. 9: 1981. https://doi.org/10.3390/biomedicines12091981
APA StyleWang, D., Xiao, J., Du, Y., Zhang, L., & Qin, X. (2024). Abnormally High Expression of DNAJB6 Accelerates Malignant Progression of Lung Adenocarcinoma. Biomedicines, 12(9), 1981. https://doi.org/10.3390/biomedicines12091981





