Decreased Protein C Pathway Activity in COVID-19 Compared to Non-COVID Sepsis: An Observational and Comparative Cohort Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Participant Enrollment and Eligibility Criteria
2.2. Study Procedures
2.3. Laboratory Analysis of Blood Samples
2.4. Statistical Analysis
3. Results
3.1. Study Population Characteristics
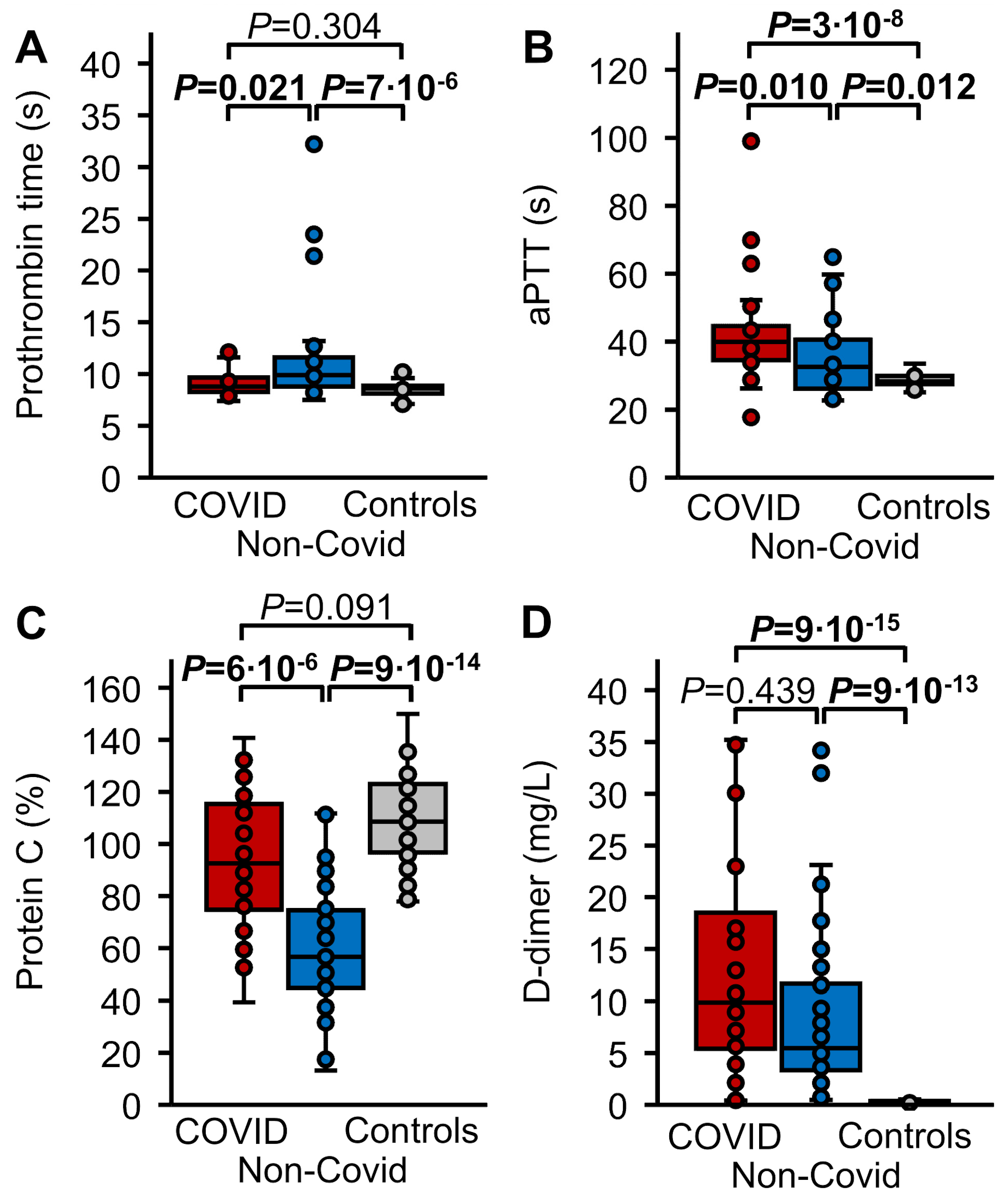
3.2. Routine Hemostasis Parameters
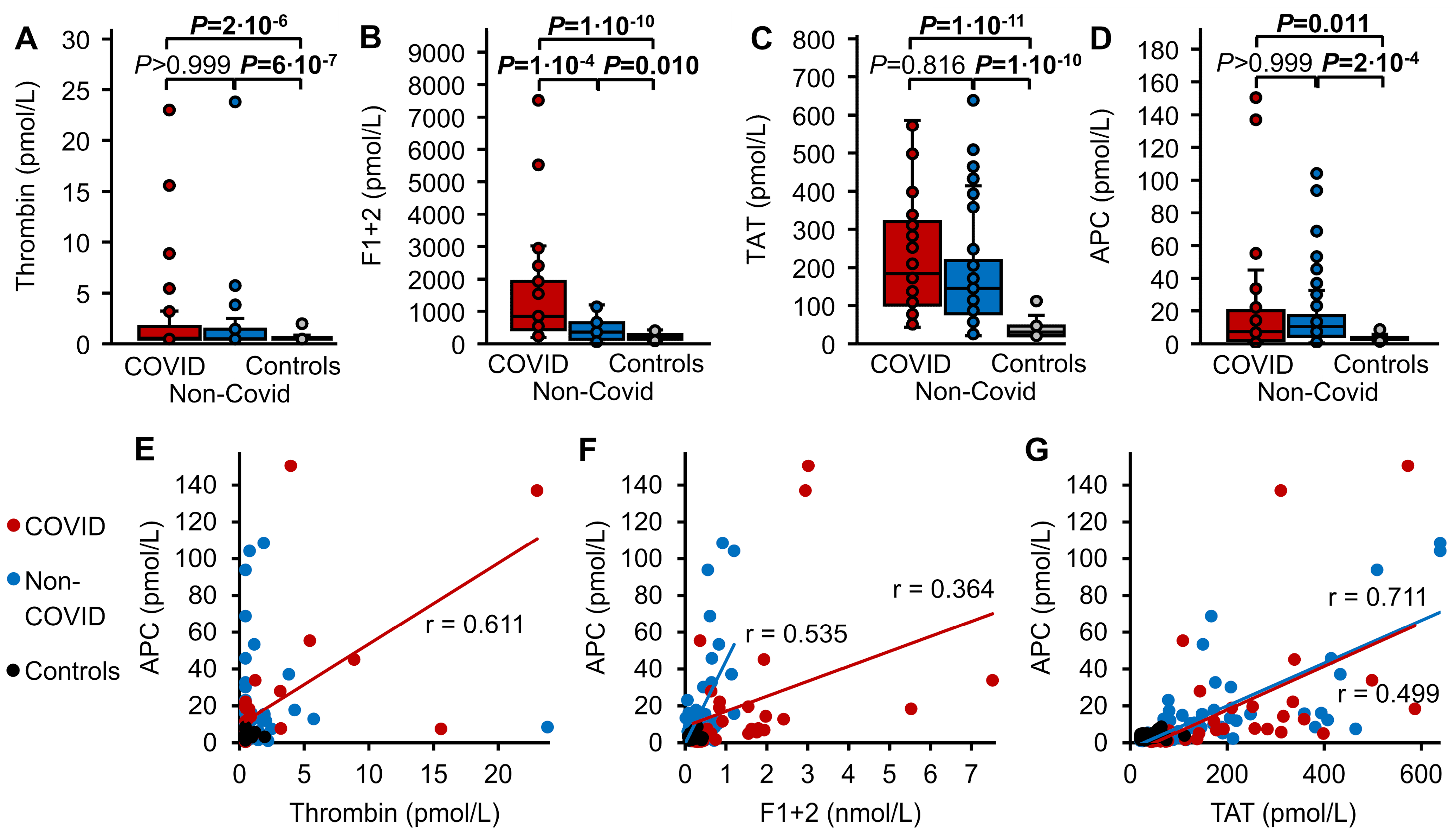
3.3. Thrombin Markers and APC
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.-D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef]
- Levi, M.; van der Poll, T. Coagulation and sepsis. Thromb. Res. 2016, 149, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Bode, C.; Weis, S.; Sauer, A.; Wendel-Garcia, P.; David, S. Targeting the host response in sepsis: Current approaches and future evidence. Crit. Care 2023, 27, 478. [Google Scholar] [CrossRef] [PubMed]
- Gando, S.; Levi, M.; Toh, C.-H. Disseminated intravascular coagulation. Nat. Rev. Dis. Prim. 2016, 2, 16037. [Google Scholar] [CrossRef] [PubMed]
- Levi, M.; Poll, T. Coagulation in Patients with Severe Sepsis. Semin. Thromb. Hemost. 2015, 41, 009–015. [Google Scholar] [CrossRef]
- Bouck, E.G.; Denorme, F.; Holle, L.A.; Middelton, E.A.; Blair, A.M.; de Laat, B.; Schiffman, J.D.; Yost, C.C.; Rondina, M.T.; Wolberg, A.S.; et al. COVID-19 and Sepsis Are Associated With Different Abnormalities in Plasma Procoagulant and Fibrinolytic Activity. Arter. Thromb. Vasc. Biol. 2020, 41, 401–414. [Google Scholar] [CrossRef]
- Juneja, G.K.; Castelo, M.; Yeh, C.H.; Cerroni, S.E.; Hansen, B.E.; Chessum, J.E.; Abraham, J.; Cani, E.; Dwivedi, D.J.; Fraser, D.D.; et al. Biomarkers of coagulation, endothelial function, and fibrinolysis in critically ill patients with COVID-19: A single-center prospective longitudinal study. J. Thromb. Haemost. 2021, 19, 1546–1557. [Google Scholar] [CrossRef]
- Hardaway, R.M.; Williams, C.H.; Vasquez, Y. Disseminated Intravascular Coagulation in Sepsis. Semin. Thromb. Hemost. 2001, 27, 577–584. [Google Scholar] [CrossRef]
- Xu, J.; Esmon, N.L.; Esmon, C.T. Reconstitution of the Human Endothelial Cell Protein C Receptor with Thrombomodulin in Phosphatidylcholine Vesicles Enhances Protein C Activation. J. Biol. Chem. 1999, 274, 6704–6710. [Google Scholar] [CrossRef]
- Griffin, J.H.; Zlokovic, B.V.; Mosnier, L.O. Activated protein C, protease activated receptor 1, and neuroprotection. Blood 2018, 132, 159–169. [Google Scholar] [CrossRef]
- Conway, E.M.; Mackman, N.; Warren, R.Q.; Wolberg, A.S.; Mosnier, L.O.; Campbell, R.A.; Gralinski, L.E.; Rondina, M.T.; van de Veerdonk, F.L.; Hoffmeister, K.M.; et al. Understanding COVID-19-associated coagulopathy. Nat. Rev. Immunol. 2022, 22, 639–649. [Google Scholar] [CrossRef] [PubMed]
- Stanne, T.M.; Pedersen, A.; Gisslén, M.; Jern, C. Low admission protein C levels are a risk factor for disease worsening and mortality in hospitalized patients with COVID-19. Thromb. Res. 2021, 204, 13–15. [Google Scholar] [CrossRef]
- Mast, A.E.; Wolberg, A.S.; Gailani, D.; Garvin, M.R.; Alvarez, C.; Miller, J.I.; Aronow, B.; Jacobson, D. SARS-CoV-2 suppresses anticoagulant and fibrinolytic gene expression in the lung. eLife 2021, 10, e64330. [Google Scholar] [CrossRef]
- Francischetti, I.M.; Toomer, K.; Zhang, Y.; Jani, J.; Siddiqui, Z.; Brotman, D.J.; Hooper, J.E.; Kickler, T.S. Upregulation of pulmonary tissue factor, loss of thrombomodulin and immunothrombosis in SARS-CoV-2 infection. eClinicalMedicine 2021, 39, 101069. [Google Scholar] [CrossRef] [PubMed]
- Bayrakci, N.; Ozkan, G.; Mutlu, L.C.; Erdem, L.; Yildirim, I.; Gulen, D.; Celikkol, A. Relationship between serum soluble en-dothelial protein C receptor level and COVID-19 findings. Blood Coagul. Fibrinolysis 2021, 32, 550–555. [Google Scholar] [CrossRef]
- Won, T.; Wood, M.K.; Hughes, D.M.; Talor, M.V.; Ma, Z.; Schneider, J.; Skinner, J.T.; Asady, B.; Goerlich, E.; Halushka, M.K.; et al. Endothelial thrombomodulin downregulation caused by hypoxia contributes to severe infiltration and coagulopathy in COVID-19 patient lungs. EBioMedicine 2022, 75, 103812. [Google Scholar] [CrossRef] [PubMed]
- Becher, T.; Müller, J.; Akin, I.; Baumann, S.; Bosch, K.; Stach, K.; Borggrefe, M.; Pötzsch, B.; Loßnitzer, D. The evolution of activated protein C plasma levels in septic shock and its association with mortality: A prospective observational study. J. Crit. Care 2018, 47, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Becher, T.; Müller, J.; Akin, I.; Baumann, S.; Stach, K.; Borggrefe, M.; Pötzsch, B.; Loßnitzer, D. Characterization of circulating thrombin in patients with septic shock: A prospective observational study. J. Thromb. Thrombolysis 2019, 50, 90–97. [Google Scholar] [CrossRef]
- Schuppert, A.; Polotzek, K.; Schmitt, J.; Busse, R.; Karschau, J.; Karagiannidis, C. Different spreading dynamics througout Germany during the second wave of the COVID-19 pandemic: A time series study based on national surveillance data. Lancet Reg. Health–Eur. 2021, 6, 100151. [Google Scholar] [CrossRef]
- Dellinger, R.P.; Levy, M.M.; Rhodes, A.; Annane, D.; Gerlach, H.; Opal, S.M.; Sevransky, J.E.; Sprung, C.L.; Douglas, I.S.; Jaeschke, R.; et al. Surviving Sepsis Campaign: International Guidelines for Management of Severe Sepsis and Septic Shock, 2012. Intensive Care Med. 2013, 41, 165–228. [Google Scholar] [CrossRef]
- Müller, J.; Becher, T.; Braunstein, J.; Berdel, P.; Gravius, S.; Rohrbach, F.; Oldenburg, J.; Mayer, G.; Pötzsch, B. Profiling of Active Thrombin in Human Blood by Supramolecular Complexes. Angew. Chem. Int. Ed. 2011, 50, 6075–6078. [Google Scholar] [CrossRef] [PubMed]
- Müller, J.; Friedrich, M.; Becher, T.; Braunstein, J.; Kupper, T.; Berdel, P.; Gravius, S.; Rohrbach, F.; Oldenburg, J.; Mayer, G.; et al. Monitoring of plasma levels of activated protein C using a clinically applicable oligonucleotide-based enzyme capture assay. J. Thromb. Haemost. 2012, 10, 390–398. [Google Scholar] [CrossRef] [PubMed]
- Billoir, P.; Leprêtre, P.; Thill, C.; Bellien, J.; Duchez, V.L.C.; Selim, J.; Tamion, F.; Clavier, T.; Besnier, E. Routine and Advanced Laboratory Tests for Hemostasis Disorders in COVID-19 Patients: A Prospective Cohort Study. J. Clin. Med. 2022, 11, 1383. [Google Scholar] [CrossRef] [PubMed]
- Hvas, C.L.; Larsen, J.B.; Adelborg, K.; Christensen, S.; Hvas, A.-M. Dynamic Hemostasis and Fibrinolysis Assays in Intensive Care COVID-19 Patients and Association with Thrombosis and Bleeding—A Systematic Review and a Cohort Study. Semin. Thromb. Hemost. 2021, 48, 031–054. [Google Scholar] [CrossRef] [PubMed]
- Rühl, H.; Berens, C.; Winterhagen, F.I.; Reda, S.; Müller, J.; Oldenburg, J.; Pötzsch, B. Increased Activated Protein C Response Rates Reduce the Thrombotic Risk of Factor V Leiden Carriers But Not of Prothrombin 20210G>A Carriers. Circ. Res. 2019, 125, 523–534. [Google Scholar] [CrossRef]
- Flaumenhaft, R.; Enjyoji, K.; Schmaier, A.A. Vasculopathy in COVID-19. Blood 2022, 140, 222–235. [Google Scholar] [CrossRef]
- Bonaventura, A.; Vecchié, A.; Dagna, L.; Martinod, K.; Dixon, D.L.; Van Tassell, B.W.; Dentali, F.; Montecucco, F.; Massberg, S.; Levi, M.; et al. Endothelial dysfunction and immunothrombosis as key pathogenic mechanisms in COVID-19. Nat. Rev. Immunol. 2021, 21, 319–329. [Google Scholar] [CrossRef]
- Renzi, S.; Landoni, G.; Zangrillo, A.; Ciceri, F. MicroCLOTS pathophysiology in coronavirus disease 2019. Korean J. Intern. Med. 2020, 38, 570–571. [Google Scholar] [CrossRef]
- Meini, S.; Zanichelli, A.; Sbrojavacca, R.; Iuri, F.; Roberts, A.T.; Suffritti, C.; Tascini, C. Understanding the Pathophysiology of COVID-19: Could the Contact System Be the Key? Front. Immunol. 2020, 11, 2014. [Google Scholar] [CrossRef]
- Nicosia, R.F.; Ligresti, G.; Caporarello, N.; Akilesh, S.; Ribatti, D. COVID-19 Vasculopathy: Mounting Evidence for an Indirect Mecahnism of Endothelial Injury. Am. J. Pathol. 2021, 191, 1374–1384. [Google Scholar] [CrossRef]
- Iba, T.; Levy, J.H.; Maier, C.L.; Connors, J.M.; Levi, M. Four years into the pandemic, managing COVID-19 patients with acute coagulopathy: What have we learned? J. Thromb. Haemost. 2024, 22, 1541–1549. [Google Scholar] [CrossRef] [PubMed]
- Vincent, J.L.; Francois, B.; Zabolotskikh, I.; Daga, M.K.; Lascarrou, J.B.; Kirov, M.Y.; Pettilä, V.; Wittebole, X.; Meziani, F.; Mercier, E.; et al. Effect of a Recombinant Human Soluble Thrombomodulin on Mortality in Patients With Sepsis-Associated Coagulopathy: The SCARLET Randomized Clinical Trial. JAMA 2019, 321, 1993–2002. [Google Scholar] [CrossRef] [PubMed]
- Seeliger, B.; Doebler, M.; Hofmaenner, D.A.; Wendel-Garcia, P.D.M.; Schuepbach, R.A.; Schmidt, J.J.; Welte, T.; Hoeper, M.M.; Gillmann, H.-J.; Kuehn, C.; et al. Intracranial Hemorrhages on Extracorporeal Membrane Oxygenation: Differences Between COVID-19 and Other Viral Acute Respiratory Distress Syndrome. Crit. Care Med. 2022, 50, e526–e538. [Google Scholar] [CrossRef] [PubMed]


| COVID-19 (n = 30) | Non-COVID | Stimulated Healthy Controls (n = 40) | p * | |
|---|---|---|---|---|
| Age in years | 63 (59–66) | 63 (52–71) | 29 (27–48) | 0.54 (3 × 10−12) |
| Male sex | 26 (87%) | 38 (81%) | 20 (50%) | 0.553 (0.007) |
| Deceased | 20 (67%) | 18 (38%) | - | 0.015 |
| Diabetes | 11 (37%) | 14 (30%) | - | 0.527 |
| Immunosuppression | 3 (10%) | 7 (15%) | - | 0.732 |
| Malignant disease | 2 (7%) | 12 (26%) | - | 0.066 |
| End-stage renal failure | 1 (3%) | 5 (11%) | - | 0.395 |
| Mechanical ventilation | 28 (93%) † | 33 (70%) | - | 0.020 |
| Therapeutic heparin | 15 (50%) | 0 | - | 4 × 10−8 |
| SOFA score | 13 (11–13) | 12 (11–14) | - | 0.932 |
| Procalcitonin | 0.5 (0.4–1.1) | 28.5 (1.6–50.0) ‡ | - | 1 × 10−7 |
| CRP (mg/L) | 186 (114–240) § | 207 (125–298) | - | 0.376 |
| WBCs (109/L) | 9.9 (8.0–16.0) | 11.6 (5.6–20.5) | 6.1 (4.9–6.8) | 0.534 (3 × 10−6) |
| Platelets (109/L) | 133 (91–184) | 181 (115–269) | 251 (215–287) | 0.204 (3 × 10−5) |
| COVID-19 (n = 30) | Non-COVID (n = 47) | Stimulated Healthy Controls (n = 40) | |
|---|---|---|---|
| APC/Thrombin | 5.5 (2.7–18.7) | 11.3 (3.9–25.4) | data |
| APC/F1+2 | 0.005 (0.003–0.014) | 0.024 (0.012–0.068) | 0.018 (0.012–0.023) |
| APC/TAT | 0.04 (0.02–0.07) | 0.06 (0.03–0.14) | 0.11 (0.07–0.14) |
| APC/PC (pmol/L) | 6.7 (2.4–23.0) * | 15.3 (5.9–39.1) † | 3.0 (2.2–4.0) |
| (APC/PC)/Thrombin | 9.0 (3.4–24.0) | 19.4 (5.9–59.0) | 5.1 (4.4–7.2) |
| (APC/PC)/F1+2 | 0.007 (0.003–0.018) | 0.041 (0.017–0.151) | 0.016 (0.012–0.024) |
| (APC/PC)/TAT | 0.04 (0.02–0.09) | 0.12 (0.05–0.28) | 0.11 (0.07–0.14) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rühl, H.; Bode, C.; Becher, T.; Eckert, S.; Mohsen, G.; McRae, H.L.; Müller, J.; Reda, S.; Loßnitzer, D.; Oldenburg, J.; et al. Decreased Protein C Pathway Activity in COVID-19 Compared to Non-COVID Sepsis: An Observational and Comparative Cohort Study. Biomedicines 2024, 12, 1982. https://doi.org/10.3390/biomedicines12091982
Rühl H, Bode C, Becher T, Eckert S, Mohsen G, McRae HL, Müller J, Reda S, Loßnitzer D, Oldenburg J, et al. Decreased Protein C Pathway Activity in COVID-19 Compared to Non-COVID Sepsis: An Observational and Comparative Cohort Study. Biomedicines. 2024; 12(9):1982. https://doi.org/10.3390/biomedicines12091982
Chicago/Turabian StyleRühl, Heiko, Christian Bode, Tobias Becher, Sebastian Eckert, Ghaith Mohsen, Hannah L. McRae, Jens Müller, Sara Reda, Dirk Loßnitzer, Johannes Oldenburg, and et al. 2024. "Decreased Protein C Pathway Activity in COVID-19 Compared to Non-COVID Sepsis: An Observational and Comparative Cohort Study" Biomedicines 12, no. 9: 1982. https://doi.org/10.3390/biomedicines12091982
APA StyleRühl, H., Bode, C., Becher, T., Eckert, S., Mohsen, G., McRae, H. L., Müller, J., Reda, S., Loßnitzer, D., Oldenburg, J., Putensen, C., & Pötzsch, B. (2024). Decreased Protein C Pathway Activity in COVID-19 Compared to Non-COVID Sepsis: An Observational and Comparative Cohort Study. Biomedicines, 12(9), 1982. https://doi.org/10.3390/biomedicines12091982








