Exploring the Relationships between Lifestyle Patterns and Epigenetic Biological Age Measures in Men
Abstract
:1. Introduction
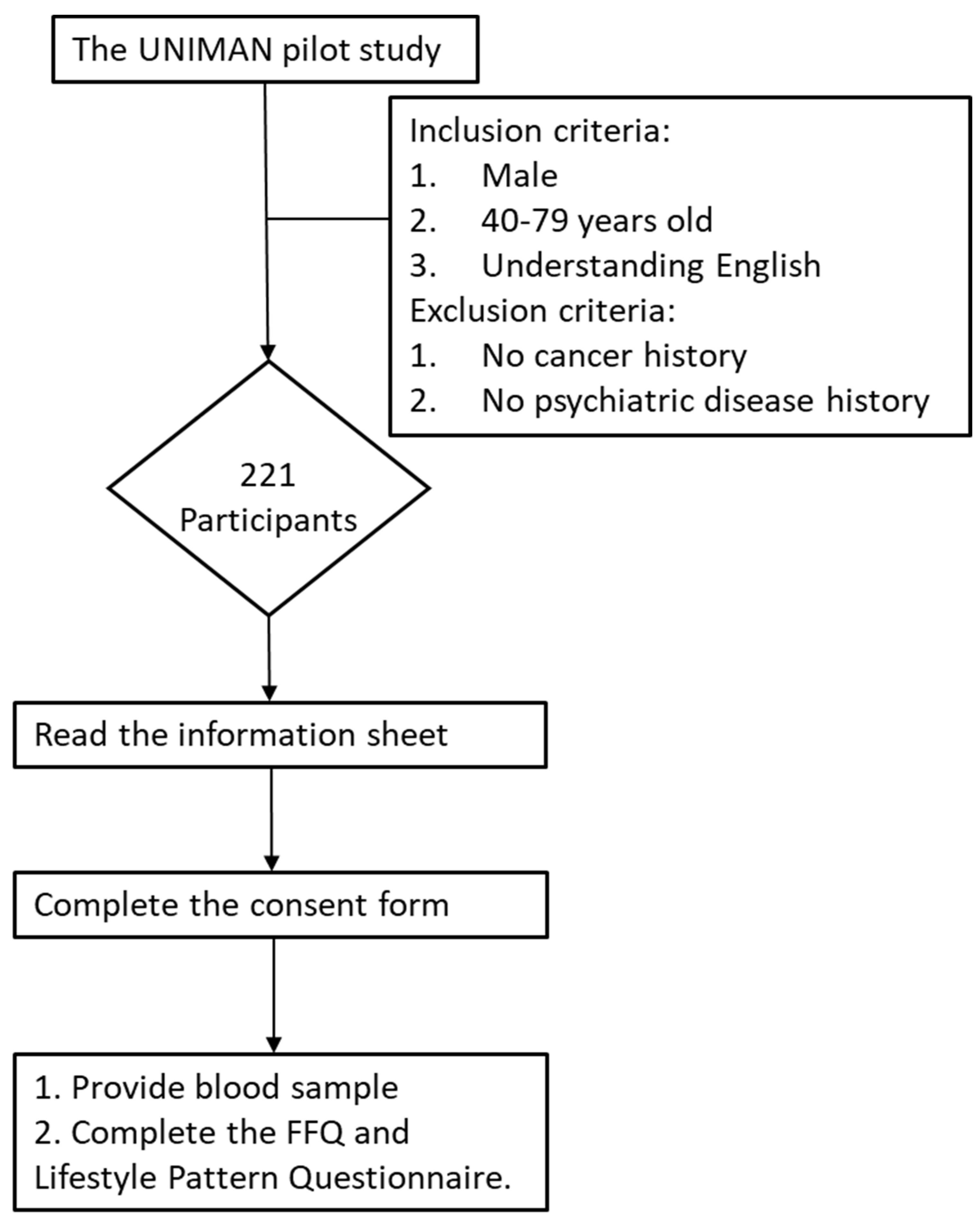
2. Materials and Methods
2.1. Study Population
2.2. Assessment of Lifestyle-Related Exposures
2.3. Blood Sample Analysis: Epigenetic Data Process and Quality Control
2.4. Data Analysis and Statistics
2.5. Ethics
3. Results
3.1. Participants in the Study
3.2. The Distribution of Lifestyle-Related Factors Analysed in Relation to Epigenetic Biological Age
| Lifestyle-Related Factors | Number of Participants |
|---|---|
| Body mass index (BMI) (n = 214) | |
| Obese (BMI ≥ 30) | 59 (27.57%) |
| Overweight (25 ≤ BMI < 30) | 94 (43.93%) |
| Normal (18.5 ≤ BMI < 25) | 61 (28.50%) |
| BMI (con) (n = 214) | 28.30 ± 5.78 (14.98–61.31) # |
| Smoking status (n = 147) | |
| Current smokers | 3 (2.04%) |
| Previous smokers | 53 (36.05%) |
| Never | 91 (61.90%) |
| Alcohol consumption (n = 139) | |
| More than 7 drinks/week † | 57 (41.01%) |
| No more than 7 drinks/week † | 76 (54.68%) |
| Never | 6 (4.32%) |
| Physical activity (n = 139) | |
| No exercise habit | 2 (1.44%) |
| Exercise occasionally | 27 (19.42%) |
| Exercise routinely (at least 3 times a week, 30 min a day) | 110 (79.14%) |
| Cholesterol (n = 154) | |
| >300 mg/day | 32 (20.78%) |
| ≤300 mg/day | 122 (79.22%) |
| Cholesterol (con) (n = 154) | 227.46 ± 113.49 (59.91–809.13) # |
| Saturated fat (n = 154) | |
| Male > 30 g/day | 41 (26.62%) |
| Male ≤ 30 g/day | 113 (73.38%) |
| Saturated fat (as continuous) (n = 154) | 26.05 ± 10.97 (9.3–79.68) # |
| Total sugar (n = 154) | |
| >90 g/day | 106 (68.83%) |
| ≤90 g/day | 48 (31.17%) |
| Total sugar (as continuous) (n = 154) | 116.74 ± 42.21 (42.07–302.34) # |
| Vitamin D (n = 154) | |
| <10 mcg/day | 134 (87.01%) |
| ≥10 mcg/day | 20 (12.99%) |
| Vitamin D (as continuous) (n = 154) | 4.98 ± 4.07 (0.66–23.28) # |
| Daily total calories (n = 154) | |
| Male > 2500 kcal/day, Female > 2000 kcal/day | 37 (24.03%) |
| Male ≤ 2500 kcal/day, Female ≤ 2000 kcal/day | 117 (75.97%) |
| Daily calories (as continuous) (n = 154) | 2129.79 ± 609.20 (1020.43–4194.43) # |
| Healthy life index (HLI) (as continuous score) (n = 133) | 18.29 ± 1.72 (14–22) # |
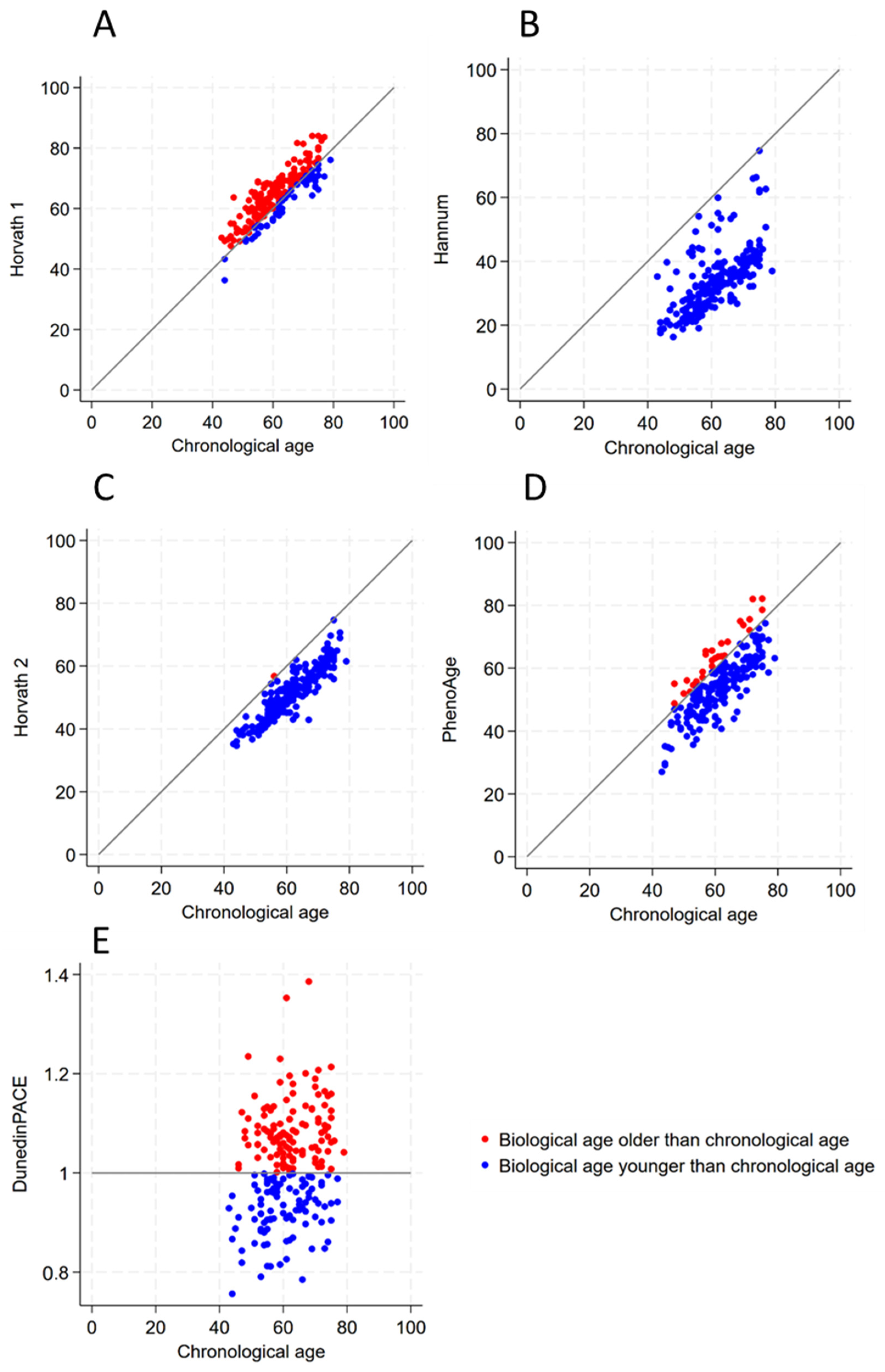
3.3. The Results of Epigenetic Markers
| Mean | SD | Minimum | Maximum | Correlation # with Chronological Age | |
|---|---|---|---|---|---|
| Biological Clocks | |||||
| Horvath 1 [16] | 64.22 | 8.18 | 36.30 | 84.04 | 0.860 * |
| Hannum [14] | 34.22 | 9.69 | 16.31 | 74.63 | 0.693 * |
| Horvath 2 (Blood & Skin Cock) [19] | 51.40 | 7.90 | 34.66 | 74.63 | 0.909 * |
| PhenoAge [20] | 54.83 | 9.64 | 27.02 | 82.16 | 0.802 * |
| DunedinPACE [21] | 1.01 | 0.10 | 0.76 | 1.39 | 0.205 * |
| Accelerated aging (AA) † | |||||
| Horvath 1 AA | 2.82 | 4.48 | −8.75 | 16.68 | - |
| Hannum AA | −27.19 | 7.68 | −42.03 | −0.37 | - |
| Horvath 2 AA | −10.00 | 3.61 | −24.07 | 0.76 | - |
| PhenoAge AA | −6.57 | 5.95 | −22.03 | 10.04 | - |
| DunedinPACE AA | - | - | - | - | - |
| Biological Clock Age Greater than Chronological Age | Biological Clock Age Less than or Equal to Chronological Age | Total Number | |
|---|---|---|---|
| Horvath 1 [16] | 158 (73.83%) | 56 (26.17%) | 214 |
| Hannum [14] | 0 (0%) | 214 (100%) | 214 |
| Horvath 2 [19] | 1 (0.47%) | 213 (99.53%) | 214 |
| PhenoAge [20] | 27 (12.62%) | 187 (87.38%) | 214 |
| DunedinPACE [21] | 116 (54.21%) | 98 (45.79%) | 214 |
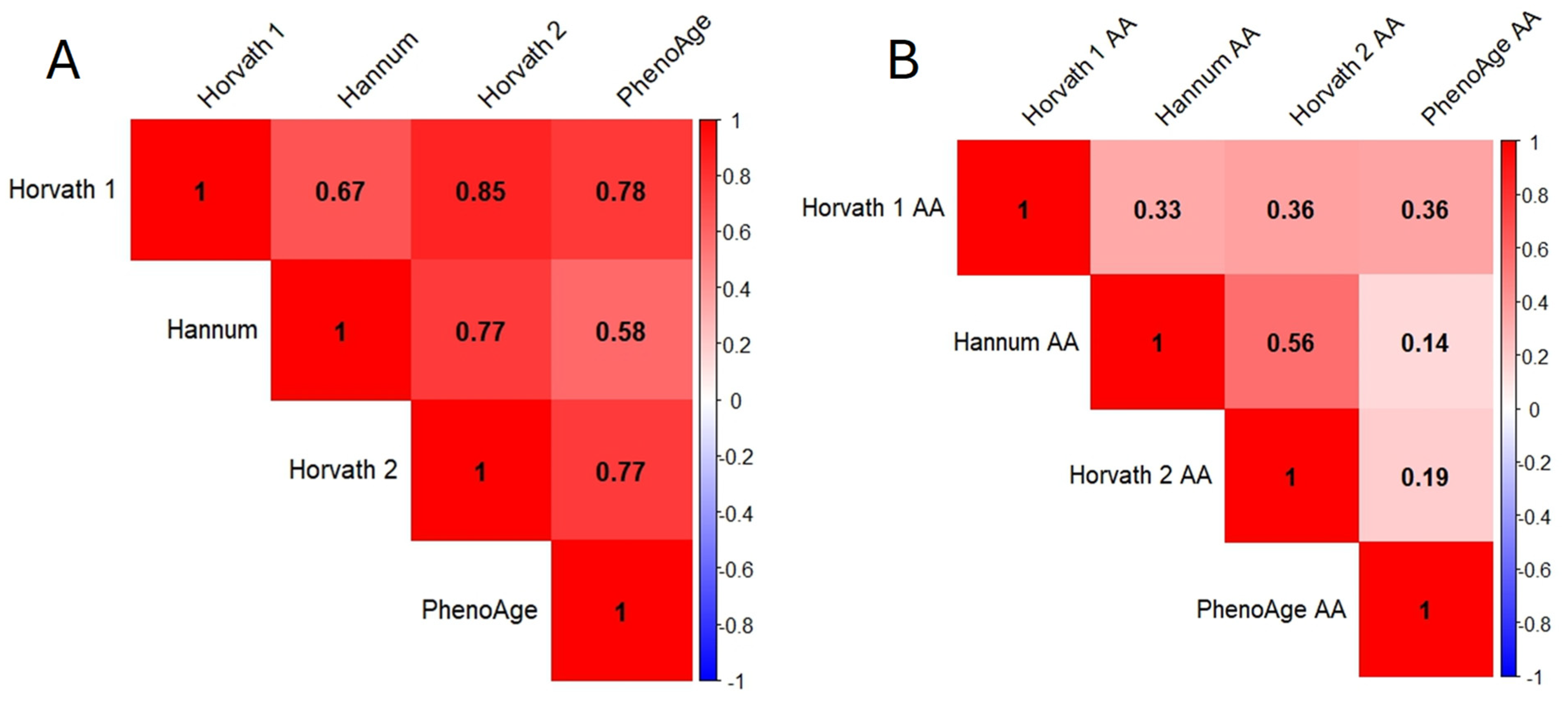
3.4. Correlation between Various Variables and Accelerated Aging (AA) for Different Biological Clocks
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Damle, S.G. Big Tobacco, Big Food, Big Alcohol “Lifestyle Diseases”. Contemp. Clin. Dent. 2018, 9, 327–329. [Google Scholar] [CrossRef] [PubMed]
- LIFESTYLE DISEASES, UNITED NNATION. Available online: https://www.un.org/en/chronicle/article/lifestyle-diseases-economic-burden-health-services (accessed on 17 May 2024).
- Noroozi, R.; Ghafouri-Fard, S.; Pisarek, A.; Rudnicka, J.; Spólnicka, M.; Branicki, W.; Taheri, M.; Pośpiech, E. DNA methylation-based age clocks: From age prediction to age reversion. Ageing Res. Rev. 2021, 68, 101314. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, K.N.; Hodges, R.; Hanes, D.; Stack, E.; Cheishvili, D.; Szyf, M.; Henkel, J.; Twedt, M.W.; Giannopoulou, D.; Herdell, J.; et al. Potential reversal of epigenetic age using a diet and lifestyle intervention: A pilot randomized clinical trial. Aging 2021, 13, 9419–9432. [Google Scholar] [CrossRef] [PubMed]
- Fiorito, G.; Caini, S.; Palli, D.; Bendinelli, B.; Saieva, C.; Ermini, I.; Valentini, V.; Assedi, M.; Rizzolo, P.; Ambrogetti, D.; et al. DNA methylation-based biomarkers of aging were slowed down in a two-year diet and physical activity intervention trial: The DAMA study. Aging Cell 2021, 20, e13439. [Google Scholar] [CrossRef] [PubMed]
- Mitteldorf, J. A Clinical Trial Using Methylation Age to Evaluate Current Antiaging Practices. Rejuvenation Res. 2019, 22, 201–209. [Google Scholar] [CrossRef]
- Moore, L.D.; Le, T.; Fan, G. DNA Methylation and Its Basic Function. Neuropsychopharmacology 2013, 38, 23–38. [Google Scholar] [CrossRef]
- Uysal, F.; Sukur, G.; Cinar, O. DNMT enzymes differentially alter global DNA methylation in a stage-dependent manner during spermatogenesis. Andrologia 2022, 54, e14357. [Google Scholar] [CrossRef]
- Jang, H.; Shin, W.; Lee, J.; Do, J. CpG and Non-CpG Methylation in Epigenetic Gene Regulation and Brain Function. Genes. 2017, 8, 148. [Google Scholar] [CrossRef]
- Nowacka-Zawisza, M.; Wiśnik, E. DNA methylation and histone modifications as epigenetic regulation in prostate cancer. Oncol. Rep. 2017, 38, 2587–2596. [Google Scholar] [CrossRef]
- Morgan, A.E.; Davies, T.J.; Mc Auley, M.T. The role of DNA methylation in ageing and cancer. Proc. Nutr. Soc. 2018, 77, 412–422. [Google Scholar] [CrossRef]
- Klutstein, M.; Nejman, D.; Greenfield, R.; Cedar, H. DNA Methylation in Cancer and Aging. Cancer Res. 2016, 76, 3446–3450. [Google Scholar] [CrossRef] [PubMed]
- Reed, R.G.; Carroll, J.E.; Marsland, A.L.; Manuck, S.B. DNA methylation-based measures of biological aging and cognitive decline over 16-years: Preliminary longitudinal findings in midlife. Aging 2022, 14, 9423–9444. [Google Scholar] [CrossRef] [PubMed]
- Hannum, G.; Guinney, J.; Zhao, L.; Zhang, L.; Hughes, G.; Sadda, S.; Klotzle, B.; Bibikova, M.; Fan, J.B.; Gao, Y.; et al. Genome-wide methylation profiles reveal quantitative views of human aging rates. Mol. Cell 2013, 49, 359–367. [Google Scholar] [CrossRef]
- Marioni, R.E.; Shah, S.; McRae, A.F.; Chen, B.H.; Colicino, E.; Harris, S.E.; Gibson, J.; Henders, A.K.; Redmond, P.; Cox, S.R.; et al. DNA methylation age of blood predicts all-cause mortality in later life. Genome Biol. 2015, 16, 25. [Google Scholar] [CrossRef] [PubMed]
- Horvath, S. DNA methylation age of human tissues and cell types. Genome Biol. 2013, 14, R115. [Google Scholar] [CrossRef] [PubMed]
- Perna, L.; Zhang, Y.; Mons, U.; Holleczek, B.; Saum, K.U.; Brenner, H. Epigenetic age acceleration predicts cancer, cardiovascular, and all-cause mortality in a German case cohort. Clin. Epigenetics 2016, 8, 64. [Google Scholar] [CrossRef] [PubMed]
- Levine, M.E.; Lu, A.T.; Quach, A.; Chen, B.H.; Assimes, T.L.; Bandinelli, S.; Hou, L.; Baccarelli, A.A.; Stewart, J.D.; Li, Y.; et al. An epigenetic biomarker of aging for lifespan and healthspan. Aging 2018, 10, 573–591. [Google Scholar] [CrossRef]
- Dhingra, R.; Nwanaji-Enwerem, J.C.; Samet, M.; Ward-Caviness, C.K. DNA Methylation Age-Environmental Influences, Health Impacts, and Its Role in Environmental Epidemiology. Curr. Environ. Health Rep. 2018, 5, 317–327. [Google Scholar] [CrossRef]
- Zhang, Y.; Wilson, R.; Heiss, J.; Breitling, L.P.; Saum, K.-U.; Schöttker, B.; Holleczek, B.; Waldenberger, M.; Peters, A.; Brenner, H. DNA methylation signatures in peripheral blood strongly predict all-cause mortality. Nat. Commun. 2017, 8, 14617. [Google Scholar] [CrossRef]
- Belsky, D.W.; Caspi, A.; Corcoran, D.L.; Sugden, K.; Poulton, R.; Arseneault, L.; Baccarelli, A.; Chamarti, K.; Gao, X.; Hannon, E.; et al. DunedinPACE, a DNA methylation biomarker of the pace of aging. Elife 2022, 11, e73420. [Google Scholar] [CrossRef]
- Bingham, S.A. Dietary assessments in the European prospective study of diet and cancer (EPIC). Eur. J. Cancer Prev. 1997, 6, 118–124. [Google Scholar]
- Gonzalez-Ramirez, L.P.; De la Roca-Chiapas, J.M.; Colunga-Rodriguez, C.; Preciado-Serrano, M.L.; Daneri-Navarro, A.; Pedroza-Cabrera, F.J.; Martinez-Arriaga, R.J. Validation of Health Behavior and Stages of Change Questionnaire. Breast Cancer 2017, 9, 199–205. [Google Scholar] [CrossRef] [PubMed]
- REDCap. Available online: https://www.hrds.manchester.ac.uk/about/redcap/ (accessed on 23 March 2024).
- Infinium MethylationEPIC v2.0 Kit. Available online: https://emea.illumina.com/products/by-type/microarray-kits/infinium-methylation-epic.html (accessed on 22 March 2024).
- Min, J.L.; Hemani, G.; Davey Smith, G.; Relton, C.; Suderman, M. Meffil: Efficient normalization and analysis of very large DNA methylation datasets. Bioinformatics 2018, 34, 3983–3989. [Google Scholar] [CrossRef] [PubMed]
- meffil. Available online: https://github.com/perishky/meffil (accessed on 24 March 2024).
- methylCIPHER. Available online: https://github.com/MorganLevineLab/methylCIPHER (accessed on 24 March 2024).
- DunedinPACE. Available online: https://github.com/danbelsky/DunedinPACE (accessed on 24 March 2024).
- R Software. Available online: https://www.r-project.org/ (accessed on 13 February 2024).
- Mishra, P.; Pandey, C.M.; Singh, U.; Gupta, A.; Sahu, C.; Keshri, A. Descriptive statistics and normality tests for statistical data. Ann. Card. Anaesth. 2019, 22, 67–72. [Google Scholar] [CrossRef]
- Chervova, O.; Chernysheva, E.; Panteleeva, K.; Widayati, T.A.; Hrbkova, N.; Schneider, J.; Maximov, V.; Ryabikov, A.; Tillmann, T.; Pikhart, H.; et al. Evaluation of Epigenetic Age Acceleration Scores and Their Associations with CVD-Related Phenotypes in a Population Cohort. Biology 2022, 12, 68. [Google Scholar] [CrossRef] [PubMed]
- Gialluisi, A.; Santoro, A.; Tirozzi, A.; Cerletti, C.; Donati, M.B.; de Gaetano, G.; Franceschi, C.; Iacoviello, L. Epidemiological and genetic overlap among biological aging clocks: New challenges in biogerontology. Ageing Res. Rev. 2021, 72, 101502. [Google Scholar] [CrossRef]
- Hazra, A.; Gogtay, N. Biostatistics Series Module 6: Correlation and Linear Regression. Indian. J. Dermatol. 2016, 61, 593–601. [Google Scholar] [CrossRef]
- Bender, R.; Lange, S. Adjusting for multiple testing--when and how? J. Clin. Epidemiol. 2001, 54, 343–349. [Google Scholar] [CrossRef]
- Benjamini, Y.; Hochberg, Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Society. Ser. B 1995, 57, 289–300. [Google Scholar] [CrossRef]
- STATA. Available online: https://www.stata.com/company/ (accessed on 13 February 2024).
- Evidence Review for Dietary Cholesterol Strategies. Available online: https://www.nice.org.uk/guidance/ng238/evidence/b-dietary-cholesterol-strategies-pdf-13253901662 (accessed on 27 March 2024).
- Fat: The Facts. Available online: https://www.nhs.uk/live-well/eat-well/food-types/different-fats-nutrition/#:~:text=Saturated%20fat%20guidelines,of%20saturated%20fat%20a%20day (accessed on 27 March 2024).
- Sugar: The Facts. Available online: https://www.nhs.uk/live-well/eat-well/food-types/how-does-sugar-in-our-diet-affect-our-health/#:~:text=The%20reference%20intake%20for%20total,more%20information%2C%20see%20Food%20labels. (accessed on 27 March 2024).
- Vitamin, D. Available online: https://www.nhs.uk/conditions/vitamins-and-minerals/vitamin-d/ (accessed on 27 March 2024).
- Calorie Counting. Available online: https://www.nhs.uk/better-health/lose-weight/calorie-counting/#:~:text=How%20many%20calories%20to%20eat,2%2C000kcal%20for%20women (accessed on 27 March 2024).
- NHS Alcohol Units. Available online: https://www.nhs.uk/live-well/alcohol-advice/calculating-alcohol-units/ (accessed on 13 February 2024).
- Statistis on Smoking. Available online: https://commonslibrary.parliament.uk/research-briefings/cbp-7648/#:~:text=In%202022%2C%20around%2012.9%25%20of,were%20current%20smokers%20in%202022 (accessed on 30 March 2024).
- Health Survey for England, 2021 Part 1: Drinking Alcohol Alcohol and Health. Available online: https://digital.nhs.uk/data-and-information/publications/statistical/health-survey-for-england/2021/part-3-drinking-alcohol (accessed on 30 March 2024).
- Adults’ Activity Levels in England Bounce Back to Pre-Pandemic Levels. Available online: https://www.sportengland.org/news/adults-activity-levels-england-bounce-back-pre-pandemic-levels (accessed on 30 March 2024).
- NHS:Cholesterol. Available online: https://www.oxfordhealth.nhs.uk/wp-content/uploads/2014/08/OP-026.14-Cholesteral.pdf (accessed on 29 March 2024).
- Crimmins, E.M.; Thyagarajan, B.; Levine, M.E.; Weir, D.R.; Faul, J. Associations of Age, Sex, Race/Ethnicity, and Education with 13 Epigenetic Clocks in a Nationally Representative U.S. Sample: The Health and Retirement Study. J. Gerontol. A Biol. Sci. Med. Sci. 2021, 76, 1117–1123. [Google Scholar] [CrossRef]
- Bell, C.G.; Lowe, R.; Adams, P.D.; Baccarelli, A.A.; Beck, S.; Bell, J.T.; Christensen, B.C.; Gladyshev, V.N.; Heijmans, B.T.; Horvath, S.; et al. DNA methylation aging clocks: Challenges and recommendations. Genome Biol. 2019, 20, 249. [Google Scholar] [CrossRef] [PubMed]
- Kresovich, J.K.; Park, Y.M.; Keller, J.A.; Sandler, D.P.; Taylor, J.A. Healthy eating patterns and epigenetic measures of biological age. Am. J. Clin. Nutr. 2022, 115, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Appel, L.J.; Moore, T.J.; Obarzanek, E.; Vollmer, W.M.; Svetkey, L.P.; Sacks, F.M.; Bray, G.A.; Vogt, T.M.; Cutler, J.A.; Windhauser, M.M.; et al. A clinical trial of the effects of dietary patterns on blood pressure. DASH Collaborative Research Group. New Engl. J. Med. 1997, 336, 1117–1124. [Google Scholar] [CrossRef] [PubMed]
- Krebs-Smith, S.M.; Pannucci, T.E.; Subar, A.F.; Kirkpatrick, S.I.; Lerman, J.L.; Tooze, J.A.; Wilson, M.M.; Reedy, J. Update of the Healthy Eating Index: HEI-2015. J. Acad. Nutr. Diet. 2018, 118, 1591–1602. [Google Scholar] [CrossRef] [PubMed]
- Chiuve, S.E.; Fung, T.T.; Rimm, E.B.; Hu, F.B.; McCullough, M.L.; Wang, M.; Stampfer, M.J.; Willett, W.C. Alternative dietary indices both strongly predict risk of chronic disease. J. Nutr. 2012, 142, 1009–1018. [Google Scholar] [CrossRef]
- Sotos-Prieto, M.; Bhupathiraju, S.N.; Mattei, J.; Fung, T.T.; Li, Y.; Pan, A.; Willett, W.C.; Rimm, E.B.; Hu, F.B. Changes in Diet Quality Scores and Risk of Cardiovascular Disease Among US Men and Women. Circulation 2015, 132, 2212–2219. [Google Scholar] [CrossRef]
- Lu, A.T.; Quach, A.; Wilson, J.G.; Reiner, A.P.; Aviv, A.; Raj, K.; Hou, L.; Baccarelli, A.A.; Li, Y.; Stewart, J.D.; et al. DNA methylation GrimAge strongly predicts lifespan and healthspan. Aging 2019, 11, 303–327. [Google Scholar] [CrossRef]
- Biemans, Y.; Bach, D.; Behrouzi, P.; Horvath, S.; Kramer, C.S.; Liu, S.; Manson, J.E.; Shadyab, A.H.; Stewart, J.; Whitsel, E.A.; et al. Identifying the relation between food groups and biological ageing: A data-driven approach. Age Ageing 2024, 53, ii20–ii29. [Google Scholar] [CrossRef]
- Galkin, F.; Kovalchuk, O.; Koldasbayeva, D.; Zhavoronkov, A.; Bischof, E. Stress, diet, exercise: Common environmental factors and their impact on epigenetic age. Ageing Res. Rev. 2023, 88, 101956. [Google Scholar] [CrossRef]
- Waziry, R.; Ryan, C.P.; Corcoran, D.L.; Huffman, K.M.; Kobor, M.S.; Kothari, M.; Graf, G.H.; Kraus, V.B.; Kraus, W.E.; Lin, D.T.S.; et al. Effect of long-term caloric restriction on DNA methylation measures of biological aging in healthy adults from the CALERIE trial. Nat. Aging 2023, 3, 248–257. [Google Scholar] [CrossRef]
- Gensous, N.; Sala, C.; Pirazzini, C.; Ravaioli, F.; Milazzo, M.; Kwiatkowska, K.M.; Marasco, E.; De Fanti, S.; Giuliani, C.; Pellegrini, C.; et al. A Targeted Epigenetic Clock for the Prediction of Biological Age. Cells 2022, 11, 4044. [Google Scholar] [CrossRef]
- Liu, A.G.; Ford, N.A.; Hu, F.B.; Zelman, K.M.; Mozaffarian, D.; Kris-Etherton, P.M. A healthy approach to dietary fats: Understanding the science and taking action to reduce consumer confusion. Nutr. J. 2017, 16, 53. [Google Scholar] [CrossRef] [PubMed]
- Cena, H.; Calder, P.C. Defining a Healthy Diet: Evidence for The Role of Contemporary Dietary Patterns in Health and Disease. Nutrients 2020, 12, 334. [Google Scholar] [CrossRef] [PubMed]
- Sillanpää, E.; Ollikainen, M.; Kaprio, J.; Wang, X.; Leskinen, T.; Kujala, U.M.; Törmäkangas, T. Leisure-time physical activity and DNA methylation age-a twin study. Clin. Epigenetics 2019, 11, 12. [Google Scholar] [CrossRef]
- Marioni, R.E.; Shah, S.; McRae, A.F.; Ritchie, S.J.; Muniz-Terrera, G.; Harris, S.E.; Gibson, J.; Redmond, P.; Cox, S.R.; Pattie, A.; et al. The epigenetic clock is correlated with physical and cognitive fitness in the Lothian Birth Cohort 1936. Int. J. Epidemiol. 2015, 44, 1388–1396. [Google Scholar] [CrossRef] [PubMed]
- Rönn, T.; Volkov, P.; Davegårdh, C.; Dayeh, T.; Hall, E.; Olsson, A.H.; Nilsson, E.; Tornberg, A.; Dekker Nitert, M.; Eriksson, K.F.; et al. A six months exercise intervention influences the genome-wide DNA methylation pattern in human adipose tissue. PLoS Genet. 2013, 9, e1003572. [Google Scholar] [CrossRef] [PubMed]
- Sailani, M.R.; Halling, J.F.; Møller, H.D.; Lee, H.; Plomgaard, P.; Pilegaard, H.; Snyder, M.P.; Regenberg, B. Lifelong physical activity is associated with promoter hypomethylation of genes involved in metabolism, myogenesis, contractile properties and oxidative stress resistance in aged human skeletal muscle. Sci. Rep. 2019, 9, 3272. [Google Scholar] [CrossRef]
- Horvath, S.; Erhart, W.; Brosch, M.; Ammerpohl, O.; von Schönfels, W.; Ahrens, M.; Heits, N.; Bell, J.T.; Tsai, P.C.; Spector, T.D.; et al. Obesity accelerates epigenetic aging of human liver. Proc. Natl. Acad. Sci. USA 2014, 111, 15538–15543. [Google Scholar] [CrossRef]
- De Toro-Martín, J.; Guénard, F.; Tchernof, A.; Hould, F.S.; Lebel, S.; Julien, F.; Marceau, S.; Vohl, M.C. Body mass index is associated with epigenetic age acceleration in the visceral adipose tissue of subjects with severe obesity. Clin. Epigenetics 2019, 11, 172. [Google Scholar] [CrossRef]
- Ambatipudi, S.; Cuenin, C.; Hernandez-Vargas, H.; Ghantous, A.; Le Calvez-Kelm, F.; Kaaks, R.; Barrdahl, M.; Boeing, H.; Aleksandrova, K.; Trichopoulou, A.; et al. Tobacco smoking-associated genome-wide DNA methylation changes in the EPIC study. Epigenomics 2016, 8, 599–618. [Google Scholar] [CrossRef]
- Joehanes, R.; Just, A.C.; Marioni, R.E.; Pilling, L.C.; Reynolds, L.M.; Mandaviya, P.R.; Guan, W.; Xu, T.; Elks, C.E.; Aslibekyan, S.; et al. Epigenetic Signatures of Cigarette Smoking. Circ. Cardiovasc. Genet. 2016, 9, 436–447. [Google Scholar] [CrossRef]
- McCartney, D.L.; Stevenson, A.J.; Hillary, R.F.; Walker, R.M.; Bermingham, M.L.; Morris, S.W.; Clarke, T.K.; Campbell, A.; Murray, A.D.; Whalley, H.C.; et al. Epigenetic signatures of starting and stopping smoking. EBioMedicine 2018, 37, 214–220. [Google Scholar] [CrossRef]
- Christiansen, C.; Castillo-Fernandez, J.E.; Domingo-Relloso, A.; Zhao, W.; El-Sayed Moustafa, J.S.; Tsai, P.C.; Maddock, J.; Haack, K.; Cole, S.A.; Kardia, S.L.R.; et al. Novel DNA methylation signatures of tobacco smoking with trans-ethnic effects. Clin. Epigenetics 2021, 13, 36. [Google Scholar] [CrossRef] [PubMed]
- Klopack, E.T.; Carroll, J.E.; Cole, S.W.; Seeman, T.E.; Crimmins, E.M. Lifetime exposure to smoking, epigenetic aging, and morbidity and mortality in older adults. Clin. Epigenetics 2022, 14, 72. [Google Scholar] [CrossRef]
- Nannini, D.R.; Joyce, B.T.; Zheng, Y.; Gao, T.; Wang, J.; Liu, L.; Jacobs, D.R.; Schreiner, P.J.; Liu, C.; Dai, Q.; et al. Alcohol consumption and epigenetic age acceleration in young adults. Aging 2023, 15, 371–395. [Google Scholar] [CrossRef] [PubMed]
- Peila, R.; Coday, M.; Crane, T.E.; Saquib, N.; Shadyab, A.H.; Tabung, F.K.; Zhang, X.; Wactawski-Wende, J.; Rohan, T.E. Healthy lifestyle index and risk of pancreatic cancer in the Women’s Health Initiative. Cancer Causes Control 2022, 33, 737–747. [Google Scholar] [CrossRef] [PubMed]
- Naudin, S.; Viallon, V.; Hashim, D.; Freisling, H.; Jenab, M.; Weiderpass, E.; Perrier, F.; McKenzie, F.; Bueno-de-Mesquita, H.B.; Olsen, A.; et al. Healthy lifestyle and the risk of pancreatic cancer in the EPIC study. Eur. J. Epidemiol. 2020, 35, 975–986. [Google Scholar] [CrossRef]
- Chudasama, Y.V.; Khunti, K.; Gillies, C.L.; Dhalwani, N.N.; Davies, M.J.; Yates, T.; Zaccardi, F. Healthy lifestyle and life expectancy in people with multimorbidity in the UK Biobank: A longitudinal cohort study. PLoS Med. 2020, 17, e1003332. [Google Scholar] [CrossRef]
- Luu, H.N.; Paragomi, P.; Wang, R.; Jin, A.; Brand, R.E.; Koh, W.P.; Yuan, J.M. Composite Score of Healthy Lifestyle Factors and the Risk of Pancreatic Cancer in a Prospective Cohort Study. Cancer Prev. Res. 2022, 15, 29–36. [Google Scholar] [CrossRef]
- Bian, Z.; Zhang, R.; Yuan, S.; Fan, R.; Wang, L.; Larsson, S.C.; Theodoratou, E.; Zhu, Y.; Wu, S.; Ding, Y.; et al. Healthy lifestyle and cancer survival: A multinational cohort study. Int. J. Cancer 2024, 154, 1709–1718. [Google Scholar] [CrossRef]
- Fitzgerald, K.N.; Campbell, T.; Makarem, S.; Hodges, R. Potential reversal of biological age in women following an 8-week methylation-supportive diet and lifestyle program: A case series. Aging 2023, 15, 1833–1839. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Chen, X.; Gill, T.M.; Ma, C.; Crimmins, E.M.; Levine, M.E. Associations of genetics, behaviors, and life course circumstances with a novel aging and healthspan measure: Evidence from the Health and Retirement Study. PLoS Med. 2019, 16, e1002827. [Google Scholar] [CrossRef] [PubMed]
- Qiu, W.; Chen, H.; Kaeberlein, M.; Lee, S.I. ExplaiNAble BioLogical Age (ENABL Age): An artificial intelligence framework for interpretable biological age. Lancet Healthy Longev. 2023, 4, e711–e723. [Google Scholar] [CrossRef] [PubMed]
- Body Mass Index from NHS. Available online: https://www.nhs.uk/common-health-questions/lifestyle/what-is-the-body-mass-index-bmi/. (accessed on 13 February 2024).
- Soliman, G.A. Dietary Cholesterol and the Lack of Evidence in Cardiovascular Disease. Nutrients 2018, 10, 780. [Google Scholar] [CrossRef]
- Dietary Guidelines for Americans 2020–2025. Available online: https://www.dietaryguidelines.gov/sites/default/files/2021-03/Dietary_Guidelines_for_Americans-2020-2025.pdf (accessed on 30 March 2024).
- Rees, K.; Takeda, A.; Martin, N.; Ellis, L.; Wijesekara, D.; Vepa, A.; Das, A.; Hartley, L.; Stranges, S. Mediterranean-style diet for the primary and secondary prevention of cardiovascular disease. Cochrane Database Syst. Rev. 2019, 2019, CD009825. [Google Scholar] [CrossRef] [PubMed]
| Variables | Number of Participants (n = 221) |
|---|---|
| Recruitment | |
| Age | 61.55 ± 8.27 (43–79) # |
| Age group | |
| 40–49 | 17 (7.69%) |
| 50–59 | 77 (34.84%) |
| 60–69 | 79 (35.75%) |
| 70–79 | 48 (21.72%) |
| Gender | |
| Male | 221 (100%) |
| Consent to join the Lifestyle Pattern and Epigenetic Biomarker Study | 214 (96.83%) |
| Food Frequency Questionnaire fill rate | 154 (69.68%) |
| Lifestyle Pattern Questionnaire fill rate | 147 (66.51%) |
| Horvath 1 AA | Hannum AA | Horvath 2 AA | PhenoAge AA | DunedinPACE | |
|---|---|---|---|---|---|
| BMI (con) | 0.067 | 0.012 | −0.138 | 0.120 | 0.371 * |
| Smoking (cat) | −0.023 | −0.234 * | −0.169 | 0.076 | 0.106 |
| Alcohol (cat) | 0.046 | 0.027 | 0.113 | 0.111 | 0.034 |
| Physical activity (cat) | −0.050 | −0.075 | 0.030 | −0.107 | 0.011 |
| Cholesterol (con) | 0.183 | 0.091 | 0.140 | 0.161 | −0.027 |
| Saturated fat (con) | 0.241 * | 0.064 | 0.199 * | 0.156 | 0.008 |
| Sugar (con) | 0.118 | −0.075 | 0.086 | 0.112 | −0.001 |
| VitD (con) | −0.072 | −0.117 | 0.008 | −0.081 | −0.199 * |
| Ekal (con) | 0.221 * | −0.011 | 0.178 | 0.167 | 0.013 |
| Horvath 1 AA | Hannum AA | Horvath 2 AA | PhenoAge AA | DunedinPACE | |
|---|---|---|---|---|---|
| Healthy lifestyle index (HLI) | −0.108 | 0.106 | 0.054 | −0.278 * | −0.261 * |
| Coefficients | SD | p-Value | 95% CI Lower | 95% CI Upper | |
|---|---|---|---|---|---|
| PhenoAge AA | −0.780 | 0.224 | 0.001 | −1.222 | −0.337 |
| DunedinPACE | −0.010 | 0.004 | 0.039 | −0.019 | −0.0005 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ke, T.-M.; Lophatananon, A.; Muir, K.R. Exploring the Relationships between Lifestyle Patterns and Epigenetic Biological Age Measures in Men. Biomedicines 2024, 12, 1985. https://doi.org/10.3390/biomedicines12091985
Ke T-M, Lophatananon A, Muir KR. Exploring the Relationships between Lifestyle Patterns and Epigenetic Biological Age Measures in Men. Biomedicines. 2024; 12(9):1985. https://doi.org/10.3390/biomedicines12091985
Chicago/Turabian StyleKe, Te-Min, Artitaya Lophatananon, and Kenneth R. Muir. 2024. "Exploring the Relationships between Lifestyle Patterns and Epigenetic Biological Age Measures in Men" Biomedicines 12, no. 9: 1985. https://doi.org/10.3390/biomedicines12091985
APA StyleKe, T.-M., Lophatananon, A., & Muir, K. R. (2024). Exploring the Relationships between Lifestyle Patterns and Epigenetic Biological Age Measures in Men. Biomedicines, 12(9), 1985. https://doi.org/10.3390/biomedicines12091985





